Abstract
Background
The levels of erythrocyte polyunsaturated fatty acids (FAs) may be associated with obesity, metabolic syndrome, and cancer. Thus, we investigated the association between erythrocyte n−3 and n−6 FA composition, body mass index (BMI), and biochemical profiles.
Methods
The body composition, dietary intake, and blood parameters, including serum lipid, glucose, insulin, adipokines, oxidative stress, and erythrocyte FA, were assessed in 66 overweight and obese women (average age, 43.4 years). We also classified the participants into the overweight, obese, and morbidly obese (MO) groups based on the BMI values of 23, 25, and 30 kg/m2, respectively. Erythrocyte FA was measured via gas chromatography.
Results
The serum glucose, triglyceride, total cholesterol, and low-density lipoprotein cholesterol levels of the participants in the overweight, obese, and MO groups were not significantly different. However, the serum insulin, high-density lipoprotein, cholesterol and leptin levels were significantly different. The erythrocyte n−6/n−3 ratios of the overweight, obese, and MO groups were 2.4, 2.5, and 2.8, respectively. These data were consistent with the dietary n−6/n−3 ratio findings. Moreover, the erythrocyte n−6/n−3 ratio was correlated with serum insulin levels.
Conclusions
As the severity of obesity increased, the levels of insulin and leptin and the ratio of dietary n−6/n−3 increased, which was consistent with erythrocyte FA. These results indicate that erythrocyte FA may be a predictive biomarker for the increased prevalence of obesity, insulin resistance, leptin resistance, and risk of developing metabolic disorders.
Keywords: Obesity, Erythrocytes, Fatty acids, Insulin resistance
INTRODUCTION
Obesity is defined as excessive fat accumulation that may cause a serious health problem,1 and is commonly diagnosed using body mass index (BMI), a ratio of weight (kg) and height (m2), which are often associated with the level of body fat.2 In the case of Asian populations, according to the World Health Organization (WHO), overweight is defined as a BMI greater than or equal to 23 kg/m2. Obesity and severe obesity are defined as a BMI greater than or equal to 25 and 30 kg/m2, respectively.2 According to the WHO, the global prevalence of obesity has nearly doubled between 1980 and 2014. Moreover, 39% of adults aged 18 years and older are overweight.1 The prevalence of adult obesity in Korea increased from 26.0% in 1998 to 33.2% in 2015.3,4 For Korean women, the prevalence rate of obesity slightly decreased from 27.3% in 2005 to 26.0% in 2015. However, the prevalence rate of severe obesity increased from 2.8% in 2005 to 4.7% in 2015.3 Obesity may be a significant risk factor for the development of type 2 diabetes mellitus, hyperlipidemia, cardiovascular diseases (CVDs), cerebrovascular diseases, osteoarthritis, non-alcoholic fatty liver disease (NAFLD), and various cancers.5,6
Several studies showed that obesity is linked to a state of chronic low-grade inflammation and high oxidative stress. Moreover, this condition causes increased levels of pro-inflammatory cytokines, such as TNF-α and interleukin-6 (IL-6), that induce insulin resistance.7 The level of leptin, an adipocytokine, is usually elevated in patients who are obese. It also plays a key role in mediating a pro-inflammatory state. Furthermore, increased levels of pro-inflammatory cytokine may induce oxidative stress.8 Reactive oxygen species (ROS), which are contributors of oxidative stress, may be related to the pathogenesis of obesity-related metabolic and CVDs, including endothelial dysfunction and atherosclerosis.9 Thus, the suppression of inflammation could be useful in improving insulin resistance and oxidative stress in individuals who are obese.
Overweight or obesity is significantly associated with an increased risk of developing several cancers and cancer-related mortality.10,11 Numerous studies have shown that a high BMI is associated with a slightly increased risk for breast and endometrium cancer in postmenopausal women.10,11 A 5 kg/m2 increase in BMI was associated with a 12% increase in breast cancer risk.10 Among postmenopausal women, those who are obese have a 20% to 40% increase in the risk of developing breast cancer compared with those who have normal weight.10 Obesity is thought to play a variety of roles in the pathogenesis of colorectal cancer, for example, its association with insulin.12,13
Essential fatty acids (FAs) have an important function in complex metabolic reactions in the body.14 A low dietary ratio of n−6/n−3 polyunsaturated fatty acid (PUFA) has been recommended to reduce the formation of pro-inflammatory eicosanoids from synthetized n−6 PUFA and increase the production of anti-inflammatory mediators from synthetized n−3 PUFA.14 Wall et al.15 reported that the reduction in the dietary ratio of n−6/n−3 FA may lower the incidence rate of numerous chronic diseases that involve inflammatory processes, which include CVDs, inflammatory bowel diseases, cancers, and rheumatoid arthritis.
