Abstract
Study Design:
Review and technical report.
Objective:
Intraoperative ultrasound has been used by spine surgeons since the early 1980s. Since that time, more advanced modes of intraoperative imaging and navigation have become widely available. Although the use of ultrasound during spine surgery has fallen out of favor, it remains the only true real-time imaging modality that allows surgeons to visualize soft tissue anatomy instantly and continuously while operating. It is our objective to demonstrate that for this reason, ultrasound is a useful adjunctive technique for spine surgeons, especially when approaching intradural lesions or when addressing pathology in the ventral spinal canal via a posterior approach.
Methods:
Using PubMed, the existing literature regarding the use of intraoperative ultrasound during spinal surgery was evaluated. Also, surgical case logs were reviewed to identify spinal operations during which intraoperative ultrasound was used. Illustrative cases were selected and reviewed in detail.
Results:
This article provides a brief review of the history of intraoperative ultrasound in spine surgery and describes certain surgical scenarios during which this technique might be useful. Several illustrative cases are provided from our own experience.
Conclusions:
Surgeons should consider the use of intraoperative ultrasound when approaching intradural lesions or when addressing pathology ventral to the thecal sac via a posterior approach.
Keywords: intraoperative ultrasound, spine surgery, intramedullary tumor, thoracic disc herniation, thoracolumbar burst fracture
Introduction
During the early 1980s, the use of intraoperative ultrasound gained popularity among cranial neurosurgeons for guiding the biopsy and resection of lesions within the brain.1,2 This technology was subsequently adopted by spine surgeons for real-time intraoperative imaging of the spinal cord.3–5 In 1978, Reid first reported the use of focused ultrasound to preoperatively evaluate the spinal cord in a patient with cystic astrocytoma of the cervical cord by asking the patient to flex his neck and looking into the spinal canal with ultrasound via an “interlaminar window.”6 Subsequently, in 1982, Dohrmann and Rubin reported using ultrasound intraoperatively during spinal surgery on 10 patients with varying diagnoses including syringomyelia, spinal cord cysts, and intramedullary and extramedullary tumors.7 They noted that by using the ultrasound intraoperatively after laminectomy, they were able to obtain higher quality images than could be obtained via the “interlaminar window” since the bone that was removed would attenuate passing ultrasound waves.7,8 In comparison to ultrasound, computed tomography (CT) and myelography—the most widely available imaging modalities at the time—were not as sensitive in visualizing soft tissue structures. Therefore, ultrasound was seen as a tool that could more accurately define the extent of intradural lesions. A number of publications were produced where intraoperative ultrasound was used to guide the resection of intramedullary and extramedullary tumors, the drainage of spinal cysts, and the placement of syringo-subarachnoid shunts.1,9–18 Intraoperative ultrasound was also used to identify and confirm decompression of pathology ventral to the thecal sac such as central disc herniations or retropulsed bone fragments.12,13,15,19
As high-resolution magnetic resonance imaging (MRI) and more advanced modes of intraoperative image acquisition such as cone-beam CT (cbCT) and intraoperative CT (iCT) have become more widely available, the use of intraoperative ultrasound has fallen out of favor among spinal surgeons.20 Based on our experience, however, intraoperative ultrasound remains a useful adjunct in a number of operative situations and, in some cases, has an advantage over newer technologies. This is particularly true when operating on intradural mass lesions or lesions that are ventral to the thecal sac since the pathology is out of direct sight of the surgeon. Although the use of intraoperative ultrasound is by definition operator dependent, we have found that this technique is very easy to use, surgeons can become proficient after 1 or 2 operations, and images can be readily interpreted without a radiologist (see Table 1).
Table 1.
Advantages of Different Intraoperative Imaging Technologies.
| Intraoperative Imaging Technology | Advantages |
|---|---|
| Ultrasound | Real time, excellent soft tissue imaging |
| Fluoroscopy | Real time, provides 2-dimensional images of bony structures |
| Cone beam CT and intraoperative CT | Provide 3-dimensional and multiplanar reconstructions, can be used with navigation systems |
| Intraoperative MRI | Multiplanar reconstructions with excellent soft tissue imaging |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
Requirements, Technique, and Normal Spinal Imaging
We have used an Aloka Prosound Alpha 5 mobile ultrasound machine (Hitachi, Wallingford, CT) for intraoperative ultrasound during spinal surgery. In general, we have found using the Aloka UST-9120 transducer probe (Hitachi, Wallingford, CT), which has a 20 mm diameter and offers a 10 to 4.4 MHz frequency range, to be the most compatible for our specifications. There are a number of comparable devices on the market, and we recommend using any modern, mobile ultrasound unit with dedicated transducers. After bony removal is complete and the dura is exposed, the surgical field should be filled with saline solution for acoustic coupling. The ultrasound probe should then be placed within the saline bath to obtain images in both the transverse and longitudinal planes. It is usually not necessary to touch the dura or the spinal cord with the probe in order to obtain images.20,21
On ultrasound, the dura appears as an echogenic membrane surrounding a space of anechoic spinal fluid. The spinal cord is located within the dura and appears as a homogenous structure with low echos surrounded by an echogenic rim that represents the physical change in density from spinal fluid to spinal cord parenchyma.8 There is also a bright central echo representing the central canal.20 The exiting nerve roots are brightly echogenic and are particularly prominent at the cauda equina.
