Abstract
Protocadherins (Pcdhs) are a large family of cadherin-related molecules. They play a role in cell adhesion, cellular interactions, and development of the central nervous system. However, their expression and role in endothelial cells has not yet been characterized. Here, we examined the expression of selected clustered Pcdhs in endothelial cells from several vascular beds. We analyzed human and mouse brain microvascular endothelial cell (BMEC) lines and primary cells, mouse myocardial microvascular endothelial cell line, and human umbilical vein endothelial cells. We examined the mRNA and protein expression of selected Pcdhs using RT-PCR, Western blot, and immunostaining. A strong mRNA expression of Pcdhs was observed in all endothelial cells tested. At the protein level, Pcdhs-gamma were detected using an antibody against the conserved C-terminal domain of Pcdhs-gamma or an antibody against PcdhgC3. Deletion of highly expressed PcdhgC3 led to differences in the tight junction protein expression and mRNA expression of Wnt/mTOR (mechanistic target of rapamycin) pathway genes as well as lower transendothelial electrical resistance. Staining of PcdhgC3 showed diffused cytoplasmic localization in mouse BMEC. Our results suggest that Pcdhs may play a critical role in the barrier-stabilizing pathways at the blood–brain barrier.
Keywords: Adhesion molecules, blood–brain barrier, endothelium, cell culture, vascular biology
Introduction
Protocadherins (Pcdhs) regulate neuronal development and survival and they are highly expressed in the central nervous system (CNS). Pcdhs belong to the cadherin superfamily of transmembrane glycoproteins which mediate calcium-dependent cell–cell adhesion.1,2 They were first identified using PCR primers amplifying the cadherin ectodomain.3 To date, more than 80 Pcdhs have been identified in vertebrates. Pcdhs are classified into the clustered Pcdhs and nonclustered Pcdhs.4 Clustered Pcdhs are organized in three large clusters, α, β, and γ, on mouse chromosome 18 and human chromosome 5, while nonclustered Pcdhs are scattered in several genome loci.4,5 Pcdhs have six or seven cadherin-like extracellular domains, transmembrane domain, and C-terminal intracellular domain.
Expression of Pcdhs has been well characterized in neurons and observed in other cell types of the CNS such as astrocytes, pericytes, or choroid plexus epithelial cells.6,7 Extracellular domains of Pcdhs have a capacity to interact with each other both homophilically and heterophilically.8–10 Pcdh-alpha and -gamma have been shown to bind to each other and to the tyrosine kinases PYK2, FAK, and Ret and be a part of large membrane-bound protein complexes.11–13 Pcdhs promote neuronal development and survival and the direct interaction of Pcdh-gamma isoforms with CCM3 (cerebral cavernous malformation protein 3; also known as PDCD10: programmed cell death 10) has been shown to mediate this process.14 Moreover, Pcdhs are involved in cellular migration and cell sorting,15–19 cell survival,20,21 and the inhibition of cell growth.22,23 In neurons, Pcdhs were demonstrated to play a role in cell adhesion and communication.17 The Pcdh-gamma knockout mice show excessive apoptosis of interneurons and a decreased number of synapses of spinal cord interneurons.20,24
Vascular endothelial cells line the blood vessels and are the only cell type in the capillaries which are surrounded by a basal lamina. Endothelial cells build organ-specific blood tissue barriers, such as highly specialized blood–brain barrier (BBB).25 The integrity of the BBB depends on the stability of cell–cell contacts and on the expression of numerous adhesion molecules.26,27 The expression and role of Pcdhs, to authors’ best knowledge, was not characterized yet in brain microvascular endothelial cells (BMECs). Some reports, however, mention the presence of Pcdhs in brain vessels.14,28 In the present study, we analyze therefore the endogenous expression of selected Pcdhs in endothelial cells from brain, heart, and umbilical vein. We demonstrate a strong expression at the mRNA and protein level in BMECs and other vascular beds tested. In addition, a deletion of one representative of Pcdh-gamma family, a PcdhgC3 in mouse BMECs using a CRISPR/Cas9 system, led to expression changes of genes encoding for tight junction proteins and genes of Wnt and mechanistic target of rapamycin (mTOR) signaling pathways. Thus, Pcdhs might play an important role in BBB-related cellular processes.
Methods
Cell cultures
Mouse microvascular cerebral (cEND) and cerebellar (cerebEND) endothelial cell lines as well as the microvascular myocardial endothelial cell line MyEND were isolated, immortalized, and cultivated as described previously.29–37 Primary mouse BMECs (PELOBiotech) were cultured in ECM Medium (PELOBiotech) according to the manufacturer’s recommendations. Human microvascular cerebral endothelial cell line hDMEC/D3 was described previously and cultivated as previously described.26,38 Primary human umbilical vein endothelial cells (HUVEC) were isolated as described previously39,40 and maintained in M199 medium (Invitrogen) supplemented with 20% FCS, 25 µg/ml sodium heparin (Sigma), and 25 µg/ml endothelial cell growth supplement (Sigma). All cultures were supplemented with 100 U/ml of penicillin and 0.1 mg/ml streptomycin (1% PEST) (Sigma). Cells were maintained in an atmosphere of 5.0% CO2 and air and at 37℃. Endothelial cell lines were grown on plates coated with collagen IV (Fluka).
For establishment of PcdhgC3 (also known as Pcdh2) knockout cEND and cerebEND cells, the cells were cotransfected upon confluence with Pcdh2 CRISPR/Cas9 KO Plasmid and Pcdh2 HDR Plasmid (sc-430015 and sc-430015-HDR, Santa Cruz Biotechnology) using Effectene Transfection Reagent (Qiagen) and selected with 3 µg/ml puromycin for four weeks. Knockout was confirmed by Western blot analysis.