Numerous studies showed that a balanced n−6/n−3 ratio is important for the health and the prevention and management of obesity-related problems, including metabolic disorders.16–18 A high level of n−6/n−3 in the umbilical cord blood erythrocyte membrane phospholipids was associated with a high subscapular skin-fold thickness and obesity at 3 years of age.16 The result of a prospective study showed that erythrocyte n−6 FA may be positively associated with obesity, and n−3 FA may also be inversely associated with weight gain in women who initially had normal weight.17 Colorectal cancer patients also showed a significantly lower mean percentage of n−3 PUFAs than the controls.18 These findings were reflected in the high ratio of n−6-/n−3 PUFA observed in obese and cancer patients. Therefore, the PUFA composition of the erythrocyte membrane and the biochemical indicators of obesity can be significant parameters in preventing metabolic diseases and cancer.
However, the correlation between obese Korean women and erythrocyte PUFA is not fully understood. Therefore, the associations between erythrocyte membrane FA composition and obesity were investigated.
MATERIALS AND METHODS
1. Participants
Sixty-six overweight or obese women were recruited. The mean age of the participants was 43.4 years. The informed consent and clinical data, including information on sex, age, height, weight, body composition, dietary intake, and blood analysis results, were obtained from the participants. The study was approved by the Institutional Review Board of Kyung Hee Medical Center (KMC IRB 1304-03-C1). The participants were divided into 3 groups according to their BMI. The number of total participants was 66, of which 21 were included in the overweight group (BMI, > 23 kg/m2), 36 in the obese group (BMI, > 25 kg/m2), and 9 in the morbidly obese (MO) group (BMI, > 30 kg/m2).
2. Anthropometric analysis
Anthropometric measurements of all the participants were obtained. Height and weight were recorded to the nearest 0.1 cm and 0.1 kg, respectively, using an automatic height and weight scale. BMI was calculated by dividing weight (kg) by height squared (m2). Body fat mass (BFM) and percent BFM (%BFM) were measured via bioimpedance analysis (InBody 3.0; Biospace, Seoul, Korea). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured after a 20 minutes rest using an automatic blood pressure monitor (TM-2654; A&D, Tokyo, Japan). Waist circumference (WC) and hip circumference (HC) were measured with a flexible measuring tape. WC was measured at the middle in the costal inferior border and iliac crest. HC was measured at the widest point of the hip. Waist-to-hip ratio (WHR) was calculated by dividing WC by HC.
3. Dietary intake assessment
A clinically trained nutritionist was tasked to keep and complete a 3-day food record of all the participants using food models (two days during weekdays and one day during the weekend). After collecting data on food intake, the data are usually converted into nutrient intake profiles, generally by using a computer-aided nutritional analysis program (CAN-Pro ver. 5.0; Korean Nutrition Society, Seoul, Korea).
4. Biochemical analysis
Blood samples were collected after a 10-hour overnight fast and centrifuged at 1,500 ×g for 15 minutes to separate the serum, which was stored at −70°C until analysis. Serum samples were analyzed for glucose, triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, alanine transaminase (ALT), and aspartate transaminase (AST) levels using enzymatic methods with commercial kits (Asan Pharmaceutical Co., Seoul, Korea). Serum insulin was measured using the Coat-A-Count Insulin Kit (Diagnostic Products Co., Los Angeles, CA, USA). Serum ROS levels were determined using the OxiSelect Intracellular ROS Assay kit (Cell Biolabs Inc., San Diego, CA, USA). Serum TNF-α and leptin levels were measured using the ELISA kits (Enzo Life Sciences, Inc. Farmingdale, NY, USA) and human leptin radioimmunoassay kit (Linco Research, St Louis, MO, USA), respectively. Erythrocyte FAs were measured with solvent extraction and gas-liquid chromatography.19
5. Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics for Windows ver. 21.0 (IBM Co., Armonk, NY, USA). The measuring variables were presented as mean ± SD. The differences among the three groups were evaluated via one-way ANOVA along with a post-hoc Tukey’s test, and a P-value < 0.05 was considered statistically significant.
