Abstract
Study Design:
Retrospective database review.
Objectives:
After the Food and Drug Administration approved bone morphogenetic protein–2 (BMP) in 2002, BMP was used off-label in the cervical spine to increase bone growth and bony fusion. Since then, concerns have been raised regarding complication rates and safety. This study was conducted to examine the use of BMP in anterior cervical discectomy and fusion (ACDF) in the Medicare population and to determine risk of complications and associated costs within 90 days of surgery.
Methods:
Patients who underwent ACDF were identified using Current Procedural Terminology (CPT) and International Classification of Diseases, Ninth Revision Procedure codes (ICD9-P). Complications were identified using ICD9 diagnostic codes. Charges were calculated as amount billed, and reimbursements were calculated as amounts paid by Medicare. Data for these analyses came from a nationwide claims database.
Results:
A total of 215 047 patients were identified who had ACDF from 2005 to 2011. For the majority of the procedures (89.0%), BMP was not used. BMP use rose from 11.84% in 2005 to a peak of 16.73% in 2007 before decreasing to 12.01% in 2011. BMP was used 16% more in women than men. BMP use was the highest in the West (13.6%) followed by Midwest (11.8%), South (10.6%), and Northeast (7.5%). There was a higher overall complication rate in the BMP group (2.1%) compared with the non-BMP group (1.9%) (odds ratio [OR] = 1.11, 95% CI = 1.01-1.22). The BMP group also had a higher rate of wound complications (0.98% vs 0.76%, OR = 1.29, 95% CI = 1.12-1.48). In this study population, there was no difference in dysphagia/hoarseness, neurologic, medical, or other complications. During the 90-day perioperative period, BMP surgeries were charged at 17.6% higher than non-BMP surgeries.
Conclusions:
The use of BMP in ACDF in the Medicare population has decreased since a peak in 2007. The rate of wound and overall complications for BMP use with ACDF was higher than without. Our results regarding dysphagia/hoarseness did not show a statistically meaningful difference, which is in contrast with many other studies. Charges associated with BMP use were higher during the 90-day perioperative period.
Keywords: anterior cervical discectomy and fusion, bone morphogenetic protein, trends
Introduction
Back and neck pain are significant contributors to morbidity and health care costs in the United States.1,2 Many patients fail the first-line treatment of non-surgical interventions and proceed to surgical treatment. Cervical arthrodesis has evolved as a treatment for neck pain caused by disc disease and herniated discs that result in bony fusion of spinal segments. Cervical arthrodesis has been associated with a complication rate of about 3.9%, increasing with patient age.3 These complications include infections, swallowing problems and dysphagia, neurological problems, and failure of bony fusion, which can lead to pain, instability, and require revision surgery.
Bone morphogenetic protein–2 (BMP) was approved by the US Food and Drug Administration (FDA) in 2002 to promote fusion in anterior lumbar surgery.4 The use of BMP has increased from 2002 to 2011 with off-label applications accounting for the majority of use.5 One of these off-label uses is in the cervical spine to increase bone growth and bony fusion while decreasing risk of pseudarthrosis and nonunion.6 Over time, concerns have been raised regarding complication rates and safety, including a public health notification from the US FDA in 2008.7-9
This study was conducted to examine the use of BMP in anterior cervical discectomy and fusion (ACDF) in the Medicare population and to determine risk of complications and associated costs within 90 days of surgery. We hypothesized that the use of BMP would increase complication rates and increase costs associated with care. As has previously been documented, we hypothesized that safety concerns regarding use of BMP would have a dramatic effect on its use for ACDF. We attempted to quantify the impact of these concerns on clinical utilization.
Methods
Records for patients who underwent ACDF were collected using the PearlDiver Patient Record Database (PearlDiver Technologies, Warsaw, IN). This is a publicly available national database of Medicare insurance records. Patients were identified by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. We performed a retrospective review over 48 million patients from January 1, 2005 through December 31, 2011. Region was defined as Midwest, Northeast, South, and West (Table 1). Charges were calculated as amount billed by the institution for each patient for care surrounding the index procedure. Our institutional review board deemed this study exempt from review, as all patient information was deidentified. Incidence was calculated as procedures per 100 000 members. P values less than .05 were considered significant. Patient data was completely deidentified therefore did not require institutional review board approval.
