Abstract
Background
Whether ASPECTS 5 and ASPECTS 6 were significantly different on clinical outcomes in acute anterior circulation ischemic stroke undergoing endovascular treatment remains unclear. We aimed to retrospectively compare the effectiveness and safety of ASPECTS 5 and ASPECTS 6 in acute anterior circulation large-artery occlusive stroke patients.
Methods
A total of 41 patients, 14 in the ASPECTS 5 group and 27 in the ASPECTS 6 group, were enrolled between January 2014 and June 2016. Modified Rankin Scale 0–2 was considered as good functional outcome. Symptomatic intracerebral hemorrhage at 72 hours and mortality at 90 days were recorded.
Results
Good functional outcome at 90 days in the ASPECTS 5 group (0% (0/14)) was significantly lower than that in the ASPECTS 6 group (25.9% (7/27)) (p = 0.04). Rates of symptomatic intracranial hemorrhage (21.4 (3/14) vs 18.5% (5/27), p = 0.83) and mortality (64.3% (9/14) vs 44.4% (12/27), p = 0.23) within 90 days were not significantly different. There is a trend for a lower rate of successful reperfusion in the ASPECTS 5 group (71.4% (10/14) for ASPECTS 5 vs 92.6% (25/27) for ASPECTS 6, p = 0.07).
Conclusions
ASPECTS 5 has very little chance to reach good functional outcome in Chinese patients with anterior circulation large-artery occlusive stroke. Future studies with large sample sizes are needed.
Keywords: Endovascular treatment, stroke, thrombectomy, ASPECTS
Introduction
Recent clinical trials1–5 demonstrated that endovascular treatment (EVT) could benefit patients with acute anterior circulation occlusive stroke more than standard medical care. Consequently, the 2015 American Heart Association (AHA)/American Stroke Association (ASA) focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding EVT recommended patients should receive EVT with a stent retriever if they had a prestroke modified Rankin Scale (mRS) score of 0 to 1, causative occlusion of the internal carotid artery (ICA) or proximal middle cerebral artery (MCA) (M1), National Institutes of Health Stroke Scale (NIHSS) score of ≥6, Alberta Stroke Program Early CT Score (ASPECTS) of ≥6, and treatment can be initiated (groin puncture) within six hours of symptom onset (Class I; Level of Evidence A).6 A recent meta-analysis even extended the time window of onset to groin puncture to 7.3 hours.7
The ASPECTS8 quantifies early ischemic changes in the MCA territory on computed tomography (CT), ranges from 0 to 10, with 8–10 indicating a small infarction core, 5–7 indicating moderate infarction, and 0–4 indicating large infarction. In the guideline, EVT with stent retrievers may be reasonable for patients with ASPECTS <6 (Class IIb; Level of Evidence B-R).6 A retrospective study by Noorian et al. comparing EVT with medical therapy in acute large-artery occlusion patients with ASPECTS 5–7 found that EVT may result in lower degrees of disability and may lessen the need for hemicraniectomy.9 However, a meta-analysis by Goyal et al. on randomized controlled trials revealed that EVT may not provide a benefit over medical therapy in a subgroup of ASPECTS 0–5.10 ASPECTS 5 as the cut-off, however, as to whether the outcomes of patients with ASPECTS 5 were significantly poorer than ASPECTS 6, has not been investigated.
Therefore, our study aims to investigate the differences of clinical outcomes between ASPECTS 5 and ASPECTS 6 in Chinese acute stroke patients with anterior circulation large-artery occlusion within 7.3 hours of stroke onset. We hypothesized that patients with ASPECTS 5 may have a significantly lower proportion of good functional outcome at 90 days, higher rate of symptomatic intracerebral hemorrhage and higher rate of mortality at 90 days when compared with ASPECTS 6.
Methods
Participants
Individuals were included from the Endovascular Treatment for Acute Anterior Circulation (ACTUAL)11 ischemic stroke registry, which enrolled acute ischemic stroke patients older than 18 years, with anterior circulation proximal large-artery occlusion who all received EVT from 21 stroke centers across 10 provinces in China between January 2014 and June 2016. Details of the ACTUAL registry were published previously.11 The registry was approved by the ethics committees of the central research institution, Nanjing Jinling Hospital, and collaborating centers.