RT-PCR
For RT- PCR, total RNA was isolated from endothelial cells or frozen mouse brain tissue (strain C57BL/6, 8 weeks old) (Harlan Laboratories) using RNeasyMini kit (Qiagen). One microgram of RNA was used for the cDNA synthesis with Verso cDNA synthesis kit (Thermo Fisher Scientific). RT-PCR was performed using the AmpliTaq Gold® DNA Polymerase (Thermo Fisher Scientific) and PCR reactions were performed in 2720 Thermal Cycler (Thermo Fisher Scientific). To amplify the distinct Pcdhs, we used the primer sequences listed in Table 1.41 Mouse primer sequences for Pcdhs-gamma were kindly provided by J. Weiner (University of Iowa, USA). The primers were synthetized by Eurofins MWG. After amplification, 10 µl of each PCR reaction was loaded on 2% agarose gel containing 0.01% ethidium bromide. The picture of the gel was taken using FluorChem FC2 Multi-imager II (Alpha Innotech).
Table 1.
Primer sequences applied for amplification of protocadherins41 and J. Weiner, University of Iowa, USA, personal communication.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Human: | ||
| PCDHG A12 | TGCTGTCAGGTGATTCGGTA | GAGAAACGCCAGTCCGTGTTG |
| PCDHG A6 | AGCGAGCCTCTTCTGATAACTCA | GAGAAACGCCAGTCCGTGTTG |
| PCDHG B2 | ACAATGCCTCTTGGGAACAAA | GAGAAACGCCAGTCCGTGTTG |
| PCDHG C3 | GATCCGGTGTTCTATAGGCAGGTG | GAGAAACGCCAGTCCGTGTTG |
| PCDHG C4 | TATGGCAGGGGAGCCTGTT | GAGAAACGCCAGTCCGTGTTG |
| PCDHG C5 | CATCCGGTCCCGCTCTAA | GAGAAACGCCAGTCCGTGTTG |
| Mouse: | ||
| Pcdhg A1 | AAATGGACTGACTGGCCTGC | Pcdhg ce2 -R: CCAAGATCATGGCTTGCAGC |
| Pcdhg A2 | TGACAACATCCCCGAAGTC | |
| Pcdhg A3 | GCTTCAGGAAATGGATTGGC | (used with individual forward primers) |
| Pcdhg A4 | TGGGTTGTCATCCTTGCCTG | |
| Pcdhg A5 | TCTGGGATTTGTTACCGTGC | |
| Pcdhg A6 | GTCCACTTCACATTTCGTGG | |
| Pcdhg A7 | GCTACAGGCTTCTTCAGGTG | |
| Pcdhg A8 | AGGGCTCCAGAAGTGGATTG | |
| Pcdhg A9 | CCTACTCTTTGTGCTAGTGC | |
| Pcdhg A10 | GCTGCAGTCTTCGAGAGATG | |
| Pcdhg A11 | TGCCTCCTTCGCACTTTGTG | |
| Pcdhg A12 | CTCCCGAAAGAGTCACTTG | |
| Pcdhg B1 | CCTGAAGTCTCAACCTGTGG | |
| Pcdhg B2 | CTCTAACCTTGCGACTGGAG | |
| Pcdhg B4 | GCTTTCAGTCGGAAGTGGTG | |
| Pcdhg B5 | TCTAAGCCTGTGGTTTTCCC | |
| Pcdhg B6 | GTTCTAAGGCTAGACCAGGG | |
| Pcdhg B7 | CTAGACCAGTACTTCCACCC | |
| Pcdhg B8 | GCTGTAAGACGAGACCAGAG | |
| Pcdhg C3 | GTGAGCTCCCTGTACCGAAC | |
| Pcdhg C4 | TCACCAGATCTCGGAGGAGG | |
| Pcdhg C5 | CTCCAGGGAGTTCTATAAGC |
PCDHGA: protocadherin gamma A; PCDHGB: protocadherin gamma B; PCDHGC: protocadherin gamma C.
Real-time PCR
Real-time PCR was performed as described previously.42 Relative quantification of mRNA expression was performed with commercially available TaqMan Gene Expression Assays (Thermo Fisher Scientific). β-actin or 18SrRNA was used as endogenous controls. Measurements were performed with StepOnePlus Real-Time PCR System (Thermo Fisher Scientific).
Western blot analysis
Mouse brain capillaries were isolated from frozen brains by mechanical separation and centrifugation with 25% BSA. Isolated capillaries and mouse brain tissue were lysed in RIPA buffer followed by Western blot analysis. Endothelial cells were plated on collagen IV-coated dishes at a density of 1.1 × 105 cells per 3.5 cm2 and grown to confluence. At confluence, cells were maintained in medium containing 1% FCS for 48 h. For Western blot analyses, cells were lysed in RIPA buffer supplemented with protease inhibitors cocktail (Roche). Protein contents were quantified by BCA protein Assay Kit (Thermo Fisher Scientific) and 20 µg of protein were loaded on SDS-polyacrylamide gels for Western blot analysis. For immunoblotting, proteins were transferred to Hybond nitrocellulose membranes (Promega) which were blocked with 10% (w/v) low fat milk in PBS and incubated overnight at 4℃ with the respective primary antibody in blocking solution. The following primary antibodies were used: rabbit anti-claudin-3, mouse anti-claudin-5, rabbit anti-ZO-1 (Thermo Fisher Scientific), guinea pig anti-occludin (Acris), goat anti-VE-cadherin (Santa Cruz Biotechnology), pan-Pcdhg antibody (PCDHGC 5A2, sc-130556) against mouse and human conserved C-terminal region of the Pcdhg gene cluster (Santa Cruz Biotechnology), rabbit anti-PcdhgC3 (generously provided by M. Frank, University of Freiburg, Germany),43 and mouse monoclonal anti-β-actin antibody (Sigma). As secondary antibody, horseradish peroxidase-labeled anti-mouse (Roche), anti-rabbit (Cell Signaling), anti-goat and anti-guinea pig (Santa Cruz Biotechnology) IgGs were used at 1:3000 in blocking solution, respectively. For blots with pan-Pcdhg primary antibody anti-mouse IgA was used as a secondary antibody (Santa Cruz Biotechnology). Images were taken using an enhanced chemiluminescence detection kit (Promega) and FluorChem FC2 Multi-imager II (Alpha Innotech) with CD camera. Intensity of protein bands was calculated with ImageJ software.
