Abstract
A nationwide survey was performed to investigate the current incidence of viral diseases in Korean sweet potatoes for germplasm and growing fields from 2011 to 2014. A total of 83.8% of the germplasm in Korea was infected with viruses in 2011. Commercial cultivars that were used to supply growing fields were infected at a rate of 62.1% in 2012. Among surveyed viruses, the incidence of five Potyvirus species that infect sweet potato decreased between 2012 and 2013, and then increased again in 2014. Representatively, the incidence of Sweet potato feathery mottle virus (SPFMV) was 87.0% in 2012, 20.7% in 2013 and then increased to 35.3% in 2014. Unlike RNA viruses, DNA viruses were shown to decrease continuously. The incidence of Sweet potato leaf curl virus (SPLCV) was 5.5% in 2003, 59.5% in 2011, and 47.4% in 2012. It then decreased continuously year by year to 33.2% in 2013, and then 25.6% in 2014. While the infection rate of each virus species showed a tendency to decline, the virus infection status was more variable in 2013 and 2014. Nevertheless, the high rate of single infections and mixed infection combinations were more variable than the survey results from 2012. As shown in the results from 2013, the most prevalent virus infection was a single infection at 27.6%, with the highest rate of infection belonging to sweet potato symptomless virus-1 (SPSMV-1) (12.9%). Compared to 2013, infection combinations were more varied in 2014, with a total of 122 kinds of mixed infection.
Keywords: occurrence survey, sweet potato, virus incidence
In Korea, sweet potato (Ipomea batatas L.), of the family Convolvulaceae, was traditionally regarded as a bearing crop to combat starvation during the winter and spring. Recently, sweet potato has gained popularity due to its high nutritional value, with high levels of vitamins A and C, iron, potassium, and fiber. Sweet potato is also an excellent source of the vitamin A precursor, beta-carotene. Moreover, many countries are expanding research projects on sweet potato because the high-starch content can be used as a biofuel (ethanol). Sweet potato production in Korea was 320 thousand tons in 2014, which was the third highest ranked cereal crop.
Viral diseases of sweet potato have become widespread, causing serious crop losses around the world. In total, more than 30 viruses have now been reported to infect sweet potato (Clark et al., 2012). Among these, 23 have been assigned a formal taxonomic position by the International Committee on Taxonomy of Viruses. The number continues to increase as virus detection methods improve. Only a few of the viruses are considered to be of major economic importance. The most severe disease in sweet potato is caused by co-infection with the whitefly-transmitted Sweet potato chlorotic stunt virus (SPCSV) and the aphid-transmitted Sweet potato feathery mottle virus (SPFMV), which results in the synergistic sweet potato virus disease (SPVD) (Gibson et al., 1998; Karyeija et al., 2000; Mukasa et al., 2006). Synergism has also been observed between SPCSV and the possibly whitefly-transmitted Sweet potato mild mottle virus (SPMMV) (Gutierrez et al., 2003). SPCSV caused synergistic diseases in sweet potato with many other sweet potato viruses (Untiveros et al., 2007). Moreover, the decline of yield due to virus infection was reported by several studies. For example, in China, losses of over 20% on average due to sweet potato virus diseases have been observed (Feng et al., 2000) which was mainly due to Sweet potato feathery mottle virus (SPFMV) and Sweet potato latent virus (SPLV). In addition, Sweet potato leaf curl virus (SPLCV) infection caused a 30% decline in production of the ‘Beauregard’ cultivar in the USA (Clark and Hoy, 2006).
In Korea, SPFMV, Sweet potato virus G (SPVG), and SPLV, all belonging to the family Potyviridae, and SPLCV, a member of the Geminiviridae, have been detected (Kwak et al., 2006). Our previous nationwide survey revealed that, in 2003, about 73% of the samples were infected with at least one of these four viruses (Kwak et al., 2006). SPFMV and SPVG were especially prevalent (40% and 16%, respectively), and co-infection with SPFMV and SPVG was detected in 11% of surveyed sweet potatoes. Although SPCSV was reported by Yun et al. (2002), it has not subsequently been detected in Korea. Moreover, in 2012, new species of sweet potato-infecting viruses were reported and surveyed. Sweet potato virus C (SPVC), Sweet potato chlorotic fleck virus (SPCFV), Sweet potato virus 2 (SPV2), and Sweet potato symptomless virus-1 (SPSMV-1) were firstly reported in Korea by Kwak et al (2014).