We included patients from the ACTUAL database if they fulfilled the following inclusion criteria: (1) time from onset to groin puncture ≤7.3 hours; (2) preoperative ASPECTS 5 or 6; (3) the culprit large vessels included ICA, M1/M2 segment of the middle cerebral artery (MCA); (4) stent retriever thrombectomy was performed; (5) premorbid modified Rankin Scale (mRS) <2. We excluded patients with dissection or aneurysm.
Baseline characteristics
We collected patients’ demographic data, medical history (hypertension, hyperlipidemia, diabetes mellitus, etc.), laboratory findings (neutrophil to lymphocyte ratio (NLR), glucose, lipid profile, etc.), neuroimaging parameters (infarct size, collaterals, recanalization, intracerebral hemorrhage), stroke causes (Trial of ORG 10172 in Acute Stroke Treatment (TOAST) types12), stroke severity (NIHSS score13), time interval (from symptom onset to groin puncture).
All imaging data were sent to the core laboratory in Jinling Hospital, and two physicians who were blinded to the clinical data assessed the images separately, with the third physician’s advice in case of disagreement. We used the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) grading on digital subtraction angiography to evaluate collateral flow. It ranges from grade 0 (no collaterals) to grade 4 (complete and rapid collateral perfusion to the ischemic bed).14 Recanalization was evaluated by the modified Thrombolysis in Cerebral Infarction (mTICI) scale; grade 2b/3 was defined as successful reperfusion.14 If recanalization of the targeting artery failed, rescue therapies, such as balloon angioplasty, stent implantation, intra-arterial thrombolysis, or intracatheter tirofiban administration, might be performed.
Outcomes
Outcome measures including mRS at 90 days, mortality in hospital and at 90 days, as well as symptomatic intracerebral hemorrhage (sICH) at 72 hours were recorded. The functional outcomes were assessed with mRS at 90 days after onset, with good functional outcome as mRS 0–2.15 Follow-up of mRS at 90 days was carried out by individual centers, either by telephone or outpatient visit. sICH was defined by the criteria of the Heidelberg Bleeding Classification.16 sICH was diagnosed if the new observed ICH was associated with any of the following conditions: (1) NIHSS score increased >4 points than that immediately before worsening; (2) NIHSS score increased >2 points in one category; (3) deterioration led to intubation, hemicraniectomy, external ventricular drain placement, or any other major interventions.
Statistical analysis
Median (interquartile range) and frequency (percentage) were used to describe continuous variable and categorical variable. Mann-Whitney U tests for numerical variables and trend tests for ordinal variables were used to compare the differences in baseline characteristics and outcomes between the ASPECTS 5 group and ASPECTS 6 group. Because of the limited sample size, logistic regression for functional dependence (mRS 0–2) could not be performed. Statistical analyses were conducted using the SPSS version 22 software package (IBM, Chicago, IL, USA), and two-tailed p values of <0.05 were considered statistically significant.
Results
Participants
We retrieved 41 patients who underwent stent retriever thrombectomy with ASPECTS 5 and 6 out of the total of 698 patients in the registry between January 2014 and June 2016. Ten of the 21 stroke centers had ASPECT 5 patients. Fourteen patients were ASPECTS 5, and 27 patients were ASPECTS 6. Regarding the inter-rater reliabilities for imaging evaluations between the two physicians, the kappa value for ASITN/SIR rating and mTICI score was 0.895 and 0.920, respectively. The intraclass correlation coefficient by single measures for ASPECTS was 0.701 (0.505–0.829).
Comparisons of baseline characteristics and outcomes
Table 1 shows comparisons of baseline characteristics between ASPECTS 5 and ASPECTS 6. The baseline characteristics including age, sex, hypertension, atrial fibrillation, hyperlipidemia, diabetes mellitus, NLR, glucose, collateral flow and procedural times were not statistically different between the two groups (p > 0.05). All patients with ASPECTS 5 had moderate to severe NIHSS at admission (≥10); three patients with ASPECTS 5 had NIHSS at admission<10. No significant difference was found on NIHSS at admission between the two groups (p = 0.81).