Intradural Mass Lesions
In our experience, intraoperative ultrasound has proved to be extremely useful during surgery for intradural pathology. Authors have previously reported using ultrasound for resection of intramedullary lesions such as tumors and cavernomas, extramedullary lesions such as posttraumatic cysts, placement of syringo-subarachnoid shunts, and also the obliteration of spinal dural arteriovenous fistulae.10–12,14,16–18,21–38 Typically, when preparing for intradural surgery, the bony exposure is planned based on a preoperative CT or MRI. A durotomy is then made by roughly approximating where the lesion should be based on adjacent landmarks. Once the lesion comes into view, the durotomy may then need to be lengthened in either the cranial or caudal direction to ensure adequate exposure. Intradural extramedullary lesions of the cauda equina can migrate rostrally in comparison to preoperative imaging, occasionally making them difficult to locate.32 If intraoperative ultrasound is used, however, the lesion can be visualized while the dura is still intact and the dural opening can be tailored exactly to the size and location of the lesion.27,32 When compared to more advanced intraoperative imaging systems such as cbCT or iCT, ultrasound provides superior soft tissue imaging of intradural structures. For example, several authors have reported that septations within spinal cord cysts can be visualized on ultrasound but not CT.1,3 Intraoperative MRI (iMRI) is available at some institutions and does provide much higher resolution soft tissue imaging.11,20,21 However, use of this technology is expensive, time consuming, and unnecessary for most intradural pathology, especially when compared to intraoperative ultrasound, which is widely available, inexpensive, and can be performed in minutes.21
Intramedullary lesions pose a significant challenge to the surgeon as resection involves the dissection through neural tracts and there is an inherent risk of postoperative neurological deficit to the patient.39 Generally accepted principles of intramedullary spinal cord surgery include minimizing dissection of normal spinal cord parenchyma, finding tissue planes when able, and resecting only as much tumor as possible without resulting in neurological deficit. The use of intraoperative ultrasound can help the surgeon to safely and effectively achieve these goals of resection by providing real-time information on tissue composition during dissection through the spinal cord.16,21,27 During surgery, Gelfoam (Pfizer, New York, NY) may also be used to discern tissue planes and to define the limits of dissection for intramedullary lesions. Gelfoam appears hyperechoic on ultrasound and does not attenuate acoustic waves so it can be identified on ultrasound and used as a surgical marker.8,15
Illustrative Case 1: Cervical Intramedullary Lesion Approached via Midline Myelotomy
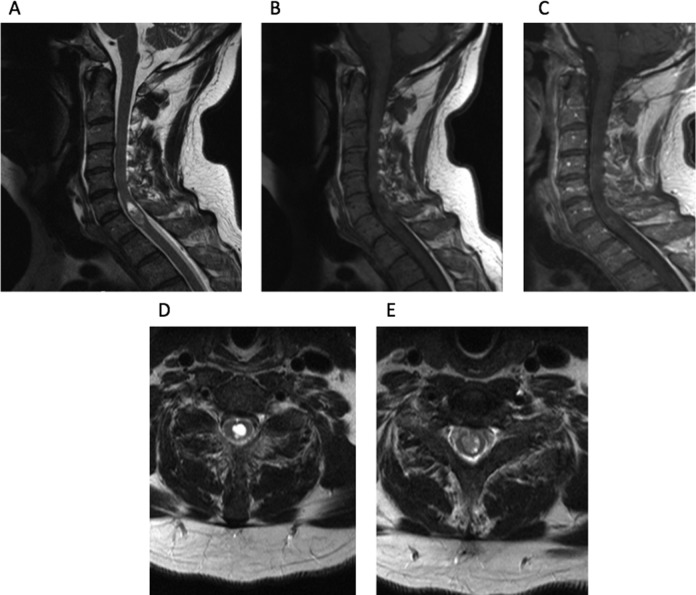
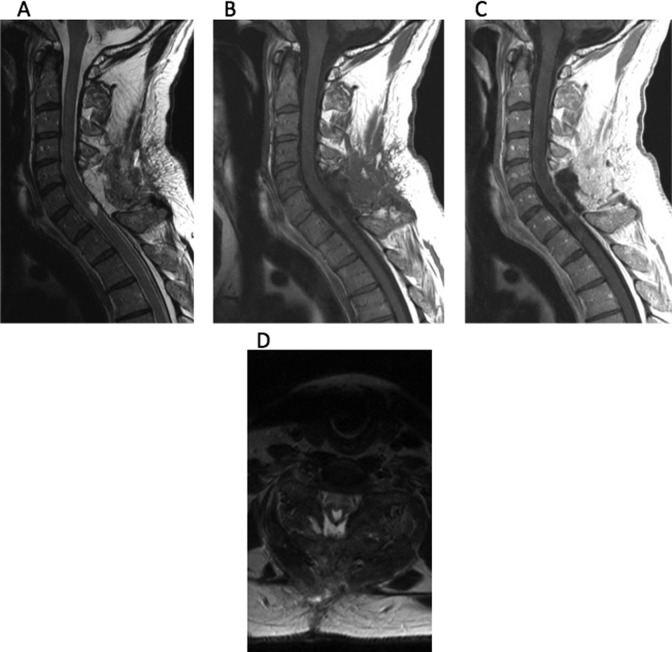
