Abstract
Background and purpose
No series reported the mid-term results of Trufill DCS Orbit and Orbit Galaxy detachable coils with independent evaluation. We present the one-year safety and efficacy of these coils in real-life routine clinical practice.
Methods
A total of 167 patients with 167 aneurysms (39.1% ruptured) were enrolled in the prospective TRULINE study. The primary endpoint was the safety, assessed by the combined morbidity-mortality rate observed since the time of the procedure and up to one-year follow-up. For safety, primary analyses were performed on intent-to-treat population (attempted coils procedure) and all adverse events have been reviewed by an independent Data Safety Monitoring Board. For efficacy, primary analyses were performed on the per-protocol population (patients treated with more than 70% of Trufill coils and not retreated during the follow-up period) and an independent core laboratory evaluated angiographic results.
Results
At one-year post-procedure, neurologic impairment was observed in 6.5% (95% confidence interval: 3.5–11.8) of the patients, and 2.6% (95% confidence interval: 1.0–6.8) had a permanent neurological deterioration. Three deaths were observed, unrelated to the procedure or coils. At one year, complete occlusion was seen in 52 aneurysms (54.2%), neck remnant in 28 aneurysms (29.2%), and aneurysm remnant in 16 aneurysms (16.7%). During the one-year follow-up, the overall incidence of recurrence was 30.2% with a mean interval of 13.8 ± 4.5 months and the retreatment for major recanalization was needed in nine patients (6.3%).
Conclusions
The TRULINE study confirms that endovascular coiling with Trufill DCS Orbit and Orbit Galaxy detachable coils is safe and effective.
Keywords: Intracranial aneurysms, coiling, endovascular treatment, recanalization
Endovascular treatment by using bare platinum coils is now an established technique for both ruptured and unruptured aneurysms.1 The Trufill DCS Orbit and Orbit Galaxy detachable coils (Codman Neurovascular, Rayhnam, Massachusetts, USA) are indicated for treatment of intracranial aneurysms. Few results are reported concerning the long-term safety and efficacy of these intracranial coils.2–6 The Trufill DCS Orbit coils were evaluated prospectively at long-term in only one study;2 however 78.9% (236/299) of patients were lost on long-term follow-up, and only 21% (60/299) of patients had a one-year angiographic follow-up, limiting the precision of the results.7 In addition, no consecutive large series reported the long-term safety and efficacy of the Trufill DCS Orbit and Orbit Galaxy. Thus, to date, the results of endovascular treatment (EVT) with these coils are not well known.
The TRULINE study was conducted in seven French centres to evaluate the long-term safety and efficacy of the Trufill DCS Orbit and Orbit Galaxy detachable coils in real-life routine clinical practice.
Material and methods
TRULINE protocol
TRULINE (TRUfill’s Line in Intracranial aNeurysm Embolization) was a prospective, consecutive, observational study involving the use of the Trufill DCS Orbit and Orbit Galaxy Detachable Coils in the treatment of intracranial aneurysms between December 2011– October 2014 in seven French centres. Adult patients with a ruptured or unruptured aneurysm considered suitable for endovascular treatment were included. Exclusion criteria included refusal to take part in the study by the patient, or a representative in case of patient’s inability, after having been informed by the investigator and having received information letter, and patient already enrolled in a clinical study involving experimental medication or device. Informed consent from the patient authorizing the collection of data was obtained. The protocol was approved by French Ministry administrations (CEPP, CCTIRS and CNIL) in compliance with the Helsinki Declaration, the French law ‘Informatique et libertés' for patient data protection and good clinical practice (GCP) as defined in ISO EN 14155-1 & 2 and ICH E9 on statistical methods.
Patients
A total of 167 patients have been included in the TRULINE study in seven initiated sites (Hôpital Neurologique Pierre Wertheimer, Lyon (90 patients); Clairval Hospital, Marseille (27 patients); Timone University Hospital, Marseille (10 patients); Pasteur Hospital, Colmar (25 patients); Pontchaillou University Hospital, Rennes (six patients); Rotchschild Fondation, Paris (five patients); Gabriel Montpied University Hospital, Clermont-Ferrand (four patients)). Among the 167 screened patients, four were not included in the intention-to-treat (ITT) population according to the core laboratory decision (patients with dissection, not aneurysms). Out of the 163 patients included patients (ITT population), 20 did not complete the study, 17 were lost to follow-up and three died (Figure 1). For these 163 patients, 39 did not respect at least one required criterion to be included in the per protocol (PP) population (five patients did not fulfil inclusion and non-inclusion criteria, nine patients had a re-treatment during the follow-up and 33 patients were not treated with at least 70% of Trufill coils (Trufill DCS Orbit and Orbit Galaxy coils). The PP analysis population was composed of 124 patients.
