Abstract
Background
Aneurysms of the perforating arteries arising from the basilar artery trunk are rare and represent a therapeutic challenge.
Methods
A basilar perforator aneurysm was initially treated by the stent-in-stent technique. Enlargement of the aneurysm was seen on follow up and the sac was secondarily coiled using two extremely soft bare coils, delivered through a one-marker microcatheter.
Results
At 6 months, the patient had a modified Rankin Scale score of 0 and cerebral arteriography demonstrated complete occlusion of the lesion.
Conclusions
We describe, as far as we are aware, the first case of basilar perforator aneurysm occlusion using extremely soft bare coils, inserted through the mesh of two stents previously placed in the basilar trunk.
Keywords: Basilar artery, perforator aneurysm, stent, endovascular coil embolization
Introduction
Aneurysms of the perforating arteries arising from basilar artery are rare.1 They represent a therapeutic challenge because they are often very small, making catheterization difficult and dangerous.2 The endovascular treatment most often used for these aneurysms involves placing one or several stents next to the origin of the perforator artery carrying the lesion.2,3,4,5 We describe, as far as we are aware, the first case in which a basilar perforator artery aneurysm has been occluded using coils inserted through the mesh of two stents, which had been previously placed over the origin of the parent artery.
Clinical case report
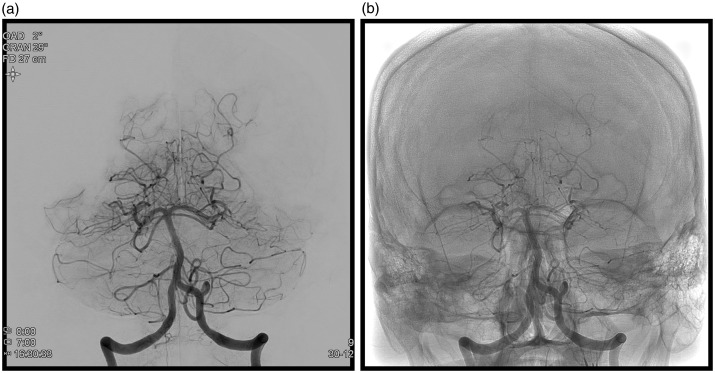
A right-handed 53-year-old male patient, with no significant prior medical history, was admitted with a headache of sudden onset accompanied by nausea. The Glasgow score was 15, the World Federation of Neurosurgical Societies score was 1 and the Hunt and Hess score was 1. A computed tomography (CT) scan without intravenous iodine contrast injection, showed a Fisher grade 4 subarachnoid hemorrhage (intraventricular hemorrhage without hydrocephalus). The CT angiogram did not show any intracranial aneurysm. Cerebral angiography performed on day 1, revealed an aneurysm, with a maximum diameter of 1.80 mm, at the origin of a left perforating branch of the upper third of the basilar artery (Figure 1(a)). On the following day, under general anesthetic, bilateral femoral access was achieved using an 8Fr sheath, followed by a bolus injection of 20 mg of Eptifibatide. After bilateral injection in the vertebral arteries, 3D image acquisition showed that the lesion had reduced in size and now measured 0.5 mm in its longest axis (Figure 1(b)). Given the impossibility of coiling this lesion, it was decided to use the stent-in-stent technique in order to reduce the pressure in the perforator branch carrying the aneurysm, while at the same time preserving its patency (which was why a flow diverter was not used). The first stent, a Leo Baby 2,5-18 (Balt Montmorency France), was placed in the basilar artery facing the perforator artery carrying the aneurysm, and then a second, a Leo Baby 2,5-12 (Balt), was placed inside the first one. Follow-up checks showed complete disappearance of the lesion (Figure 2(a–b)). On awakening, the patient’s clinical status was unchanged. A loading dose of 60 mg prasugrel orally, plus 250 mg acetylsalicylic acid IV was given, followed by 10 mg prasugrel plus 75 mg acetylsalicylic acid per day, orally.
Figure 1.
(a) 1.8 mm aneurysm of a left lateral basilar perforator, day 0; (b) reduction in size of the aneurysm (0.5 mm) on day 1 (white arrows).
Figure 2.
Occlusion of the aneurysm (day 1) after placing of two stents. (a) frontal image with subtraction; (b) no subtraction.
