Abstract
Study Design:
Retrospective study.
Objectives:
Recombinant human bone morphogenetic protein-2 (rhBMP-2) has been widely used in spinal fusion surgery, but there is little information on rhBMP-2 utilization in single-level posterior lumbar interbody fusion (PLIF). The purpose of our study was to evaluate the trends and demographics of rhBMP-2 utilization in single-level PLIF.
Methods:
Patients who underwent single-level PLIF from 2005 to 2011 were identified by searching ICD-9 diagnosis and procedure codes in the PearlDiver Patient Records Database, a national database of orthopedic insurance records. The year of procedure, age, gender, and region of the United States were recorded for each patient. Results were reported for each variable as the incidence of procedures identified per 100 000 patients searched in the database.
Results:
A total of 2735 patients had single-level PLIF. The average rate of single-level PLIF with rhBMP-2 maintained at a relatively stable level (28% to 31%) from 2005 to 2009, but decreased in 2010 (9.9%) and 2011 (11.8%). The overall incidence of single-level PLIF without rhBMP-2 (0.68 cases per 100 000 patients) was statistically higher (P < .01) compared to single-level PLIF with rhBMP-2 (0.21 cases per 100 000 patients). The average rate of single-level PLIF with rhBMP-2 utilization was the highest in West (30.1%), followed by Midwest (26.9%), South (20.5%), and Northeast (17.8%). The highest incidence of single-level PLIF with rhBMP-2 was observed in the age group <65 years (0.3 per 100 000 patients).
Conclusions:
To our knowledge, this is the first study to report on the demographics associated with rhBMP-2 use in single-level PLIF. There was a 3-fold increase in the rate of PLIF without rhBMP-2 compared to PLIF with rhBMP-2, with both procedures being mainly done in patients less than 65 years of age.
Keywords: recombinant human bone morphogenetic protein-2, rhBMP-2, posterior lumbar interbody fusion, PLIF, demographics, single-level, gender, age
Introduction
Posterior lumbar interbody fusion (PLIF) was initially introduced by Cloward, and it is used for various spinal conditions. Successful fusion rates (>90%) have been reported for degenerative disc disease and spondylolisthesis.1–4 An important objective of the PLIF procedure is to achieve solid arthrodesis of the spinal segment and to restore segmental lordosis at the involved level.5,6 Recombinant human bone morphogenetic protein-2 (rhBMP-2) belongs to a family of growth factors that promote bone formation and remodeling.7 In 2002, the Food and Drug Administration (FDA) approved the use of rhBMP-2 for anterior lumbar spine fusion.8 Potential benefits of rhBMP-2 are an increase in fusion rates and a reduction in morbidity from the bone graft harvest. However, use of rhBMP-2 might be associated with complications such as heterotopic ossification, radiculitis, prevertebral soft-tissue swelling, and bone resorption.9–12
The main objective of this study was to define the epidemiology of rhBMP-2 utilization in single-level PLIF using a national private insurance database for the period between 2005 and 2011.
Materials and Methods
Patients undergoing single-level PLIF with and without rhBMP-2 were identified using the PearlDiver Patient Records Database (Pearl-Diver Technologies, Fort Wayne, IN). Medicare database between 2005 and 2011 was used for the purpose of this study. Patients were identified using Current Procedural Terminology (CPT) code 22 630 (“Arthrodesis, posterior interbody technique, including laminectomy and/or discectomy to prepare interspace [other than for decompression], single interspace; lumbar”) and combination or exclusion of International Classification of Diseases, Ninth Edition (ICD-9) code 84.52 (“Insertion of recombinant bone morphogenetic protein”), as listed in the Table 1. Patients with ICD-9 codes 81.30 to 81.39 for “correction of pseudarthrosis of spine, refusion of spine” and CPT code 22 585 for “each additional interspace” were excluded. The search was defined to exclude the same code being counted more than once for the same patient. Patients were stratified by gender, geographic region (Midwest, Northeast, South, and West), and age group (less than 65, 65-69, 70-74, 75-79, 80-84, and more than 84 years). In this study, PLIF included transforaminal lumbar interbody fusion and minimally invasive transforaminal lumbar interbody fusion. Our study was deemed exempt from institutional review board review as all patient information was de-identified.