Figure 1.
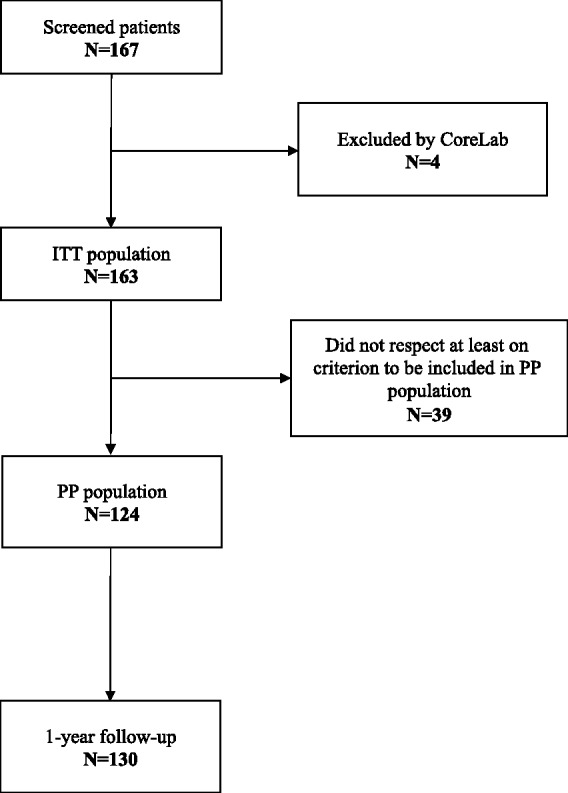
Flow chart. PP: per protocol.
There were 109 women and 54 men (37.5% of the patients had arterial hypertension, 21.3% dyslipidemia, 6.9% diabetes mellitus and 39.1% of patients were current smokers) with a mean age of 53.8 years, harbouring 163 aneurysms. WFNS (World Federation Neurological Score) and modified Rankin Scale (mRS) values are presented in Table 1.
Table 1.
Baseline clinical status.
| Scale and score | PP population |
ITT population (n = 163) | |
|---|---|---|---|
| Yes (n = 124) | No (n = 39) | ||
| WFNS (ruptured only) – n (%) | |||
| Missing | 3 | 1 | 4 |
| I | 11 (25) | 5 (33.3) | 16 (27.1) |
| II | 16 (36.4) | 4 (26.7) | 20 (33.9) |
| III | 10 (22.7) | 0 | 10 (16.9) |
| IV | 1 (2.3) | 4 (26.7) | 5 (8.5) |
| V | 6 (13.6) | 2 (13.3) | 8 (13.6) |
| mRS – n (%) | |||
| Missing | 3 | 2 | 5 |
| 0 | 60 (49.6) | 21 (56.8) | 81 (51.3) |
| 1 | 31 (25.6) | 9 (24.3) | 40 (25.3) |
| 2 | 11 (9.1) | 1 (2.7) | 12 (7.6) |
| 3 | 10 (8.3) | 1 (2.7) | 11 (7) |
| 4 | 14 | 5.0 | 3 (1.9) |
| 5 | 8 (6.6) | 3 (8.1) | 11 (7) |
| 6 | 0 | 0 | 0 |
Baseline neurological status was evaluated by two of the World Federation Neurological Surgeons (WFNS) and the modified Rankin Scale (mRS) for per-protocol (PP) population and intention-to-treat (ITT) population.
Aneurysms
Baseline aneurysm characteristics are summarized in Table 2. Among the 163 target aneurysms treated, 98 aneurysms (60.9%) were non-ruptured and 63 (39.1%) were acutely ruptured (≤30 days). Aneurysms were mostly located in the anterior circulation (88.3%) and mainly the anterior communicating artery (30.7%). The average size of aneurysms was 6.5 ± 3.1 mm (sac height), 6.0 ± 3.7 mm (sac width), 3.4 ± 2.4 mm (neck), dome-to-neck ratio was 2.1 (±0.9) and 54.0% were considered as wide neck (neck width ≥ 4 mm).
Table 2.
