Abstract
Background
Anthocyanin-rich foods and preparations have been reported to reduce the risk of life-style related diseases, including cancer. The SL222 sweet potato, a purple-fleshed cultivar developed in New Zealand, accumulates high levels of anthocyanins in its storage root.
Methods
We examined the chemopreventative properties of the SL222 sweet potato in the C57BL/6J-APCMIN/+ (APCMIN) mouse, a genetic model of colorectal cancer. APCMIN and C57BL/6J wild-type mice (n=160) were divided into four feeding groups consuming diets containing 10% SL222 sweet potato flesh, 10% SL222 sweet potato skin, or 0.12% ARE (Anthocyanin rich-extract prepared from SL222 sweet potato at a concentration equivalent to the flesh-supplemented diet) or a control diet (AIN-76A) for 18 weeks. At 120 days of age, the mice were anaesthetised, and blood samples were collected before the mice were sacrificed. The intestines were used for adenoma enumeration.
Results
The SL222 sweet potato-supplemented diets reduced the adenoma number in the APCMIN mice.
Conclusions
These data have significant implications for the use of this sweet potato variant in protection against colorectal cancer.
Keywords: Anthocyanins, Sweet potato, Colorectal cancer, APCMIN mice, cancer Protection
INTRODUCTION
Cancer burden, commonly measured in disability adjusted lifestyle years (DALY), is increasing in westernized countries, such as New Zealand.1 The incidence of colorectal cancer (CRC) is lower in Maori, although this population presents with more advanced disease than do non-Maori populations.2,3 In the early 2000s, the comparative rates of adenomas among New Zealand Europeans and Maori were 16.7% and 8.7%, respectively.4 However, National cancer statistics indicate that this gap has more recently closed, with New Zealand Europeans recording an age standardized CRC rate of 42.8 per 100,000 compared to 33.3 per 100,000 among New Zealand Maori, and both populations showing similar death rates due to CRC.5 Indeed, it is of concern that Maori populations have increasing rates of CRCs, and the DALY burden for Maori men is almost equal (99%) to that of Caucasian groups, while that of females is 77% of Caucasian DALY estimates.6 The increase of these rates in recent years may reflect changes in lifestyle and particularly diet.2 The traditional Maori diet follows a pattern different from the current eating habits.7 Among the currently consumed tropical crops is the sweet potato or kumara (Ipomea batatas (L) Lam.), and we have previously suggested that sweet potatoes may have cancer protective properties.8
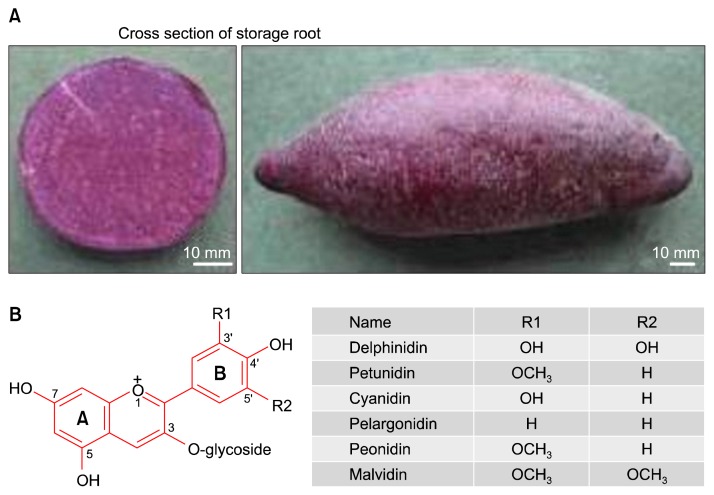
The sweet potato is considered the world’s seventh most important food crop on a fresh-weight basis, after wheat, rice, maize, potato, barley, and cassava. More than 123 million metric tons are produced internationally per year, and Asia is the world’s largest sweet potato producing region.9 The average yearly per capita consumption of fresh roots greatly varies within countries. In Oceania, including New Zealand, this consumption is estimated as 73 kg (very high consumption), in Asia; 18 kg (high consumption), in Latin America, Japan, and Africa: 5, 7 and 9 kg, respectively (moderate consumption) and in USA with 2 kg (low consumption).10 In contrast to potato, the per capita consumption of sweet potato in Canada, Europe, and Australia is limited.11 Of considerable interest are purple-fleshed new sweet potato varieties with enhanced anthocyanin content.12 One such variety, SN 99N1/222 has been developed by Plant and Food Research in New Zealand, and has now been released onto the commercial market (Fig. 1A).
Figure 1.
SL222 purple sweet potato. (A) Storage root. (B) Chemical structures of anthocyanidine.
The basic chemical structure of anthocyanins is a diphenylpropanoid–C6 (A-ring)-C3 (C-ring)-C6 (B-ring)–based polyphenolic ring structure with one or more sugar molecules bonded at different 3, 5, and 7 hydroxylated positions of the basic structure (Fig. 1B). The differences between individual anthocyanins are related to the number of hydroxyl groups; the nature, number, and location of sugars attached to the molecule; and the number and nature of aliphatic or aromatic acids attached to the sugar.13 Both chemical structure and cellular location may be important in determining the biological activity of anthocyanins.11
The multistep colorectal carcinogenesis process is complex and characterized by molecular and cellular alterations that result in a particular precursor lesion, i.e., the adenomatous polyp.14 The conversion from normal mucosa to adenoma and consequent progression to carcinoma are extended events, possibly for decades, to develop invasive cancer from normal tissue that provides opportunities for preventive interventions. In principle, CRC can be prevented at the molecular level by impeding carcinogenesis through modification of behavior and diet or by using chemical agents.15 Many presumed chemopreventive compounds have been recognized and generally categorized as ‘cancer-blocking’ or ‘cancer-suppressing’ agents according to the site along the carcinogenesis pathway at which these compounds exert their actions.16 Thus, chemoprevention has the potential to become a major component of CRC control.
Various mouse models are available to test the effects of food components on the risk of CRC.17 Among these models, the multiple intestinal neoplasia (MIN) mouse model, with a point mutation in the adenomatous polyposis coli (APC) gene, is particularly appropriate for studying inflammation-associated intestinal tumors.18,19 APCMIN+/− mutant mice are an inbred strain of C57BL/6J mice carrying an autosomal dominant heterozygous nonsense mutation of the mouse APC gene, located on mouse chromosome 18b1, that results in a truncated APC protein.20–22 This gene is homologous to the human APC gene that is mutated in most cases of CRC.20 APCMIN+/− mice develop adenomas within a few weeks of birth, and the primary phenotype of mice carrying this mutation is the development of multiple intestinal adenomatous polyps at 9 to 12 weeks of age.22 Intestinal adenomas progress to adenocarcinomas of the intestine in older mice, which are unlikely treatable, reflecting the higher tumor burden in this model compared with that in humans.18 The APCMIN+/− mouse was originally developed in 1986 by chemical carcinogenesis using N-ethyl-N-nitrosourea,21,23,24 including a germ-line mutation at codon 850 of the APC gene.24 APCMIN−/− homozygous mice are not viable,25 and 100% heterozygous APCMIN+/− raised on a high-fat diet develop an excess of approximately 45 adenomas throughout the intestinal tract.26 These mice rarely live beyond 150 days.23
Mutations in the APC tumor suppressor gene in humans occur in nearly all CRC patients and are detected in a majority of sporadic colorectal tumors and in familial adenomatous polyposis, a hereditary form of intestinal cancer.27 Among the tumor-suppressing activities of the APC protein is the down-regulation of β-catenin-induced transcriptional activity.28 The APCMIN+/− mouse has been utilized to improve the basic understanding of intestinal tumor formation and presents an opportunity to study the pathogenesis of neoplasia in which the initial genetic defect is the same in humans and mice.29 Moreover, the multistep progression of an adenomatous polyp to invasive cancer is well elucidated, thus the APCMIN+/− mouse is ideal for testing dietary and chemical cancer-preventive agents. For these reasons, we used this model to examine the chemopreventive properties of a new sweet potato variety.