Transendothelial electrical resistance (TEER) measurements
Wild-type cerebEND and PcdhgC3-knockout cells were grown on 24-well transwells (0.4 µm pores, Greiner) coated with collagen IV to confluence. TEER measurements were performed with chopstick electrode (World Precision Instruments Inc.) at day 7.
Immunocytochemistry
For visualization of claudin-5 and PcdhgC3 in control and knockout cerebEND cells, cells were grown on glass slides for five days and were differentiated in medium containing 1% FCS for one day. The cells were washed with PBS and fixed for 10 min with ice cold methanol. All glass slides were incubated in PBS/BSA for 15 min and then for 1 h in 5% normal swine serum at room temperature. The cells were incubated overnight at 4℃ with PcdhgC3-Biotin labeled antibody (Stress Marq) and claudin-5-Alexa Fluor 488 antibody (Thermo Fisher Scientific) at a dilution of 1:100 or 1:200, respectively. In case of biotin-labeled antibody, an additional blocking step with Streptavidin/Biotin Blocking Kit (Vector Laboratories) was performed. CF594 Streptavidin (Biotium) has been used to detect PcdhgC3-biotin. Incubations without primary antibodies served as a background control. DAPI staining was used to visualize the nuclei. After incubation, coverslips were mounted in Vectashield (Vector Laboratories). Images were generated with a Keyence BZ9000 microscope (Keyence).
Statistical analysis
For statistical comparison of two groups, we performed two-sided Student’s t-test with same variances; p values less than 0.05 were considered significant.
Results
Endothelial cells from different vascular beds express Pcdh mRNA
In order to investigate whether endothelial cells from brain, heart, and umbilical vein express selected Pcdhs, we first analyzed their mRNA expression. We analyzed the mouse microvascular endothelial cell lines of brain (primary BMEC, cEND, and cerebEND) or heart (MyEND) origin for the expression of Pcdh-gamma. We used the samples from mouse brain as a positive control (Figure 1(a)). We detected expression of all Pcdh-gamma in mouse brain. Also, all endothelial cell cultures were shown to express Pcdh-gamma. Only PcdhgA5 was not present in cEND, primary BMEC, and MyEND as well as the PcdhgB5 in MyEND. For expression analysis of selected human Pcdh-gamma, we used human BMEC line hCMEC/D3 and the primary HUVECs (Figure 1(b)). We detected mRNA expression of PCDHGA12, PCDHGA6, PCDHGB2, PCDHGC3, PCDHGC4, and PCDHGC5 in hCMEC/D3 cells. No product could be amplified for PCDHGB3 in these cells. HUVECs expressed mRNA for PCDHGA12, PCDHGB2, PCDHGC3, PCDHGC4, and PCDHGC5. We could not detect PCDHGA6 and PCDHGB3 expression in these cells. Expression of mRNA for these selected Pcdhs suggests that endothelial cells from different vascular beds may express a variety of Pcdh isoforms, similar to that observed in neurons.
Figure 1.
Endothelial cells from different vascular beds express high levels of protocadherins-gamma. (a) Messenger RNA expression of protocadherins-gamma (Pcdhg) in mouse endothelial cells. Total RNA was isolated from mouse brain, mouse primary brain microvascular endothelial cells (BMEC), endothelial cell lines cEND, cerebEND, and MyEND. After reverse transcription, PCR amplification was performed using specific primers for Pcdhg (Table 1) followed by the separation of PCR products in 2% agarose gel. (b) Messenger RNA expression of selected protocadherins-gamma (PCDHG) in human endothelial cells. Total RNA was isolated from human brain microvascular endothelial cell line hCMEC/D3 and human umbilical vein endothelial cells (HUVEC), reverse transcribed and subjected to PCR using specific primers for individual PCDHG (Table 1). The PCR products were separated in 2% agarose gel.
Endothelial cells from different vascular beds express high levels of Pcdh-gamma proteins
Having shown the expression of Pcdh-gamma on mRNA level, we proceeded with the detection of protein expression in endothelial cells (Figure 2). Lysates of mouse brain, mouse isolated capillaries, primary BMEC, cEND, cerebEND, and MyEND cells as well as human hCMEC/D3 and HUVECs were analyzed with a pan Pcdh-gamma antibody recognizing a constant cytoplasmic domain of mouse and human Pcdh-gamma (Figure 2(a)) or an antibody recognizing mouse and human PcdhgC3 (Figure 2(b)). We detected specific bands of molecular weight between 100 and 130 kDa corresponding to different Pcdh-gamma family proteins in accordance with the literature43 (Figure 2(a)). A single band of 130 kDa was detected with anti-PcdhgC3 antibody in all lysates (Figure 2(b)). The lowest level of Pcdh-gamma and PcdhgC3 was detected in HUVECs (Figure 2(a) and (b)). An additional band of about 25 kDa was also detected. According to literature, this band corresponds to the C-terminal fragment of Pcdh-gamma.44 β-actin was used as a loading control.
Figure 2.
