Abstract
Early prognostication in patients with a devastating brain injury is not always accurate and can lead to inappropriate decisions. We present case histories to support the recent recommendations of the Neurocritical Care Society that treatment withdrawal decisions should be delayed by up to 72 h in these patients. Development of pathways incorporating these recommendations can improve prognostication, enhance end of life care given to these patients and their families, and increase the opportunities to explore the donation wishes of more patients. They may also standardise the approach to decision making in the same way as the recommendations for management of patients after out of hospital cardiac arrest have done.
Keywords: Devastating brain injury, critical care management, neurocritical care, end of life care, organ donation
Introduction
A devastating brain injury (DBI) has recently been defined by the Neurocritical Care Society as either a neurological injury posing an immediate threat to life or a severe neurological insult where early limitation of therapy is being considered.1 Patients with a DBI will frequently die while others may survive with severe neurological impairment, the precise numbers of patients doing so being dependent on many factors such as the cause of DBI (trauma, subarachnoid haemorrhage, stroke, hypoxic injury, etc.), the severity of the neurological insult, the presence of co-morbidities and the criteria used by clinicians to recommend the withdrawal of life sustaining treatments (WLST). Some patients, however, may have better functional outcomes than originally expected. Inaccuracies in prognostication may result in early and inappropriate withdrawal of life sustaining treatments so creating a self-fulfilling prophecy of death or survival with severe disability. The Neurocritical Care Society recommends that patients with a DBI undergo repeated neurological examination to increase confidence in initial prognostication. They also recommend that physiological stability is maintained and repeated neurological examination undertaken for a period of up to 72 h after the neurological insult to allow sufficient opportunity for prognostic evaluation, end of life care planning, and consideration of organ donation, even when early limitation of aggressive treatment is being considered.1 We were aware of five patients admitted with DBI to Emergency Departments (ED) in the past 2 years, where the WLST was delayed because the family had agreed to organ donation. Three patients were admitted to our neurocritical care unit and two to neighbouring neurocritical care units. All the patients survived: two returned to independence and employment, and three survived with moderate disability. As a result of these cases and following discussion with ED and neurosurgical colleagues we have developed a pathway for patients with DBI, incorporating the recommendations of the Neurocritical Care Society1, as well as NICE guidance2 and NHSBT’s strategy3 on best practice in the identification and referral of potential organ donors (Figure 1). In this paper, we will outline some scenarios that arise and our experiences since altering our practice in light of the Neurocritical Society’s recommendations. We suggest that this practice should be more widely considered. Consent for publication was obtained from the patient himself in scenario 1 and from the next of kin of the patients in scenarios 2 and 3.
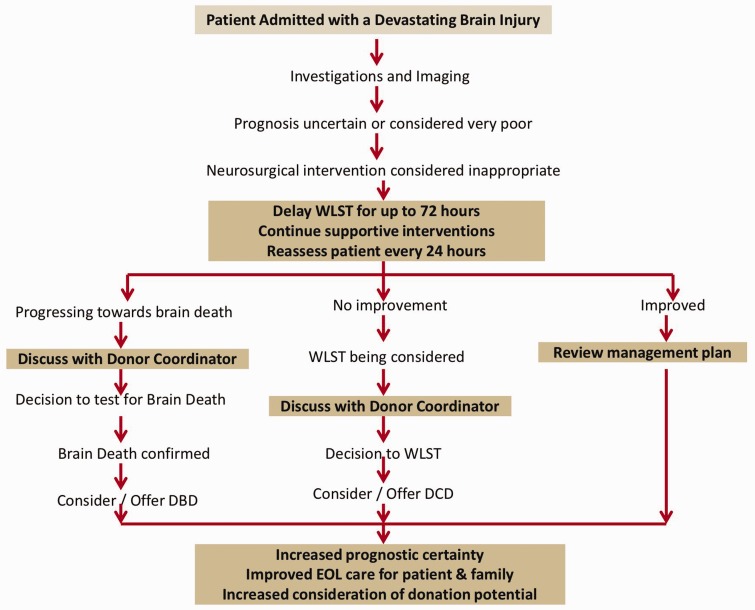
Figure 1.
Suggested pathway for intubated patients with Devastating Brain Injury incorporating the recommendations of the Neuro-critical Care Society1, as well as NICE guidance2 and NHSBT's strategy3 on best practice on identification and referral of potential organ donors.