A 54-year-old male with no significant past medical history presented with a 1-month history of fever of unknown origin. Although he was neurologically intact, as part of his workup, he had a cervical spine MRI that revealed a large intramedullary mass centered at C6 with heterogeneous T2 signal at the lower aspect and fluid signal at the top with very subtle enhancement after contrast (Figure 1). The mass remained constant in size after 1 month of surveillance imaging. An extensive workup was performed to search for other possible causes of fever without success. Due to the need for a definitive diagnosis, the patient was taken to the operating room for C5-7 laminectomy and resection of the lesion. Motor evoked potentials (MEPs) and somatosensory evoked potentials (SSEPs) were obtained during the case. After exposing the dura, the ultrasound was then used to localize the lesion and plan the durotomy (Figure 2). The dura was opened in the midline sharply and the dural leaflets were stitched to the adjacent paraspinal muscles. Next, the arachnoid was opened and attached to the overlying dura using vessel clips. The midline of the spinal cord was identified by observing the drainage of the septal veins and by using microstimulation to identify the location of phase reversal of the SSEPs.40 The pia was then opened sharply and a microdissector was used to deepen the dissection through the dorsal median raphe. The ultrasound was used frequently to guide the surgical trajectory through the spinal cord until the grayish tumor was visualized. At the very cranial part of the tumor, a cyst was encountered as expected from the preoperative MRI. To confirm that this truly was the most cranial aspect of the tumor, a small piece of Gelfoam was placed in the resection cavity and, once again, the ultrasound was used to confirm that this indeed was the limit of the tumor (Figure 2). The tumor capsule was dissected free from the spinal cord and the tumor was then removed in a piecemeal fashion. The ultrasound was used to identify residual tumor, which was subsequently removed. After complete resection of the lesion, the pia and dura were closed. It was noted that MEPs and SSEPs remained unchanged throughout the case. Postoperatively, the patient returned to his neurological baseline. His fever resolved after the removal of the tumor. The final pathological diagnosis returned World Health Organization grade II ependymoma and an MRI obtained 2 months later confirmed a complete resection (Figure 3).
Figure 1.
(A) Sagittal T2-weighted MRI reveals lesion centered at C5-7, with an associated fluid collection at the most rostral part of the lesion. (B) Sagittal T1-weighted MRI of lesion. (C) Sagittal contrast-enhanced MRI reveals scant rim enhancement. (D) Axial T2-weighted MRI centered on the fluid collection. (E) Axial T2-weighted MRI at more caudal part of the lesion.
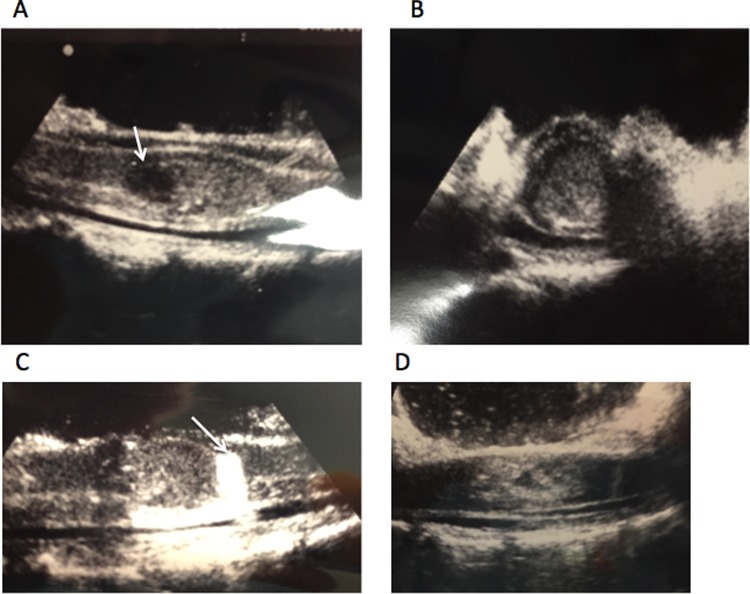
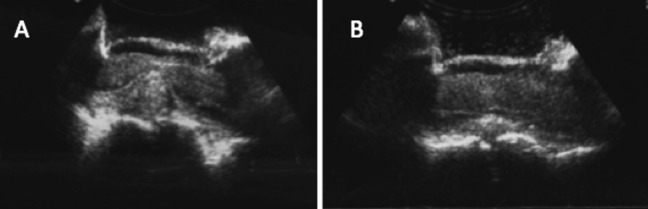
Figure 2.