Baseline aneurysms characteristics.
| Aneurysm characteristics | PP population |
ITT population (n = 163) | |
|---|---|---|---|
| Yes (n = 124) | No (n = 39) | ||
| Side – n (%) | |||
| Left | 45 (36.3) | 20 (51.3) | 65 (39.9) |
| Medial | 14 (11.3) | 7 (17.9) | 21 (12.9) |
| Right | 64 (51.6) | 12 (30.8) | 76 (46.6) |
| Unknown | 1 (0.8) | 0 | 1 (0.6) |
| Location – n (%) | |||
| Anterior cerebral | 8 (6.5) | 1 (2.6) | 9 (5.5) |
| Anterior communicating | 37 (29.8) | 13 (33.3) | 50 (30.7) |
| Basilar artery | 7 (5.6) | 5 (12.8) | 12 (7.4) |
| Termination carotid | 12 (9.7) | 1 (2.6) | 13 (8) |
| Cerebellar | 2 (1.6) | 1 (2.6) | 3 (1.8) |
| Middle cerebral artery bifurcation | 24 (19.4) | 7 (17.9) | 31 (19) |
| Middle cerebral artery pre-bifurcation | 2 (1.6) | 1 (2.6) | 3 (1.8) |
| Ophthalmic portion internal carotid artery | 5 (4) | 5 (12.8) | 10 (6.1) |
| Posterior cerebral artery | 2 (1.6) | 0 | 2 (1.2) |
| Posterior communicating | 18 (14.5) | 3 (7.7) | 21 (12.9) |
| Supraclinoid internal carotid artery | 5 (4) | 2 (5.1) | 7 (4.3) |
| Vertebral artery | 2 (1.6) | 0 | 2 (1.2) |
| Circulation – n (%) | |||
| Anterior circulation | 111 (89.5) | 33 (84.6) | 144 (88.8) |
| Posterior circulation | 13 (10.5) | 6 (15.4) | 19 (11.7) |
| Sac height (mm) | |||
| n | 118 | 38 | 156 |
| Mean (±SD) | 5.8 (3.5) | 6.8 (4.6) | 6 (3.7) |
| Min-max | 2–16 | 2–22 | 2–22 |
| Sac width (mm) | |||
| n | 90 | 23 | 113 |
| Mean (±SD) | 5.8 (3.5) | 6.8 (4.6) | 6 (3.7) |
| Min-max | 2–19 | 2.1–18 | 2–19 |
| Neck (mm) | |||
| n | 110 | 34 | 144 |
| Mean (±SD) | 3.4 (2.6) | 3.4 (1.5) | 3.4 (2.4) |
| Min-max | 1.3–24 | 1.7–9.3 | 1.3–24 |
| Dome-to-neck ratio | |||
| n | 110 | 34 | 144 |
| Mean (±SD) | 2.1 (0.9) | 2.1 (0.8) | 2.1 (0.9) |
| Min-max | 0.5–6 | 0.8–4.2 | 0.5–6 |
| Wide neck (≥4 mm) | 61 (55.5) | 17 (50) | 78 (54.2) |
| Aneurysm status – n (%) | |||
| Unruptured | 77 (62.2) | 21 (56.8) | 98 (60.9) |
| Ruptured (≤30 days) | 47 (37.9) | 16 (43.2) | 63 (39.1) |
ITT: intention to treat; PP: per-protocol; SD: standard deviation.
Endovascular procedures
All endovascular procedures were performed under general anaesthesia and systemic heparinization. For the treatment of the target aneurysms, five coils were used on average. According to the investigators, aneurysm filling was performed with at least 70% of Trufill coils (total length) in 80% of patients and additional techniques were used in 38% of cases (balloon-assisted coiling: 28.2%; stent-assisted coiling: 10.4%). Among the 1027 coils used for the treatment of the target aneurysms (Supplementary Material, Table 1), 73.3% of them were Trufill coils, with a majority of Orbit Galaxy coils (92%). Only 2% of procedures have not been completed with satisfactory results for the impossibility to deploy additional coils.
