Abstract
Introduction
Glycaemic control is an important predictor of mortality in sepsis. Various international organizations including the Surviving Sepsis campaign recommend glycaemic control in critical illness with a glucose target between 6.1-10 mmol/L. The NICE-SUGAR Trial in 2009 was a landmark in the debate over tight versus liberal glycaemic control in the critically ill and subsequent guidelines have been adjusted to reflect a move towards moderate glycaemic control.
Methods
We conducted a nation-wide study comparing glucose targets used in intensive care units in the United Kingdom in 2007 with those used in 2014 to 2015 to see the impact of the NICE-SUGAR study and subsequent guideline changes.
Results
We received a combined response from 81% of intensive care units in the UK. There was an increase in the average median glucose target in 2014/2015 compared with 2007 (7.8 versus 7.2; p < 0.01). However, there is still much variability in glucose targets used in critical care in the UK.
Conclusions
There is an overall trend towards using a more moderate glucose target in critical care in the UK reflecting changes in international guidelines. However, it is likely that controversies, which still exist in the literature, are reflected in the variability of glycaemic control targets. It is possible that the advent of closed-loop or continuous glucose monitoring may have a further impact on this.
Keywords: NICE-SUGAR, glucose control, critical care, diabetes, sepsis
Introduction
Hyperglycaemia is part of a normal response to physiological stress. However, extreme hyperglycaemia and hypoglycaemia worsen mortality in the critically ill.1 Therefore, various international organisations including the American Diabetes Association, American Heart Association, the French Societies of Anaesthesia and Intensive care (SFAR and SRLF) as well as the global Surviving Sepsis campaign recommend glycaemic control in critical illness with a target range usually between 6.1 and 10 mmol/L.2–5
Several clinical trials and meta-analyses have investigated optimal targets for glycaemic control with some conflicting results. A Belgian trial in 2001 using a tight glycaemic control protocol, now known as the Leuven protocol, demonstrated a mortality benefit from tight versus lenient glycaemic control in a surgical intensive care unit.6 In this study, the target range was 4.4–6.1 mmol/L in the treatment group and 10–11.1 mmol/L in the control arm. This initial trial involved mostly post-cardiac surgical patients (63%) and showed astounding reductions in in-hospital mortality (34%), septicaemia (46%), acute renal failure requiring dialysis (41%) and critical illness polyneuropathy (44%). The Leuven protocol was subsequently trialed in a medical ICU by the same investigators in 2006.7 In the second trial, there was no demonstrable benefit from tight versus conventional glucose control.
In the wake of the trials of the Leuven protocol, multiple attempts at replicating these results in other centers failed to show any mortality benefit in tight versus conventional glycaemic control, and many demonstrated an increased risk of hypoglycaemia in the tight glycaemic control cohort. The NICE-SUGAR study in 2009 was the largest randomised controlled trial comparing the intensive (target 4.5–6.0 mmol/L) and conventional (target < 10 mmol/L) glycaemic control.8 This study showed a significantly higher incidence of hypoglycaemia in the intensive versus conventional group (6.8% vs. 0.5%) and a higher mortality (OR 1.14; 95% CI 1.28–1.02; p = 0.02). Further studies including the glucocontrol study and a meta-analysis including NICE-SUGAR in 2009 by Griesdale et al. showed no mortality benefit to intensive glycaemic control and an increased risk of hypoglycaemia.9,10 As such, most international guidelines have increased their target for glycaemic control since 2009 to reflect this.
We conducted a survey in 2007 before NICE-SUGAR was first published and then again in 2014 to 2015 to see what impact this trial and subsequent guidelines have had on practices in the intensive care units in the United Kingdom.
Methods
We undertook a national telephone survey of adult mixed medical and surgical intensive care units in the United Kingdom in 2007 and 2014–2015 to investigate practices in glycaemic control in the critically ill patients. When calling each unit, we asked to speak to the nurse in charge or the most senior doctor available. They were only asked what the target glucose range was used in their unit protocol.
We included all general medical and surgical intensive care units of all sizes. We excluded specialist intensive care units e.g. neurointensive care units, cardiothoracic, paediatric, liver and renal intensive care units. While this does not guarantee a homogenous population in all intensive care units, we assume that all general intensive care units have a mixed medical and surgical population with a range of presenting conditions. We telephoned 259 hospitals and were able to collect data from 213 hospitals (81%) using a maximum of 2 phone calls due to time constraints. The data coverage in 2007 was 100% but only those hospitals covered on both occasions were included in this analysis. There is no reason to believe that those units lost to follow-up represent a group with a special attitude towards glucose control that would skew the distribution of data gathered in 2014/2015.