DBD: donation after brain death; DCD: donation after circulatory death; NHSBT: National Health Service Blood and Transplant; NICE: National Institute for Health and Care Excellence; WLST: withdrawal of life sustaining treatment.
Scenario 1: Improving prognostication by delaying WLST
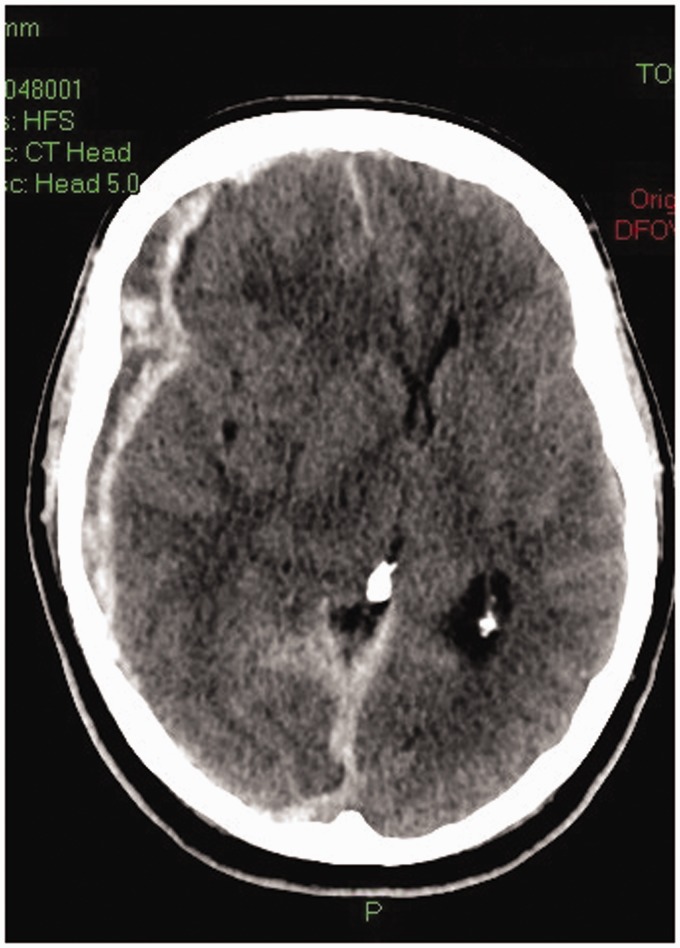
A 65-year-old man presented to his local ED complaining of a headache and increased somnolence five days after a fall. A CT head revealed a small right subdural haematoma with no mass effect. Neurological examination was normal and he was not taking any anticoagulant or anti platelet medication. Neurosurgical advice was for a period of in-patient observation. A repeat CT head on day 5 showed slight enlargement of the haemorrhage but no mass effect and the patient was discharged home with no neurological deficit. Three days later, he was admitted to the ED having collapsed. His Glasgow Coma Score (GCS) was 3 (E1 V1 M1), his right pupil was 2 mm larger than the left and both pupils were unreactive to light. His trachea was intubated and mechanical ventilation commenced prior to a CT head. This demonstrated marked enlargement of the subdural haematoma with mass effect and midline shift (Figure 2). Since his prognosis was considered to be poor, the opinion of the tertiary neurosurgical service was that no surgical intervention was indicated. WLST was considered the most appropriate course of action by the team in the ED and the patient was referred to the Intensive Care Unit (ICU) and the Specialist Nurse-Organ Donation (SNO-D). His family understood the devastating nature of the injury and likely poor outcome. Once the family had accepted the neurological prognosis, they gave consent for the patient to become an organ donor after WLST and cardio-respiratory arrest. The kidneys, lungs and liver were provisionally accepted by the regional transplant centre. No sedative medication had been administered since admission to the ICU. The patient was admitted to ICU 5 h after hospital admission. Twelve hours after ICU admission, while awaiting the arrival of the retrieval team, the patient’s GCS improved to E1 V(t) M4. He was given a further dose of mannitol, the decision to WLST was suspended and his GCS further improved to E2 V(t) M5. Neurosurgical opinion was sought and the patient was transferred to the regional neurosurgical unit. He underwent evacuation of the haematoma and a decompressive craniectomy. Following a prolonged ICU admission, the patient was discharged back to his local hospital with a GCS of E4 V4 M6. After a further 2 months, he was discharged to a community hospital, and subsequently home with a good functional outcome (able to live independently).