Table 1.
Regional Breakdown of States.
| Region | States |
|---|---|
| Midwest | IA, KS, MN, MO, NE, IL, IN, MI, WI, OH, NO, SD |
| Northeast | CT, MA, ME, NH, NJ, PA, RI, NY, VT |
| South | AL, AR, DC, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV, PR |
| West | AK, AZ, CA, CO, ID, MT, NM, NV, OR, UT, WA, WY, HI |
Patients were eligible if they had ACDF from January 1, 2005 to October 2, 2011. Patients who underwent primary ACDF were identified by use of ICD-9 code for arthrodesis of C2 level or below: anterior (interbody) technique anterolateral technique (ICD-9 81.02). Use of BMP2 was identified by ICD-9 code 84.52.
Complications were identified using ICD-9 and CPT codes for each patient 90 days following their index procedure, from January 1, 2005 to December 31, 2011. The following complications were identified: dysphagia/hoarseness (478.30, 478.31, 478.32, 478.33, 478.34, 784.4, and 787.2), nervous system complications (997.0, 997.00, 997.01, 997.09), wound complications (998.1, 998.11, 998.12, 998.13, 998.3, 998.31,998.32, 998.83, 998.5, 999.3, 998.51, 998.59, 998.83, and 999.3), medical complications (997.1-997.3, 410.0-410.9, 415.1, 998.0), and other complications (998.81, 998.89, 998.9, 999.9).
Unadjusted relative risk (RR) and 95% confidence intervals (CIs) were used to determine patient characteristics and complications from BMP use. Student’s t tests and chi-square tests were used for cost comparisons. P values less than .05 were considered significant.
Results
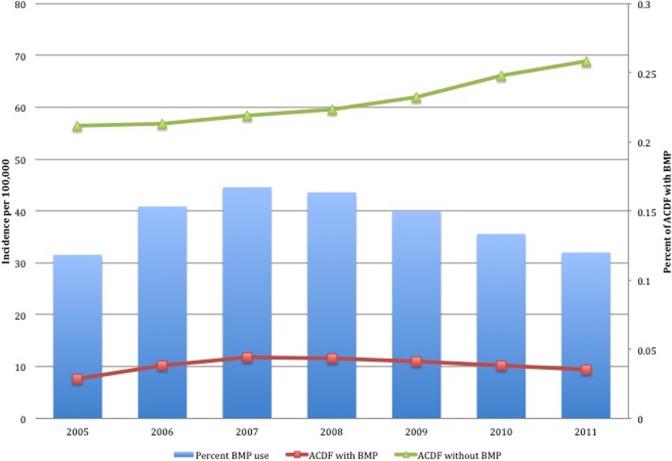
A total of 215 047 patients were identified who underwent primary ACDF. For the majority of the procedures (89.0%, n = 191 421), BMP was not used. BMP use rose from 11.84% (n = 3222) of all ACDFs within 2005 to a peak of 16.73% (n = 5198) in 2007 before decreasing to 12.01% (n = 4595) in 2011 (Figure 1). The number of ACDF with BMP also rose from 3222 to 4595 over the same time period, but incidence of ACDF with BMP paralleled percent use; it increased from 7.58 in 2005 with a peak in 2007 with 11.74 before decreasing to 9.41 in 2011. The number of total cases of ACDF without BMP increased steadily throughout, from 23 996 in 2005 to 33 677 in 2011. Incidence of ACDF without BMP also increased from 56.46 in 2005 to 68.94 in 2011.
Figure 1.
Incidence of anterior cervical discectomy and fusion (ACDF) with and without use of bone morphogenetic protein (BMP) from 2005 to 2011.
There were differences among BMP use according to sex, age, and region (Table 2). Use of BMP was highest in the 70- to 74-year age group compared with <65-year age group (RR = 1.06, 95% CI = 1.02-1.09). BMP use was least in the >84-year age group (RR = 0.81, 95% CI = 0.73-0.90) followed by the 80- to 84-year old age group (RR = 0.90, 95% CI = 0.85-0.96). Women were more likely to receive BMP than men (RR = 1.16, 95% CI = 1.13-1.19). BMP use was highest in the West compared with Midwest (RR = 1.15, 95% CI = 1.11-1.19) and lowest in the Northeast compared with Midwest (RR = 0.63, 95% CI = 0.60-0.66).