Intraoperative ultrasound of spinal cord after laminectomy reveals lesion. (A) Fluid collection can be seen at the left (white arrow). (B) Looking at axial perspective, one can see lesion encompassing most of the spinal cord. (C) Using a piece of Gelfoam (white arrow), one is able to make sure that most caudal aspect of lesion is exposed during intramedullary dissection. (D) Ultrasound after resection of lesion reveals resolution of mass effect.
Figure 3.
(A) Sagittal T2-weighted MRI taken 2 months postoperatively reveals complete resection of tumor. (B) T1-weighted MRI without contrast and (C) contrast-enhanced reveals complete resection.
Lesions Ventral to the Thecal Sac
We also found the use of intraoperative ultrasound very helpful when addressing lesions located ventral to the thecal sac from a posterior approach, particularly in the cervical and thoracic spine where the thecal sac cannot be mobilized freely due to the risk of iatrogenic spinal cord injury. Intraoperative ultrasound has been reported to be useful in the resection of intervertebral disc herniations, the reduction of thoracolumbar burst fractures, the resection of ventrally located extradural tumors, and to determine whether posterior decompression alone is adequate for treatment of spinal canal stenosis due to ossification of the posterior longitudinal ligament.19,22,41–51 Anterior approaches to the ventral spinal canal can provide a better corridor for direct visualization of this region; however, transthoracic, thoracoabdominal, and retroperitoneal approaches are associated with increased blood loss, operative time, and surgical morbidity. These disadvantages can be avoided by using a posterior approach with ultrasound guidance to maneuver angled instruments beneath the thecal sac without the need to retract the thecal sac, spinal cord, or nerve roots.
For example, when approaching a calcified thoracic disc via a posterior pedicle sparing transfacet approach, a down-angled curette can be safely and placed beneath the thecal sac under intraoperative ultrasound visualization in order to decompress the canal.41 Likewise, in the case of a thoracolumbar burst fracture, intraoperative ultrasound can be used to identify bone fragments invading into the canal and a boot-shaped impactor or modified surgical hook can be safely placed beneath the thecal sac to reduce the fracture.46
Illustrative Case 2: Symptomatic Thoracic Disc Herniation Resected via Posterior Pedicle Sparing Transfacet Approach
A 73-year-old female presented with a several-month history of worsening gait dysfunction, spasticity, and bilateral lower extremity numbness. On neurological exam she did not have any motor weakness but was noted to be extremely myelopathic with marked clonus, 4+ lower extremity muscle stretch reflexes, and a wide-based, staggering gait. CT and MRI demonstrated a large, noncalcified T10-11 intervertebral disc herniation with spinal cord compression (Figure 4). She underwent a right-sided T10-11 hemilaminectomy, facetectomy, and pedicle-sparing microdiscectomy along with T9-11 instrumented fusion. After the spinal dura was exposed during surgery, intraoperative ultrasound was used to identify the disc herniation (Figure 5A). The disc annulus was then opened with a scalpel and a pituitary rongeur was used to create a central cavity. A down-angled curette was then carefully inserted ventral to the thecal sac and used to push the herniated disc fragments previously identified on ultrasound into the disc space. These disc fragments were then removed again with a pituitary rongeur. The ultrasound was used frequently to evaluate the decompression and this process was repeated until the entire disc herniation was excised (Figure 5B). Without the use of ultrasound, it is possible that compressive disc fragments might have been left behind. Postoperatively she returned to her neurological baseline, and by 1-month follow up, all of her preoperative symptoms had resolved.
Figure 4.
(A) Sagittal and (B) axial T2-weighted MRI images demonstrating a large T10-11 disc herniation resulting in severe spinal cord compression.
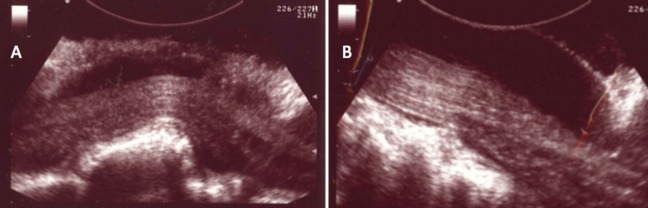
Figure 5.
Longitudinal intraoperative ultrasound images demonstrating (A) a large disc herniation displacing the spinal cord and thecal sac and (B) complete spinal cord decompression at the conclusion of surgery.