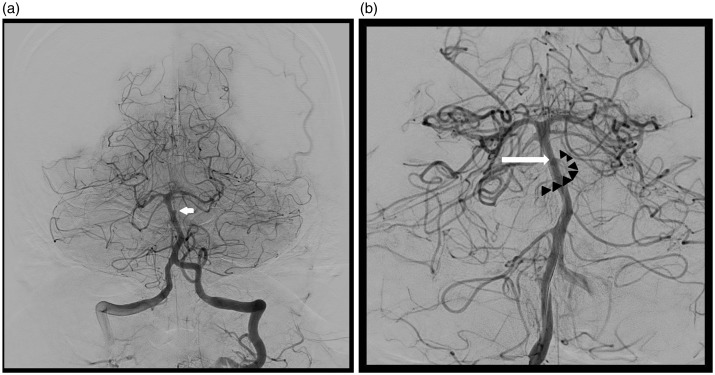
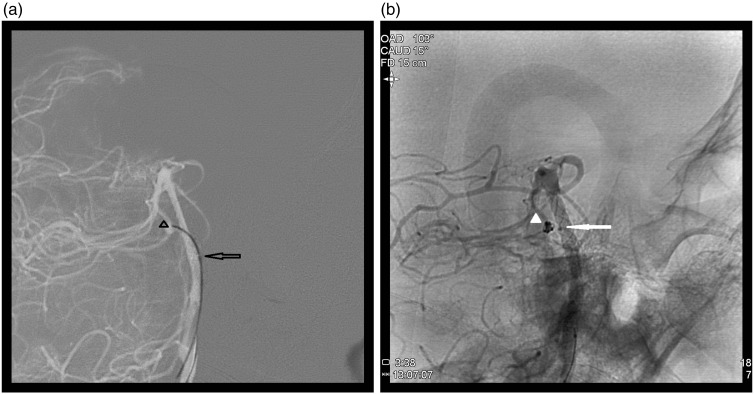
On day 8, cerebral angiography was performed due to clinical signs of vasospasm; an aneurysm growth was detected (Figure 3(a–b)). On day 9, under general anesthesia, using bilateral femoral approach, a Specter XC 4-11 (MicroVention Europe Saint Germain en Laye France) balloon catheter was placed at the end of the right vertebral artery. Several unsuccessful attempts were made to introduce a catheter through the stent meshes into the aneurysm using an UltraFlow (eV3) microcatheter mounted on different microguides [Radifocus Guidewire GT, angled at 45°, double angle, 0.012’’ (Terumo Europe Leuven Belgium); Hybrid007 et Hybrid008 (Balt)]. Only the Hybrid008 (Balt) guide could crossover the stent mesh into the aneurysm. It was eventually possible to position a Headway Duo (Microvention) microcatheter next to the neck of the aneurysm, after having inserted a Hybrid008 (Balt) guide wire into the aneurysm (Figure 4(a)). Via this microcatheter, two coils - ED Coil10 Extrasoft 2-2 and 1.5-1 (Kaneka Kanagawa Japan) were placed in the aneurysm, without any microcatheter kickback. An attempt to place a third coil, Nano 1.5-1 (Target, Stryker Pusignan, France) did result in microcatheter kickback, and this coil and the microcatheter were then withdrawn. Angiographic control confirmed aneurysm occlusion (Figure 4(b)). On awakening, the patient had right-sided hemiplegia and central facial nerve paralysis. Emergency magnetic resonance imaging revealed a left medial pontine ischemic lesion. After 6 hours, neurological signs had completely regressed. At 6 months after his subarachnoid hemorrhage, the patient had a modified Rankin Scale score of 0 and a follow-up angiogram showed aneurysm complete occlusion.
Figure 3.
(a) At day 8, re-opacification of 2 mm diameter aneurysm (arrow); (b) At day 9, 2.5 mm aneurysm. Aneurysm: arrow, perforating artery arising from the dome of the aneurysm (arrow head).
Figure 4.
(a) Tip of the Hybrid008 guidewire in the aneurysm (arrowhead), Duo microcatheter marker (arrow); (b) complete occlusion of the aneurysm (arrowhead), microcatheter marker (arrow) ‘sitting on’ the mesh of the stents.
Discussion
Aneurysms of the basilar perforating arteries are rare and usually have a diameter less than 2 mm.1,2 Coiling is difficult for aneurysms of such a small size; because of the kickback phenomenon it carries a high risk of perforation.6
Microcatheter kickback is thought to be due to counterforce exerted against the catheter by the prolapsed coil tail. This counterforce is due either to lack of available space for coil insertion, or a loss of freedom at the catheter tip because of the stiffness at the terminal joint with the delivery wire.7 Kanenaka et al.8 compared the microcatheter kickback phenomenon during insertion of different types of coil, using a 1.5 mm × 1.5 mm silicone aneurysm model. They showed that the EDC-10 ES was the only coil for which the entire 2 cm length could be placed within the aneurism with negligible microcatheter kickback. Their second experiment investigated the influence of the delivery wire system, and compared the repelling forces on the microcatheter. The softer the delivery wire, the lower the degree of microcatheter rebound; greater softness appears to contribute to stability during embolization. The results showed that there was only very slight microcatheter rebound with HypersoftER and EDC-10 ES. The rebound was greater with the other coils. The clinical case presented here demonstrates that the EDC-10 ES coil is characterized by a high degree of softness and less microcatheter kickback. There was no kickback phenomenon during placement of the two first coils. Use of a soft coil probably leads to a reduction in the rupture rate of very small (<3 mm) aneurysms. Harada et al.9 reported just one case of perforation (1.1%) when using EDC-10 ES as a finishing coil for 92 aneurysms treated.