MATERIALS AND METHODS
1. SL222 purple sweet potato
SL222 (99N1/222) sweet potato cultivars were kindly supplied by Dr. Steve Lewthwaite of the New Zealand Institute for Crop and Food Research limited (currently Plant and Food), New Zealand.
2. Diet materials
Four experimental diet groups were prepared: a basal-modified American Institute of Nutrition diet, number 76A (AIN-76TMA) (Bio-Serve Inc., Frenchtown, NJ, USA) containing 35% corn flour in the control and extract diet group, or this same diet with 25% corn flour and the remaining 10% supplied by the flesh or skin of the SL222 purple sweet potato. The AIN-76TMA diet was prepared from the following materials: casein (Alacid; 730-30; Riverlea Dairies, Auckland, New Zealand), corn flour (Edmond Fielder’s corn flour; Bluebird Foods Ltd., Manukau, New Zealand), sugar (Chelsea white sugar pure natural sugar cane; Foodtown Supermarket, Auckland, New Zealand), safflower oil (Healthy Food Shops, Auckland, New Zealand), lard (Lard-kiwi lard; Kiwi Bacon Company Ltd., Auckland, New Zealand), AIN vitamin mix (Bio-Serve-A Holton Industries Co., Frenchtown, NJ, USA), AIN mineral mix (Bio-Serve-A Holton Industries Co., Frenchtown, NJ, USA), DL-Methionine (Sigma-Aldrich Chemie, Gmbh-Steinheim, Germany), Choline bitartrate, approximately 99% (Sigma-Aldrich Chemie), and the fresh purple SL222 (99N1/222) sweet potato cultivar. Table 1 shows the composition of these diets, which were prepared dry. All ingredients were thoroughly mixed together in water (60 mL/kg diet) to generate dough, followed by rolling into small balls (approximately 20 g each) and freezing at −5°C until further required.
Table 1.
Composition (g/kg diet) of the experimental diets
| Ingredient | Control diet | Flesh diet | Skin diet | Extract diet |
|---|---|---|---|---|
| Casein (g) | 200 | 200 | 200 | 200 |
| Cornflour (g) | 350 | 250 | 250 | 348.7 |
| Sugar (g) | 200 | 200 | 200 | 200 |
| Safflower oil (g) | 100 | 100 | 100 | 100 |
| Lard (g) | 100 | 100 | 100 | 100 |
| AIN vitamin mix (g) | 10 | 10 | 10 | 10 |
| AIN mineral mix (g) | 35 | 35 | 35 | 35 |
| DL-methionine (g) | 3 | 3 | 3 | 3 |
| Choline bitartrate (g) | 2 | 2 | 2 | 2 |
| Purple sweet potato (flesh) (g) | - | 100 | - | - |
| Purple sweet potato (skin) (g) | - | - | 100 | - |
| Anthocyanin-rich extract (g) | - | - | - | 1.21 |
Approximately 20 kg of each diet was prepared and consumed by animals over the course of the study.
The sweet potato storage roots were washed and cooked in a microwave for 10 minutes at 700 W for each 800 g, and 100-g skin samples were collected by light peeling. These samples were mixed with 900 g total of other ingredients listed in Table 1 in a blender. To prepare the flesh diet, the microwaved storage roots without skin were chopped into small pieces, and 100 g of this sample was mixed with 900 g of the other ingredients listed in Table 1 in a blender. The extract diet was prepared by mixing all of the ingredients of the control diet and subsequently, 1.21 g of the dry powder of the anthocyanin-rich extract (ARE) was added to 1 kg of each diet. The amount of ARE added was estimated from the proportional amounts in SL222 storage roots. The caloric intake of the four experimental diets was equivalent, reflecting the substitution of the 100-g carbohydrate source (corn flour) with another carbohydrate source (100 g flesh or skin) in each 1 kg of diet.
3. Animals
The animals were obtained from the Animal Research Unit at the Faculty of Medical and Health Sciences, University of Auckland and the research was conducted with ethical approval of the University of Auckland Animal Ethics Committee, Permit (AEC No. R222). Four heterozygous male C57BL/6J-APCMin/+ mice (APCMIN) and eight wild-type female C57BL/6J (Apc+/+) mice were mated at five weeks of age to maintain an inbred breeding colony from which experimental animals were generated. The animals were transferred to a housing room, and distributed into four plastic cages with filter tops at a density of three mice per cage; one male APCMIN+/− was housed with two wild-type females to maximize the reproductive potential of C57BL/6J-APCMin/+. The animals in the first cage were fed control diet; the high-fat, semi-purified, American Institute of Nutrition diet, number 76A (AIN-76A). The animals in the second cage were fed the purple sweet potato flesh diet (SL222). The animals in the third cage were fed the purple sweet potato skin diet, and the animals in the fourth cage were fed the purple sweet potato ARE diet (Table 1). The resulting offspring were weaned at three weeks of age, and placed on diets corresponding to their respective breeding pairs until 18 weeks of age. Prior to being sacrificed for the collection of blood and organs, the mice were weighed.
Laboratory conditions were controlled to maintain a 12-hour light/dark cycle at 50% relative humidity and an environmental temperature of 21°C ± 1°C. The mice were fed the control or different experimental diets until termination of the study, i.e., 18 weeks. The mice were monitored for health and weighed weekly. Food and water were freely available at all times. Breeding trios were maintained on the diet for their breeding life (each female gave birth to 4–6 litters). During the weaning period (~3 weeks), the four APCMIN males were transferred to four new cages and maintained on the control or other experimental diets. The pups at weaning were sexed to normalize the distribution of males and females and avoid the clustering of individual mice from single litters. Because the APCMIN mutation is maintained in the heterozygous state, all progeny was identified and screened for the Min/+ genotype at five weeks of age using PCR assay (discussed below). The littermates were transferred at this stage to new cages according to the four diet groups and maintained on the appropriate diet for their lifetime (from the time of weaning to the time of death, maximum 18 weeks). All four APCMIN males were returned to mate with the normal females in the original cages to start a new generation of progeny. This procedure was repeated three times. However, to obtain a total of 40 mice in each experimental group in the present study, four new MIN males were mated to eight new normal females in four new cages. The breeding colonies were examined and followed up regularly for signs of ill health, such as weight loss or lethargy, which might indicate intestinal obstruction or anemia, or any other evidence of distress and poor feeding. These symptoms were indicators for immediate sacrifice.