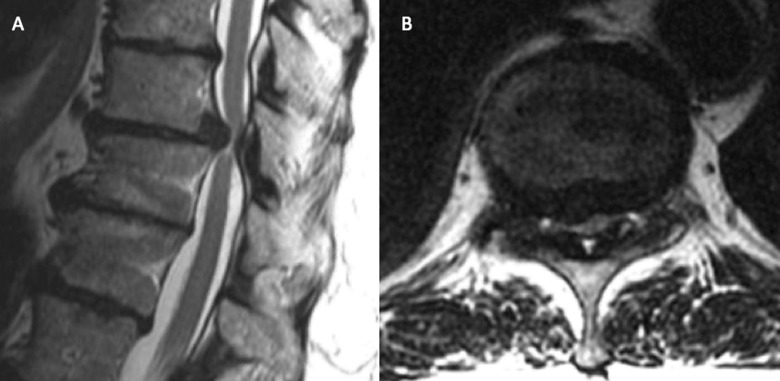
Illustrative Case 3: Reduction of a Lumbar Burst Fracture
A 57-year-old female with a history of metastatic appendiceal cancer presented with mechanical back pain and acute-onset left anterior thigh pain. One month prior she underwent balloon kyphoplasty at L1 and L2 for treatment of a pathologic compression fractures. On examination, she did not have motor deficits but was found to have decreased sensation to light touch over her left thigh. An MRI was performed, which demonstrated a pathological L2 burst fracture (Figure 6). She was taken to the operating room the following day where she underwent an L1-L2 laminectomy, left transpedicular reduction of the fracture, and T12-L3 instrumented posterolateral fusion. After the laminectomy was completed the left L2 pedicle was drilled flush with the vertebral body. The L2 nerve root was seen to be draped over a loose bone fragment, which was removed. The field was filled with saline solution for acoustic coupling and ultrasound was used to examine the thecal sac. Using ultrasound we found a large bone fragment in the ventral spinal canal displacing the thecal sac that could not be directly visualized (Figure 7A). A boot-shaped impactor was then used to reduce this retropulsed fragment. The ultrasound was then brought back into the field to confirm adequate decompression (Figure 7B). Postoperatively, the patient returned to her neurological baseline with improvement of her symptoms and was discharged home several days later.
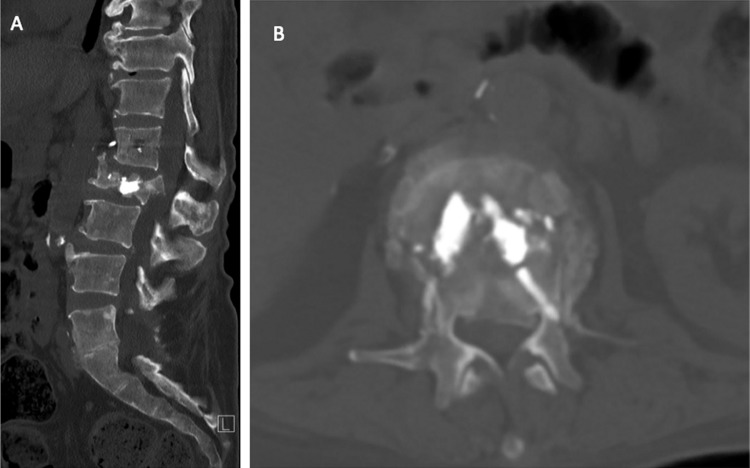
Figure 6.
(A) Sagittal and (B) axial CT demonstrating a pathological L2 burst fracture.
Figure 7.
Longitudinal intraoperative ultrasound images demonstrating (A) a retropulsed bone fragment in the ventral spinal canal deforming the thecal sac at the level of the conus medullaris and (B) complete reduction and decompression of the spinal canal. The right side of the image is cranial and the left side is caudal.