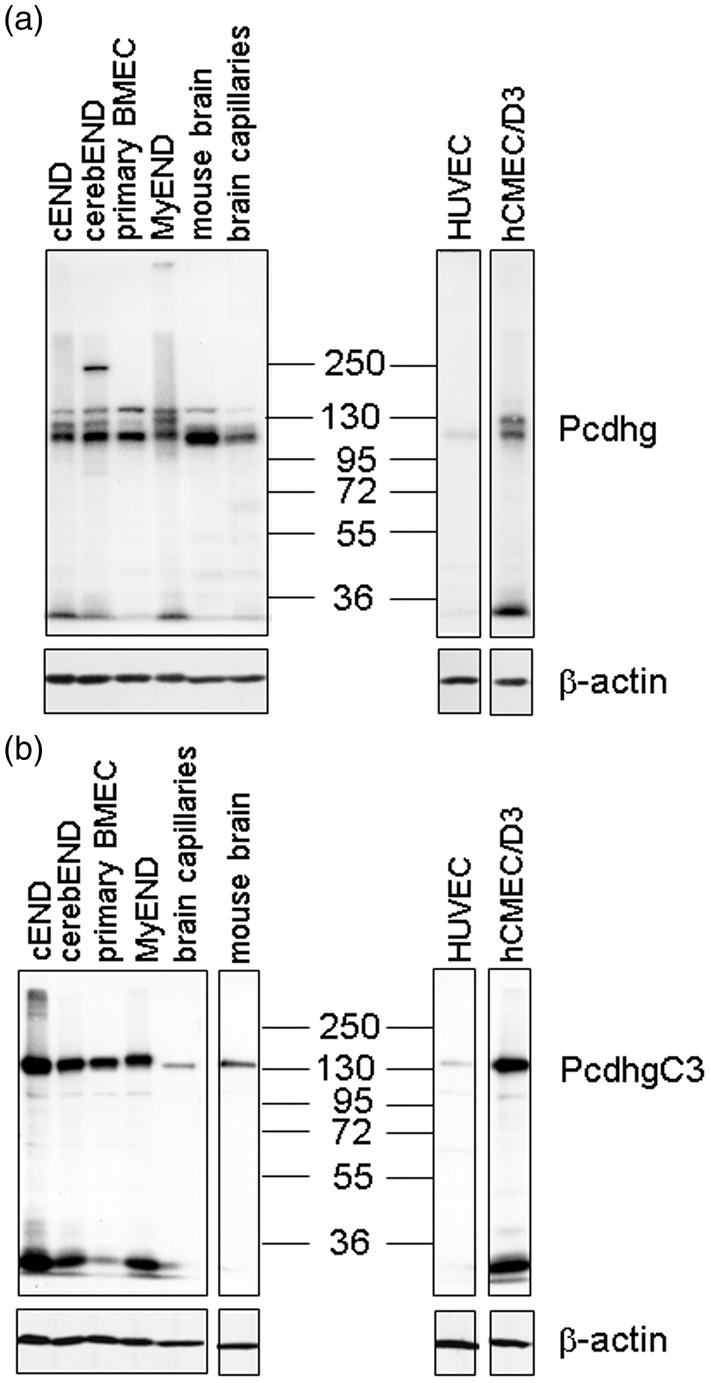
Protein levels of protocadherin-gamma (Pcdhg) family in endothelial cells. (a) Protein levels of mouse brain, isolated mouse brain capillaries, primary brain microvascular endothelial cells (BMEC) and cell lines cEND, cerebEND, MyEND as well as human primary umbilical vein endothelial cells (HUVEC) and immortalized human brain microvascular endothelial cell line hCMEC/D3 were analyzed in Western blot using a monoclonal antibody detecting 22 members of Pcdhg protein family that share an identical C-terminal cytoplasmic domain. β-actin detection was used as a loading control. (b) Protein levels of PcdhgC3 in endothelial cells. Lysates listed in Figure 2(a) were subjected to Western blot analysis with anti-PcdhgC3 antibody. β-actin detection was used as a loading control.
Knockout of PcdhgC3 leads to tight junctions protein upregulation
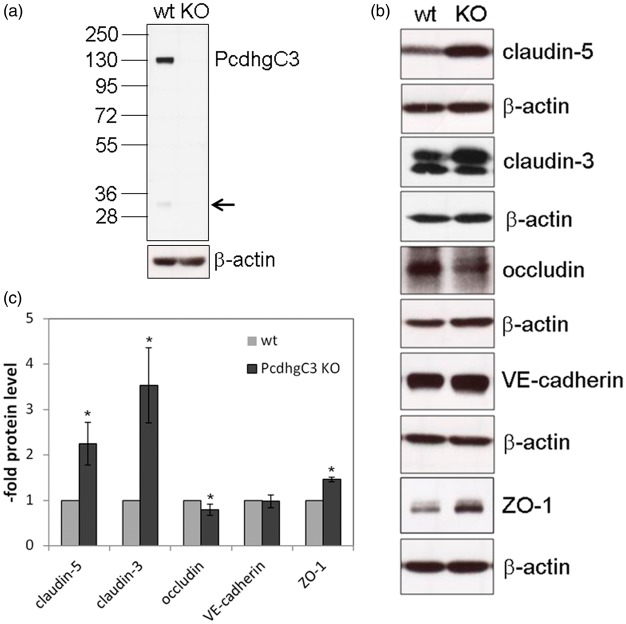
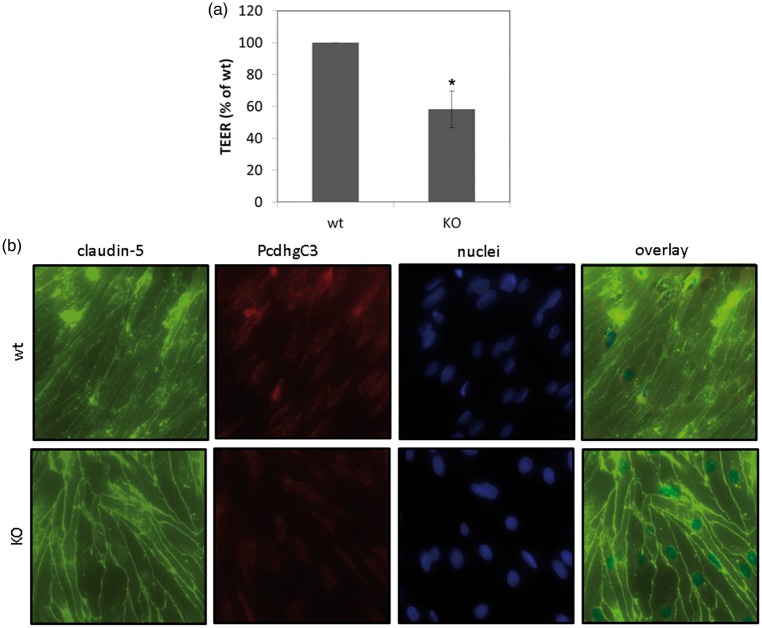
Pcdhs have been reported to play a role in cell differentiation and blockage of cell growth.22,23 In order to study cellular functions of Pcdhs in endothelial cells, we generated a stable knockout cerebEND cell line deficient for PcdhgC3 expression. The knockout efficiency in cerebEND was analyzed by Western blot (Figure 3(a)). CerebEND cell line appeared to be deficient for PcdhgC3 in comparison to control wild-type cells. The 130 kDa band corresponding to full length protein as well as the lower band of about 25 kDa corresponding to the C-terminal fragment of PcdhgC3 was not detectable in knockout cells (Figure 3(a)). Next, we analyzed the protein level of tight and adherens junctions proteins, claudin-5, claudin-3, occludin, ZO-1, and VE-cadherin in knockout and control cerebEND cells from the four following passages. We detected significantly increased protein levels of claudin-5 and claudin-3 as well as ZO-1. Interestingly, the level of occludin was lower in PcdhgC3 knockout cells (Figure 3(b) and (c)). However, despite the increased protein level of claudin-3, -5, and ZO-1, the TEER values of PcdhgC3 knockout cells were significantly lower in comparison to wild-type cells (Figure 4(a)). In addition, the tight junctions of cerebEND cells were stained with anti-claudin-5 antibody and biotin-labeled anti-PcdhgC3 antibody (Figure 4(b)). PcdhgC3 knockout cells showed enhanced claudin-5 staining with more evenly formed tight junctions, which was in accordance with Western blot results. In control cerebEND cells, PcdhgC3 was stained in the cytoplasm (Figure 4(b)).
Figure 3.