4. Preparation of anthocyanin-rich extract
SL222 sweet potato was washed, peeled, finely chopped and placed in an extraction thimble (Quickfit; Whatman International Ltd., Maidstone, England). The thimble was placed in a Soxhlet apparatus and methanol containing 1% formic acid boiled under reflux through the Soxhlet, until the solution draining from the extraction thimble was colorless. The methanolic solution-containing compounds extracted from SL222 sweet potato, including anthocyanins pigments, was sequentially filtered through 90 mm #54 hardened filter paper (Whatman International Ltd.), GF/D and GF/F glass microfiber filters (Whatman International Ltd.), followed by rotary evaporation to dryness (Büchi Rotavapor R-200; Büchi Laboratoriums-Technik AG, Flawil, Switzerland) at a constant evaporation temperature of < 60°C.
A C18 silica column (100 g Octadecyl-functionalized silica gel; Sigma-Aldrich, MI, USA) was packed in a 3.5 cm glass column housing to a final (conditioned) height of 19 cm. The column was conditioned by washing under vacuum (Büchi Laboratorium-Technik AG) initially with excess methanol and then with excess Milli-Q H2O.
The dried extract was resuspended in Milli-Q water and loaded onto the preconditioned column until saturation and the eluent began to show coloration. The column was washed with excess Milli-Q H2O and the retained compounds, including the anthocyanin’ pigments, were eluted with methanol containing 1% formic acid until the eluent ran colorless. The eluent was rotary evaporated to dryness as described above and the ARE powder was stored at room temperature.
The ARE extraction procedure was repeated 3 times, processing a total of 170 g of sweet potato, which yielded a total of 1.7 g of ARE. To produce sufficient ARE for the ARE-supplemented diet, the extraction process was repeated multiple times until a total of > 24 g was obtained. This ARE was subsequently added to the AIN-76A control diet at 1.21 g/kg to generate an anthocyanin concentration equivalent to that in the flesh-supplemented diet.
Since 170 g of sweet potato yielded 1.7 g of ARE, this concentration is equivalent to 1.84 μg of anthocyanins extracted from each gram of sweet potato using the Soxhlet method-based high-performance liquid chromatography (HPLC) of an ARE solution. Thus, the extraction efficiency of the Soxhlet method was 82.5%.
5. High-performance liquid chromatography analysis of sweet potato anthocyanins
HPLC analysis was performed using the Agilent 1100 series instrument equipped with a diode array detector, a thermostated autosampler, and Agilent chemostation software. All solvents were filtered through 0.45-μm Gelman hydrophilic polypropylene membrane filters before HPLC injection. Anthocyanins were separated on a 5-μm Luna C18 (2) analytical column (100 × 4.6 mm; Phenomenex Co., Torrance, CA, USA) fitted with a Luna C18 guard column (Phenomenex Co.). The mobile phase comprised the following solvents: (A) 1% formic acid in water containing 6% acetonitrile. (B) Acetonitrile containing 6% of 1% formic acid in water. Separation was achieved using a flow rate of 1.0 mL/min and the following linear gradient elution protocol: 100% mobile phase A: 0% mobile phase B (0 minutes) → 85% mobile phase; A: 15% mobile phase B (30 minutes) → 15% mobile phase; A: 85% mobile phase B (40 minutes) → 100% mobile phase; and A: 0% mobile phase B (50 minutes). A sample injection volume of 50 μL was used, and spectral information over the wavelength range 190 to 950 nm was collected throughout the separation time.
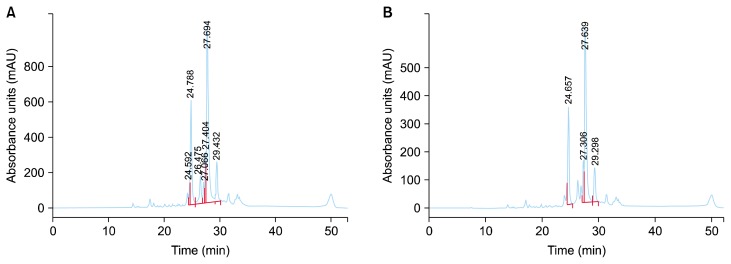
The first aqueous extract prepared and filtered through 0.2 μm syringe filter and subjected to HPLC analysis. Similarly, 625 μg/mL of ARE was analyzed using the same HPLC protocol (Fig. 2), showing that neither the extraction process nor the storage affected the anthocyanin profile of ARE compared with that of fresh SL222 sweet potato.
Figure 2.
High-performance liquid chromatography analysis. (A) Anthocyanine profile of fresh aqueous extract from SL222 sweet potato. (B) Anthocyanine profile of anthocyanin-rich extract after several weeks of storage.
6. Animal experimental procedure
At 120 days of age, the mice were anesthetized using halothane, and a blood sample was collected through cardiac puncture for further DNA protection studies to understand the anthocyanin effect in animal models. The mice were subsequently euthanized through cervical dislocation, and the tissues, including the spleen, liver, large and small intestine, were removed for analysis. Gastrointestinal tracts (from the esophagus to the distal rectum) were excised, flushed with PBS and placed in small Petri dishes containing PBS for immediate adenoma enumeration.
7. Adenoma enumeration
The small intestine, caecum, and colon from each mouse were dissected to determine the number and size of adenomatous polyps. Intestinal sections were opened longitudinally using fine iris scissors and extensively rinsed with PBS to remove the intestinal contents. The tissue was spirally rolled around a plastic tube and examined under a dissecting microscope with 5 × magnification to obtain adenomatous polyp counts. These numbers were determined for each intestinal section and classified as small (diameter, ≤ 2 mm) and/or large (diameter, > 2 mm). An individual blinded to the experimental groups and the genetic status of the mice counted the adenomas.
8. Genotyping procedure
The presence of the mutant allele was detected in the DNA extracted from a tissue sample (2–4 mm from the tail tip), obtained from weaner mice under anesthesia at 5 weeks of age. The genotyping procedure was based on a modified protocol from Jackson Laboratories (Bar Harbor, ME, USA). A short fragment of the APC gene encompassing the site of the APCMIN mutation was PCR amplified using the forward primer: 5′-TCT CGT TCT GAG AAA GAC AGA AGC T-3′ and the reverse primer: 5′-TGA TAC TTC TTC CAA AGC TTT GGC TAT-3′. This fragment was digested with Hind III restriction enzyme, which cuts the fragment only if the mutation is not present. DNA from the restriction digest was subjected to electrophoresis on a 3% agarose gel, and subsequent ethidium bromide staining before imaging under UV light. Therefore, compared with digested fragments from APCMIN, digested fragments from wild-type alleles produce two shorter fragments. The shortest digested fragment from wild-type DNA runs beyond the gel upon electrophoresis and leaves only one shorter fragment on the gel. Therefore, a single short band on the gel indicates a C57BL/6J APCMIN+/+ (wild-type) mouse, whereas two bands indicate a heterozygous C57BL/6J APCMIN+/− mouse.