Conclusion
Although ultrasound is one of the most primitive modalities of intraoperative image acquisition used during spinal surgery, it continues to maintain several advantages over newer technologies. When used appropriately, intraoperative ultrasound provides valuable information about soft tissue structures that could not otherwise be visualized by the operating surgeon. Ultrasound images can then be interpreted in light of preoperative MRI studies to help the surgeon better understand local anatomy. Neither routine fluoroscopy, cbCT, nor iCT provide adequate soft tissue resolution for this purpose. Furthermore, ultrasound remains the only true real-time intraoperative imaging modality for soft tissue visualization. It is inexpensive, widely available, easy to use, and does not expose the patient to ionizing radiation. For these reasons, we believe that the use of intraoperative ultrasound should be considered during posterior approach for intradural mass lesions or for lesions located within the ventral spinal canal. The use of ultrasound and interpretation of images should be incorporated into residency teaching programs for spinal surgery.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Montalvo BM, Quencer RM. Intraoperative sonography in spinal surgery: current state of the art. Neuroradiology. 1986;28:551–590. [DOI] [PubMed] [Google Scholar]
- 2. Rubin JM, Dohrmann GJ. Use of ultrasonically guided probes and catheters in neurosurgery. Surg Neurol. 1982;18:143–148. [DOI] [PubMed] [Google Scholar]
- 3. Raymond CA. Brain, spine surgeons say yes to ultrasound. JAMA. 1986;255:2258–2259. [PubMed] [Google Scholar]
- 4. Rubin JM, Dohrmann GJ. The spine and spinal cord during neurosurgical operations: real-time ultrasonography. Radiology. 1985;155:197–200. doi:10.1148/radiology.155.1.3883416. [DOI] [PubMed] [Google Scholar]
- 5. Machi J, Sigel B, Jafar JJ, et al. Criteria for using imaging ultrasound during brain and spinal cord surgery. J Ultrasound Med. 1984;3:155–161. [DOI] [PubMed] [Google Scholar]
- 6. Reid MH. Ultrasonic visualization of a cervical cord cystic astrocytoma. AJR Am J Roentgenol. 1978;131:907–908. doi:10.2214/ajr.131.5.907. [DOI] [PubMed] [Google Scholar]
- 7. Dohrmann GJ, Rubin JM. Intraoperative ultrasound imaging of the spinal cord: syringomyelia, cysts, and tumors—a preliminary report. Surg Neurol. 1982;18:395–399. [DOI] [PubMed] [Google Scholar]
- 8. Quencer RM, Montalvo BM. Normal intraoperative spinal sonography. AJR Am J Roentgenol. 1984;143:1301–1305. doi:10.2214/ajr.143.6.1301. [DOI] [PubMed] [Google Scholar]
- 9. Braun IF, Raghavendra BN, Kricheff II. Spinal cord imaging using real-time high-resolution ultrasound. Radiology. 1983;147:459–465. doi:10.1148/radiology.147.2.6340159. [DOI] [PubMed] [Google Scholar]
- 10. Hutchins WW, Vogelzang RL, Neiman HL, Fuld IL, Kowal LE. Differentiation of tumor from syringohydromyelia: intraoperative neurosonography of the spinal cord. Radiology. 1984;151:171–174. doi:10.1148/radiology.151.1.6701310. [DOI] [PubMed] [Google Scholar]
- 11. Juthani RG, Bilsky MH, Vogelbaum MA. Current management and treatment modalities for intramedullary spinal cord tumors. Curr Treat Options Oncol. 2015;16:39 doi:10.1007/s11864-015-0358-0. [DOI] [PubMed] [Google Scholar]
- 12. Knake JE, Gabrielsen TO, Chandler WF, Latack JT, Gebarski SS, Yang PJ. Real-time sonography during spinal surgery. Radiology. 1984;151:461–465. doi:10.1148/radiology.151.2.6709919. [DOI] [PubMed] [Google Scholar]
- 13. Montalvo BM, Quencer RM, Green BA, Eismont FJ, Brown MJ, Brost P. Intraoperative sonography in spinal trauma. Radiology. 1984;153:125–134. doi:10.1148/radiology.153.1.6473773. [DOI] [PubMed] [Google Scholar]
- 14. Pasto ME, Rifkin MD, Rubenstein JB, Northrup BE, Cotler JM, Goldberg BB. Real-time ultrasonography of the spinal cord: intraoperative and postoperative imaging. Neuroradiology. 1984;26:183–187. [DOI] [PubMed] [Google Scholar]
- 15. Quencer RM, Montalvo BM, Eismont FJ, Green BA. Intraoperative spinal sonography in thoracic and lumbar fractures: evaluation of Harrington rod instrumentation. AJR Am J Roentgenol. 1985;145:343–349. doi:10.2214/ajr.145.2.343. [DOI] [PubMed] [Google Scholar]