Effects of PcdhgC3 knockout on tight junction protein level in cerebEND. (a) Efficiency of PcdhgC3 knockout in cerebEND. β-actin served as a loading control. The C-terminal fragment of PcdhgC3 of about 25 kDa in wild-type cerebEND is indicated by the arrow. (b) Western blot of tight junction proteins in cerebEND and PcdhgC3 knockout cerebEND. Lysates of the four following passages of cerebEND (wt) and knockout cerebEND (KO) were subjected to Western blot and detected with respective antibodies. Representative blots are shown. (c) Densitometric analysis of Western blot presented in (b).
Figure 4.
Transendothelial electrical resistance and intracellular localization of PcdhgC3. (a) Transendothelial electrical resistance (TEER) measurement. Wild-type cerebEND (wt) and PcdhgC3 knockout cerebEND (KO) were seeded on collagen IV coated transwells. TEER was measured at day 7. The results of three independent experiments are presented as a percentage of the control wild-type cells, which were set as 100%. (b) Wild-type cerebEND (wt) and PcdhgC3 knockout cerebEND (KO) were seeded on collagen IV coated glass slices, grown to confluence and differentiated in serum-reduced medium for one day. The cells were then fixed and stained with PcdhgC3 and claudin-5 antibodies. DAPI was used to visualize the nuclei. Images were taken at 400× magnification. DAPI: 4′,6-Diamidino-2-Phenylindole.
Knockout of PcdhgC3 leads to changed gene expression in cerebEND
We analyzed on mRNA level the expression of genes encoding for tight and adherens junctions proteins, mesenchymal markers, mTOR pathway, and Wnt pathway (Table 2). We detected changes in genes encoding for tight and adherens junctions proteins, genes of mTOR, and Wnt pathway, suggesting that PcdhgC3 might play a role in cellular processes mediated by these pathways.
Table 2.
Effects of PcdhgC3 knockout on gene expression in mouse brain microvascular endothelial cell line cerebEND.
| Gene symbol | PcdhgC3KO relative to wild-type cells set as 1 |
|---|---|
| Tight junctions: | |
| Cldn3 | 0.7 ± 0.1 (*) |
| Cldn5 | 4.4 ± 1.71 (*) |
| Cdh5 | 2.2 ± 0.77 |
| Ocln | 0.2 ± 0.07 (*) |
| Tjp1 | 2.6 ± 0.79 (*) |
| Mesenchymal markers: | |
| Fn-1 | 1.0 ± 0.38 |
| Snail | 1.0 ± 0.18 |
| Vim | 1.4 ± 0.27 |
| mTOR pathway: | |
| mTOR | 0.8 ± 0.1 (*) |
| Sqstm-1 | 0.4 ± 0.19 (*) |
| Wnt pathway: | |
| Axin-1 | 0.7 ± 0.08 (*) |
| Fzd-1 | 1.4 ± 0.2 (*) |
| Gsk3b | 0.8 ± 0.12 (*) |
| Lrp-5 | 0.6 ± 0.12 (*) |
| Pard3 | 0.6 ± 0.18 (*) |
Cdh5: cadherin 5 (VE-cadherin); Cldn: claudin; Fn-1: fibronectin-1; Fzd-1: Wnt/fizzled; Gsk3b: glycogen synthase kinase 3 beta; Lrp-5: LDL receptor-related protein 5; mTOR: mechanistic target of rapamycin; Ocln: occludin; Pard3: Par-3 family cell polarity regulator; Snail: snail family zinc finger 1; Sqstm-1: sequestosome 1; Tjp1: tight junction protein 1 (ZO-1); Vim: vimentin.