RESULTS
1. Body weights of animals
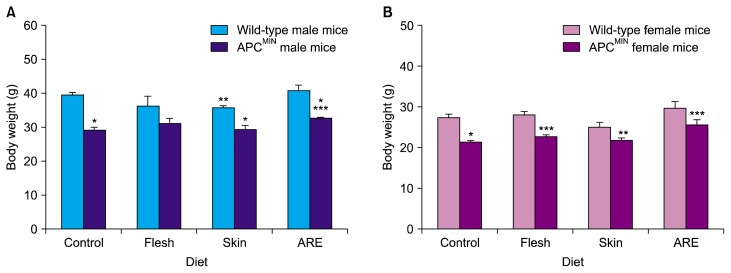
Male mice weighed more than the female mice, and wild-type mice weighed more than APCMIN mice of the same sex. For the control diet, wild-type males and females weighed 39.5 ± 2.4 g and 27.4 ± 2.6 g, respectively, whereas APCMIN males and females on the control diet weighed 29.1 ± 2.9 g and 21.3 ± 1.3 g (Fig. 3).
Figure 3.
Body weights of animals. (A) Body weight of male wild-type and APCMIN mice for the indicated diet groups at 18 weeks. Data represent mean ± SD. ARE, anthocyanin-rich extract. *P < 0.0001 vs. wild-type mice with the same diet, **P < 0.001 vs. control diet in wild-type mice, ***P < 0.01 vs. control diet in APCMIN. (B) Body weight of female wild-type and APCMIN mice for the indicated diet groups at 18 weeks. Data represent mean ± SD. *P < 0.0001 vs. wild-type mice, **P < 0.001 vs. control diet in wild-type mice, ***P < 0.01 vs. control diet in APCMIN.
This weight loss in APCMIN mice could be partially abrogated in both males and females by the diet containing ARE; compared with APCMIN mice on the control diet and female mice on the flesh-supplemented diet (to a lesser extent, P = 0.047), APCMIN mice showed statistically significant (P < 0.01) improvements in weight. Slight, but non-significant, improvements were also observed in the weights of male APCMIN mice on a flesh-supplemented diet, relative to those on the control diet.
In wild-type mice, most of the sweet potato supplemented diets did not result in a statistically significant deviation from the weight of mice on the control diet. The exception was the skin-supplemented diet in male wild-type mice, which caused a moderate reduction in body weight (P = 0.002).
No adverse clinical signs were observed in the wild-type mice in any of the diet groups throughout the 18-week study. However, melena (occult blood in the feces), frank intestinal hemorrhage and splenomegaly with marked anemia were prominent towards the end of the experiment in APCMIN mice consuming control diets. Data for the body weights of the mice are shown in Figure 3.
2. Incidence and size of intestinal adenomatous polyps
No statistically significant differences were observed between male and female APCMIN mice for incidence of adenomatous polyps or size for individual diets (data not shown). Therefore, the results for males and females have been combined for each diet to increase statistical power.
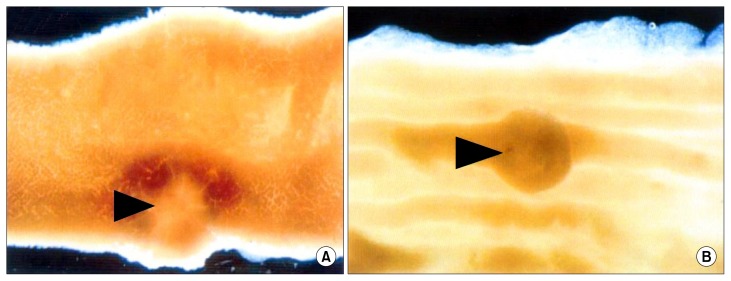
All APCMIN mice maintained on a control diet (n = 20) developed adenomatous polyps, and most of these polyps (> 90%) occurred in the small intestine. On average, these mice developed a total of 35.0 ± 8.2 polyps, with 32.6 ± 8.8 polyps in the small intestine and 2.4 ± 1.5 polyps in the colon (7.4 ± 4.7% of total) (Table 2). Under the dissecting microscope, the polyps in the small intestinal of APCMIN mice were generally sessile, whereas those in the caecum and colon were typically polypoid in appearance (Fig. 4).
Table 2.
Adenomatous polyp numbers in APCMIN mice detected in total and differentiated by location in the small intestine or colon
| Variable | Control (n=20) | Flesh (n=21) | Skin (n=21) | Extract (n=20) |
|---|---|---|---|---|
| Small intestinal (n) | 32.6 ± 8.8 | 10.4 ± 2.4*** | 10.6 ± 2.1*** | 7.5 ± 1.2*** |
| Colon (n) | 2.4 ± 1.5 | 1.2 ± 0.9** | 1.6 ± 1.3 | 1.3 ± 1.0** |
| Colonic proportion (%) | 7.4 ± 4.7 | 11.2 ± 10.0 | 13.4± 10.5* | 14.6± 12.3* |
| Total polyp (n) | 35.0 ± 8.2 | 11.6 ± 1.9*** | 12.2 ± 1.7*** | 8.8 ± 1.2*** |
| Reduction from control diet (%) | 66.9 | 65.1 | 74.9 |
Values are presented as mean ± SD. Colonic proportion was calculated as the mean of the number of polyps in the colon as a percentage of the total for individual mice. The percentage reduction was calculated from the total polyp number relative to that seen in mice fed on control diet.
P < 0.05,
P < 0.01,
P < 0.001 vs. control diet.
Figure 4.
Typical appearance of intestinal adenomas under a dissecting microscope in the APCMIN mouse (5× magnification). (A) A polyp in the small intestine, which is sessile in appearance (arrowhead). (B) A polyp in the colon, which is polypoid in appearance (arrowhead).
Flesh-, skin-, and ARE-supplemented diets caused a significant reduction (P < 0.001) in total polyp numbers to 11.6 ± 1.9, 12.2 ± 1.7, and 8.8 ± 1.2, respectively, from 35.0 ± 8.2 in the control diet (Fig. 5). While the total mean polyp numbers were reduced in both the small intestine and colon in response to flesh-, skin- and ARE-supplemented diets, the % proportion of polyps in the colon increased to 11.2 ± 10.0, 13.4 ± 10.5, and 14.6 ± 12.3, respectively, from 7.4 ± 4.7 in the control diet, although statistical significance was reached only with skin (P = 0.025) and ARE (P = 0.019)-supplemented diets (Table 2).