Table 1.
Comparisons of baseline characteristics between ASPECTS 5 and 6 groups.
| ASPECTS 5 (N = 14) | ASPECTS 6 (N = 27) | p value | |
|---|---|---|---|
| Age, median (IQR), years | 64.5 (55.25–75.25) | 67 (61–74) | 0.44 |
| Sex, male, n (%) | 7 (50.0) | 14 (51.9) | 0.91 |
| Coronary heart disease | 5 (35.7) | 9 (33.3) | 0.88 |
| Atrial fibrillation | 7 (50.0) | 11 (40.7) | 0.58 |
| Hypertension | 9 (64.3) | 18 (66.7) | 0.88 |
| Diabetes mellitus | 1 (7.1) | 8 (29.6) | 0.10 |
| Hyperlipidemia | 1 (7.1) | 2 (7.4) | 0.98 |
| SBP>140 mmHg | 7 (50.0) | 18 (66.7) | 0.31 |
| NLR, median (IQR) | 9.97 (5.16–15.13) | 12.82 (5.64–17.22) | 0.58 |
| Glucose, median (IQR), mmol/l | 6.96 (5.57–8.84) | 7.45 (6.76–11.39) | 0.31 |
| Baseline NIHSS score | 0.81 | ||
| <10 | 0 (0) | 3 (11.1) | |
| 10–20 | 10 (71.4) | 12 (44.4) | |
| >20 | 4 (28.4) | 12 (44.4) | |
| Stroke causes, n (%) | 0.45 | ||
| Atherosclerotic | 5 (35.7) | 13 (48.1) | |
| Cardioembolic | 9 (48.1) | 14 (51.9) | |
| Collateral status (ASITN/SIR), n (%) | 0.88 | ||
| 0–1 | 5 (35.7) | 9 (33.3) | |
| 2–3 | 9 (64.3) | 18 (66.7) | |
| Occlusion sites | 0.54 | ||
| ICA | 6 (42.8) | 10 (37.0) | |
| MCA M1 | 7 (50.0) | 13 (48.1) | |
| MCA M2 | 1 (7.1) | 4 (14.8) | |
| Prior intravenous rt-PA, n (%) | 8 (57.1) | 13 (48.1) | 0.59 |
| Onset to groin puncture, mins | 252 (221–320) | 268 (193–319) | 0.95 |
| Door to imaging, mins | 20 (9–30.25) | 27 (15–33) | 0.45 |
| Puncture to first revascularization, mins | 75 (49–100) | 70.5 (45.75–121) | 0.81 |
| Imaging to first revascularization, mins | 185.5 (133.5–226.3) | 135 (107–162) | 0.10 |
| Puncture to end of revascularization, mins | 113.5 (81–129.75) | 103 (66–140) | 0.92 |
| Rescue therapy | 7 (50.0) | 10 (37.0) | 0.43 |
| Passes of stent retriever | |||
| 0–2 | 10 (71.4) | 16 (59.3) | 0.45 |
| >2 | 4(28.6) | 11 (40.7) |
IQR: interquartile range; SBP: systolic blood pressure; ICA: internal carotid artery; MCA: middle cerebral artery; NLR: neutrophil to lymphocyte ratio; NIHSS: National Institutes of Health Stroke Scale; ASPECTS: the Alberta Stroke Program Early Computed Tomography Score; mRS: modified Rankin Scale; ASITN/SIR: the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; rt-PA: recombinant tissue-type plasminogen activator.