RESULTS
1. General characteristics
Anthropometrics parameters are presented in Table 1. Body weight, BMI, BFM, %BFM, and HC of the obese group were significantly higher than those of the overweight group. The body composition of the MO group were remarkably higher than that of the obese group. The WC and WHR of the obese and MO groups were significantly higher than those of the overweight group. However, no significant difference was observed between the WC and WHR of the obese and MO groups. The height, SBP, and DBP of the three groups were not significantly different as well.
Table 1.
Anthropometric parameters of overweight, obese, and morbidly obese Korean women
| Variable | Group | ||
|---|---|---|---|
|
| |||
| Overweight (n = 21) | Obese (n = 36) | Morbidly obese (n = 9) | |
| Age (yr) | 43.76 ± 8.83 | 43.36 ± 9.70 | 42.44 ± 11.92 |
| Weight (kg) | 60.48 ± 3.83a | 68.38 ± 6.37b | 79.47 ± 7.82c |
| Height (cm) | 159.60 ± 5.62 | 159.36 ± 6.02 | 158.53 ± 4.00 |
| BMI (kg/m2) | 23.73 ± 0.59a | 26.89 ± 1.60b | 31.58 ± 2.51c |
| BFM (kg) | 19.70 ± 1.56a | 24.92 ± 4.04b | 33.96 ± 5.30c |
| %BFM | 32.66 ± 2.72a | 36.96 ± 3.27b | 42.69 ± 4.34c |
| WC (cm) | 84.67 ± 4.63a | 91.54 ± 5.32b | 92.87 ± 5.98b |
| HC (cm) | 96.60 ± 3.02a | 100.92 ± 4.66b | 108.76 ± 5.61c |
| W/H ratio | 0.88 ± 0.05a | 0.91 ± 0.05b | 0.92 ± 0.05b |
| SBP (mmHg) | 111.33 ± 10.55 | 115.94 ± 13.99 | 113.11 ± 8.68 |
| DBP (mmHg) | 67.67 ± 7.35 | 73.22 ± 9.54 | 70.67 ± 8.59 |
Values are presented as mean ± SD. Means in the same row not sharing a common letter are significantly different at P < 0.05. The groups classified the participants into the overweight, obese, and morbidly obese groups based on the BMI values of 23, 25, and 30 kg/m2, respectively. BMI, body mass index; BFM, body fat mass; %BFM, percent BFM; WC, waist circumference; HC, hip circumference; W/H ratio, waist and hip circumference ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure.
2. Dietary intake assessment
Data on daily energy and nutrient intake are presented in Table 2. The caloric intake of the overweight group was higher than that of the obese and MO groups. However, no significant difference was observed among the three groups. In the MO group, the amount of carbohydrate intake was high but the difference was not significant. In the overweight group, the amount of fat, fiber, and cholesterol intake was also high, but not significant. The ratio of PUFA : monounsaturated FA : saturated FA and PUFA n−6/n−3 in the MO group was the highest among the three groups.
Table 2.
Dietary intakes of overweight, obese, and morbidly obese Korean women
| Variable | Group | ||
|---|---|---|---|
|
| |||
| Overweight (n = 21) | Obese (n = 36) | Morbidly obese (n = 9) | |
| Energy (kcal) | 1,642.90 ± 356.96 | 1,592.15 ± 599.40 | 1,352.10 ± 492.86 |
| CHO : Fat : Pro | 58 : 26 : 16 | 60 : 25 : 15 | 62 : 23 : 14 |
| Fiber (g) | 18.81 ± 5.30 | 18.80 ± 7.33 | 18.07 ± 7.85 |
| Cholesterol (mg) | 269.28 ± 101.59 | 249.28 ± 167.73 | 235.98 ± 44.23 |
| P : M : S | 0.9 : 1.2 : 1.0 | 1.0 : 1.4 : 1.0 | 1.3 : 1.4 : 1.0 |
| PUFA n−6 : n−3 | 6.1 : 1.0 | 8.2 : 1.0 | 11.5 : 1.0 |
Values are presented as mean ± SD or ratio. The groups classified the participants into the overweight, obese, and morbidly obese groups based on the body mass index values of 23, 25, and 30 kg/m2, respectively. CHO, carbohydrate; Pro, protein; P : M : S, polyunsaturated fatty acid : monounsaturated fatty acid : saturated fatty acid; PUFA, polyunsaturated fatty acid.