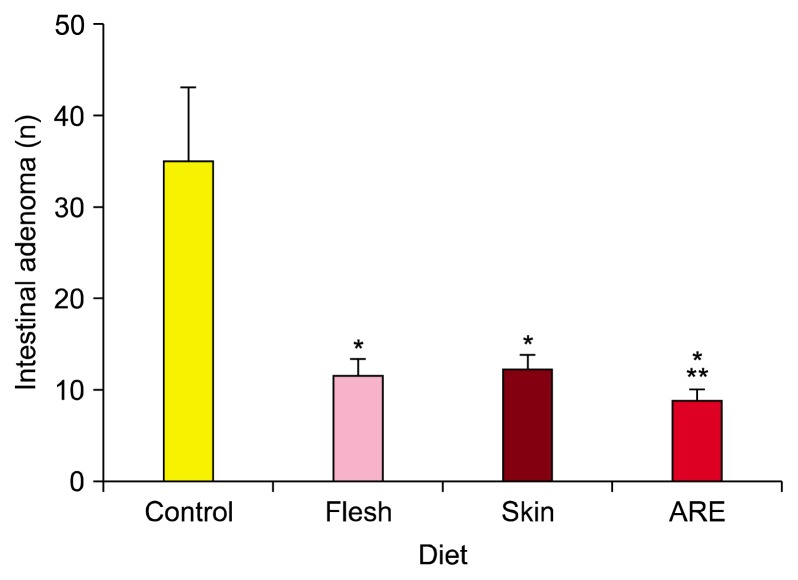
Figure 5.
Effect of anthocyanin-rich diets on the number of intestinal adenomas in APCMIN mice. Mice were fed the indicated diets in utero for 18 weeks before polyps were enumerated in the small intestine and colon. Data represent mean ± SD. ARE, anthocyanin-rich extract. *P < 0.0001 vs. control diet, **P < 0.001 vs. diet with flesh or skin.
There was no statistically significant difference in total polyp number between animals fed the flesh diet and those fed the skin diet (P = 0.263). However, the ARE-supplemented diet was significantly more effective in reducing total polyp numbers than the flesh- or skin-supplemented diets (Fig. 5). The reduction of polyp number in APCMIN mice on supplemented diets was reflected in an improvement in the severity of polyp-associated anemia, with the spleens of mice on supplemented diets being much closer in weight to those of wild-type mice fed control diets (data not shown).
The diameters of the adenomas were measured in the small intestine and the colon under the dissecting microscope. Table 3 summarizes the effect of control and flesh-, skin-, and ARE-supplemented diets on polyp size and number in the small intestine and the colon of APCMIN mice. The reduction in polyps ≤ 2 mm in diameter in both the small intestine and colon largely reflects a reduction in total polyp number, with significant reductions in the small intestine of mice on all three supplemented diets and significant reductions in the colon of mice on flesh- and ARE-supplemented diets. The supplemented diets were less effective at reducing the number of polyps > 2 mm in diameter, with significant reductions observed only in the small intestine of APCMIN mice on flesh- and ARE-supplemented diets, and no significant reductions in the number of larger polyps in the colon were observed with any of the supplemented diets.
Table 3.
The influence of SL222 sweet potato anthocyanin-rich diets on tumor size and number in the small intestine and the colon of APCMINmice
| Tumor size | Control (n=20) | Flesh (n=21) | Skin (n=21) | ARE (n=20) |
|---|---|---|---|---|
| Small intestine | ||||
| ≤2 mm | 27.0 ± 11.5 | 8.6 ± 2.9*** | 7.6 ± 3.0*** | 5.3 ± 2.0*** |
| >2 mm | 5.6 ± 6.0 | 1.8 ± 1.3** | 3.1 ± 1.9 | 2.2 ± 1.7* |
| Colon | ||||
| ≤2 mm | 1.9 ± 1.2 | 0.7 ± 0.9** | 1.0 ± 1.5 | 0.9 ± 0.6** |
| >2 mm | 0.6 ± 0.5 | 0.5 ± 0.5 | 0.6 ± 0.5 | 0.4 ± 0.8 |
| Average number/group | 35.0 ± 8.2 | 11.6 ± 1.9 | 12.2 ± 1.7 | 8.8 ± 1.2 |
Values are presented as mean ± SD. ARE, anthocyanin-rich extract.
P < 0.05,
P < 0.01,
P < 0.001 vs. control diet.
DISCUSSION
Previous studies with anthocyanin-rich foods have shown anticancer effects both in tissue culture30 and animal models.18,31,32 Typically, these reports have studied the effects of berry fruits, whereas here, the effects of SL222 purple sweet potato were investigated. Sweet potato is a staple food and thus represents a more viable method of incorporating high levels of anthocyanins into the diet.8 The effect of the SL222 purple sweet potato ARE and anthocyanin-rich diets on polyp numbers in APCMIN mice has been examined.
In this study, breeding pairs consisting of a C57BL/6J wild-type female and an APCMIN male were set up and fed either AIN-76A control diet, or diets supplemented with SL222 sweet potato flesh (10%), skin (10%), or ARE (10%). The resulting offspring were weaned at three weeks of age, and placed on diets corresponding to their respective breeding pairs until 18 weeks of age. SL222 purple sweet potato flesh and skin were separately investigated, as previous studies have reported that the contents of anthocyanin and other potential chemopreventative compounds, such as fiber, are highest in the skin. The ARE-supplemented diet was formulated so that the resulting diet had the same anthocyanin content as the flesh diet.
As expected, at 18 weeks of age, female mice were smaller and weighed less than male mice. The weight of wild-type mice consuming a control diet was higher than that of APCMIN mice fed the same diet, likely reflecting increased demands on body resources resulting from melena, intestinal hemorrhage, and splenomegaly with marked anemia, which are typical in APCMIN mice of this age.18 However, no changes in body weight were observed in wild-type mice of either sex consuming flesh- and ARE-supplemented diets relative to mice consuming control diets, although a moderate reduction was observed in male wild-type mice on skin-supplemented diets. The lack of any increases in the body weights of wild-type mice on supplemented diets over wild-type mice on control diets suggests that potential improvements in the body weights of APCMIN mice on supplemented diets would not reflect increased calories in the supplemented diets or the increased consumption of supplemented diets compared with that of the control diet. In fact, the slight reduction observed in the weight of male wild-type mice on skin-supplemented diets suggests reduced calories in this diet, which is possible as skin lacks much of the starch present in the flesh and displaces starch relative to the control diet, or skin-supplemented diets may be less palatable to the mice, resulting in lower consumption.
Except for the skin-supplemented diet, the lack of weight loss in wild-type mice suggests a lack of toxicity associated with these anthocyanin-rich diets. This finding is supported by a lack of evidence for genotoxic effects (mice were exposed since in utero), generally healthy appearance and activity levels at the end of the experiment, no teratogenic effects and/or apparent abnormalities of the internal organs following dissection and substantial improvement in the melena and intestinal hemorrhage normally observed in APCMIN mice.