Angiographic follow-up
Anatomic results were systematically evaluated on digital subtraction angiography. The rate of aneurysm occlusion was assessed at the end of the procedure and at one-year (12–18 months) post-procedure follow-up independently by the core laboratory (Hubert Desal, Interventional Neuroradiology, Nord Laennec Hospital, Nantes, France and Georges Rodesch, Interventional Neuroradiology, Foch Hospital, Paris, France). Two three-point scales are used to classify the treated aneurysms according to whether they are adequately occluded or not: by using the simplified three-point Jean Raymond classification scale (complete occlusion, neck remnant, and aneurysm remnant),8 and by using the Ferns scale (100% occlusion, occlusion from 90 to 99%, and occlusion <90%).9
Bleeding/retreatment
Bleeding or rebleeding occurrence during the period of follow-up was evaluated. Retreatment of aneurysms during follow-up was also recorded.
Data analysis
Clinical and procedural data were collected using an electronic case report forms (e-CRFs) under the supervision of the principal investigator (Francis Turjman, Neurologic Hospital, Lyon, France). For safety, analyses were performed on the ITT population (attempted Trufill coils procedure) and for efficacy, analyses were performed on the PP population (patients treated with more than 70% of Trufill coils and not retreated during the follow-up period).
All adverse events have been reviewed by the Data Safety Monitoring Board Composition (DSMB; Evelyne Emery, Caen University Hospital, France; and Laurent Derex, FHU IRIS, Neurologic University Hospital, France) who adjudicated on the severity and relationship of the event to the device, the procedure and/or the disease. In case of disagreement between investigator and DSMB’s evaluation, the investigator was informed and had the possibility of modifying his initial evaluation. If the disagreement persisted, the investigator’s advice would have been taken into account for the final data analysis.
Morbidity and mortality of treatment were evaluated before and after treatment, at hospital discharge, at 30 days, and at one year (12–18 month) follow-up. The primary endpoint was the long-term safety of the coiling procedure, assessed by the combined morbidity-mortality rate observed since the time of the procedure up to one-year follow-up visit. The combined morbidity-mortality rate is defined by the proportion of patients for which a deterioration of their neurological status is observed between the pre-operative baseline measurement and any time until the one-year follow-up visit. Neurological status is evaluated with the mRS, and a deterioration of the neurological status is defined by: an increase of the mRS between 2–6 if the pre-operative mRS is equal to zero, or any increase of the mRS if the pre-operative mRS is equal or higher to one.
The rate of aneurysm recanalization up to one-year post-treatment was defined as the percentage of aneurysms in which recanalization occurs at any time up to and including the one-year post-procedure follow-up. Recanalization is defined as any increase in the size of the remnant or defined as a change of classification of the anatomic result.
Results
Clinical results
Clinical outcomes of patients are summarized in Table 3. Out of the 163 patients of the ITT population, 6.5% (95% CI: 3.5–11.8) of patients (n = 10) had a deterioration of their neurological status from the index procedure to the one-year follow-up visit. Permanent neurologic deterioration was found in 2.6% (95% CI: 1.0–6.8) of patients at 12 months. From these patients, 5.0% (95% CI: 2.5–9.8) of the patients ((n = 8) had a deterioration of their neurological status in the 30 days following the procedure leading to a permanent neurologic deterioration 1.9% (95% CI: 0.6–5.7). One late event has been collected at 29 months. Three deaths were observed, unrelated to the procedure or Trufill coils.
Table 3.
Evolution of clinical status between baseline and one-year follow-up.
| Modified Rankin Scale | Pre-procedure (n = 158) | Discharge (n = 157)a | 30 days (n = 147) | 1-year (n = 130)b |
|---|---|---|---|---|
| 0 | 81 (51.3) | 101 (64.3) | 85 (57.8) | 94 (72.3) |
| 1 | 40 (25.3) | 29 (18.5) | 50 (34) | 27 (20.8) |
| 2 | 12 (7.6) | 14 (8.9) | 8 (5.4) | 3 (2.3) |
| 3 | 11 (7.0) | 8 (5.1) | 2 (1.4) | 2 (1.5) |
| 4 | 3 (1.9) | 2 (1.3) | 1 (0.7) | 2 (1.5) |
| 5 | 11 (7) | 1 (0.6) | 1 (0.7) | 2 (1.5) |
| 6 | 0 | 2 (1.3) | 0 | 0 |
The median duration between the procedure date and the patient discharge date was six days; bthe median duration between the procedure date and the patient one-year follow-up date was 12.5 months.