3. Glycemic indices and lipid profiles
The parameters of the glycemic indices and lipid profiles are shown in Table 3. Serum glucose levels of the overweight, obese, and MO groups were not significantly different. However, the serum insulin levels of the obese and MO groups were significantly higher than those of the overweight group. Serum TG, TC, and LDL cholesterol levels did not significantly differ among the three groups. Although the serum lipid levels did not significantly differ among the three groups, the HDL cholesterol level of the MO group was the highest, while that of the overweight group was the lowest. In addition, the obese group had an intermediate HDL cholesterol level. Serum AST and ALT activities were not significantly different among the three groups.
Table 3.
Biochemical parameters of overweight, obese, and morbidly obese Korean women
| Variable | Group | ||
|---|---|---|---|
|
| |||
| Overweight (n = 21) | Obese (n = 36) | Morbidly obese (n = 9) | |
| Glucose (mg/dL) | 93.67 ± 10.75 | 95.17 ± 9.04 | 96.67 ± 7.58 |
| Insulin (μU/mL) | 6.84 ± 4.38a | 7.56 ± 4.17b | 10.96 ± 3.67c |
| TG (mg/dL) | 92.29 ± 32.06 | 121.74 ± 49.75 | 102.89 ± 45.54 |
| TC (mg/dL) | 190.24 ± 39.24 | 190.28 ± 28.38 | 188.44 ± 39.25 |
| LDL-C (mg/dL) | 117.29 ± 36.54 | 116.19 ± 25.68 | 124.11 ± 37.61 |
| HDL-C (mg/dL) | 60.29 ± 12.88a | 51.86 ± 11.20b | 49.22 ± 9.87c |
| AST (U/L) | 24.67 ± 8.38 | 21.28 ± 8.55 | 20.44 ± 5.20 |
| ALT (U/L) | 17.81 ± 12.01 | 17.81 ± 11.75 | 16.67 ± 8.05 |
Values are presented as mean ± SD. Means in the same row not sharing a common letter are significantly different at P < 0.05. The groups classified the participants into the overweight, obese, and morbidly obese groups based on the body mass index values of 23, 25, and 30 kg/m2, respectively. TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; AST, aspartate transaminase; ALT, alanine transaminase.
4. Reactive oxygen species and adipocytokines
The parameters of oxidative stress and adipocytokines are presented in Table 4. The ROS and TNF-α levels were very similar among the three groups. The serum leptin level of the MO group was the highest, followed by that of the obese group and then that of the overweight group.
Table 4.
Levels of ROS and adipokines of overweight, obese, and morbidly obese Korean women
| Variable | Group | ||
|---|---|---|---|
|
| |||
| Overweight (n = 21) | Obese (n = 36) | Morbidly obese (n = 9) | |
| ROS (nM) | 147.02 ± 18.63 | 157.69 ± 34.54 | 160.95 ± 28.78 |
| Leptin (ng/mL) | 12.71 ± 6.23a | 17.35 ± 8.60b | 28.34 ± 11.72c |
| TNF-α (pg/mL) | 199.26 ± 19.65 | 200.07 ± 18.87 | 212.28 ± 26.53 |
Values are presented as mean ± SD. Means in the same row not sharing a common letter are significantly different at P < 0.05. The groups classified the participants into the overweight, obese, and morbidly obese groups based on the body mass index values of 23, 25, and 30 kg/m2, respectively. ROS, reactive oxygen species.