The aim of the stent-in-stent technique is to increase coverage of the vessel wall to reduce the pressure in the perforating artery, leading to thrombosis of the aneurysm, while preserving the patency of the perforator artery. Several authors had described this technique, with different stents2–5,10,11 allowing aneurysmal occlusion in almost all cases, but two. On one case a third stent was placed inside the first two, and in this case the remaining aneurism was embolized with coils. The choice of stent depends on its porosity; the less porous is the stent, the greater is the intrasaccular hemodynamic effect but also the greater is the risk of occlusion of a perforator artery.12,13 Thus, Philips14 reports 14% perforator territory infarctions when placing a flow diverter stent in the treatment of posterior circulation aneurysms.
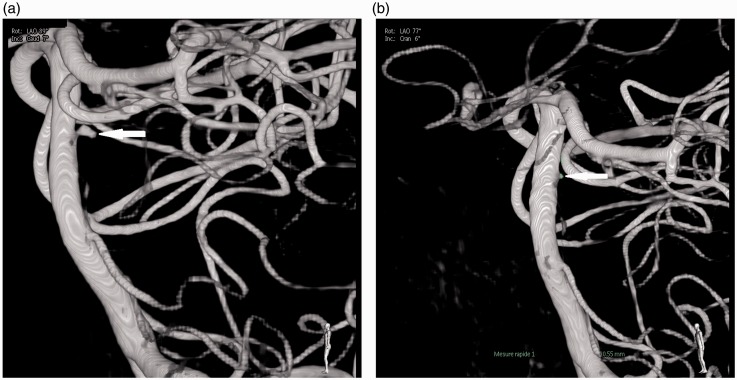
The vessel wall percentage coverage with the Leo+ stent is 17%,12 the surface area of the hole is 0.79 mm2, and its porosity is intermediate, lower than other open-cell stents but higher than a flow diverter.13 When two Leo+ stents are overlapped, the largest hole area varies between 0.2 mm2 and 0.8 mm2 (Figure 5). Probably the initial treatment was inefficient because the hole area near the perforator artery origin was close to 0.8 mm2. The distal cross-sectional area of the Headway Duo microcatheter is 0.15 mm2 and its internal diameter is 0.0165 inches. According to Heit et al.,15 the Duo microcatheter exhibits hybrid properties of a wire-directed and flow-directed microcatheter. The Marathon has already been used to deliver coils, including EDC-10 ES which are the only ones for which correct positioning of the detachment point is indicated by an audible alarm, thereby obviating the need for a proximal marker.16 Though the trans-cell catheterization through the mesh of overlapping stent have been descripted,17 to our knowledge, the clinical case reported here is the first description of aneurysm embolization using EDC-10 ES coils delivered via a Duo microcatheter through the mesh of two previously placed stents. Adding a flow diverter inside the two previous stents was not a contemplated solution. This would induce a significantly diminished porosity with greater risk of perforator artery thrombosis.18 The disadvantage of stenting is the need to start a double anti-aggregant therapy during the bleeding period. Optional treatment for this rare pathology has been described: surgery (selective aneurismal exclusion or sacrifice of the perforator artery) or endovascular (simple coiling, Onyx occlusion of the perforator or a placement of a flow diverter in the basilar artery); finally, conservator treatment has been proposed by several authors.1Nevertheless, considering the rarity of this pathology, the therapeutic decision and the modality of choice for the treatment will depend on local multi-disciplinary discussion.
Figure 5.
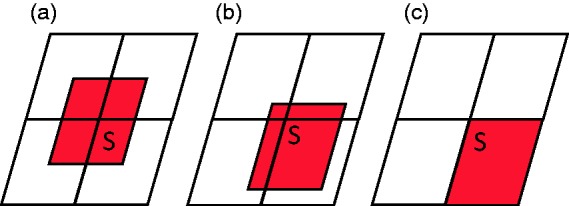
Variation in surface area (S) of the largest aperture after overlapping of two stents (holes in the first stent shown in white, those in the second stent in red). (a) minimum surface area (0.2 mm2); (b) surface area between 0.2 and 0.8 mm2; (c) maximum surface area (0.8 mm2).