At 12-month follow-up, for the PP population of 124 patients, 8.6% (95% CI: 4.7–15.4) of patients ((n = 10) had a deterioration of their neurological status. For the 98 patients with non-ruptured target aneurysm, 3.4% (95% CI: 1.1–10.3; (n = 3) had a deterioration of their neurological status at 12-month follow-up, and 1.1% (95% CI: 0.1–7.2; (n = 1) at one month. For the 63 patients with ruptured target aneurysm, 11.4% (95% CI: 5.6–22.4; (n = 7) had neurologic deterioration at 12-month, and 11.4% (95% CI: 5.6–22.4; (n = 7) at one month.
Angiographic results
Angiographic outcomes are summarized in Table 4. During the 12-month follow-up out of the 124 patients of the PP population, a complete aneurysm occlusion was seen in 52 aneurysms (54.2%), neck remnant in 28 aneurysms (29.2%), and aneurysm remnant in 16 aneurysms (16.7%).
Table 4.
Evolution of angiographic results between baseline and one-year follow-up.
| Angiographic scale | PP population |
ITT population (n = 163) | |
|---|---|---|---|
| Yes (n = 124) | No (n = 39) | ||
| Raymond scale – end of procedure – n (%) | |||
| Complete occlusion | 66 (53.2) | 20 (51.3) | 86 (52.8) |
| Residual neck | 38 (30.6) | 10 (25.6) | 48 (29.4) |
| Residual aneurysm | 20 (16.1) | 9 (23.1) | 29 (17.8) |
| Raymond scale – one-year post-procedure – n (%) | |||
| Complete occlusion | 52 (54.2) | 12 (37.5) | 64 (50) |
| Neck remnant | 28 (29.2) | 7 (21.9) | 35 (27.3) |
| Residual aneurysm | 16 (16.7) | 13 (40.6) | 29 (22.7) |
| Ferns scale – end of procedure – n (%) | |||
| 100% occluded | 66 (53.2) | 20 (51.3) | 86 (52.8) |
| Occlusion from 90 to 99% | 41 (33.1) | 10 (25.6) | 51 (31.3) |
| Occlusion <90% | 17 (13.7) | 9 (23.1) | 26 (16) |
| Ferns scale – one-year post-procedure – n (%) | |||
| 100% occluded | 52 (54.2) | 11 (34.4) | 63 (49.2) |
| Occlusion from 90 to 99% | 28 (29.2) | 7 (21.9) | 35 (27.3) |
| Occlusion <90% | 16 (16.7) | 14 (43.8) | 30 (23.4) |
At one year, for the secondary population of 163 patients (ITT population), complete occlusion was seen in 64 aneurysms (50.0%), neck remnant in 35 aneurysms (27.3%), and aneurysm remnant in 29 aneurysms (22.7%).
Aneurysm recanalization
During the one-year follow-up period, out of the 124 patients of the PP population, the overall incidence of recurrence was seen in 30.2% of the aneurysms with a mean interval of 13.8 ± 4.5 months. In the ITT population, the overall incidence of recurrence was seen in 33.6% of the aneurysms with a mean interval of 13.5 ± 4.4 months.
Retreatment
Aneurysm retreatment could be only analysed in ITT population (patient with retreatment were excluded from the PP population). During the one-year follow-up period, out of the 163 patients of the ITT population, 6.3% (95% CI: 3.3–11.8) of patients ((n = 9) had an aneurysm retreatment. For the retreated patients, none had a deterioration of his neurological status at one year.
Among the 98 patients with non-ruptured target aneurysm, 4.6% (95% CI: 1.7–11.7) of patients (n = 4) had an aneurysm retreatment at 12-month follow-up. Among the 63 patients with ruptured target aneurysm, 9.5% (95% CI: 4.0–21.3) of patients ((n = 5) had an aneurysm retreatment at 12-month follow-up
Bleeding/rebleeding
During the procedure, 6.4% (95% CI: 3.1–11.5]) of patients ((n = 10) had a subarachnoid haemorrhage (SAH; five before coiling and five during coiling), none leading to a modification of the initial clinical status. No recurrent SAH has been observed.
Seven of the 10 patients experiencing SAH already had an acute ruptured aneurysm before coiling; three of them had a non-ruptured aneurysm before coiling and the SAH was related to the procedure. Among the seven patients with acute ruptured aneurysm, five were declared as having an SAH before coiling and two of them, belonging to the same centre, were declared by investigator as having experienced SAH during the procedure. The discrepancy here leads in the way of filling the e-CRF data by investigators: for five patients with ruptured aneurysm, the SAH is declared as pre-existing before coiling while for the two other, the SAH is declared as observed during coiling which in fact corresponds to the pre-existing SAH (no serious adverse event declared for new SAH, no modification to the initial procedure, satisfactory results).