The weight loss in APCMIN mice was partially abrogated by flesh and ARE-supplemented diets, likely reflecting the observed reduction in symptoms normally observed in APCMIN at 18 weeks of age. The lack of significant net weight gain in APCMIN mice fed skin-supplemented diet likely reflects a balance between improved symptoms and the weight loss observed in wild-type mice on this diet. Interestingly, the greatest improvements in weight relative to APCMIN mice fed the control diet was observed in APCMIN mice fed the ARE-supplemented diet, indicating that activity was not lost by the removal of the extract from the biological matrix of the purple sweet potato. In fact, improved bioavailability may explain why the greatest effects are observed with the ARE-supplemented diet over those supplemented with the whole food. In addition, the retention or even improvement of activity in the ARE-supplemented diet, where the proportional content of anthocyanins increased relative to other sweet potato constituents, is consistent with anthocyanins as the causative agent, although the possibility that another compound that co-purified with the anthocyanins is responsible for this effect cannot be ruled out. However, similar results on weight gain in APCMIN mice have been reported in response to other anthocyanic foods, including Kang et al.,31 who demonstrated the increased body weight of APCMIN mice consuming tart cherries. Additionally, Bobe et al.33 observed an increase in body weight for APCMIN mice fed tart cherry and sulindac. Comparable effects of red grape pomace extract (oenocyanin) in APCMIN mice have also been recorded.34
The main interest in this study was testing the potential of anthocyanin-rich diets from SL222 sweet potato to inhibit or suppress adenomatous polyp development in APCMIN mice, as has previously reported with flavonoids or anthocyanins from other dietary sources. In the present study, APCMIN mice consuming control diets had many adenomatous polyps. In contrast, all three supplemented diets resulted in significant reductions in total polyp numbers of approximately two-thirds or more. As with body weight, the ARE-supplemented diet showed the greatest improvement in the polyp numbers in both male (reduced by 74.3%) and female (reduced by 75.8%) APCMIN mice. The ability to detect a statistically significant improvement (P < 0.001) of the ARE-supplemented diet over the flesh- and skin-supplemented diets may reflect the improved bioavailability of the extract in the gastrointestinal tract over the whole food.
A majority of polyps in APCMIN mice were observed in the small intestine, irrespective of diet, and reductions in polyp numbers were observed at both sites in mice on supplemented diets. However, the proportion of polyps in the colon relative to the total was higher in APCMIN mice on supplemented diets, suggesting that the supplemented diets have the greatest effect in the small intestine. Similarly, Kang et al.31 reported that the small intestinal, but not colonic, adenoma numbers in APCMIN mice were influenced by consuming tart cherry anthocyanins, an effect attributed to the degradation of anthocyanins by elevated pH in the intestinal lumen and bacterial metabolism.
The reductions observed in polyp numbers differentiated by size reflect an overall reduction in polyp numbers, except reductions larger than 2 mm in the colon, possibly due to a very low incidence at this site and the insufficient power of this study to detect a difference. However, Kang et al.31 also reported that colonic adenoma volumes in APCMIN mice were not influenced by consuming tart cherry anthocyanins. Therefore, this effect may also be attributed to degradation of anthocyanins by elevated pH in the intestinal lumen and bacterial metabolism. Although, the numbers of polyps of all sizes were decreased, there were proportionally higher numbers of larger polyps in both the small intestine and colon of APCMIN mice fed experimental diets, implying that supplemented diets show a maximal effect in the prevention of polyp formation, rather than polyp growth.
Lim et al.35 showed that a purple-fleshed sweet potato clone, supplemented at levels between 10% and 30% in the diet, significantly suppressed the azoxymethane-induced formation of aberrant crypt foci in the colons of CF-1 mice. These authors also conducted in vitro mechanistic studies, and the results suggested that the anthocyanin-enriched sweet potato P40 may protect against CRC by inducing cell-cycle arrest, antiproliferative, and apoptotic mechanisms. Hagiwara et al.16 showed that anthocyanin-enriched extracts from a purple sweet potato and red cabbage inhibited associated colorectal carcinogenesis in F344/DuCrj rats initiated with 2-dimethylhydrazine and received 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine in the diet.15 However, the present study is the first to demonstrate a chemopreventative effect of purple sweet potato in the APCMIN mouse, a model that may be more germane to human CRC, where the APC mutation is often inherited or occurs sporadically.
Many studies have reported chemoprevention in the APCMIN mouse with a variety of phenolic- or anthocyanin-rich foods. Kim et al.32 reported that indole-3-carbinol, a constituent of cruciferous vegetables produced an 8.4% reduction in polyp numbers in the small intestine of APCMIN mice, although this effect was not statistically significant, and a 39.3% reduction in polyp numbers in the colons of these mice. Green tea and white tea (1.5% w/v, in drinking water), which are rich in catechins, suppressed intestinal tumorigenesis in APCMIN mice by 45% and 57.5%, respectively,36 and the major tea catechins epigallocatechin-3-gallate (EGCG) (0.16% in drinking water) reduced polyp numbers by 47%.37 Tart cherry anthocyanin extract reduced the number of small intestinal adenomas in APCMIN mice by 28%,31 although the small group sizes used in this study suggest that this difference was not statistically significant.
The effects of continuously administering an ARE from Chinese purple sweet potato were studied on the growth of Sarcoma S180 cells in Institute of Cancer Research (ICR) mice.38 Compared with mice consuming control diets, mice consuming the purple sweet potato extracts formed significantly fewer (69% fewer at the highest concentration) and smaller sarcomas.38
Other studies have investigated chemoprevention in the APCMIN mouse using pharmaceuticals, particularly nonsteroidal anti-inflammatory drugs. Sulindac has been investigated in a number of studies and can produce up to a 50% reduction in polyp numbers in APCMIN mice36,39 and rofecoxib (Vioxx), a COX-2 selective inhibitor, markedly reduced polyp numbers by 55% in APCΔ716 mice.40,41
All the quoted studies were performed under slightly different conditions, with different feeding regimes, control diets and time spans; thus, direct comparisons are not possible. However, notably, all three SL222 purple sweet potato diets resulted in reductions in polyp numbers greater than any others reported in the APCMIN mouse, including pharmaceutical agents. Sulindac has been implicated in the regression of polyps in human patients,42,43 through the trials were carried out based on studies in the APC mutant mouse.44 Bobe et al.33 stated that the dietary combination of tart cherry anthocyanins and sulindac is more protective against colon cancer than sulindac alone.
Vioxx entered clinical trial as a chemopreventative agent for CRC (Adenomatous Polyp Prevention on Vioxx [APPROVe] trial). However, this trial reported associations with thrombotic vascular events.45,46 Therefore, the incorporation of SL222 sweet potato into the diet may represent a viable alternative for CRC prevention in humans, particularly when the APC mutation has been confirmed. Mohanraj and Sivasankar10 have reviewed the biological activities of various isolated sweet potato compounds, and suggested that anthocyanins may have plausible medicinal applications. The present study may reinforce this suggestion.