Figure 2.
CT of head of a patient showing the subdural haematoma with mass effect and midline shift.
This case highlights the potential for error in early prognostication; had consent for organ donation not been obtained it is likely that the patient would have undergone WLST in the ED. The delay in WLST, on this occasion to explore the possibility of organ donation, made the prognosis clearer. As a result, the patient made a good functional recovery.
Scenario 2: Enhanced end of life care that also facilitated organ donation
An 82-year-old man presented to the ED with a sudden onset of severe headache and confusion. He had chronic atrial fibrillation and was taking dabigatran for stroke prevention. His GCS rapidly reduced to 5 (E1 V1 M3). His trachea was intubated and mechanical ventilation commenced before transfer for a CT head. This demonstrated a right parietal intraparenchymal haemorrhage extending into the subdural space, subarachnoid space and ventricular system. There was associated midline shift and uncal herniation with compression of the midbrain and upper brainstem. Neurosurgical opinion was that the injury was ‘unsurviveable’ and that WLST should be considered. At the time, only the patient’s wife was in the ED. The nature of the DBI and prognosis was discussed with her and the likelihood of progression to brain death explained. The patient was admitted to ICU for neurological observation, to allow for a planned WLST and to allow time for his daughters and grandson to travel to the hospital. No sedative medication was administered after admission to ICU. After 12 h, the patient was apnoeic, unresponsive, had unreactive pupils and no cough or gag reflex. By this time, the patient’s two daughters and grandson had managed to travel to the hospital from abroad and from other parts of the country to see the patient before his death and also to support the wife. The family were informed of the need to undertake tests to confirm the patient’s death using neurological criteria. Family members opted to witness the tests which confirmed death. Once the family had accepted the patient’s death, a collaborative approach to discuss organ donation was undertaken4. The patient was not registered on the organ donor register but the family believed that the altruistic act of organ donation was consistent with the patient’s values. Consent to proceed with donation after brain death (DBD) was obtained resulting in both kidneys and the liver being successfully transplanted. Had treatment been withdrawn in the ED donation is unlikely to have proceeded as only donation after circulatory death (DCD) would have been possible at that point and this would have been contraindicated given the patient’s age. WLST in the ED would also have meant that the wife would have had little time to come to terms with the reason for the decision, and would not have had family support at this time.
Scenario 3: Enhanced end-of-life care without facilitating organ donation
A 73-year-old man collapsed at home and was admitted to his local ED with a GCS of 5 (E1 V1 M3). Both pupils were unreactive to light. He had suffered a fall a week ago and was taking warfarin. His trachea was intubated and he was mechanically ventilated. A CT head showed a large left-sided acute subdural haematoma, complete effacement of the left lateral ventricle, midline shift and left uncal herniation. He was given prothrombin complex concentrate and vitamin K to correct the prothrombin time. His daughter and sister were informed that the prognosis was extremely poor. He was transferred to the ICU for observation and end of life care. After 16 h, his GCS reduced to 3 (E1 V1 M1) and he became hypoxic due to aspiration pneumonia. The potential for organ donation was considered to be small given his multiple co-morbidities. This was confirmed by the SN-OD. His other two sisters, brothers-in-law, nephews and nieces were given the time to travel and visit him and the opportunity to discuss the futility of the situation before a decision to WLST was reached. The patient died 26 h after admission to ICU. The family were thankful for the care he received and appreciated the opportunity to be present at the time of the patient’s death
Experience since establishing the DBI pathway
The DBI pathway shown in Figure 1 is currently being assessed by the ICU at Southmead Hospital. We are more comfortable with a definition of DBI as “a severe neurological insult where early limitation of therapy is being considered”. We do not find the definition “a neurological injury posing an immediate threat to life” as helpful since many of such patients are treated aggressively and not simply observed and stabilised. Only intubated patients who have been stabilised are admitted to the ICU for further observation. The relatives are told that the expectation is that the patient will almost certainly die, possibly by progression to brain death but that a further period of observation would increase the certainty of the prognostication. In effect, the patients are observed and neurological examinations performed repeatedly while physiological stability maintained with fluids, vasoactive agents and mechanical ventilation while a ceiling of treatment is defined. This typically means a Do Not Attempt CPR decision, no intracranial pressure monitoring and no additional invasive organ support such as dialysis.