Discussion
TRULINE is an observational, prospective and multicentre study conducted in France to evaluate the long term safety and effectiveness of the Trufill DCS Orbit® and Orbit Galaxy coils for endovascular treatment of ruptured and unruptured intracranial aneurysms. In this study, only 20 patients (12.2%) were lost to follow-up at 12 months. The use of a DSMB to review all the adverse events and device related complications add to the quality of the final results. In addition, angiographic results were systematically evaluated by an independent core laboratory. In a recent meta-analysis, Rezek et al. demonstrated that core laboratories tend to report higher rates of unfavourable outcomes compared with self-reporting centers.7
One-year safety of the Trufill DCS coils
In the series of Bendok et al. dealing with 299 patients harbouring 313 aneurysms (43% ruptured aneurysms), six patients (2%) presented a post-operative neurological deficit and 10 patients died postoperatively; all deaths were judged to be unrelated to the device.2 However, only 21% (63/299) of patients treated have been clinically evaluated at one-year follow-up. We observed similar findings since 2.6% of the patients had a permanent deterioration of the neurological status at one-year post-procedure and 1.9% of patients had this permanent deterioration of the neurological status at 30 days post-procedure.
Compared with other series, morbi-mortality rates observed in our series are relatively similar. In the large prospective, multicentre, French ATENA study including 694 patients, the one-month morbidity and mortality rates were 1.7% and 1.4%, respectively (3.1 % combined morbidity-mortality rate).10 In accordance with the data of the literature, our results confirm the safety of EVT using coils with a very low rate of treatment-related morbi-mortality.1,10,11
One-year efficacy of the Trufill coils
In our series, we found that at one-year post-procedure follow-up, complete aneurysm occlusion was seen in 54.2% of the aneurysms treated with at least 70% of Trufill coils. In their series of 99 aneurysms treated with Trufill coils, Hirsch et al. showed a quite similar rate of 49.5% of complete aneurysm occlusion at one year. At mid-term, Bendok et al. reported that, of 174 aneurysms in 167 patients with angiographic follow-up, 137 (79%) remained stable or improved, nine (5%) showed aneurysm regrowth, 26 (15%) showed compaction.2,3 But, one-year follow-up angiographic results were obtained in only 59 aneurysms (34%) treated with Trufill coils, and angiographic results were not evaluated by an independent core laboratory to insure uniformity in reporting data.
In their systematic review, Ferns et al. showed a recanalization rate of 20.8% at a mean follow-up ranging from 4.7–38 months9 and Koltz et al.6 showed an overall recurrence of 26% at seven months. With an overall incidence of recurrence of 30.2% observed at a mean interval of 13.8 ± 4.5 months, our results are relatively higher. This variance could be explained by the anatomic characteristics of target aneurysms since there was an increasing reopening rates with increasing proportions of aneurysms >10 mm. The rate of ruptured aneurysms could be also a risk factor of recanalization. Furthermore, it is difficult to compare the present results with those from a previous series because the degree of angiographic occlusion is frequently overestimated by the neuroradiologist treating the patient. In fact, in a recent meta-analysis, Rezek7 suggested that core laboratory interpretation was significant for unfavourable outcomes (odds ratio (OR) 5.60; 95% CI: 2.01–15.60; p = 0.001). TRULINE study was designed taking into account these parameters, which reduce the bias.
In addition, the main goal of coiling is the protection against bleeding or rebleeding. In this series, no bleeding or rebleeding were reported during follow-up after coiling. Ten patients presented a SAH, but five of them before coiling and five during coiling.
Retreatment
During the follow-up out of the 163 patients of the ITT population, 6.3% of patients had an aneurysm retreatment at 12 months. In the unique series assessing the long-term anatomical results of aneurysms treated exclusively with Trufill coils, Bendok et al.2 and Hirsch et al.3 showed similar rates with 6% and 5.1% respectively. In a systematic review dealing with 8161 coiled aneurysms, Ferns reported a relatively higher rate with 10.3%.9
Conclusion
We have reported mid-term safety and efficacy results of the largest series to date using Trufill DCS detachable coils for intracranial aneurysm treatment. Endovascular coiling with Trufill DCS coils showed a favourable rate of permanent occlusion with an acceptable combined morbi-mortality rates of 2.6% (95% CI: 1.0–6.8) per ITT analysis. Among the three patients who died during the study, no death was related to the device or procedure. The Trufill DCS coils demonstrated efficacy that is comparable to published series of intracranial aneurysms treated with bare platinum coils.