Table 2.
| Patient Characteristics.Characteristics | No BMP (n = 191 421), n (%) | BMP (n = 23 626), n (%) | Relative Risk of BMP Use (95% CI) |
|---|---|---|---|
| Age group, years | |||
| <65 | 73 639 (38.5) | 9862 (41.7) | Reference |
| 65-69 | 50 617 (26.4) | 6604 (28.0) | 0.98 (0.95-1.01) |
| 70-74 | 33 160 (17.3) | 4734 (20.0) | 1.06 (1.02-1.09) |
| 75-79 | 19 891 (10.4) | 2691 (11.4) | 1.01 (0.97-1.05) |
| 80-84 | 9035 (4.7) | 1075 (4.6) | 0.90 (0.85-0.96) |
| >84 | 3301 (1.7) | 349 (1.5) | 0.81 (0.73-0.90) |
| Sex | |||
| Male | 90 038 (47.0) | 10 160 (43.0) | Reference |
| Female | 98 784 (51.6) | 13 191 (55.8) | 1.16 (1.13-1.19) |
| Region | |||
| Midwest | 40 909 (21.4) | 5480 (23.2) | Reference |
| Northeast | 20 905 (10.9) | 1692 (7.2) | 0.63 (0.60-0.66) |
| South | 98 119 (51.3) | 11 654 (49.3) | 0.90 (0.88-0.93) |
| West | 31 557 (16.5) | 4971 (21.0) | 1.15 (1.11-1.19) |
Abbreviation: BMP, bone morphogenetic protein.
There was an 11% higher overall complication rate in the BMP group (2.1%) compared with the non-BMP group (1.9%) (odds ratio [OR] = 1.11, 95% CI = 1.01-1.22). The BMP group also had a higher rate of wound complications (0.98% vs 0.76%, OR = 1.29, 95% CI = 1.12-1.48). In this study population, there was no difference in dysphagia/hoarseness, neurologic, medical, or other complications (Table 3).
Table 3.
Complications With and Without BMP in ACDF Within 90 Days.
| Complication | No BMP (n = 191 421), n (%) | BMP (n = 23 626), n (%) | Odds Ratio (95% CI) |
|---|---|---|---|
| Any | 3650 (1.9) | 497 (2.1) | 1.11 (1.01-1.22) |
| Dysphagia or hoarseness | 986 (0.52) | 127 (0.54) | 1.04 (0.87-1.26) |
| Wound | 1461 (0.76) | 232 (0.98) | 1.29 (1.12-1.48) |
| NS | 98 (0.05) | 11 (0.05) | 0.91 (0.49-1.70) |
| Medical | 794 (0.41) | 85 (0.36) | 0.87 (0.69-1.08) |
| Other | 311 (0.16) | 42 (0.18) | 1.09 (0.79-1.51) |
Abbreviations: ACDF, anterior cervical discectomy and fusion; BMP, bone morphogenetic protein; NS, neurologic symptoms.
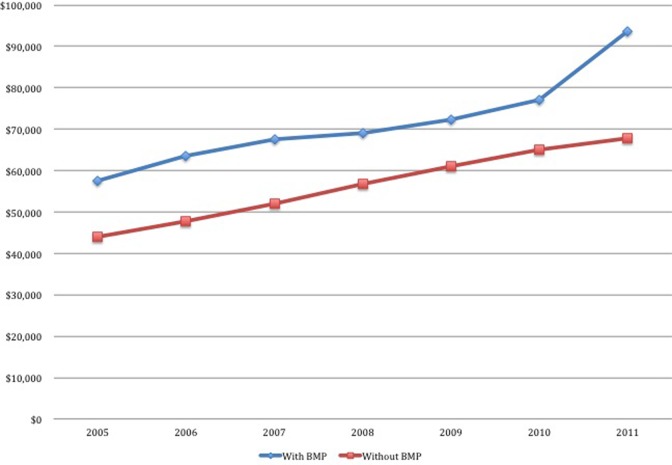
Averages charges for ACDF with BMP ($61 838) were significantly higher than those without BMP ($57 245) (P < .0001) (Table 4). There were significant differences in costs for the BMP and non-BMP in every demographic subgroup with the exception of patients aged older than 84 years. ACDF charges were significantly higher for males compared with females without and with BMP use ($62 302 and $73 913 compared with $52 811 and $60 552, P < .001). Charges for both groups increased over time (Figure 2). In 2005, ACDF without BMP averaged $43 927 and with BMP $57 927 for a difference of $13 528. By 2011, ACDF without BMP was $67 690 and with $93 532 for a difference of $25 842. The differences remained significant throughout P < .0001.