Patients who are not intubated in the ED continue to be transferred to a medical ward for further observation and ward based end of life care, i.e., ‘elective intubation and ventilation’ is not practiced. The patients have a Do Not Attempt Resuscitation order in place and are not considered for any escalation in support unless they show evidence of improvement.
In the 6 months since introducing the DBI pathway, 12 patients with DBI for whom WLST was recommended were admitted to the ICU for further observation. Two patients improved and the decision to WLST was reversed and the patients underwent ICP monitoring, surgery and full active management. Both patients subsequently died. The other ten patients also died, all within 48 hours of admission to ICU. None required the full 72 h specified in the pathway either because they had progressed to brain death (four patients) or because repeated clinical examination had shown further neurological deterioration leading to a decision to WLST before 72 h (six patients), as was the case in the third scenario presented. Four patients proceeded to organ donation (three DBDs and one DCD). None of the three patients who proceeded to DBD would have successfully become DBDs if WLST was undertaken in ED. None met the criteria for neurological testing as brain stem reflexes persisted, and one patient (scenario 2 in this report) would not even have been considered for DCD as he was above the age limit for consideration of DCD. The relatives of all patients were complimentary about the care received by their relatives and most appreciated the time for relatives to be present at the time of the patient’s death and the time to understand the decision making.
Discussion
Currently, there is a lack of national consensus or guidance on how patients with DBI should be managed, leading to potential for errors in initial prognostication, inconsistencies in end of life care and missed opportunities to explore the patient’s and family’s views on organ donation after they have been given enough time to come to terms with the most likely outcomes. The recent publication by the Neurocritical Society1 has addressed these issues and some of their recommendations should be considered for adoption in the United Kingdom since they may have benefits for patients with DBI, their relatives and the wider community. These issues can be addressed if all intubated patients with a DBI who present to the ED follow a pathway based on these recommendations, such as the one we are currently assessing in our hospital (Figure 1). Those who are not intubated should continue with further observation and end of life care on a medical ward in line with current practice. Some hospitals in the United Kingdom already admit all such patients to the ICU routinely, but it is more common for these patients only to be admitted to ICU if the relatives have agreed to organ donation. The approach recommended by the Neurocritical Care Society does, however, raise some legitimate questions and concerns amongst intensive care practitioners, which need further discussion within the profession. These include the following:
Is prognostication inaccurate?
We believe that for the vast majority of patients with DBI, the initial prognosis will prove to be correct and a move to the provision of end of life care will be required. However the Neurocritical Care Society estimates that as many as 3% of patients admitted with a DBI go on to make a good neurological recovery. It is easy to criticise their recommendations as not evidence based and anecdotal, but current practice results in a self-fulfilling prophesy whereby early WLST will always result in the death of the patient, perpetuating the view that none of these patients may survive with a good outcome. Scoring systems used for patients suffering DBI usually use a combination of physiological variables and clinical and CT features at the time of hospital admission to develop predictive models based on the observed population outcomes. It is noteworthy that the observed mortality used in the development of many predictive models includes the deaths that follow the WLST. It has been shown that hospital mortality after intracerebral haemorrhage is significantly influenced by the rate of use of DNAR orders, even after adjustment for case mix.5 The same scoring system was associated with a reduction in its predictive accuracy when applied to populations in whom the WLST is much less frequent.6 One of the main difficulties is that there are no standardised criteria for making a decision to WLST in patients with DBI. While prognostic scoring systems may be helpful in identifying populations of patients with a poor prognosis, their use in an individual patient basis is fraught with difficulties. Perhaps this is best illustrated in the patient in the first scenario where the CRASH model predicted a 96.7% risk of death at 14 d and a 98.1% risk of an unfavourable outcome at 6 months7 and yet the patient went on to make a good recovery.