For each range of target glucose used by different hospitals, a median was calculated and used for further analysis. In cases where only an upper limit was specified e.g. <10 mmol/L, the upper limit value was used. This approach was used as a way of estimating the most likely single numerical goal within the range given. For a range 5 to 8mmol/L for example, we assume that nurses will attempt to maintain a patient s serum glucose around 6.5mmol/L. However, for those ranges specified as < 10mmol/L one would assume that nurses would keep the glucose as close to 10mmol/L as possible as there is no need to lower it beyond that.
Due to the high response rate, nearly all the data for the population in question has been presented here. Therefore we have used descriptive rather than inferential statistical methods. Both ranges and medians of ranges have been represented graphically for analysis and comparison.
Tight glycaemic control is defined here as a range of 4.5–6.0 mmol/L or median target glucose of 6 mmol/L or less. Moderate glycaemic control is defined as a range of 6.1–10 mmol/L or a median of 8 mmol/L and liberal glycaemic control is defined as 10–12 mmol/L with a median of 11 mmol/L. This is to reflect ranges studied in NICE-SUGAR and the Leuven protocol.
Results
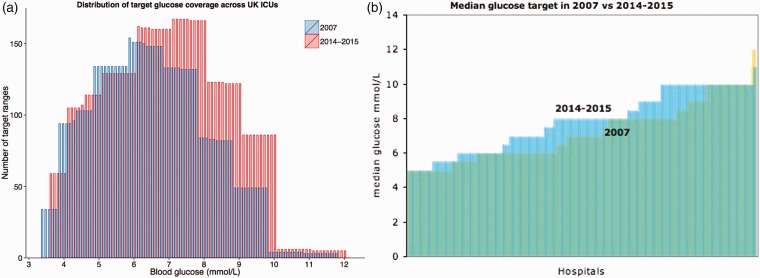
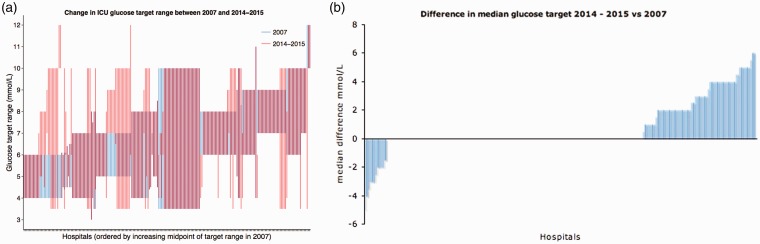
There is a general trend towards increasing target glucose ranges in intensive care units in the UK. Figure 1a shows an increase in the use of higher target ranges in 2014–2015 compared with 2007. Figure 1b also shows an increase in the median glucose targets in 2014–2015 compared with 2007. Figure 2a shows how each intensive care unit has changed its target glucose range between 2007 and 2014–2015 with the majority of change seen in the units which had low target ranges in 2007. However, as seen more clearly in Figure 2b, the majority of hospitals had not changed their median glucose targets between these two dates and a small proportion of hospitals have decreased their glucose targets in this time. Of the units surveyed, 40% practiced tight glycaemic control in 2007, which has reduced to 23% in 2014–2015. Of units which practiced tight glycaemic control in 2007, no unit decreased their median target glucose and 46% of hospitals increased their target. In the liberal glycaemic control arm from 2007, 9 hospitals have become stricter, 73% have not changed and the remaining have increased their median target glucose. Those units that decreased their target ranges were a mix of district general and teaching hospitals. The exact case mix for each of these hospitals was not investigated.
Figure 1.
(a) Each bar represents the frequency with which each glucose target value occurs within the ranges of each intensive care unit or the degree of overlap of ranges i.e. glucose values from 7 to 8 mmol/L were the most frequently seen values in 2014–2015 and 6 to 7 mmol/L in 2007. (b) Median glucose targets from different hospitals. The yellow bars represent glucose targets in 2007; the blue bars represent glucose targets in 2014–2015.
Figure 2.
(a) Glucose target ranges in 2007 and 2014-2015. Each overlapping blue and red bar represents target ranges for each hospital in 2007 and then 2014-2015 respectively. (b) Difference in median glucose target in 2014-2015 versus 2007. Negative bars represent a decrease in the median glucose target in 2014/2015 compared with 2007, a positive bar represents an increase in the glucose target and no bar represents no change in glucose target.
The data represented above show a gradual trend towards a more liberal approach to glycaemic control in the intensive care setting. However, there is still great variability in the median target glucose within the intensive care units around the UK and most units have not changed at all in the intervening years.
Discussion
For this particular study the authors only investigated the glucose ranges used in intensive care units in the UK and compared those in 2007 with 2014. No attempt was made to qualitatively investigate why each unit chose their target ranges. While this would be interesting to know, it is a complex issue and outside the scope of this particular review. While much of the current literature encourages a more moderate approach to glycaemic control in the critically unwell and warns of the dangers of hypoglycaemia, it appears the jury is still out on the optimal glucose target in the intensive care setting. This is likely to be due to the many controversies and misunderstandings around the available trial data.