The uniqueness of this study is that the parents were administered control or test diets since three weeks of age, and the offspring were fed the same diets soon after weaning at three weeks. Therefore, theoretically, the offspring have had anthocyanin supplement through maternal feeding until weaning and thereafter with direct test diets for a total of 18 weeks. A more recent soybean-based anthocyanin extract fed to APCMIN mice showed lesion reduction from 41.3 ± 8.14 in control diet to 34.7 ± 14.51 with 0.2% ARE and 28.3 ± 11.50 with 0.5% ARE diets. These mice were monitored from 5 weeks weaning to 7 weeks of testing.47 Comparatively, the present study monitored mice from weaning at three weeks to a total of 18 weeks, which better mimics aged colorectal issues in humans. Cai et al.34 fed APCMIN mice an anthocyanin-rich red grape extract and monitored these mice for 16 weeks, observed that the adenoma numbers were halved. However, in the present study, colonic adenoma volumes showed greater reductions than did small intestinal adenoma volumes; and the medium and large adenomas showed the highest reduction, while smaller adenomas showed worsening with supplementation. These results could suggest that various anthocyanins derived from various sources have different mechanisms of action. Thomasset et al.48 treated 25 CRC patients with a bilberry anthocyanin extract mirtocyan for seven days before surgery. Through surgical specimens these authors detected mirtocyan and methyl and glucuronide metabolites in plasma, colorectal tissue and urine, but not in the liver. The mechanisms associated with anthocyanin benefits includes Ki-67 and Akt reduction34 and a reduction in Insulin-like growth factor 148, providing further evidence of the benefits of these natural ingredients.
Collectively, dietary administration of SL222 purple sweet potato anthocyanin-rich diets, specifically flesh, skin, or ARE diets, significantly reduced the incidence and number of intestinal adenomas in APCMIN mice, suggesting anticancer properties in animal models. In addition, understanding the chemopreventive mechanisms of action of SL222 sweet potato anthocyanins will emphasize the development of preventive and practical strategies towards the design of chemoprevention trials in humans.
ACKNOWLEDGMENTS
We thank Dr. Steve Lewthwaite, Plant and Food Research, for providing the sweet potato (kumara). This work was financially supported by both the Auckland and National Division of the Cancer Society of New Zealand.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Beckett EL, Yates Z, Veysey M, Duesing K, Lucock M. The role of vitamins and minerals in modulating the expression of microRNA. Nutr Res Rev 2014;27:94–106. 10.1017/S0954422414000043 [DOI] [PubMed] [Google Scholar]
- 2.Caldararo N. The Maori, behavior, modern diets and colorectal cancers. Asian Pac J Cancer Prev 2012;13:1711–2. 10.7314/APJCP.2012.13.4.1711 [DOI] [PubMed] [Google Scholar]
- 3.Blair V, Kahokehr A, Sammour T. Cancer in Māori: lessons from prostate, colorectal and gastric cancer and progress in hereditary stomach cancer in New Zealand. ANZ J Surg 2013;83:42–8. 10.1111/ans.12042 [DOI] [PubMed] [Google Scholar]
- 4.Dickson G, Cunningham CW, Parry S. The prevalence of colorectal adenomas in Maori and New Zealand Europeans parallels colorectal cancer rates. N Z Med J 2010;123:45–9. [PubMed] [Google Scholar]
- 5.Ministry of Health. Cancer: new registrations and deaths 2012. Wellington, Ministry of Health, 2015. [Google Scholar]
- 6.Costilla R, Tobias M, Blakely T. The burden of cancer in New Zealand: a comparison of incidence and DALY metrics and its relevance for ethnic disparities. Aust N Z J Public Health 2013;37:218–25. 10.1111/1753-6405.12062 [DOI] [PubMed] [Google Scholar]
- 7.Cambie RC, Ferguson LR. Potential functional foods in the traditional Maori diet. Mutat Res 2003;523–524:109–17. 10.1016/S0027-5107(02)00344-5 [DOI] [PubMed] [Google Scholar]
- 8.Philpott M, Ferguson LR, Gould KS, Harris PJ. Anthocyanidin-containing compounds occur in the periderm cell walls of the storage roots of sweet potato (Ipomoea batatas). J Plant Physiol 2009;166:1112–7. 10.1016/j.jplph.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 9.Food and Agriculture Organization of the United Nations. Report on the Inter-Centre Review of Root and Tuber Crops Research in the CGIAR. Appendix 4 - Global production and consumption of roots and tubers. Consultative group on international agricultural research technical advisory committee. Washington, D.C., Consultative Group on International Agricultural Research Technical Advisory Committee, 1997. [Google Scholar]
- 10.Mohanraj R, Sivasankar S. Sweet potato (Ipomoea batatas [L.] Lam): a valuable medicinal food: a review. J Med Food 2014; 17:733–41. 10.1089/jmf.2013.2818 [DOI] [PubMed] [Google Scholar]
- 11.Philpott M, Gould KS, Markham KR, Lewthwaite SL, Ferguson LR. Enhanced coloration reveals high antioxidant potential in new sweetpotato cultivars. J Sci Food Agric 2003;83:1076–82. 10.1002/jsfa.1504 [DOI] [Google Scholar]
- 12.He W, Zeng M, Chen J, Jiao Y, Niu F, Tao G, et al. , Identification and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in China by UPLC-PDA and UPLC-QTOF-MS/MS. J Agric Food Chem 2016;64:171–7. 10.1021/acs.jafc.5b04878 [DOI] [PubMed] [Google Scholar]
- 13.Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry 2003;64:923–33. 10.1016/S0031-9422(03)00438-2 [DOI] [PubMed] [Google Scholar]
- 14.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell 1996;87:159–70. 10.1016/S0092-8674(00)81333-1 [DOI] [PubMed] [Google Scholar]
- 15.Lung MS, Trainer AH, Campbell I, Lipton L. Familial colorectal cancer. Intern Med J 2015;45:482–91. 10.1111/imj.12736 [DOI] [PubMed] [Google Scholar]
- 16.Hagiwara A, Yoshino H, Ichihara T, Kawabe M, Tamano S, Aoki H, et al. Prevention by natural food anthocyanins, purple sweet potato color and red cabbage color, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-associated colorectal carcinogenesis in rats initiated with 1,2-dimethylhydrazine. J Toxicol Sci 2002;27:57–68. 10.2131/jts.27.57 [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 18.Jackstadt R, Sansom OJ. Mouse models of intestinal cancer. J Pathol 2016;238:141–51. 10.1002/path.4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zhang Q. Application of the Apc(Min/+) mouse model for studying inflammation-associated intestinal tumor. Biomed Pharmacother 2015;71:216–21. 10.1016/j.biopha.2015.02.023 [DOI] [PubMed] [Google Scholar]