Table 1.
ICD-9 Diagnosis Codes and CPT Procedure Codes Searched.
| Code | Diagnosis/Procedure |
|---|---|
| CPT code 22 630 | Arthrodesis, posterior interbody technique, including laminectomy and/or discectomy to prepare interspace (other than for decompression), single interspace; lumbar |
| ICD-9 code 84.52 | Insertion of recombinant bone morphogenetic protein (rhBMP) |
Abbreviations: CPT, Current Procedural Terminology; ICD-9, International Classification of Diseases, Ninth Edition.
Results were reported as incidence, number of single-level PLIF cases with/without rhBMP-2 identified per every 100 000 patients searched in a particular year, gender, age group, or region. This was done to account for differences in the number of patients in the database for a given variable. We used χ2 analysis to determine the statistical significance, and linear regression was performed to test the significance of trends over time. The level of significance was P < .05.
Results
A total of 2735 patients undergoing single-level PLIF were identified. There were 1711 women and 1024 men. There were 2094 patients undergoing single-level PLIF without rhBMP-2 and 641 patients undergoing single-level PLIF with rhBMP-2. The overall incidence of single-level PLIF without rhBMP-2 (0.68 cases per 100 000 patients) was 3 times higher than the incidence of single-level PLIF with rhBMP-2 (0.21cases per 100 000 patients; P < .01; Figure 1). Looking at the annual increase, incidence of PLIF without rhBMP-2 had a steady increase from 2005 to 2011 (Figure 2). Interestingly, single-level PLIF with rhBMP-2 maintained at a relatively stable level (0.22 to 0.28 cases per 100 000) from 2005 to 2009, but sharply decreased in 2010 and 2011 (Figure 2).
Figure 1.
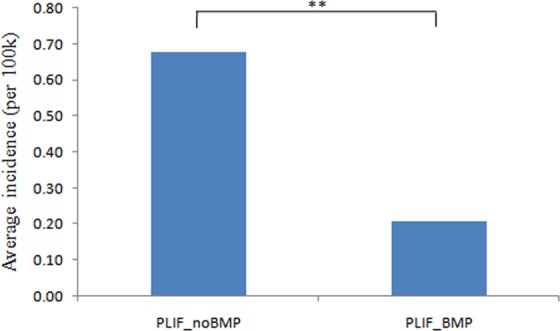
Average incidence of patients undergoing single-level PLIF with/without rhBMP-2 from 2005 to 2011 (per 100 000 patients).
Figure 2.
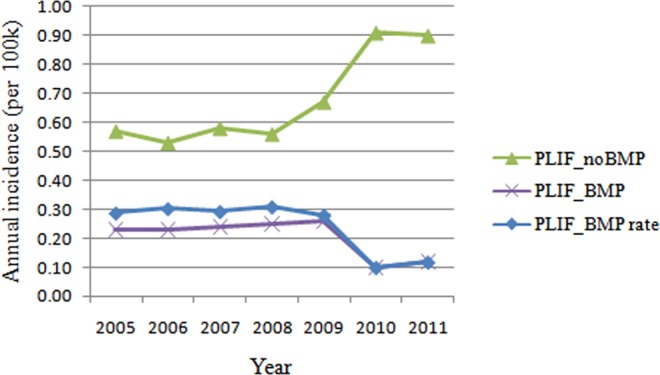
Annual incidence of patients undergoing single-level PLIF with/without rhBMP-2 from 2005 to 2011 (per 100 000 patients).
Gender
In 2010, the incidence of patients undergoing single-level PLIF with rhBMP-2 decreased by 61.9% in males and decreased by 64.5% in females (Figure 3). The incidence of female patients undergoing single-level PLIF with/without rhBMP-2 (P < .05) was higher than male patients undergoing the same procedure (Figure 4).
Figure 3.