- 16. Quencer RM, Montalvo BM, Green BA, Eismont FJ. Intraoperative spinal sonography of soft-tissue masses of the spinal cord and spinal canal. AJR Am J Roentgenol. 1984;143:1307–1315. doi:10.2214/ajr.143.6.1307. [DOI] [PubMed] [Google Scholar]
- 17. Quencer RM, Morse BM, Green BA, Eismont FJ, Brost P. Intraoperative spinal sonography: adjunct to metrizamide CT in the assessment and surgical decompression of posttraumatic spinal cord cysts. AJR Am J Roentgenol. 1984;142:593–601. doi:10.2214/ajr.142.3.593. [DOI] [PubMed] [Google Scholar]
- 18. Rubin JM, Dohrmann GJ. Work in progress: intraoperative ultrasonography of the spine. Radiology. 1983;146:173–175. doi:10.1148/radiology.146.1.6849042. [DOI] [PubMed] [Google Scholar]
- 19. McGahan JP, Benson D, Chehrazi B, Walter JP, Wagner FC., Jr Intraoperative sonographic monitoring of reduction of thoracolumbar burst fractures. AJR Am J Roentgenol. 1985;145:1229–1232. doi:10.2214/ajr.145.6.1229. [DOI] [PubMed] [Google Scholar]
- 20. Sosna J, Barth MM, Kruskal JB, Kane RA. Intraoperative sonography for neurosurgery. J Ultrasound Med. 2005;24:1671–1682. [DOI] [PubMed] [Google Scholar]
- 21. Toktas ZO, Sahin S, Koban O, Sorar M, Konya D. Is intraoperative ultrasound required in cervical spinal tumors? A prospective study. Turk Neurosurg. 2013;23:600–606. doi:10.5137/1019-5149.jtn.7199-12.1. [DOI] [PubMed] [Google Scholar]
- 22. Harel R, Knoller N. Intraoperative spine ultrasound: application and benefits. Eur Spine J. 2016;25:865–869. doi:10.1007/s00586-015-4222-5. [DOI] [PubMed] [Google Scholar]
- 23. Prada F, Vetrano IG, Filippini A, et al. Intraoperative ultrasound in spinal tumor surgery. J Ultrasound. 2014;17:195–202. doi:10.1007/s40477-014-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vetrano IG, Prada F, Nataloni IF, Bene MD, Dimeco F, Valentini LG. Discrete or diffuse intramedullary tumor? Contrast-enhanced intraoperative ultrasound in a case of intramedullary cervicothoracic hemangioblastomas mimicking a diffuse infiltrative glioma: technical note and case report. Neurosurg Focus. 2015;39:E17 doi:10.3171/2015.5.focus15162. [DOI] [PubMed] [Google Scholar]
- 25. Lanzino G, Morales-Valero SF, Krauss WE, Campero M, Marsh WR. Resection of cervical ependymoma. Neurosurg Focus. 2014;37(suppl 2):Video 14. doi:10.3171/2014.v3.focus14378. [DOI] [PubMed] [Google Scholar]
- 26. Shamov T, Eftimov T, Kaprelyan A, Enchev Y. Ultrasound-based neuronavigation and spinal cord tumour surgery-marriage of convenience or notified incompatibility? Turk Neurosurg. 2013;23:329–335. doi:10.5137/1019-5149.jtn.6639-12.2. [DOI] [PubMed] [Google Scholar]
- 27. Zhou H, Miller D, Schulte DM, et al. Intraoperative ultrasound assistance in treatment of intradural spinal tumours. Clin Neurol Neurosurg. 2011;113:531–537. doi:10.1016/j.clineuro.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 28. Bozinov O, Burkhardt JK, Woernle CM, et al. Intra-operative high frequency ultrasound improves surgery of intramedullary cavernous malformations. Neurosurg Rev. 2012;35:269–275. doi:10.1007/s10143-011-0364-z. [DOI] [PubMed] [Google Scholar]
- 29. Sciubba DM, Liang D, Kothbauer KF, Noggle JC, Jallo GI. The evolution of intramedullary spinal cord tumor surgery. Neurosurgery. 2009;65:84–91. doi:10.1227/01.NEU.0000345628.39796.40. [DOI] [PubMed] [Google Scholar]
- 30. Regelsberger J, Fritzsche E, Langer N, Westphal M. Intraoperative sonography of intra-and extramedullary tumors. Ultrasound Med Biol. 2005;31:593–598. doi:10.1016/j.ultrasmedbio.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 31. Iacopino DG, Conti A, Giusa M, Cardali S, Tomasello F. Assistance of intraoperative microvascular doppler in the surgical obliteration of spinal dural arteriovenous fistula: cases description and technical considerations. Acta Neurochir. 2003;145:133–137. doi:10.1007/s00701-002-1039-x. [DOI] [PubMed] [Google Scholar]
- 32. Friedman JA, Wetjen NM, Atkinson JL. Utility of intraoperative ultrasound for tumors of the Cauda equina. Spine (Phila Pa 1976). 2003;28:288–290. doi:10.1097/01.brs.0000042271.75392.e4. [DOI] [PubMed] [Google Scholar]
- 33. Tekula F, Pritz MB, Kopecky K, Willing SJ. Usefulness of color Doppler ultrasound in the management of a spinal arteriovenous fistula. Surg Neurol. 2001;56:304–307. [DOI] [PubMed] [Google Scholar]