5. Polyunsaturated fatty acid composition of the erythrocyte membranes
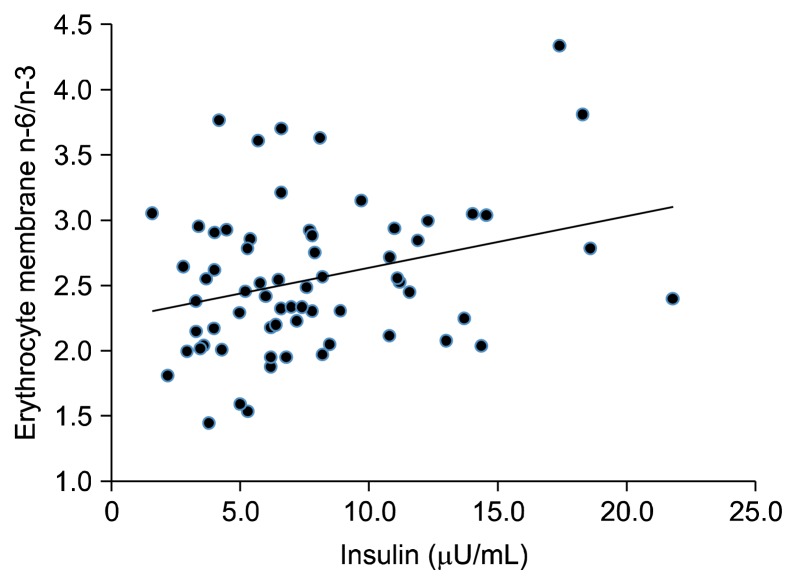
The FA profiles of the erythrocyte membranes are summarized in Table 5. No significant difference was observed among the saturated FA, monounsaturated FA, PUFA, PUFA n−6, and PUFA n−3 values of the three groups. Total PUFA, PUFA n−6, and PUFA n−3 of the MO group tended to decrease. However, the difference was not significant. As the severity of obesity increases, the n−6/n−3 ratio of the erythrocyte membrane also increases, whereas the total PUFA, PUFA n−6, and PUFA n−3 of the MO group tends to decrease. Moreover, the erythrocyte n−6/n−3 ratio was correlated with serum insulin levels (Fig. 1).
Table 5.
Fatty acid profiles of erythrocyte membranes in overweight, obese, and morbidly obese Korean women
| Variable | Group | ||
|---|---|---|---|
|
| |||
| Overweight (n = 21) | Obese (n = 36) | Morbidly obese (n = 9) | |
| SAT (%) | 40.84 ± 0.88 | 41.15 ± 1.18 | 42.49 ± 4.28 |
| MONO (%) | 14.49 ± 0.87 | 14.94 ± 0.94 | 14.59 ± 0.98 |
| PUFA (%) | 43.68 ± 1.46 | 42.98 ± 1.72 | 41.84 ± 4.85 |
| PUFA n−6 (%) | 30.84 ± 2.21 | 30.46 ± 2.36 | 30.66 ± 3.66 |
| PUFA n−3 (%) | 12.84 ± 1.74 | 12.52 ± 2.13 | 11.19 ± 2.00 |
| n−6/n−3 | 2.46 ± 0.50a | 2.53 ± 0.63a | 2.80 ± 0.48b |
Values are presented as mean ± SD. Means in the same row not sharing a common letter are significantly different at P < 0.05. The groups classified the participants into the overweight, obese, and morbidly obese groups based on the body mass index values of 23, 25, and 30 kg/m2, respectively. SAT, saturated fatty acid; MONO, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; PUFA n−6, omega-6 polyunsaturated fatty acids; PUFA n−3, omega-3 polyunsaturated fatty acids.
Figure 1.
Correlation of polyunsaturated fatty acid n−6/n−3 with serum insulin.
DISCUSSION
The increasing severity of obesity has become a major public health concern worldwide. Obesity is associated with a variety of diseases, including insulin resistance, diabetes mellitus, CVDs, dyslipidemia, NAFLD, and cancers.20 We investigated the association between biochemical parameters of obesity and FA compositions of the erythrocyte membrane in obese Korean women. The anthropometric parameters, dietary intake, blood metabolic profiles, and FA compositions of the erythrocyte membrane were measured. Results showed that body weight, BMI, BFM, %BFM, and HC of the MO group were the highest among the three groups. However, no significant difference was observed between the WC measurement and WHR of the obese and MO groups.
In the 2015 Korea National Health and Nutrition Examination Survey (KNHANES),21 the mean caloric intake of women was 1,768 kcal. However, the intake of the overweight, obese, and MO groups was lower in the present study (1,642.90 ± 356.96 kcal, 1,592.15 ± 599.40, and 1,352.10 ± 492.86 kcal, respectively). When the carbohydrate (C), fat (F), and protein (P) intake was compared based on the 2015 KNHANES, the ratio was 63.7 : 21.8 : 14.5. In this study, the average daily intake of C, F, and P ratio of the overweight, obese, and MO groups was 58 : 26 : 16, 60 : 25 : 15, and 62 : 23 : 14, respectively. These data showed the low consumption of C and P and the high consumption of F of the study participants compared to those of Korean adults. Based on the PUFA n−6/n−3 ratio, the ratio of these FAs should be close to 4 : 1 to 5 : 1 and must not exceed 10 : 1.22 In this study, the dietary ratios of n−6/n−3 PUFAs of the overweight, obese, and MO groups were 6.1 : 1.0, 8.2 : 1.0, and 11.5 : 1.0, respectively.