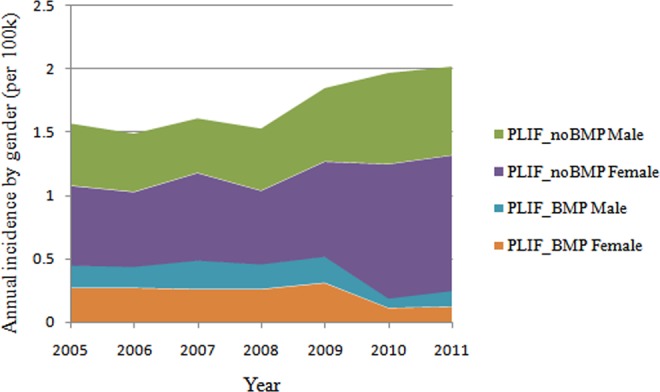
Annual incidence of patients undergoing single-level PLIF with/without rhBMP-2 by gender from 2005 to 2011 (per 100 000 patients).
Figure 4.
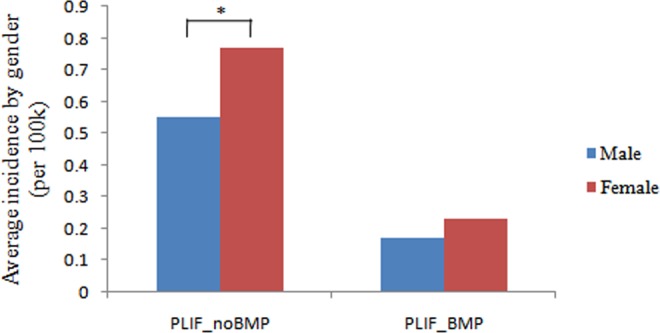
Average incidence of patients undergoing single-level PLIF with/without rhBMP-2 by gender from 2005 to 2011 (per 100 000 patients).
Region
The average rate of single-level PLIF with rhBMP-2 was the highest in West (30.1%), followed by Midwest (26.9%), South (20.5%), and Northeast (17.8%). The incidence of patients undergoing single-level PLIF with rhBMP-2 declined dramatically in 2010 in all regions, especially in the West (by 78.1%) and South (by 57.1%; Figure 5). Overall, the incidence of patients undergoing single-level PLIF with rhBMP-2 was the lowest in Northeast (0.08 cases per 100 000 patients) compared to South (0.25 cases per 100 000 patients, P < .001), Midwest (0.25 cases per 100 000 patients, P < .001), and West (0.22 cases per 100 000 patients, P = .002; Figure 6). As for the incidence of patients undergoing single-level PLIF without rhBMP-2, there were significant differences between Midwest and Northeast, Midwest and South, Northeast and South, and South and West (P < .01; Figure 6).
Figure 5.
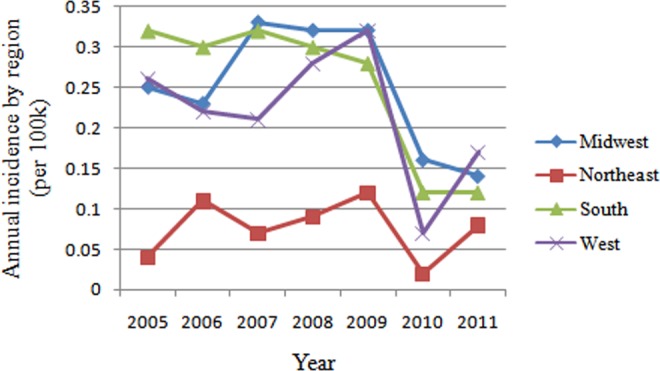
Annual incidence of patients undergoing single-level PLIF with/without rhBMP-2 by region from 2005 to 2011 (per 100 000 patients).
Figure 6.
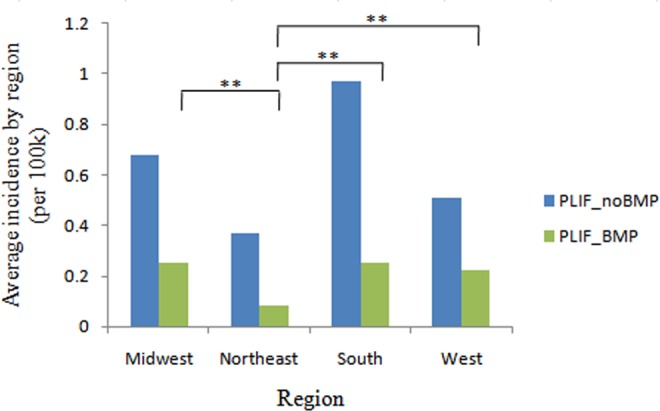
Average incidence of patients undergoing single-level PLIF with/without rhBMP-2 by region from 2005 to 2011 (per 100 000 patients). *P < .05, **P < .01.