- 34. Lunardi P, Acqui M, Ferrante L, Fortuna A. The role of intraoperative ultrasound imaging in the surgical removal of intramedullary cavernous angiomas. Neurosurgery. 1994;34:520–523. [DOI] [PubMed] [Google Scholar]
- 35. Matsuzaki H, Tokuhashi Y, Wakabayashi K, Toriyama S. Clinical values of intraoperative ultrasonography for spinal tumors. Spine (Phila Pa 1976). 1992;17:1392–1399. [DOI] [PubMed] [Google Scholar]
- 36. Kawakami N, Mimatsu K, Kato F. Intraoperative sonography of intramedullary spinal cord tumours. Neuroradiology. 1992;34:436–439. [DOI] [PubMed] [Google Scholar]
- 37. Epstein FJ, Farmer JP, Schneider SJ. Intraoperative ultrasonography: an important surgical adjunct for intramedullary tumors. J Neurosurg. 1991;74:729–733. doi:10.3171/jns.1991.74.5.0729. [DOI] [PubMed] [Google Scholar]
- 38. Platt JF, Rubin JM, Chandler WF, Bowerman RA, DiPietro MA. Intraoperative spinal sonography in the evaluation of intramedullary tumors. J Ultrasound Med. 1988;7:317–325. [DOI] [PubMed] [Google Scholar]
- 39. Harrop JS, Ganju A, Groff M, Bilsky M. Primary intramedullary tumors of the spinal cord. Spine (Phila Pa 1976). 2009;34(22 suppl):S69–S77. doi:10.1097/BRS.0b013e3181b95c6f. [DOI] [PubMed] [Google Scholar]
- 40. Simon MV, Chiappa KH, Borges LF. Phase reversal of somatosensory evoked potentials triggered by gracilis tract stimulation: case report of a new technique for neurophysiologic dorsal column mapping. Neurosurgery. 2012;70:E783–E788. doi:10.1227/NEU.0b013e31822e0a76. [DOI] [PubMed] [Google Scholar]
- 41. Nishimura Y, Thani NB, Tochigi S, Ahn H, Ginsberg HJ. Thoracic discectomy by posterior pedicle-sparing, transfacet approach with real-time intraoperative ultrasonography: clinical article. J Neurosurg Spine. 2014;21:568–576. doi:10.3171/2014.6.spine13682. [DOI] [PubMed] [Google Scholar]
- 42. Tian W, Weng C, Liu B, et al. Intraoperative 3-dimensional navigation and ultrasonography during posterior decompression with instrumented fusion for ossification of the posterior longitudinal ligament in the thoracic spine. J Spinal Disord Tech. 2013;26:E227–E234. doi:10.1097/BSD.0b013e318286ba39. [DOI] [PubMed] [Google Scholar]
- 43. Seichi A, Chikuda H, Kimura A, et al. Intraoperative ultrasonographic evaluation of posterior decompression via laminoplasty in patients with cervical ossification of the posterior longitudinal ligament: correlation with 2-year follow-up results. J Neurosurg Spine. 2010;13:47–51. doi:10.3171/2010.3.spine09680. [DOI] [PubMed] [Google Scholar]
- 44. Aoyama T, Hida K, Akino M, Yano S, Iwasaki Y. Detection of residual disc hernia material and confirmation of nerve root decompression at lumbar disc herniation surgery by intraoperative ultrasound. Ultrasound Med Biol. 2009;35:920–927. doi:10.1016/j.ultrasmedbio.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 45. Tokuhashi Y, Matsuzaki H, Oda H, Uei H. Effectiveness of posterior decompression for patients with ossification of the posterior longitudinal ligament in the thoracic spine: usefulness of the ossification-kyphosis angle on MRI. Spine (Phila Pa 1976). 2006;31:E26–E30. [DOI] [PubMed] [Google Scholar]
- 46. Mueller LA, Degreif J, Schmidt R, et al. Ultrasound-guided spinal fracture repositioning, ligamentotaxis, and remodeling after thoracolumbar burst fractures. Spine (Phila Pa 1976). 2006;31:E739–E746. doi:10.1097/01.brs.0 000 237012.83128.80. [DOI] [PubMed] [Google Scholar]
- 47. Matsuyama Y, Kawakami N, Yanase M, et al. Cervical myelopathy due to OPLL: clinical evaluation by MRI and intraoperative spinal sonography. J Spinal Disord Tech. 2004;17:401–404. [DOI] [PubMed] [Google Scholar]
- 48. Bose B. Thoracic extruded disc mimicking spinal cord tumor. Spine J. 2003;3:82–86. [DOI] [PubMed] [Google Scholar]
- 49. Lazennec JY, Saillant G, Hansen S, Ramare S. Intraoperative ultrasonography evaluation of posterior vertebral wall displacement in thoracolumbar fractures. Neurol Med Chir (Tokyo). 1999;39:8–14. [DOI] [PubMed] [Google Scholar]
- 50. Blumenkopf B, Daniels T. Intraoperative ultrasonography (IOUS) in thoracolumbar fractures. J Spinal Disord. 1988;1:86–93. [PubMed] [Google Scholar]
- 51. Randel S, Gooding GA, Dillon WP. Sonography of intraoperative spinal arteriovenous malformations. J Ultrasound Med. 1987;6:539–544. [DOI] [PubMed] [Google Scholar]