Table 4.
Charges for ACDF With and Without BMP.
| Without BMP | With BMP | ||||||
|---|---|---|---|---|---|---|---|
| Mean ($) | SD ($) | n | Mean ($) | SD ($) | n | P | |
| Total | 57 245 | 72 034 | 191 421 | 61 838 | 58 914 | 23 626 | <.0001 |
| Age group, years | |||||||
| <65 | 52 645 | 70 197 | 73 639 | 67 461 | 64 871 | 9862 | <.0001 |
| 65-69 | 55 830 | 69 134 | 50 617 | 72 564 | 63 726 | 6604 | <.0001 |
| 70-74 | 58 095 | 64 640 | 33 160 | 74 797 | 62 846 | 4734 | <.0001 |
| 75-79 | 63 990 | 75 935 | 19 891 | 78 232 | 77 031 | 2691 | <.0001 |
| 80-84 | 74 319 | 92 678 | 9035 | 85 107 | 81 253 | 1075 | .0003 |
| >84 | 93 625 | 119 054 | 3301 | 89 370 | 75 319 | 349 | .5132 |
| Region | |||||||
| Midwest | 48 236 | 48 380 | 40 909 | 64 706 | 50 672 | 5480 | <.0001 |
| Northeast | 59 822 | 95 313 | 20 905 | 64 808 | 67 473 | 1692 | .0349 |
| South | 52 571 | 55 030 | 98 119 | 65 481 | 53 341 | 11 654 | <.0001 |
| West | 81 827 | 28 414 | 31 557 | 98 100 | 95 598 | 4971 | <.0001 |
| Sex | |||||||
| Female | 52 811 | 57 489 | 98 784 | 70 709 | 60 552 | 13 191 | <.0001 |
| Male | 62 302 | 85 370 | 90 038 | 73 913 | 73 803 | 10 160 | <.0001 |
Abbreviations: ACDF, anterior cervical discectomy and fusion; BMP, bone morphogenetic protein.
Figure 2.
Charges for anterior cervical discectomy and fusion (ACDF) over time.
Discussion
This data shows that the rate of use of BMP in ACDF in the Medicare population increased from 2005 to 2007 and then decreased thereafter. By 2011, BMP was being used in just over 12% of ACDF’s in this population. This trend temporally matches the US FDA Public Health Notification indicating “life-threatening complications associated with BMP in cervical spine fusion,” which was released in 2008.9 This announcement, in addition to a growing body of literature warning against potential adverse effects of BMP, including radiculitis, soft-tissue swelling, dysphasia, heterotopic ossification, hematoma, seroma, and cancer may have led to this decrease in utilization.3,6,7,10 This decrease in BMP use is consistent with other trends of physician use following the US FDA advisory.11 Still, utilization of 12% four years after the announcement is potentially concerning given the known serious adverse effects. Further study as to what the utilization and complication rate today are warranted.
We found that BMP utilization in ACDF to be highest in the Western region followed by Midwest, South, and Northeast. Overall use was lowest in the Northeast. Lao et al12 found similar results; that BMP use in single level anterior interbody fusion was highest in the West and lowest in the Northeast. Singh et al5 reported that overall BMP use in all spine surgery was highest in the South and lowest in the Northeast. Use of BMP was highest in the 70- to 74-year-old age group and least in the >84-year-old group. The lower use in the older age group may be due to the fact that these patients likely have lower life expectancy compared with younger patients; therefore, lifetime risk of pseudarthrosis, which BMP would help prevent, is decreased. It is unclear why the 70- to 74-year-old patients would have the highest rate of BMP use. Women were more likely to receive BMP, as has been found in other studies.7 This may be because of women, especially elderly women, having lower bone density than men,13,14 which creates greater concern for pseudarthrosis.