Age
The highest incidence of single-level PLIF with rhBMP-2 (0.3 per 100 000 patients) was observed in the age group less than 65 years and was statistically significant compared to the 75 to 79, 80 to 84, more than 84 age groups (P < .05; Figure 7). The age group more than 84 years had the lowest incidence of single-level PLIF with rhBMP-2 (0.02 per 100 000 patients), which was significant compared to the age group 65 to 79 years, P < .001. The highest incidence of single-level PLIF without rhBMP-2 was observed in the age group less than 65 years (1.03 per 100 000 patients, P < .05). The age group more than 84 years had the lowest incidence of single-level PLIF without rhBMP-2 when compared to other age groups (0.07 per 100 000 patients, P < .05).
Figure 7.
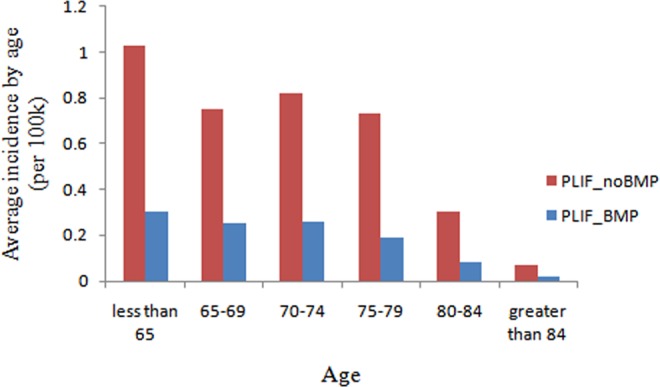
Average incidence of patients undergoing single-level PLIF with/without rhBMP-2 by age from 2005 to 2011 (per 100 000 patients).
Discussion
In our study, the rate of rhBMP-2 utilization in single-level PLIF remained stable between 2005 and 2009, but sharply decreased in 2010 and 2011.
The utilization of rhBMP-2 in spine surgery gained momentum due to the advantage of inducing bone formation without the morbidity associated with iliac crest bone graft harvest. Those beneficial characteristics prompted off-label application of rhBMP-2 that led to certain complications.10,13–15 At the same time, several studies reported nonsignificantly increased risk for cancer or no association after adjusting for comorbidities, demographics, and levels of procedure.16,17 Reduction in rhBMP-2 use in our study is very similar to the previous report for posterior lumbar fusion18,19 and could be potentially influenced by the 2008 Food and Drug Administration notification on rhBMP-2 use in cervical fusion.
The average rate of single-level PLIF with rhBMP-2 was the highest in West (30.1%), followed by Midwest (26.9%), South (20.5%), and Northeast (17.8%). Interestingly, the incidence of patients undergoing single-level PLIF with rhBMP-2 declined dramatically in 2010 in all regions, especially in West and South. Similarly, Singh and colleagues observed that for various fusion procedures the utilization rate of rhBMP-2 was the highest in South and the lowest in Northeast.20
We found the highest rhBMP-2 incidence in patients less than 65 years of age and the lowest in the age group more than 84 years of age. From 2005 to 2011, the overall incidence of single-level PLIF increased significantly in patients older than 65 years of age. These results may reflect the aging population in the United States; people older than 65 years of age are expected to represent nearly 20% of the population by 2030, compared to 12.4% in 2000.20 As previously reported, PLIF has been increasingly used for degenerative lumbar disorders in the older patient population (>70 years of age), and the surgical outcomes were very similar to those in younger patients.21–24
There are several limitations to our study. This is a retrospective study of a large database searched by ICD-9 and CPT codes and therefore is subject to errors in coding. There are also inherent limitations with gathering information from the PearlDiver database. The patient population included in our study represents a relatively small number of orthopedic patients in the United States. Furthermore, the regional distribution of patients is uneven in the database due to the variations in regional penetration of insurance carriers. This may introduce bias regarding regional trends. Despite these limitations, we believe that the results in our study reflect the real situation of PLIF and rhBMP-2 use in the United States.