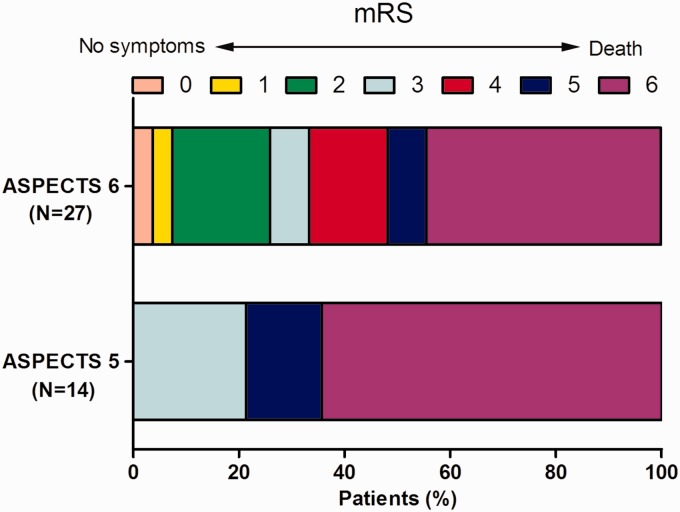
Table 2 shows the comparisons of clinical outcomes between these two groups. Patients with ASPECTS 5 had a significantly higher proportion of mRS score of 3–6 (100% (14/14)) than that of patients with ASPECTS 6 (74.1% (20/27)) (p = 0.04). The odds ratio for ASPECTS 6 for good functional outcome over ASPECTS 5 was 1.36 (95% confidence interval: 1.08–1.69). Figure 1 shows the distribution of mRS score between the two groups. The ASPECTS 5 group also had a trend for lower successful recanalization rate (71.4% (10/14) for ASPECTS 5 vs 92.6% (25/27) for ASPECTS 6, p = 0.07). Rates of sICH (21.4 (3/14) vs 18.5% (5/27), p = 0.83) and mortality (64.3% (9/14) vs 44.4% (12/27), p = 0.23) within 90 days were not significantly different. Regarding complications, ASPECTS 5 patients tended to have a higher rate of cerebral hernia (50.0% (7/14) vs 22.2% (16/27), p = 0.07); the extracranial hemorrhage and pneumonia rates were not statistically different (Table 2).
Table 2.
Comparisons of clinical and safety outcomes between the ASPECTS 5 and 6 groups.
| ASPECTS 5 (N = 14) | ASPECTS 6 (N = 27) | p value | |
|---|---|---|---|
| mTICI, n (%) | |||
| 0–2a | 4 (28.6) | 2 (7.4) | 0.07 |
| 2b–3 | 10 (71.4) | 25 (92.6) | |
| Symptomatic ICH, n (%) | 3 (21.4) | 5(18.5) | 0.83 |
| mRS at 90 days, n (%) | |||
| 0–2 | 0 (0) | 7 (25.9) | 0.04 |
| 3–6 | 14 (100) | 20 (74.1) | |
| In-hospital death | 8 (57.1) | 11 (40.7) | 0.32 |
| Death at 90 days | 9 (64.3) | 12 (44.4) | 0.23 |
| Complications | |||
| Brain hernia | 7 (50.0) | 6 (22.2) | 0.07 |
| Extracranial hemorrhage | 0 (0) | 1 (3.7) | 1.00 |
| Pneumonia | 7 (50.0) | 8 (29.6) | 0.205 |
ASPECTS: the Alberta Stroke Program Early Computed Tomography Score; mRS: modified Rankin Scale; mTICI: modified Thrombolysis in Cerebral Infarction; ICH: intracerebral hemorrhage.
Figure 1.
The distribution of mRS between ASPECTS 5 and ASPECTS 6 groups.
mRS: modified Rankin Scale; ASPECTS: Alberta Stroke Program Early Computed Tomography Score.
Discussion
Based on a large retrospective registry, our study found that no patients with ASPECTS 5 had good functional outcome (mRS 0–2) at 90 days. Patients with ASPECTS 5 had a significantly higher rate of poor functional outcome (mRS 3–6) than patients with ASPECTS 6. No differences were found between these two groups on sICH or mortality in hospital or at 90 days.