Values represent at least four different experiments and data are presented as mean ± SD. Statistical significance was evaluated using t-test and p values lower than 0.05 were considered as statistically significant (*).
Discussion
To date, the expression of Pcdhs was described in the brain in neurons, astrocytes, and epithelial cells of choroid plexus.6,7,43 Since the BBB integrity is an important factor in brain development and in numerous neurological disorders, we decided to characterize the expression of selected Pcdhs in these highly specialized endothelial cells and representative endothelial cells from other vascular beds. Moreover, we generated endothelial knockout of one representative of the Pcdh-gamma family, PcdhgC3. We showed effects of PcdhgC3 knockout on tight junctions’ protein expression as well as on genes of Wnt- and mTOR-signaling pathways.
RT-PCR analysis showed high expression of Pcdh-gamma in mouse and human in vitro BBB models as well as in primary BMEC. The expression of all tested Pcdhs has been previously described in the brain. We used the mouse brain tissue as a positive control in RT-PCR and in Western blot.
We detected at the mRNA level the expression of clustered Pcdh-gamma in mouse cells and the expression of selected PCDHG in human cells. We used gene-specific primers, which have been previously published by other groups and were proven to give specific products. For Western blot, we used an antibody against the common C-terminal domain of Pcdh-gamma, which allows detection of 22 different members of Pcdh-gamma protein family or against one representative of Pcdhg family, PcdhgC3. Pcdhgs share a constant C-terminal domain but differ in molecular weight. As expected, we detected multiple bands corresponding to proteins of 100–130 kDa molecular weight. The Pcdh-gamma and PcdhgC3 proteins were detected in all endothelial cell lines, primary BMEC, mouse brain, and isolated mouse brain capillaries, which indicated high endogenous Pcdh-gamma protein family levels. HUVECs expressed the lowest level of Pcdh-gamma proteins, which is consistent with the low or absent expression of Pcdh mRNA in these cells. Intriguingly, the lower band corresponding to the C-terminal fragment of Pcdh-gamma showed differential protein levels in analyzed samples. Higher levels of C-terminal fragment were detected in immortalized cell lines cEND, cerebEND, MyEND, and hCMEC/D3 while primary BMEC, HUVEC, isolated capillaries, and mouse brain lysates showed low C-terminal fragment levels. One possible correlation between higher C-terminal fragment expression and lower barrier properties of immortalized cell lines in comparison to the primary BMEC or in situ brain environment could be drawn. Pcdhs are proteolytically cleaved by γ-secretase complex. The cleaved intracellular C-terminal fragments are soluble and have a potential to enter the nucleus.9,44 Other known γ-secretase targets exert multiple cellular functions, e.g. Notch and amyloid precursor protein.45,46 Distinct roles for the C-terminal fragment of Pcdhg-gamma were proposed. The mice lacking the functional intracellular domain of Pcdh-gamma show neonatal lethality and express lower levels of Pcdh-gamma.47 The C-terminal fragment increased the promoter activity and the expression of Pcdh-gamma showing transcription-regulating function.47 However, more research is needed to elucidate the role of the Pcdh-gamma C-terminal fragment in endothelial cells.
Measurements of TEER revealed lower barrier properties in PcdhgC3 knockout cells in comparison to wild-type cells. This result was unexpected because knockout cells had higher expression of major tight junction proteins claudin-3, -5, and ZO-1. One possible explanation could be that the deletion of PcdhgC3 triggers some unknown regulators of barrier properties, which result in lower TEER values despite the higher tight junction protein expression. On the other side, the overexpression of claudin-3 is observed in numerous epithelial cancers, e.g. colon, breast, pancreas, and prostate and has been associated with higher motility, invasion, and survival.48,49 Immunostaining with anti-PcdhgC3 antibody was diffuse in the cytoplasm of cerebEND cells with no staining of the nucleus. In previous reports, plasma membrane localization has been described for PcdhgC3 overexpressed as fusion with green fluorescent protein in human embryonic kidney cells (HEK293) and in v-Fos transformed fibroblasts.43,50 In addition, the localization at the plasma membrane and cell junctions has been previously reported for nonclustered Pcdh, Pcdh12 in bEND.3.51 However, no membrane staining was observed for PcdhgC3 in cerebEND. Claudin-5 tight junctions appeared thicker in PcdhgC3 knockout cerebEND cells.
It has been demonstrated that Pcdhs play a role in cell growth inhibition.22,23 In neuronal cells Pcdh-alpha and Pcdh-gamma undergo differentiation-induced phosphorylation, indicating the involvement of Pcdhs in signaling pathways during neuronal differentiation.12 Using a PcdhgC3 knockout cerebEND cells we could show that PcdhgC3 might be involved in processes of tight and adherens junctions protein regulation. Moreover, the changes in expression of genes encoding for proteins of Wnt and mTOR signaling pathways suggest the more global role of PcdhC3 in endothelial cell physiology. Wnt signaling pathway has been shown to play a role in the development and maintenance of the BBB, while mTOR signaling pathway is a therapeutic target of brain metastases and cerebral ischemia–reperfusion injury.52–54 PcdhgC3 has been proposed to have tumor suppressor capabilities in human colorectal cancer.55 Changes in Wnt and mTOR pathway due to downregulation of Pcdhg have been demonstrated in colorectal cancer cell lines and in Wilms’ tumors.41,55 In addition, a recent study showed a direct interaction of PcdhgC3 with Axin1, a key component of the canonical Wnt pathway.56 Tight and adherens junctions protein changes due to PcdhgC3 could also be relevant for tumor research, as most of the epithelial tumors show changes in tight junctions organization.49 However, more research is needed to elucidate these effects in detail.