SPFMV genus Potyvirus is the most common sweet potato virus worldwide. Certain isolates in the USA and Japan cause a great deal of economic damage by inducing cracking or internal corkiness in some cultivars. Many strains of SPFMV, isolates, variants and serotypes of SPFMV have been reported, such as the russet crack (RC), C, S, C1 (from Peru), and strain 835 (from Guatemala), among others (Colinet and Kummert, 1993; Karyeija et al., 2001; Loebenstein and Thottappilly, 2009). It was suggested that SPFMV-C might be a distinct virus (Tairo et al., 2005). Evidence on recombination events between viruses from different strain groups of SPFMV have been reported recently (Untiveros et al., 2008). According to a previous study, Korean SPFMV strains were identified as the RC strain and O strain through coat protein and full genome analysis. However, SPFMV-EA strains did not exist in Korea (Kwak et al., 2007).
Research on the other species members of sweet potato-infecting Potyvirus – SPVG, SPLV, and SPV2 – has been limited. Recently, their full genome sequences were characterized and then analyzed with SPFMV and other Potyvirus species (Ateka et al., 2007; Li et al., 2012; Rodríguez et al., 2012; Wang et al., 2013). In addition, sweet potato-infecting potyviruses underwent full genome characterization, and their phylogenetic relationships were recently analyzed in Korea (Kwak et al., 2015).
Together with SPFMV, the most actively studied virus is SPLCV, which is a species member of Begomovirus, Geminiviridae. SPLCV was first reported in Japan and Taiwan (Moyer and Salazar, 1989). After that, SPLCV was reported in Kenya, USA, Brazil, Mexico, China, Puerto Rico, and Peru (Fuentes and Salazar 2003; Kwak et al., 2006; Lotrakul et al., 1998). Sweet potato-infecting Begomovirus species including SPLCV and others that were already characterized their full genome sequence, and all of these species have been categorized as belonging to the subgenus of Begomovirus referred to as Sweepovirus (Banks et al., 1999; Fauquet and Stanley 2003; Lotrakul and Valverde, 1999; Lotrakul et al., 2002; Lozano et al., 2009; Paprotka et al., 2010).
Typical symptoms of SPLCV include curling of young leaves and the swallowing of leaf veins. However, those symptoms decrease or disappear completely following their initial growth. SPLCV infection has caused a production decline up to 30%. As seen with other Begomovirus species, SPLCV is transmitted by Bemisia tabaci in a persistent manner. Vine grafting was also the transmission route for SPLCV. However, the spread of SPLCV was mostly due to vegetative propagation of already infected sweet potato. A recent study reported that SPLCV can be transmitted via seed (Kim et al., 2015). Korean isolates of SPLCV were characterized and their full genome sequences were analyzed (Choi et al., 2012; Park et al., 2011). Recently, Sweet potato golden vein associated virus was characterized and reported (Kil et al., 2014).
In this study, we sought to provide nationwide survey results for the virus infection status of the sweet potato germplasm, as well as the virus infection status of sweet potatoes from growing fields over a 3-year period.
Materials and Methods
Survey and sample collection
From 2011 to 2014, sweet potato leaves (including petiole and stem) showing virus-like symptoms were collected for virus infection status surveys. Sampling areas and the number of samples are listed in Table 1. Survey data from 2003 and 2012 were obtained for comparison from previous reports by Kwak et al (2006, 2014). The collected samples were treated with insecticides to remove potential insect vectors and maintained in pots in a greenhouse at 20–25°C. All samples were inspected for disease symptoms for at least 30 days and analyzed for virus infection by reverse transcription – polymerase chain reaction (RT-PCR).
Table 1.
Collected sweetpotato samples in this study
| 2011 | 2012** | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||
| Region | No. samples collected | Region | No. samples collected | Region | No. samples collected | Region | No. samples collected |
| Muan* | 452 | Yeongam | 40 | Yeongam | 34 | Yeongam | 29 |
| Haenam | 10 | Haenam | 127 | Haenam | 377 | ||
| Muan | 37 | Iksan | 117 | Muan | 31 | ||
| Iksan | 16 | Kimje | 62 | Iksan | 139 | ||
| Kimje | 14 | Nonsan | 77 | Kimje | 93 | ||
| Nonsan | 13 | Cheongju | 184 | Nonsan | 299 | ||
| Boryeong | 4 | Yeoju | 212 | Cheongju | 134 | ||
| Cheongju | 7 | Yeoju | 126 | ||||
| Yeoju*** | 549 | ||||||
| Sancheong | 3 | ||||||
| Sacheon | 10 | ||||||
|
| |||||||
| Total | 452 | 703 | 813 | 1,228 | |||
Collected from the “Bioenergy Crop Research Institute”.
Fields survey results were obtained from reports of Kwak et al (2014).