The rate of overall complications for ACDF was higher with BMP than without, consistent with many other studies.8,15 Our data indicated that wound complications occurred at a higher rate for patients treated with BMP than without (0.98% vs 0.76%). It is unclear whether this is due to BMP itself or selection bias of patients who had BMP used on them. Patients with risk factors suggestive of poor healing may be more likely to receive BMP. Our data regarding dysphagia/hoarseness did not show a statistically meaningful difference. Studies on this topic have had conflicting results. Lu et al16 demonstrated that use of BMP2 increases severity of dysphasia while not affecting overall incidence of dysphasia. Singh et al17 concluded a systematic review of the literature in 2014 and concluded that that rates of dysphagia were not affected by BMP. Several other studies have found a higher dysphasia rate with use of BMP.18,19
In 2011, Carragee et al18 published the under reporting of adverse events related to BMP use in clinical trials, which had been underreported. Our data contributes to the growing body of literature that use of BMP contributes to perioperative morbidity and suggest that use of BMP is decreasing.
The differences in costs are not fully explained by the higher cost of BMP as the magnitude of the difference is much larger than the cost of BMP. Therefore, other factors such as increased complication rate probably contribute to the difference in cost. It is possible surgeons chose to used BMP in patients in with higher risk of complications. Our data shows that the >84-year-old age group, whom presumably would be a higher risk group for medical comorbidities did not have a significant cost differences. This is an area for further study. While charges increased for both groups over time, the difference between the 2 groups increased from $13 528 to $25 842, almost doubling, for reasons that are unclear.
There are several limitations to this study. The study is retrospective and based on medical coding, therefore subject to billing and coding errors. In addition, although it encompasses a large database, the Medicare population is not necessarily representative of the population at large. The <65-year age group covered by Medicare is a special population with end-stage renal disease or severe disability, therefore may be predisposed to risks compared with the >65-year group Medicare population who qualify for coverage based on age alone.20 Our complication outcomes were not risk adjusted, therefore we were not able to identify whether patients who had BMP used were at inherently higher risk of complications.
Appendix
Complications by International Classification of Diseases, Ninth Revision (ICD-9) Code
| Dysphagia, vocal cord paralysis | |
| 478.30-34 784.4 787.2 |
Paralysis of vocal cords or larynx Voice and resonance disorder Dysphagia |
| Nerve system complications | |
| 997.0 997.00 997.01 997.09 |
Nervous system complication Nervous system complication, unspecified Central nervous system complication Other nervous system complication |
| Wound complication | |
| 998.1 998.11 998.12 998.13 998.3 998.31 998.32 998.5 998.51 998.59 998.83 999.3 |
Hemorrhage or hematoma or seroma complicating a procedure Hemorrhage complicating a procedure Hematoma Seroma Disruption Disruption of internal surgical wound Disruption of external operation wound Postoperative infection Infected postoperative seroma Other postoperative infection Nonhealing surgical wound Other infection |
| Medical complications | |
| 997.1 997.2 997.3 410.0-410.9 415.1 998.0 |
Cardiac complication Peripheral vascular complication Respiratory complication Myocardial infarction Pulmonary embolism and infarction Postoperative shock |
| Other complications | |
| 998.8 998.89 998.9 999.9 |
Other specified complication of procedure, not elsewhere classified Other specified complication Unspecified complication of procedure, not elsewhere classified Other and unspecified complication of medical care, not elsewhere classified |
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The financial activities of the authors are as follows: S. Tim Yoon—Bioment, Stryker, Nuvasive, Medyssey, Meditech, Phygen, Alphatec, Medtronic; Jim A. Yousseff—Nuvasive, Integra, Amedica, HealthTrust, Osprey Biomedical, Vertiflex, Benvenue, Paradigm Spine, Promethean Surgical, ISD, Spinicity, Spinal Ventures, Providence Medical, Globus Medical; Darrel S. Brodke—Amedica, Depuy Synthes, Medtronic; Jeffrey C. Wang—Fziomed, Alphatech, Promethean Spine, Paradigm Spine, Benevenue, NexGen, Amedica, Vertiflex, Electrocore, Surgitech, VG Innovations, Corespine, Expanding Orthopaedics, Osprey, Bone Biologics, Curative Biosciences, Pearldiver, Stryker, Osprey, Aesculap, Biomet, Amedica, Seaspine, Synthes, North American Spine Society, North American Spine Foundation, Cervical Spine Research Society, AO Spine/AO Foundation, Collaborative Spine Research Foundation.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding provided by AOSpine to Dr. Jeffrey C. Wang.