Although the EVT proved to be effective for acute stroke patients with anterior circulation large artery occlusion, the indication of patients with ASPECTS<6 had not yet been fully investigated. The Multicenter Randomized Clinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands (MR CLEAN) study1 involved patients with all ASPECTS ranges. It was the only positive trial that permitted enrollment in patients with ASPECTS <6. The treatment effect in that trial favored intervention in all three ASPECTS subgroups of 0 to 4 (28 patients), 5 to 7 (92 patients), and 8 to 10 (376 patients).1 The Trial and Cost Effectiveness Evaluation of Intra-arterial Thrombectomy in Acute Ischemic Stroke (THRACE) study17 also enrolled all ranges of ASPECTS patients. In the ASPECTS 5–7 subgroup, EVT was not superior to intravenous recombinant tissue-type plasminogen activator (rt-PA) with an odds ratio of 0.5 (95% confidence interval: 0.05–4.98).17 The outcomes on the subgroups of ASPECTS 5 and ASPECTS 6 have not yet been reported. Other clinical trials (Endovascular Treatment for Small Core and Anterior Circulation Proximal Occlusion with Emphasis on Minimizing CT to Recanalization Times (ESCAPE),3 Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-arterial (EXTEND-IA),2 Solitaire™ With the Intention For Thrombectomy as PRIMary Endovascular Treatment (SWIFT PRIME)4 and Randomized Trial of Revascularization with Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting within Eight Hours of Symptom Onset (REVASCAT)5) enrolled patients ≥ASPECTS 6. Our study was the first study to directly compare the outcomes of ASPECTS 5 and ASPECTS 6. It revealed that none of the patients with ASPECTS 5 achieved good functional outcome. Patients with ASPECTS 5 had a significantly higher rate of poor functional outcome than those with ASPECTS 6. It is reasonable that patients with a larger infarct core may have a lower rate of successful recanalization and higher risk for sICH. Although both recanalization rate and sICH were not statistically significant, numerical recanalization rate was lower and sICH was higher in the ASPECTS 5 group, which may be associated with its higher functional dependence rate.
There is a trend that ASPECTS 5 has a lower rate of successful recanalization due to the limited sample size. The ASPECTS may be a reflection of collateral flow and occlusion site, which were associated with recanalization as well. Although these variables were balanced between ASPECTS 5 and ASPECTS 6, the proportion of good collateral flow was relatively numerically lower in ASPECTS 5, whereas the proportion of ICA was numerically higher in ASPECTS 5, which may explain the relatively lower rate of successful recanalization in ASPECTS 5.
The strengths of our study lie in its multicenter setting and relatively intact data. However, certain limitations require attention. First, as this is a retrospective study, there was no united protocol for treatment in these centers, and neurointerventionists’ skills may also affect the results. Second, the sample size was relatively small. However, most centers treat patients according to guidelines; patients with ASPECT 5 were not given EVT, thus very limited patients with ASPECT 5 were involved in the study. Therefore, our study was currently the largest study to involve this group of patients. Moreover, this is the first study to compare the outcomes in patients with ASPECT 5 and patients with ASPECT 6.
In conclusion, ASPECTS 5 has very little chance to reach good functional outcome in Chinese patients with anterior circulation large-artery occlusive stroke. Further studies with large sample sizes are needed.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project is supported by the National Natural Science Foundation of China (No. 81400898 and 81671172).
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 5.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015; 46: 3020–3035. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 8.Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet 2000; 355: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 9.Noorian AR, Rangaraju S, Sun CH, et al. Endovascular therapy in strokes with ASPECTS 5–7 may result in smaller infarcts and better outcomes as compared to medical treatment alone. Interv Neurol 2015; 4: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 11.Hao Y, Yang D, Wang H, et al. Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke 2017; 48: 1203–1209. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989; 20: 864–870. [DOI] [PubMed] [Google Scholar]
- 14.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013; 44: 2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 16.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: Classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 17.Bracard S, Ducrocq X, Mas JL, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): A randomised controlled trial. Lancet Neurol 2016; 15: 1138–1147. [DOI] [PubMed] [Google Scholar]
- 18.Goyal M, Demchuk AM, Menon BK, et al. SAME AS 3.
- 19.Campbell BC, Mitchell PJ, Kleinig TJ, et al. SAME AS 2.
- 20.Saver JL, Goyal M, Bonafe A, et al. SAME AS 4.
- 21.Jovin TG, Chamorro A, Cobo E, et al. SAME AS 5.