To our knowledge, this is the first study to report a trend of rhBMP-2 use in single-level PLIF. The incidence of rhBMP-2 utilization in single-level PLIF decreased in 2010 and 2011. The West region had the highest incidence of rhBMP-2 use.
Footnotes
Declaration of Conflicting Interests: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: ZB—Xenco Medical (consultancy), AO Spine (consultancy, past). HJM—Dr Meisel is consultant (money paid to institution) – Regenerate Life Sciences GmbH for DiFusion (ongoing), Co.don (past); royalties from: Medtronic, Fehling Aesculap (past); stocks (money paid to institution) - Regenerate Life Sciences GmbH in DiFusion. STY—Dr Yoon owns stock in Phygen, Alphatec; Meditech, royalties Meditech Advisors, Stryker Spine (Paid directly to institution/employer), grant from AOSpine (Paid directly to institution/employer), research support from Biomet (Research support given to AREF), nonfinancial research support from Nuvasive and Medtronic. JAY—Royalties: NuVasive, Osprey Medical, Amedica, Integra; Stock Ownership: Benvenue Medical, Paradigm Spine, Promethean Surgical Devices, Spinal Ventures, VertiFlex, Spinicity, ISD, Providence Medical; Private Investments: Amedica, VertiFlex, Benvenue, NuVasive; Consulting: Integra, NuVasive, Amedica, HealthTrust; Board of Directors: Durango Orthopedic Associates (None); Research Support (Staff and/or Materials): Globus Medical (Paid directly to institution/employer), NuVasive (Paid directly to institution/employer), VertiFlex (Paid directly to institution/employer), Integra (Paid directly to institution/employer). DB—Consultant – Vallum, Royalties – America, DePuy Synthes, Medtronic, Fellowship Support – AOSpine (paid directly to institution). JCW—Royalties: Aesculap, Biomet, Amedica, Seaspine , Synthes ; Stock Ownership: Fziomed; Private Investments: Promethean Spine, Paradigm spine, Benevenue, NexGen, Vertiflex, electrocore, surgitech, expanding orthopaedics, osprey, bone biologics, curative biosciences, pearldiver; Board of Directors: North American Spine Society (non-financial, reimbursement for travel for board meetings, courses, etc.), North American Spine Foundation (non-financial), Cervical Spine Research Society (non-financial, reimbursement for travel for board meetings), AO Spine/AO Foundation (honorariums for board position); Fellowship Support: AO Foundation (spine fellowship funding paid to institution).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by AOSpine and departmental funds. AOSpine is a clinical division of the AO Foundation—an independent medically guided nonprofit organization. The AOSpine Knowledge Forums are pathology focused working groups acting on behalf of AOSpine in their domain of scientific expertise. Each forum consists of a steering committee of up to 10 international spine experts who meet on a regular basis to discuss research, assess the best evidence for current practices, and formulate clinical trials to advance spine care worldwide. Study support is provided directly through AOSpine’s Research Department.
References
- 1. Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I: indications, operative technique, after care. J Neurosurg. 1953;10:154–168. [DOI] [PubMed] [Google Scholar]
- 2. McLaughlin MR, Haid RW, Jr, Rodts GE, Jr, Subach BR. Posterior lumbar interbody fusion: indications, techniques, and results. Clin Neurosurg. 2000;47:514–527. [PubMed] [Google Scholar]
- 3. DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. J Am Acad Orthop Surg. 2008;16:130–139. [DOI] [PubMed] [Google Scholar]
- 4. Yan DL, Pei FX, Li J, Soo CL. Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J. 2008;17:1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brislin B, Vaccaro AR. Advances in posterior lumbar interbody fusion. Orthop Clin North Am. 2002;33:367–374. [DOI] [PubMed] [Google Scholar]
- 6. Ames CP, Acosta FL, Jr, Chi J, et al. Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion performed at 1 and 2 levels. Spine (Phila Pa 1976). 2005;30:E562–E566. [DOI] [PubMed] [Google Scholar]
- 7. Urist MR, Huo YK, Brownell AG, et al. Purification of bovine bone morphogenetic protein by hydroxyapatite chromatography. Proc Natl Acad Sci U S A. 1984;81:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food & Drug Administration. InFUSE Bone Graft/LT-CAGE Lumbar Tapered FusionDevice-P000058. https://www.fda.gov/ohrms/dockets/ac/02/briefing/3828b1_01_IIA.pdf. Accessed May 13, 2014.