One major confusion in the debate over the optimal target glucose is to do with the definitions of tight or intensive, moderate, liberal or conventional glycaemic control and the glucose ranges that each of these terms implies. In the 2001 Leuven study, the target glucose range in the control arm was significantly higher than in the NICE-SUGAR study. According to our definition mentioned above, the NICE-SUGAR study intensive insulin arm had a tight glucose target and its control arm had a moderate glucose target. In the Leuven protocol, the intensive insulin arm has a tight glucose target and its control arm has a liberal glucose target. This makes the two trials difficult to directly compare and skews our interpretation of the relative benefits of tight and liberal glycaemic control. In fact, in a systematic review in 2011, Kansagara et al.11 reported that trials which showed a benefit from tight glycaemic control had high glucose targets in the control arm (10–11.1 mmol/L) whereas those that did not show a benefit had moderate glucose targets in the control arm (6.0–10 mmol/L).
This systematic review also found that a target glucose <6.7 mmol/L was independent associated with a significantly increased risk of hypoglycaemia irrespective of the insulin infusion protocol used. However, some might argue that tight glycaemic control may be beneficial if hypoglycaemia can be avoided (as appeared to be the case in the original Leuven protocol trial in 2001). One current focus of research on the topic of insulin therapy in the critically ill is the use of continuous glucose monitoring and closed-loop glycaemic control systems to minimize the risk of hypoglycaemia. This technology may enable tight glycaemic control to be conducted more safely and perhaps a fairer comparison between tight versus moderate glycaemic control can be made.
The Leuven trial in 2001 remains the only large-scale study to demonstrate a mortality benefit for tight versus conventional glycaemic control. The NICE-SUGAR and subsequent trials have since turned the tide in the debate over the optimal target for glycaemic control in favour of a moderate approach. Several further trials, systematic reviews and meta-analyses have subsequently shown no benefit to tight glycaemic control over moderate with a greater incidence of hypoglycaemia with tight control. Consequently, most international bodies currently recommend a moderate target glucose of ≤10 mmol/L and it is evident that the intensive care units in the United Kingdom are following suit.
Conclusion
While the overall trend in the United Kingdom is toward moderate glycaemic control, we have observed great variability in the practices around the United Kingdom regarding insulin therapy in the critically ill and varying responses to recent trial evidence and changes in international guidelines. This may be due to confusion about the available trial evidence, controversies about the existing data or concerns over the risks of hyperglycaemia in the critically unwell. One promising development that may change the way we treat hyperglycaemia in the future could be continuous glucose monitoring in the intensive care setting. Until this is more available and widely used, however, we recommend moderate glycaemic control with a target glucose <10 mmol/L in keeping with the current international guidelines.
Key messages
– International guidelines recommend moderate glycaemic control with a glucose target of 6.1–10 mmol/L in the critically ill patients.
– Current available literature points towards a moderate approach to glycaemic control as the safest and most effective in critical care.
– In the United Kingdom, intensive care units are moving towards moderate glycaemic control but there is a great variability in the glucose targets used.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Authors’ contributions
AD: Data gathering, interpretation, preparation of manuscript. JL: Majority of data gathering. HS: Statistical analysis and visualizations SS: Review of data, supervision of preparation of manuscript.
References
- 1.Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. JAMA 2002; 288: 2167–2169. [DOI] [PubMed] [Google Scholar]
- 2.Moghissi ES, Korytkowski MT, Dinardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control. Diabetes Care 2009; 32: 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peberdy MA, Callaway CA, Neumar RW, et al. Part 9: Post–Cardiac Arrest Care 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122: S768–S786. [DOI] [PubMed] [Google Scholar]
- 4.Ichai C, Preiser JC. International recommendations for glucose control in adult non diabetic critically ill patients. Crit Care 2010; 14: R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International Guidelines for Management of Severe Sepsis and Septic Shock, 2012. Intensive Care Med 2013; 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berghe GVB, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345: 1359–1367. [DOI] [PubMed] [Google Scholar]
- 7.Berghe GVB, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354: 449–461. [DOI] [PubMed] [Google Scholar]
- 8.The NICE-SUGAR Study investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009; 360: 1283–1297. [DOI] [PubMed] [Google Scholar]
- 9.Preiser JC, Devos P, Ruiz-Santana S. A prospective randomized multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: The glucocontrol study. Intensive Care Med 2009; 35: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 10.Griesdale DEG, de Souza RJ, van Dam RM, et al. Intensive insulin therapy and mortality among critically ill patients: A meta-analysis including NICE-SUGAR study data. CMAJ 2009; 180: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kansagara D, Fu R, Freeman M, et al. Intensive insulin therapy in hospitalized patients: A systematic review. Ann Intern Med 2011; 154: 268–282. [DOI] [PubMed] [Google Scholar]