The use of repeated assessments and measurements to improve prognostication is not new in intensive care practice: an increase in the sequential assessment of organ dysfunction (SOFA) score during the first 48 h in the ICU predicts a mortality rate of at least 50%, irrespective of the initial score.8 The greater the number of observations made over time, the lesser the risk of results being affected by regression to the mean and the greater the confidence and accuracy in the predicted result.9 The sensitivity, specificity and predictive values of almost all coma scales commonly used in neurocritical care are poor, and prognostication based on later assessments is more accurate than that based on an assessment at admission.10
Paradoxically, it is recent practice of admitting such patients to explore the potential for organ donation that has started to bring these unexpected survivors to our attention, and we suspect that other parts of the country have similar experiences that go unreported. As illustrated in case report 1, patients deemed to have a hopeless neurological prognosis can survive with a good functional outcome. Admitting these patients to an ICU for a period of neurological observation allows for the possibility, however remote, of potential for neurological improvement. The survival rate and the quality of survival in patients whose relatives refused organ donation and who underwent early WLST remain unknown. The development of a pathway for patients with DBI that prevents the early WLST will allow a better assessment of the prognosis of such patients and is, therefore, in their best interests; admission of DBI patients should become routine irrespective of any consideration for organ donation. The suggested approach is in effect no different to the development of pathways for patients who are admitted to the ED following successful restoration of the circulation after an out of hospital cardiac arrest. Such patients are now virtually always admitted to ICU for a minimum of 72 h of stabilisation and observation before prognosticating, irrespective of whether the cause of the arrest is primarily cardiac or not, since the primary concern remains whether the patient will recover from their DBI, in this instance hypoxic brain injury.
A large recent study from Japan reported the outcome of over 14,000 patients admitted after an out of hospital cardiac arrest of non-cardiac origin.11 The underlying diagnosis in 1114 of these patients was a stroke of whom 4.9% survived at one month and 1.5% survived with a good outcome. It is not unreasonable to expect patients with other causes of DBI who did not have a cardiac arrest before ICU admission to have better outcomes.
Have we got the resources?
An inevitable concern in the UK is whether there is sufficient ICU capacity to admit all stable, intubated patients with a DBI for whom no neurosurgical intervention is indicated. The number of affected patients is likely to be small and the impact on local ICUs minimal: an audit conducted in the South West of England found that on average 12 patients with DBI per annum died in ED following the WLST in the neuroscience centres. In the non-neurosurgical hospitals, the number of these patients was shown to be 3–4 per annum. The audit did not capture patients who had WLST in ED and were subsequently discharged to the ward. Our own data of 12 patients in 6 months in a neuroscience centre serving a population of 2.5 million suggests that neuroscience centres of a similar size can expect about 24 extra admissions per annum and non-neuro centres 6–8 extra patients per annum with an average length of stay of 36–48 h in the non-survivors. The period of neurological observation could be undertaken in a local ICU and if the patient deteriorates or fails to improve then end of life care can be provided locally. However, if there were to be a change in the patient’s neurological condition, further discussions could be held with the local neurosurgical centre and a decision made as to whether transfer and neurosurgical intervention is now indicated. The concerns about the impact of the DBI pathway on an individual ICU’s resources also needs to be balanced by its effects on the wider NHS and the general population at large. It has been shown recently that the admission of a dying patient to the ICU for end of life care and possibly organ donation yields on average seven times the quality-adjusted life years (QALYs) in transplant recipients per ICU bed-day compared with the average benefit for the admission of an ICU patient expected to survive.12
On the other hand, the concerns go beyond ICU resources as some believe that this approach has an inherent risk of resulting in survivors with significant neurological impairment who require a lifetime of costly care. We anticipate that most patients with DBI would either progress to brain death or have WLST within a maximum of 72 h and subsequently die. The pathway in effect often delays the WLST and most will still die because the option to WLST remains for those who continue to deteriorate neurologically or who show no signs of improvement. On the other hand, those who show signs of neurological improvement have the potential to make good recoveries. Separating these salvageable patients from the majority who die is not possible on admission to the ED and further time is needed for observation.
The DBI pathway should not affect families who adopt a ‘survival at any cost’ position for their relative, since early WLST is unlikely in this situation. If anything the pathway gives these families additional time to come to terms with the diagnosis and prognosis and further time for their opinions to be heard. These views are just one consideration of many when clinicians are making a judgment as to the patient’s overall best interests.
Is end of life care enhanced?