- 9. Chrastil J, Low JB, Whang PG, Patel AA. Complications associated with the use of the recombinant human bone morphogenetic proteins for posterior interbody fusions of the lumbar spine. Spine (Phila Pa 1976). 2013;38:E1020–E1027. [DOI] [PubMed] [Google Scholar]
- 10. Hoffmann MF, Jones CB, Sietsema DL. Recombinant human bone morphogenetic protein-2 (rhBMP-2) in posterolateral lumbar spine fusion: complications in the elderly. J Orthop Surg Res. 2013;8:1 doi:10.1186/1749-799X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein-2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine (Phila Pa 1976). 2011;36:1849–1854. [DOI] [PubMed] [Google Scholar]
- 12. Simmonds MC, Brown JV, Heirs MK, et al. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013;158:877–889. [DOI] [PubMed] [Google Scholar]
- 13. Behrbalk E, Uri O, Parks RM, Musson R, Soh RC, Boszczyk BM. Fusion and subsidence rate of standalone anterior lumbar interbody fusion using PEEK cage with recombinant human bone morphogenetic protein-2. Eur Spine J. 2013;22:2869–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302:58–66. [DOI] [PubMed] [Google Scholar]
- 15. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–491. [DOI] [PubMed] [Google Scholar]
- 16. Latzman JM, Kong L, Liu C, Samadani U. Administration of human recombinant bone morphogenetic protein-2 for spine fusion may be associated with transient postoperative renal insufficiency. Spine (Phila Pa 1976). 2010;35:E231–E237. [DOI] [PubMed] [Google Scholar]
- 17. Veeravagu A, Cole T, Jiang B, Ratliff JK, Gidwani RA. The use of bone morphogenetic protein in thoracolumbar spine procedures: analysis of the Marketscan Longitudinal Database. Spine J. 2014;14:2929–2937. [DOI] [PubMed] [Google Scholar]
- 18. Lao L, Cohen JR, Lord EL, Buser Z, Wang JC. Trends analysis of rhBMP utilization in single-level posterior lumbar fusion (PLF) in the United States. Eur Spine J. 2016;25:783–788. [DOI] [PubMed] [Google Scholar]
- 19. Carragee EJ, Chu G, Rohatgi R, et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am. 2013;95:1537–1545. [DOI] [PubMed] [Google Scholar]
- 20. Singh K, Nandyala SV, Marquez-Lara A, Fineberg SJ. Epidemiological trends in the utilization of bone morphogenetic protein in spinal fusions from 2002 to 2011. Spine (Phila Pa 1976). 2014;39:491–496. [DOI] [PubMed] [Google Scholar]
- 21. Administration on Aging. Aging statistics. https://aoa.acl.gov/Aging_Statistics/index.aspx. Accessed May 13, 2014.
- 22. Okuda S, Oda T, Miyauchi A, Haku T, Yamamoto T, Iwasaki M. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. J Bone Joint Surg Am. 2006;88:2714–2720. [DOI] [PubMed] [Google Scholar]
- 23. Carreon LY, Puno RM, Dimar JR, 2nd, Glassman SD, Johnson JR. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am. 2003;85:2089–2092. [DOI] [PubMed] [Google Scholar]
- 24. Ragab AA, Fye MA, Bohlman HH. Surgery of the lumbar spine for spinal stenosis in 118 patients 70 years of age or older. Spine (Phila Pa 1976). 2003;28:348–353. [DOI] [PubMed] [Google Scholar]