The composition of PUFA in the serum and erythrocyte phospholipids, which depends on endogenous metabolism controlled by genetic polymorphisms and dietary intake, is an important factor for health maintenance and prevention of metabolic diseases.14 An imbalance in the ratio of n−6/n−3 PUFAs causes prothrombotic and pro-inflammatory effects, which contribute to the development of atherosclerosis and obesity.22 Eicosanoids obtained from arachidonic acid (C20 : 4 n−6, AA) have been associated with tumor promotion and progression. Eicosapentaenoic acid (20 : 5 n−3, EPA) and docosahexaenoic acid (22 : 6 n−3, DHA) are also potent angiogenesis inhibitors, which suppress the production of angiogenic mediators, such as VEGF, platelet-derived growth factor, COX-2, NF-κB, and nitric oxide.23 Thus, a balanced consumption of n−6/n−3 is crucial for the health and maintenance of normal ratio through diet control for an effective treatment of obesity and its complications.
The serum insulin levels of the MO group were significantly higher than those of the overweight and obese groups. The serum HDL cholesterol level of the MO group was significantly lower than that of the overweight and obese groups. However, no significant difference was observed among the three groups. Insulin resistance is an underlying major pathophysiologic process in the development of metabolic disorders among obese patients.24 Excess fat in the body decreases insulin sensitivity and, thus, increases the symptoms associated with insulin resistance.25
The major cause of metabolic diseases that are associated with excessive body fat is dyslipidemia,26 which is characterized by increased TG, free fatty acid (FFA), and LDL cholesterol levels and decreased HDL cholesterol levels.27,28 The decrease in HDL cholesterol levels is linked to the increased risk of developing coronary artery diseases.24 Our study showed that the HDL cholesterol level of the MO group was the highest among the three groups, whereas the serum TG and TC levels did not significantly differ among the three groups. The serum LDL levels of the MO group tended to slightly increase. However, no significant difference was observed. Data on TC and LDL cholesterol were in accordance of those of previous studies that show the correlation between serum leptin levels and fat mass in Korean women.29
An increase in the amount of fat in the body, particularly visceral fat, induces the secretion of secretes FFA, adipocytokines, such as leptin and adiponectin, and pro-inflammatory cytokines, such as TNF-α and IL-6.30 The size of the visceral adipocyte is associated with systemic insulin resistance and increased expression of cytokines by the immune cells in the tissues.31 Thus, the increase in the amount of visceral fat in the body and the secretion of cytokines that impair insulin signaling may significantly contribute to systemic insulin resistance. Adipose tissue does not only store TGs but also produces adipokines,32 which induce the production of ROS, causing oxidative stress.9
Leptin, which is produced by adipocytes, regulates food intake, body weight, and energy expenditure.33 In obese individuals, leptin signaling is reduced, leading to hyperleptinemia and leptin resistance.34 Hyperleptinemia itself can contribute to the development of leptin resistance by downregulating the cellular response to leptin.35 Chen et al.’s study36 showed that serum leptin level of lean Chinese women (BMI, < 25 kg/m2) was 7.85 ± 3.60 ng/mL, whereas that of obese women (BMI, ≥ 25 kg/m2) was 16.59 ± 6.92 ng/mL. In this study, the serum leptin levels of the MO group were significantly higher (28.34 ± 11.72 ng/mL) compared to those of the overweight group (12.71 ± 6.23 ng/mL) and obese group (17.35 ± 8.60 ng/mL), which was consistent with the results of a previous study.
In this study, the higher severity of obesity increased the n−6/n−3 ratio of the erythrocyte membrane. Erythrocytes have a longer half-life compared to other blood components and better reflect the long-term dietary intake of FA than serum levels.37 The data on the n−6/n−3 ratio of erythrocytes tended to have a similar dietary n−6/n−3 ratio. In addition, a positive correlation was observed between serum insulin and n−6/n−3 ratio of the erythrocyte membrane in Korean obese women. In the case of obese children, the EPA levels in the erythrocyte membrane of the obese group was lower compared to those of the control group.38 Kuriki et al.39 showed that the decreased and increased risks for colorectal cancer are related to PUFA and SFA compositions of the erythrocyte membranes, respectively. Colorectal cancer patients also showed a significantly lower mean percentage of n−3 PUFAs than the controls.18 The total n−3 PUFAs in the plasma of patients with type 2 diabetes mellitus who had abnormal lipid levels was lower, and the n−6/n−3 ratio was higher compared to those of healthy participants.32 The low levels of n−3 in the plasma and erythrocytes were observed in patients with pancreatic, lung, and prostate cancer and non-Hodgkin lymphoma.40–42 Thus, the erythrocyte membrane n−6/n−3 PUFA ratio may be a predictive biomarker of the increasing severity of obesity and metabolic disorders.