To our best knowledge, we demonstrated for the first time a strong endogenous expression of different Pcdh-gamma in endothelial cells from brain, heart, and umbilical vein at mRNA and protein level. Knockout of one representative of pcdh-gamma family, a PcdhgC3 led to changes in cell–cell contacts as well as signaling pathways suggesting a critical role of Pcdhs in endothelial homeostasis.
Acknowledgments
The authors are grateful to Elisabeth Wilken for excellent technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by institutional/departmental sources.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
CD acquired and analyzed data and was involved in writing of the manuscript, MB designed the study, analyzed and interpreted the data, and wrote the manuscript. NR and CYF contributed essential material and critically revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol 2000; 148: 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huntley GW. Dynamic aspects of cadherin-mediated adhesion in synapse development and plasticity. Biol Cell 2002; 94: 335–344. [DOI] [PubMed] [Google Scholar]
- 3.Sano K, Tanihara H, Heimark RL, et al. Protocadherins: a large family of cadherin-related molecules in central nervous system. EMBO J 1993; 12: 2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SY, Yasuda S, Tanaka H, et al. Non-clustered protocadherin. Cell Adh Migr 2011; 5: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank M, Kemler R. Protocadherins. Curr Opin Cell Biol 2002; 14: 557–562. [DOI] [PubMed] [Google Scholar]
- 6.Lobas MA, Helsper L, Vernon CG, et al. Molecular heterogeneity in the choroid plexus epithelium: the 22-member gamma-protocadherin family is differentially expressed, apically localized, and implicated in CSF regulation. J Neurochem 2012; 120: 913–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrett AM, Weiner JA. Control of CNS synapse development by {gamma}-protocadherin-mediated astrocyte-neuron contact. J Neurosci 2009; 29: 11723–11731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obata S, Sago H, Mori N, et al. Protocadherin Pcdh2 shows properties similar to, but distinct from, those of classical cadherins. J Cell Sci 1995; 108: 3765–3773. [DOI] [PubMed] [Google Scholar]
- 9.Reiss K, Maretzky T, Haas IG, et al. Regulated ADAM10-dependent ectodomain shedding of gamma-protocadherin C3 modulates cell-cell adhesion. J Biol Chem 2006; 281: 21735–21744. [DOI] [PubMed] [Google Scholar]
- 10.Murata Y, Hamada S, Morishita H, et al. Interaction with protocadherin-gamma regulates the cell surface expression of protocadherin-alpha. J Biol Chem 2004; 279: 49508–49516. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Lu Y, Meng S, et al. Alpha- and gamma-protocadherins negatively regulate PYK2. J Biol Chem 2009; 284: 2880–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schalm SS, Ballif BA, Buchanan SM, et al. Phosphorylation of protocadherin proteins by the receptor tyrosine kinase Ret. Proc Natl Acad Sci USA 2010; 107: 13894–13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han MH, Lin C, Meng S, et al. Proteomics analysis reveals overlapping functions of clustered protocadherins. Mol Cell Proteomics 2010; 9: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin C, Meng S, Zhu T, et al. PDCD10/CCM3 acts downstream of {gamma}-protocadherins to regulate neuronal survival. J Biol Chem 2010; 285: 41675–41685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroda H, Inui M, Sugimoto K, et al. Axial protocadherin is a mediator of prenotochord cell sorting in Xenopus. Dev Biol 2002; 244: 267–277. [DOI] [PubMed] [Google Scholar]
- 16.Medina A, Swain RK, Kuerner KM, et al. Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation. EMBO J 2004; 23: 3249–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aamar E, Dawid IB. Protocadherin-18a has a role in cell adhesion, behavior and migration in zebrafish development. Dev Biol 2008; 318: 335–346. [DOI] [PubMed] [Google Scholar]
- 18.Bononi J, Cole A, Tewson P, et al. Chicken protocadherin-1 functions to localize neural crest cells to the dorsal root ganglia during PNS formation. Mech Dev 2008; 125: 1033–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakao S, Platek A, Hirano S, et al. Contact-dependent promotion of cell migration by the OL-protocadherin-Nap1 interaction. J Cell Biol 2008; 182: 395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Weiner JA, Levi S, et al. Gamma protocadherins are required for survival of spinal interneurons. Neuron 2002; 36: 843–854. [DOI] [PubMed] [Google Scholar]
- 21.Emond MR, Jontes JD. Inhibition of protocadherin-alpha function results in neuronal death in the developing zebrafish. Dev Biol 2008; 321: 175–187. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki N, Takahashi N, Kojima S, et al. Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation. Carcinogenesis 2002; 23: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 23.Yu JS, Koujak S, Nagase S, et al. PCDH8, the human homolog of PAPC, is a candidate tumor suppressor of breast cancer. Oncogene 2008; 27: 4657–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiner JA, Wang X, Tapia JC, et al. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA 2005; 102: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 26.Forster C, Burek M, Romero IA, et al. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J Physiol 2008; 586: 1937–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haseloff RF, Dithmer S, Winkler L, et al. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin Cell Dev Biol 2015; 38: 16–25. [DOI] [PubMed] [Google Scholar]