References
- 1. Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. [DOI] [PubMed] [Google Scholar]
- 2. Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang MC, Chan L, Maiman DJ, Kreuter W, Deyo RA. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine (Phila Pa 1976). 2007;32:342–347. [DOI] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration. InFUSE Bone Graft/LT-CAGE lumbar tapered fusion device. 2009. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm083423.htm.pdf. Accessed 2016.
- 5. Singh K, Nandyala SV, Marquez-Lara A, Fineberg SJ. Epidemiological trends in the utilization of bone morphogenetic protein in spinal fusions from 2002 to 2011. Spine (Phila Pa 1976). 2014;39:491–496. [DOI] [PubMed] [Google Scholar]
- 6. Burkus JK, Sandhu HS, Gornet MF, Longley MC. Use of rhBMP-2 in combination with structural cortical allografts: clinical and radiographic outcomes in anterior lumbar spinal surgery. J Bone Joint Surg Am. 2005;87:1205–1212. [DOI] [PubMed] [Google Scholar]
- 7. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58–66. [DOI] [PubMed] [Google Scholar]
- 8. Goode AP, Richardson WJ, Schectman RM, Carey TS. Complications, revision fusions, readmissions, and utilization over a 1-year period after bone morphogenetic protein use during primary cervical spine fusions. Spine J. 2014;14:2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. US Food and Drug Administration. Life-threatening complications associated with recombinant human bone morphogenetic protein in cervical spine fusion. 2008. http://www.fda.gov/cdrh/safety/070108-BMP.html. Accessed 2016.
- 10. Benglis D, Wang MY, Levi AD. A comprehensive review of the safety profile of bone morphogenetic protein in spine surgery. Neurosurgery. 2008;62(5 suppl 2):ONS423–ONS431. [DOI] [PubMed] [Google Scholar]
- 11. Mckie J, Qureshi S, Iatridis J, Egorova N, Cho S, Hecht A. Trends in bone morphogenetic protein usage since the U.S. Food and Drug Administration advisory in 2008: what happens to physician practices when the Food and Drug Administration issues an advisory? Global Spine J. 2014;4:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lao L, Cohen JR, Lord EL, Buser Z, Wang JC. Trends analysis of rhBMP utilization in single-level posterior lumbar fusion (PLF) in the United States. Eur Spine J. 2016;25:783–788. [DOI] [PubMed] [Google Scholar]
- 13. Dvorak MF, Pitzen T, Zhu Q, Gordon JD, Fisher CG, Oxland TR. Anterior cervical plate fixation: a biomechanical study to evaluate the effects of plate design, endplate preparation, and bone mineral density. Spine (Phila Pa 1976). 2005;30:294–301. [DOI] [PubMed] [Google Scholar]
- 14. Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9:182. [DOI] [PubMed] [Google Scholar]
- 15. Epstein NE. Complications due to the use of BMP/INFUSE in spine surgery: the evidence continues to mount. Surg Neurol Int. 2013;4(suppl 5):S343–S352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu DC, Tumialan LM, Chou D. Multilevel anterior cervical discectomy and fusion with and without BMP-2: a comparison of dysphagia rates and outcomes in 150 patients. J Neurosurg Spine. 2013;18:43–49. [DOI] [PubMed] [Google Scholar]
- 17. Singh K, Ahmadinia K, Park DK, et al. Complications of spinal fusion with utilization of bone morphogenetic protein: a systematic review of the literature. Spine (Phila Pa 1976). 2014;39:91–101. [DOI] [PubMed] [Google Scholar]
- 18. Carragee EJ, Hurwitz El, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. [DOI] [PubMed] [Google Scholar]
- 19. Singh K, Marquez-Lara A, Nandyala SV, Patel AA, Fineberg SJ. Incidence and risk factors for dysphagia after anterior cervical fusion. Spine (Phila Pa 1976). 2013;38:1820–1825. [DOI] [PubMed] [Google Scholar]
- 20. Centers for Medicare & Medicaid Services. Signing up for Medicare: special conditions. 2015. http://www.medicare.gov/sign-up-change-plans/get-parts-a-and-b/special-conditions/special-conditions.html. Accessed 2016.