While the number of unexpected survivors may be small, the potential for improving the end of life care for these patients is a further benefit of the DBI pathway. It could be argued that the pathway is unnecessarily prolonging the dying process, but a minority of patients are not actually dying as they have the potential to survive. They are being given the time and opportunity to improve. For the patients that go on to die, admission to the ICU will offer the patient and their family quality end of life care. The Emergency Department is generally extremely busy and the staff face many competing pressures that limit the time they can make available for end of life care. The environment of a busy ED is also not conducive to undertaking WLST in an unhurried fashion that gives the family both time and privacy. Admission to ICU allows for the timely delivery of high quality end of life care in an appropriate environment. It also provides relatives with further time to understand and accept the catastrophic nature of the brain injury and the likelihood of their relative’s death. In doing so, we may be easing the transfer from hope of recovery to the beginnings of a grief process amongst relatives. The additional time provided may also allow time for relatives to travel to the hospital either nationally or as in our experience, internationally. This is important not only because family members can support one another but also because it gives them the opportunity to be present at the time of WLST and death or to witness neurological testing if they wish. In our experience this unhurried approach has been universally welcomed by families.
A shared approach to end-of-life decision making is recommended by professional critical care bodies internationally.13 This DBI pathway allows the extra time required for better exploration of the patient’s values and preferences with their relatives, and implementing a more personalised end of life care plan, ideally incorporating those wishes and values.14
Is this simply a measure to increase organ donation?
The primary objectives of the DBI pathway are to enhance end of life care and increase the accuracy of neurological prognostication in these patients. Any potential increase in organ donation would be a consequence of trying to meet those two objectives. The GMC guidance on end of life care makes it clear that doctors have a duty to explore the potential for organ donation when a patient is close to death and that they should follow any national procedures for identifying potential organ donors.15 Facilitating organ donation from the ED complies with this guidance. It is possible that many of those who became organ donors after admission to ICU may still have donated their organs after the WLST in the ED, particularly as DCDS, since few patients will fulfil the criteria for neurological testing in the ED. However, delaying the WLST and admission to ICU for prognostication and end of life care can also better meet the wishes of those who choose to donate their organs after death.
First, a number of patients progress to neurological death following ICU admission allowing them to donate as DBD donors rather than DCD donors. In the UK, it has recently been estimated that 28% of actual DCD donors have the potential to progress to neurological death and, therefore, DBD if WLST had been delayed by 36 h.16 The significance of a shift from DCD to DBD is that mean number of organs retrieveed from a DBD donor is 3.8 compared with 2.7 from a DCD.17 DBD may also be associated with better tranplant outcomes, particularly liver transplantation.18,19
Second, the admission to ICU gives the family more time to understand and accept the prognosis. It also allows for timely referral of the patient to the SN-OD.2,3 It means that the conversations regarding the prognosis and decision making can be ‘decoupled’ from the approach for organ donation. The ICU medical staff and SN-OD can plan a collaborative approach for organ donation once they are satisfied that the family understand and accept the inevitability of death if WLST is planned, or the diagnosis or death using neurological criteria. All these aspects are considered to be best practice in the family approach for organ donation.4
One consequence of the proposed DBI pathway is that organ donation should become a routine consideration in end of life planning so allowing the adoption of best practice in terms of the identification and referral of potential donors along with a collaborative approach to families.
Conclusion
There is a need to consider an alternative approach to the management of patients admitted with a DBI. Admission to ICU allows time to confirm or refute the initial prognostication, to increase consistency in end of life care and to avoid missed opportunities to explore the potential for organ donation. There are patients with a DBI who demonstrate neurological improvement and following appropriate interventions have not only survived but have done so with good functional outcome. These patients should be allowed the time needed to demonstrate their potential for improvement in the same way as those admitted following the return of spontaneous circulation after an out of hospital cardiac arrest. We welcome the recommendations made by the Neurocritical Society and suggest that further consideration is given to their adoption nationally.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Manara is the Regional Clinical Lead for Organ Donation for the South West Region. Drs Thomas and Harding are Clinical Leads for Organ Donation in their Trusts
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Souter MJ, Blissitt PA, Blosser S, et al. Recommendations for the critical care management of devastating brain injury: prognostication, psychosocial, and ethical management. A position statement for healthcare professionals from the neurocritical care society. Neurocritical Care. 2015; 23: 4–13. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Clinical Excellence (December 2011). Organ donation for transplantation: improving donor identification and consent rates for deceased organ donation. Available at: http://www.nice.org.uk/guidance/cg135/resources/organ-donation-for-transplantation-improving-donor-identification-and-consent-rates-for-deceased-organ-donation-35109512048581 (accessed 31st January 2016).