Conclusion
Basilar perforator aneurysms are rare. Their treatment is challenging. This clinical case underscores the need for early follow-up checks if occlusion of an aneurysm by coiling has not been possible, and shows that a lesion can be coiled through the mesh of two previously placed stents using a microcatheter with a thin profile and coils with weak kickback properties.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Finitsis S, Derelle AL, Tonnelet R, et al. A dissecting aneurysm of a basilar perforating artery. Basilar perforator aneurysms: presentation of 4 cases and review of the literature. World Neurosurg 2017; 97: 366–373. [DOI] [PubMed] [Google Scholar]
- 2.Buell TJ, Ding D, Raper DM, et al. Posterior circulation perforator aneurysms: a proposed management algorithm. Neurointerv Surg. Epub ahead of print 6 January 2017. DOI: 10.1136/neurintsurg-2016-012891. [DOI] [PubMed] [Google Scholar]
- 3.Fiorella D, Albuquerque FC, Deshmukh VR, et al. Endovascular reconstruction with the Neuroform stent as monotherapy for the treatment of uncoilable intradural pseudoaneurysms. Neurosurgery 2006; 59: 291–300. [DOI] [PubMed] [Google Scholar]
- 4.Deshaies EM, Jacobsen W, Krishnamurthy S. Enterprise stent-within-stent embolization of a basilar artery perforator aneurysm. World J Neurosci 2011; 1: 45. [Google Scholar]
- 5.Nyberg EM, Chaudry MI, Turk AS, et al. Report of two cases of a rare cause of subarachnoid hemorrhage including unusual presentation and an emerging and effective treatment option. J Neurointerv Surg 2013; 5: e30. [DOI] [PubMed] [Google Scholar]
- 6.Van Rooij WJ, Keeren GJ, Peluso JP, et al. Clinical and angiographic results of coiling of 196 very small (≤3 mm) intracranial aneurysms. Am J Neuroradiol 2009; 30: 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyachi S, Izumi T, Matsubara N, et al. The mechanism of catheter kickback in the final stage of coil embolization for aneurysms: the straightening phenomenon. Interv Neuroradiol 2010; 16: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanenaka N, Sato H, Hiraoka F, et al. Comparative examination of finishing coils available in Japan. J Endovasc Ther 2016; 10 : 2,88–92. [Google Scholar]
- 9.Harada K, Morioka J. Initial experience with an extremely soft bare platinum coil, ED coil-10 Extra Soft, for endovascular treatment of cerebral aneurysms. J Neurointerv Surg 2013; 5: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satti SR, Vance AZ, Fowler D, et al. Basilar artery perforator aneurysms (BAPAs): review of the literature and classification. J Neurointerv Surg. Epub ahead of print 14 June 2016. DOI: 10.1136/neurintsurg-2016-012407. [DOI] [PubMed] [Google Scholar]
- 11.Kim YJ, Ko JH. Sole stenting with large cell stents for very small ruptured intracranial aneurysms. Interv Neuroradiol 2014; 20: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krischek O, Miloslavski E, Fischer S, et al. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo+, Enterprise]. Minim Invasive Neurosurg 2011; 54: 21–28. [DOI] [PubMed] [Google Scholar]
- 13.Bouillot P, Brina O, Ouared R, et al. Particle imaging velocimetry evaluation of intracranial stents in sidewall aneurysm: hemodynamic transition related to the stent design. Lovblad KO, Farhat M, Pereira VM. PLoS One 2014; 9: e113762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips TJ, Wenderoth JD, Phatouros CC, et al. Safety of the pipeline embolization device in treatment of posterior circulation aneurysms. Am J Neuroradiol 2012; 33: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heit JJ, Faisal AG, Telischak NA, et al. Headway Duo microcatheter for cerebral arteriovenous malformation embolization with n-BCA. J Neurointerv Surg 2016; 8: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 16.Beckett JS, Duckwiler GR, Tateshima S, et al. Coil embolization through the Marathon microcatheter: Advantages and pitfalls. Interv Neuroradiol 2016; 23: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J, Oh S, Kim MJ, et al. Endovascular treatment of ruptured blood blister-like aneurysms with multiple (≥3) overlapping Enterprise stents and coiling. Acta Neurochir (Wien) 2016; 158: 803–809. [DOI] [PubMed] [Google Scholar]
- 18.Peschillo S, Caporlingua A, Cannizzaro D, et al. Flow diverter stent treatment for ruptured basilar trunk perforator aneurysms. Neurointerv Surg 2016; 8: 190–196. [DOI] [PubMed] [Google Scholar]