Supplementary Material
Acknowledgments
The authors are grateful to all the investigators and coordinators for their support and hard work in enrolling patients, completing data collection forms, and participating in the study. They wish to thank the company RCTs (Lyon-France) for the Statistical and Data Management support. They are are grateful to all the investigators and coordinators for the support and hard work in enrolling patients, completing data collection forms, and participating in the study. They thank Zahir Boutoub and Arnaud Nicolas for statistical analysis.
Author contribution
The following contributions were made to authorship: B Gory: manuscript writing; L Huot: project development, data collection; R Riva: data collection; PE Labeyrie: data collection; O Levrier: project development, data collection; P Lebedinsky: project development, data collection; H Brunel: project development, data collection; J-Y Gauvrit: project development, data collection; R Blanc: project development, data collection; E Chabert: project development, data collection; L Derex: project development; E Emery: project development; A Nicolas: project development, data collection; G Rodesch: project development; F Turjman: project development, data collection, manuscript writing.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: F Turjman is a consultant for Codman, Medtronic, and Stryker; H Desal and G Rodesch were consultants for Codman (Study Corelab); L Derex and E Emery were consultants for Codman (Study Data Safety Monitoring Board); A Nicolas is an employee of Codman Neuro. The other authors have no personal, financial or institutional interest in any of the drugs, materials or devices described in this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Codman Neuro, which provided research support to the investigators or their institutions.
References
- 1.Gory B, Turjman F. Endovascular treatment of 404 intracranial aneurysms treated with Nexus detachable coils: Short-term results from a prospective, consecutive, European multicenter Study. Acta Neurochir (Wien) 2014; 156: 831–837. [DOI] [PubMed] [Google Scholar]
- 2.Bendok BR, Rahme RJ. Complex Registry Group. Complex shaped detachable platinum coil system for the treatment of cerebral aneurysms: The Codman Trufill DCS and Trufill DCS Orbit detachable coil system COMPLEX registry final results. J Neurointerv Surg 2013; 5: 54–61. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch JA, Bendok BR, Paulsen RD, et al. Midterm clinical experience with a complex-shaped detachable platinum coil system for the treatment of cerebral aneurysms: Trufill DCS Orbit detachable coil system registry interim results. J Vasc Interv Radiol 2007; 18: 1487–1494. [DOI] [PubMed] [Google Scholar]
- 4.Higashida RT, Cognard C, Bracard S. Initial clinical experience with a new complex-shaped detachable platinum coil system for the treatment of intracranial cerebral aneurysms. The Cordis Trufill DCS detachable coil system. Interv Neuroradiol 2006; 12: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan B, de Rochement Rdu M, Raabe A, et al. Single-center experience with TruFill platinum coils for the embolization of cerebral aneurysms. Neuroradiology 2006; 48: 264–268. [DOI] [PubMed] [Google Scholar]
- 6.Koltz MT, Chalouhi N, Tjoumakaris S, et al. Short-term outcome for saccular cerebral aneurysms treated with the Orbit Galaxy detachable coil system. J Clin Neurosci 2014; 21: 148–152. [DOI] [PubMed] [Google Scholar]
- 7.Rezek I, Mousan G, Wang Z, et al. Effect of core laboratory and multiple-reader interpretation of angiographic images on follow-up outcomes of coiled cerebral aneurysms: A systematic review and meta-analysis. AJNR Am J Neuroradiol 2013; 34: 1380–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003; 34: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 9.Ferns SP, Sprengers ME, van Rooij WJ, et al. Coiling of intracranial aneurysms: A systematic review on initial occlusion and reopening and retreatment rates. Stroke 2009; 40: 523–529. [DOI] [PubMed] [Google Scholar]
- 10.Pierot L, Spelle L, Vitry F. ATENA investigators. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: Results of the ATENA trial. Stroke 2008; 39: 2497–2504. [DOI] [PubMed] [Google Scholar]
- 11.Pierot L, Spelle L, Vitry F. ATENA investigators. Similar safety in centers with low and high volumes of endovascular treatments for unruptured intracranial aneurysms: Evaluation of the analysis of treatment by endovascular approach of nonruptured aneurysms study. AJNR Am J Neuroradiol 2010; 31: 1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