- 20.Haines J, Dunford R, Moody J, Ellender M, Cox R, Silver A. Loss of heterozygosity in spontaneous and X-ray-induced intestinal tumors arising in F1 hybrid min mice: evidence for sequential loss of APC(+) and Dpc4 in tumor development. Genes Chromosomes Cancer 2000;28:387–94. [DOI] [PubMed] [Google Scholar]
- 21.Hughes SA, Carothers AM, Hunt DH, Moran AE, Mueller JD, Bertagnolli MM. Adenomatous polyposis coli truncation alters cytoskeletal structure and microtubule stability in early intestinal tumorigenesis. J Gastrointest Surg 2002;6:868–74; discussion 875. 10.1016/S1091-255X(02)00065-3 [DOI] [PubMed] [Google Scholar]
- 22.Luongo C, Gould KA, Su LK, Kinzler KW, Vogelstein B, Dietrich W, et al. Mapping of multiple intestinal neoplasia (Min) to proximal chromosome 18 of the mouse. Genomics 1993;15:3–8. 10.1006/geno.1993.1002 [DOI] [PubMed] [Google Scholar]
- 23.Mutoh M, Teraoka N, Takasu S, Takahashi M, Onuma K, Yamamoto M, et al. Loss of adiponectin promotes intestinal carcinogenesis in Min and wild-type mice. Gastroenterology 2011;140:2000–8, 2008.e1–2. 10.1053/j.gastro.2011.02.019 [DOI] [PubMed] [Google Scholar]
- 24.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990;247:322–4. 10.1126/science.2296722 [DOI] [PubMed] [Google Scholar]
- 25.Shoemaker AR, Luongo C, Moser AR, Marton LJ, Dove WF. Somatic mutational mechanisms involved in intestinal tumor formation in Min mice. Cancer Res 1997;57:1999–2006. [PubMed] [Google Scholar]
- 26.Moser AR, Shoemaker AR, Connelly CS, Clipson L, Gould KA, Luongo C, et al. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev Dyn 1995;203:422–33. 10.1002/aja.1002030405 [DOI] [PubMed] [Google Scholar]
- 27.Pettan-Brewer C, Morton J, Mangalindan R, Ladiges W. Curcumin suppresses intestinal polyps in APC Min mice fed a high fat diet. Pathobiol Aging Age Relat Dis 2011;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997;275:1787–90. 10.1126/science.275.5307.1787 [DOI] [PubMed] [Google Scholar]
- 29.Pan P, Skaer CW, Wang HT, Stirdivant SM, Young MR, Oshima K, et al. Black raspberries suppress colonic adenoma development in ApcMin/+ mice: relation to metabolite profiles. Carcinogenesis 2015;36:1245–53. 10.1093/carcin/bgv117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamei H, Kojima T, Hasegawa M, Koide T, Umeda T, Yukawa T, et al. Suppression of tumor cell growth by anthocyanins in vitro. Cancer Invest 1995;13:590–4. 10.3109/07357909509024927 [DOI] [PubMed] [Google Scholar]
- 31.Kang SY, Seeram NP, Nair MG, Bourquin LD. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett 2003;194:13–9. 10.1016/S0304-3835(02)00583-9 [DOI] [PubMed] [Google Scholar]
- 32.Kim DJ, Shin DH, Ahn B, Kang JS, Nam KT, Park CB, et al. Chemoprevention of colon cancer by Korean food plant components. Mutat Res 2003;523–524:99–107. 10.1016/S0027-5107(02)00325-1 [DOI] [PubMed] [Google Scholar]
- 33.Bobe G, Wang B, Seeram NP, Nair MG, Bourquin LD. Dietary anthocyanin-rich tart cherry extract inhibits intestinal tumorigenesis in APC(Min) mice fed suboptimal levels of sulindac. J Agric Food Chem 2006;54:9322–8. 10.1021/jf0612169 [DOI] [PubMed] [Google Scholar]
- 34.Cai H, Marczylo TH, Teller N, Brown K, Steward WP, Marko D, et al. Anthocyanin-rich red grape extract impedes adenoma development in the Apc(Min) mouse: pharmacodynamic changes and anthocyanin levels in the murine biophase. Eur J Cancer 2010;46: 811–7. 10.1016/j.ejca.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 35.Lim S, Xu J, Kim J, Chen TY, Su X, Standard J, et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol Nutr Food Res 2013;57:1908–17. 10.1002/mnfr.201300040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orner GA, Dashwood WM, Blum CA, Díaz GD, Li Q, Dashwood RH. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of beta-catenin signaling by a combination of tea plus sulindac. Carcinogenesis 2003;24:263–7. 10.1093/carcin/24.2.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res 2005;65:10623–31. 10.1158/0008-5472.CAN-05-1949 [DOI] [PubMed] [Google Scholar]
- 38.Zhao JG, Yan QQ, Lu LZ, Zhang YQ. In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato. Nutr Res Pract 2013;7:359–65. 10.4162/nrp.2013.7.5.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu CH, McEntee MF, Whelan J. Sulindac causes rapid regression of preexisting tumors in Min/+ mice independent of prostaglandin biosynthesis. Cancer Res 1997;57:4267–73. [PubMed] [Google Scholar]
- 40.Evans JF. Rofecoxib (Vioxx), a specific cyclooxygenase-2 inhibitor, is chemopreventive in a mouse model of colon cancer. Am J Clin Oncol 2003;26:S62–5. 10.1097/01.COC.0000074159.05087.50 [DOI] [PubMed] [Google Scholar]
- 41.Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, et al. Chemoprevention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res 2001;61:1733–40. [PubMed] [Google Scholar]
- 42.Kim KY, Jeon SW, Park JG, Yu CH, Jang SY, Lee JK, et al. Regression of colonic adenomas after treatment with sulindac in familial adenomatous polyposis: a case with a 2-year follow-up without a prophylactic colectomy. Ann Coloproctol 2014;30:201–4. 10.3393/ac.2014.30.4.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waddell WR, Loughry RW. Sulindac for polyposis of the colon. J Surg Oncol 1983;24:83–7. 10.1002/jso.2930240119 [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Kingsley PJ, Marnett LJ, Eling TE. The role of NAG-1/GDF15 in the inhibition of intestinal polyps in APC/Min mice by sulindac. Cancer Prev Res (Phila) 2011;4:150–60. 10.1158/1940-6207.CAPR-10-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron JA, Sandler RS, Bresalier RS, Lanas A, Morton DG, Riddell R, et al. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet 2008;372:1756–64. 10.1016/S0140-6736(08)61490-7 [DOI] [PubMed] [Google Scholar]
- 46.Głuszko P, Bielińska A. Non-steroidal anti-inflammatory drugs and the risk of cardiovascular diseases: are we going to see the revival of cyclooxygenase-2 selective inhibitors? Pol Arch Med Wewn 2009;119:231–5. [PubMed] [Google Scholar]
- 47.Park MY, Kim JM, Kim JS, Choung MG, Sung MK. Chemopreventive action of anthocyanin-rich black soybean fraction in APC (Min/+) intestinal polyposis model. J Cancer Prev 2015;20: 193–201. 10.15430/JCP.2015.20.3.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomasset S, Berry DP, Cai H, West K, Marczylo TH, Marsden D, et al. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev Res (Phila) 2009;2:625–33. 10.1158/1940-6207.CAPR-08-0201 [DOI] [PubMed] [Google Scholar]