- 3.Timely Identification and Referral of Potential Organ Donors. A strategy for implementation of best practice. NHS Blood and Transplant 2012. Available at: http://www.odt.nhs.uk/pdf/timely-identification-and-referral-potential-donors.pdf (accessed 31st January 2016).
- 4.NHS Blood and Transplant 2013. Approaching the families of potential organ donors. Best practise guidance. Available at: http://www.ics.ac.uk/easysiteweb/getresource.axd?assetid=998&type=0&servicetype=1 (accessed 31st January 2016).
- 5.Hemphill JC, 3rd, Newman J, Zhao S, et al. Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke 2004; 35: 1130–1134. [DOI] [PubMed] [Google Scholar]
- 6.Wang W, Lu J, Wang C, et al. Prognostic value of ICH score and ICH-GS score in Chinese intracerebral hemorrhage patients: Analysis from the China National Stroke Registry (CNSR). PLoS One 2013; 8: e77421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Online Calculator by Sealed Envelope Ltd. CRASH head injury prognosis http://www.crash2.lshtm.ac.uk/Risk%20calculator/index.html (accessed 31st January 2016).
- 8.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. J Am Med Assoc 2001; 286: 1754–1758. [DOI] [PubMed] [Google Scholar]
- 9.Morton V, Torgerson DJ. Effect of regression to the mean on decision making in health care. BMJ 2003; 326: 1083–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aulmann C, Steudl WI, Feldmann U. Validation of the prognostic accuracy of neurosurgical admission scales after rupture of cerebral aneurysms. Zentralbl Neurochir 1998; 59(3): 171–180. [PubMed] [Google Scholar]
- 11.Kitamura T, Kiyohara K, Sakai T, et al. Epidemiology and outcome of adult out-of-hospital cardiac arrest of non-cardiac origin in Osaka: a population-based study. BMJ Open 2014; 4: e006462, doi:10.1136/bmjopen-2014-006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunnink L, Cook DA. Palliative ICU beds for potential organ donors: an effective use of resources based on quality-adjusted life-years gained. Crit Care Resusc 2016; 18: 37–42. [PubMed] [Google Scholar]
- 13.Thompson BT, Cox PN, Antonelli M, et al. American Thoracic Society; European Respiratory Society; European Society of Intensive Care Medicine; Society of Critical Care Medicine Sociètède Rèanimation de Langue Française: Challenges in end-of-life care in the ICU: Statement of the 5th International Consensus Conference in Critical Care: Brussels, Belgium, April 2003: Executive summary. Crit Care Med 2004; 32: 1781–1784. [DOI] [PubMed] [Google Scholar]
- 14.Manara A. Bespoke end-of-life decision making in ICU: Has the tailor got the right measurements? Crit Care Med 2015; 43: 909–910. [DOI] [PubMed] [Google Scholar]
- 15.General Medical Council. Treatment and care towards the end of life: Good practice in decision making, 2010. Available from http://www.gmc-uk.org/static/documents/content/Treatment_and_care_towards_the_end_of_life_-_English_1015.pdf (accessed 31st January 2016).
- 16.Broderick AR, Manara A, Bramhall S, et al. A donation after circulatory death program has the potential to increase the number of donors after brain death. Crit Care Med. 2016; 44: 352–359. [DOI] [PubMed] [Google Scholar]
- 17.NHS Blood and Transplant. Organ Donation and Transplantation Activity Report 2014–2015. Available at http://nhsbtmediaservices.blob.core.windows.net/organ-donation-assets/pdfs/activity_report_2014_15.pdf (accessed 31st January 2016).
- 18.Abt PL, Desai NM, Crawford MD, et al. Survival following liver transplantation from non heartbeating donors. Ann Surg 2004; 239: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abt P, Crawford M, Desai N, et al. Liver transplantation from controlled non heart-beating donors: an increased incidence of biliary complications. Transplantation 2003; 75: 1659–1663. [DOI] [PubMed] [Google Scholar]