- 28.Garrett AM, Schreiner D, Lobas MA, et al. gamma-protocadherins control cortical dendrite arborization by regulating the activity of a FAK/PKC/MARCKS signaling pathway. Neuron 2012; 74: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forster C, Silwedel C, Golenhofen N, et al. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol 2005; 565: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silwedel C, Forster C. Differential susceptibility of cerebral and cerebellar murine brain microvascular endothelial cells to loss of barrier properties in response to inflammatory stimuli. J Neuroimmunol 2006; 179: 37–45. [DOI] [PubMed] [Google Scholar]
- 31.Golenhofen N, Ness W, Wawrousek EF, et al. Expression and induction of the stress protein alpha-B-crystallin in vascular endothelial cells. Histochem Cell Biol 2002; 117: 203–209. [DOI] [PubMed] [Google Scholar]
- 32.Burek M, Salvador E, Forster CY. Generation of an immortalized murine brain microvascular endothelial cell line as an in vitro blood brain barrier model. J Vis Exp 2012; 66: e4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blecharz KG, Burek M, Bauersachs J, et al. Inhibition of proteasome-mediated glucocorticoid receptor degradation restores nitric oxide bioavailability in myocardial endothelial cells in vitro. Biol Cell 2014; 106: 219–235. [DOI] [PubMed] [Google Scholar]
- 34.Helms HC, Abbott NJ, Burek M, et al. In vitro models of the blood-brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab 2016; 36: 862–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forster C, Waschke J, Burek M, et al. Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J Physiol 2006; 573: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blecharz KG, Frey D, Schenkel T, et al. Autocrine release of angiopoietin-2 mediates cerebrovascular disintegration in Moyamoya disease. J Cereb Blood Flow Metab. Epub ahead of print 5 July 2016. DOI: 10.1177/0271678X16658301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuhaus W, Burek M, Djuzenova CS, et al. Addition of NMDA-receptor antagonist MK801 during oxygen/glucose deprivation moderately attenuates the upregulation of glucose uptake after subsequent reoxygenation in brain endothelial cells. Neurosci Lett 2012; 506: 44–49. [DOI] [PubMed] [Google Scholar]
- 38.Weksler BB, Subileau EA, Perriere N, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 2005; 19: 1872–1874. [DOI] [PubMed] [Google Scholar]
- 39.Burek M, Maddika S, Burek CJ, et al. Apoptin-induced cell death is modulated by Bcl-2 family members and is Apaf-1 dependent. Oncogene 2006; 25: 2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tas PW, Stobetael C, Roewer N. The volatile anesthetic isoflurane inhibits the histamine-induced Ca2+ influx in primary human endothelial cells. Anesth Analg 2003; 97: 430–435. table of contents. [DOI] [PubMed] [Google Scholar]
- 41.Dallosso AR, Hancock AL, Szemes M, et al. Frequent long-range epigenetic silencing of protocadherin gene clusters on chromosome 5q31 in Wilms’ tumor. PLoS Genet 2009; 5: e1000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burek M, Haghikia A, Gold R, et al. Differential cytokine release from brain microvascular endothelial cells treated with dexamethasone and multiple sclerosis patient sera. J Steroids Hormon Sci 2014; 5: 128. [Google Scholar]
- 43.Frank M, Ebert M, Shan W, et al. Differential expression of individual gamma-protocadherins during mouse brain development. Mol Cell Neurosci 2005; 29: 603–616. [DOI] [PubMed] [Google Scholar]
- 44.Haas IG, Frank M, Veron N, et al. Presenilin-dependent processing and nuclear function of gamma-protocadherins. J Biol Chem 2005; 280: 9313–9319. [DOI] [PubMed] [Google Scholar]
- 45.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 1998; 393: 382–386. [DOI] [PubMed] [Google Scholar]
- 46.Weidemann A, Konig G, Bunke D, et al. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell 1989; 57: 115–126. [DOI] [PubMed] [Google Scholar]
- 47.Hambsch B, Grinevich V, Seeburg PH, et al. {gamma}-Protocadherins, presenilin-mediated release of C-terminal fragment promotes locus expression. J Biol Chem 2005; 280: 15888–15897. [DOI] [PubMed] [Google Scholar]
- 48.Walther W, Petkov S, Kuvardina ON, et al. Novel Clostridium perfringens enterotoxin suicide gene therapy for selective treatment of claudin-3- and -4-overexpressing tumors. Gene Ther 2012; 19: 494–503. [DOI] [PubMed] [Google Scholar]
- 49.Salvador E, Burek M, Forster CY. Tight junctions and the tumor microenvironment. Curr Pathobiol Rep 2016; 4: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGarry LC, Winnie JN, Ozanne BW. Invasion of v-Fos(FBR)-transformed cells is dependent upon histone deacetylase activity and suppression of histone deacetylase regulated genes. Oncogene 2004; 23: 5284–5292. [DOI] [PubMed] [Google Scholar]
- 51.Telo P, Breviario F, Huber P, et al. Identification of a novel cadherin (vascular endothelial cadherin-2) located at intercellular junctions in endothelial cells. J Biol Chem 1998; 273: 17565–17572. [DOI] [PubMed] [Google Scholar]
- 52.Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res 2014; 355: 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chi OZ, Mellender SJ, Barsoum S, et al. Effects of rapamycin pretreatment on blood-brain barrier disruption in cerebral ischemia-reperfusion. Neurosci Lett 2016; 620: 132–136. [DOI] [PubMed] [Google Scholar]
- 54.Winkler F, Osswald M, Blaes J, et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res 2016; 22(24): 6078–6087. [DOI] [PubMed] [Google Scholar]
- 55.Dallosso AR, Oster B, Greenhough A, et al. Long-range epigenetic silencing of chromosome 5q31 protocadherins is involved in early and late stages of colorectal tumorigenesis through modulation of oncogenic pathways. Oncogene 2012; 31: 4409–4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mah KM, Houston DW and Weiner JA. The gamma-Protocadherin-C3 isoform inhibits canonical Wnt signalling by binding to and stabilizing Axin1 at the membrane. Sci Rep 2016; 6: 31665. [DOI] [PMC free article] [PubMed]