In summary, as the severity of obesity increased, corresponding increases in insulin and leptin levels as well as dietary n−6/n−3 ratio were observed; whereas, HDL cholesterol levels were decreased. These data were consistent with erythrocyte FA findings. Moreover, erythrocyte FA may be a biomarker of metabolic diseases that are associated with obesity.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2016R1D1A1B03935660).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.World Health Organization. Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ Accessed October 28, 2016.
- 2.World Health Organization Western Pacific Region, International Association for the Study of Obesity, and International Obesity Task Force. The Asian-Pacific perspective: redefining obesity and its treatment. Geneva, WHO Western Pacific Region, 2000. [Google Scholar]
- 3.Korea Centers for Disease Control and Prevention. National nutrition survey report. Cheongju, Korea Centers for Disease Control and Prevention, 2015. [Google Scholar]
- 4.Statistics Korea. The statistics of mortality and the causes. Daejeon, Statistics Korea, 2015. [Google Scholar]
- 5.Oh SW, Shin SA, Yun YH, Yoo T, Huh BY. Cut-off point of BMI and obesity-related comorbidities and mortality in middle-aged Koreans. Obes Res 2004;12:2031–40. 10.1038/oby.2004.254 [DOI] [PubMed] [Google Scholar]
- 6.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533–47. 10.1158/1055-9965.EPI-07-0708 [DOI] [PubMed] [Google Scholar]
- 7.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm 2010;2010:289645. 10.1155/2010/289645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Maskari MY, Alnaqdy AA. Correlation between serum leptin levels, body mass index and obesity in omanis. Sultan Qaboos Univ Med J 2006;6:27–31. [PMC free article] [PubMed] [Google Scholar]
- 9.Higdon JV, Frei B. Obesity and oxidative stress: a direct link to CVD? Arterioscler Thromb Vasc Biol 2003;23:365–7. 10.1161/01.ATV.0000063608.43095.E2 [DOI] [PubMed] [Google Scholar]
- 10.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to post-menopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev 2014;36:114–36. 10.1093/epirev/mxt010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569–78. 10.1016/S0140-6736(08)60269-X [DOI] [PubMed] [Google Scholar]
- 12.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 1996;45: 633–8. 10.2337/diab.45.5.633 [DOI] [PubMed] [Google Scholar]
- 13.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 2012;12:159–69. [DOI] [PubMed] [Google Scholar]
- 14.Ristić-Medić D, Vučić V, Takić M, Karadžić I, Glibetić M. Polyun-saturated fatty acid in health and disease. J Serbian Chem Soc 2013;78:1269–89. 10.2298/JSC130402040R [DOI] [Google Scholar]
- 15.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 2010;68:280–9. 10.1111/j.1753-4887.2010.00287.x [DOI] [PubMed] [Google Scholar]
- 16.Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Am J Clin Nutr 2011;93: 780–8. 10.3945/ajcn.110.005801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Manson JE, Rautiainen S, Gaziano JM, Buring JE, Tsai MY, et al. A prospective study of erythrocyte polyunsaturated fatty acid, weight gain, and risk of becoming overweight or obese in middle-aged and older women. Eur J Nutr 2016;55:687–97. 10.1007/s00394-015-0889-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coviello G, Tutino V, Notarnicola M, Caruso MG. Erythrocyte membrane fatty acids profile in colorectal cancer patients: a preliminary study. Anticancer Res 2014;34:4775–9. [PubMed] [Google Scholar]
- 19.Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res 1965;6:428–31. [PubMed] [Google Scholar]
- 20.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association scientific statement on obesity and heart disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113:898–918. 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health and Welfare. 2015 National nutrition survey report. https://knhanes.cdc.go.kr/knhanes/sub04/sub04_03.do?class-Type=7 Accessed December 21, 2016.
- 22.Russo GL. Dietary n−6 and n−3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol 2009;77:937–46. 10.1016/j.bcp.2008.10.020 [DOI] [PubMed] [Google Scholar]
- 23.Bradley TG, Vargas FP. Eicosapentaenoic acid: sources, health effects and role in disease prevention. New York, Nova Science Publishers, 2012. [Google Scholar]
- 24.Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes 2012;19:81–7. 10.1097/MED.0b013e3283514e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473–81. 10.1172/JCI10842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol 2013;7:304–83. 10.1016/j.jacl.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 27.Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 2013;5:1218–40. 10.3390/nu5041218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rashid S, Genest J. Effect of obesity on high-density lipoprotein metabolism. Obesity (Silver Spring) 2007;15:2875–88. 10.1038/oby.2007.342 [DOI] [PubMed] [Google Scholar]
- 29.Kim MJ, Choi YS, Kim JA, Kim SM, Cho KH, Hong MH, et al. The correlation between plasma leptin concentration and adiposity in obesity. J Korean Acad Fam Med 2003;24:360–4. [Google Scholar]
- 30.Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes 2011;60:56–63. 10.2337/db10-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy OT, Perugini RA, Nicoloro SM, Gallagher-Dorval K, Puri V, Straubhaar J, et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg Obes Relat Dis 2011;7:60–7. 10.1016/j.soard.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ristić Medić D, Ristić V, Arsić A, Postić M, Ristić G, Blazencić Mladenović V, et al. Effects of soybean D-LeciVita product on serum lipids and fatty acid composition in type 2 diabetic patients with hyperlipidemia. Nutr Metab Cardiovasc Dis 2006;16: 395–404. 10.1016/j.numecd.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 33.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995;269:540–3. 10.1126/science.7624776 [DOI] [PubMed] [Google Scholar]
- 34.Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 2010;21:643–51. 10.1016/j.tem.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One 2010;5:e11376. 10.1371/journal.pone.0011376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen M, Gu W, Tang J, Li F, Zhu D, Wang G, et al. Serum leptin levels and adiposity in adult Chinese: a preliminary observation. Chin Med J (Engl) 1999;112:801–4. [PubMed] [Google Scholar]
- 37.Orchard TS, Ing SW, Lu B, Belury MA, Johnson K, Wactawski-Wende J, et al. The association of red blood cell n−3 and n−6 fatty acids with bone mineral density and hip fracture risk in the women’s health initiative. J Bone Miner Res 2013;28:505–15. 10.1002/jbmr.1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mustafa G, Kursat FM, Ahmet T, Alparslan GF, Omer G, Sertoglu E, et al. The relationship between erythrocyte membrane fatty acid levels and cardiac autonomic function in obese children. Rev Port Cardiol 2017;36:499–508. [DOI] [PubMed] [Google Scholar]
- 39.Kuriki K, Wakai K, Hirose K, Matsuo K, Ito H, Suzuki T, et al. Risk of colorectal cancer is linked to erythrocyte compositions of fatty acids as biomarkers for dietary intakes of fish, fat, and fatty acids. Cancer Epidemiol Biomarkers Prev 2006;15:1791–8. 10.1158/1055-9965.EPI-06-0180 [DOI] [PubMed] [Google Scholar]
- 40.Cvetković Z, Vucić V, Cvetković B, Petrović M, Ristić-Medić D, Tepsić J, et al. Abnormal fatty acid distribution of the serum phospholipids of patients with non-Hodgkin lymphoma. Ann Hematol 2010;89:775–82. 10.1007/s00277-010-0904-6 [DOI] [PubMed] [Google Scholar]
- 41.Murphy RA, Bureyko TF, Mourtzakis M, Chu QS, Clandinin MT, Reiman T, et al. Aberrations in plasma phospholipid fatty acids in lung cancer patients. Lipids 2012;47:363–9. 10.1007/s11745-011-3641-2 [DOI] [PubMed] [Google Scholar]
- 42.Zuijdgeest-van Leeuwen SD, van der Heijden MS, Rietveld T, van den Berg JW, Tilanus HW, Burgers JA, et al. Fatty acid composition of plasma lipids in patients with pancreatic, lung and oesophageal cancer in comparison with healthy subjects. Clin Nutr 2002;21:225–30. 10.1054/clnu.2001.0530 [DOI] [PubMed] [Google Scholar]