Collected from the “Yeoju-si Agriculture Technical Center”. * and *** both of them were surveyed virus infection status for the sweetpotato germplasm of Korea.
Total nucleic acid extraction and virus detection by RT-PCR
Total nucleic acids were extracted total nucleic acids from the abnormal symptom showing leaf, petiole and stem samples using the Viral gene-spinTM viral DNA/RNA extaction kit (iNtRON, Korea), according to the manufacturer’s instructions. Typical RT-PCR assays were carried out using the primers shown in Supplementary Table 1 in a two-step procedure using AMV reverse transcriptase (Promega, USA) for RT and Go Taq DNA polymerase (Promega) for PCR, as described by Kwak et al (2014). Multiplexed RT-PCR assays were performed using two-step RT-PCR as follows. RT reactions were carried out at 42°C for 30 min in a final 5 μl reaction volume obtained by combining 0.5 μl of total RNA (approx. 0.5 μg), 0.5 μl of a mixture of equal amounts of 32 μM reverse primers for four viruses, 1 μl of 5X RT reaction buffer, 0.5 μl of 2.5 μM dNTP, 0.1 μl of bovine serum albumin (10 mg/ml), 8 U of RNase inhibitor, 0.5 U of AMV reverse transcriptase (Promega), and sufficient distilled water to bring the total volume up to 5 μl. RT reactions were terminated by heating at 95°C for 5 min. When RT was completed, we added 20 μl of a solution comprising 0.5 μl of a mixture of equal amounts of 32 μM forward primers for four viruses, 5 μl of 5X PCR reaction buffer, 2.5 μl of 25 mM MgCl2, 0.4 μl of BSA (10 mg/ml), 1 U of Go-taq DNA polymerase (Promega), and dH2O to the tube containing the RT products. PCR was performed in a thermal cycler (Bio-Rad, USA) with the following conditions: pre-denaturing step at 94°C for 30s; an annealing step at 55°C for 30s; an extension step at 72°C for 60s; and a final extension at 72°C for 10 min. PCR products were analyzed by electrophoresis on a 1.5% agarose gel at 100 V for 90 min, stained with ethidium bromide and amplified DNA bands were visualized using a UV trans-illuminator.
Results
Virus infection status of sweet potato germplasm of Korea
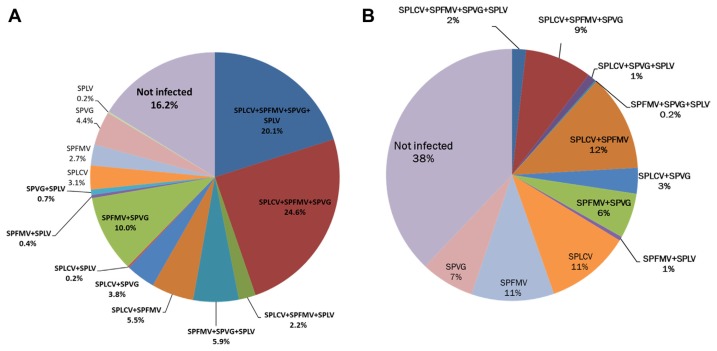
In 2011, sweet potatoes were collected from germplasm in the Bioenergy Crop Research Institute. A total of 452 samples representing each line were collected and virus infection status was tested by RT-PCR. The virus infection status was tested for only four virus species – SPFMV, SPLCV, SPVG, and SPLV. Among all tested samples, the incidence of virus-infected samples was 83.8%, consisting of both multiple (73.4%) and single (10.4%) infection (Fig. 1A).
Fig. 1.
Virus infection status of sweet potatoes from germplasm in Korea. (A) Virus infection status of 452 sweet potato lines from the Bioenergy Research Center of RDA in 2011. (B) Virus infection status of 64 commercial growing cultivars from the Yeoju-si Agricultural Technical Center in 2011. Not infected status and all kinds of multiple virus infection status were bold characterized. Both of germplasm were infected more than half of tested samples were co-infected viruses. SPFMV – Sweet potato feathery mottle virus, SPVG – Sweet potato virus G, SPLCV – Sweet potato leaf curl virus, SPLV – Sweet potato latent virus
The rate of multiple virus infection status revealed to be quadruple (SPLCV+SPFMV+SPVG+SPLV) infection was 20.1%, whereas the triple virus infection rate was 32.7%, in the following combinations: SPLCV+SPFMV+SPVG, 24.6%; SPFMV+SPVG+SPLV, 5.9%; and SPLCV+ SPFMV+SPLV, 2.2%, respectively. In addition, the double infection rate was 26.5% with the following combinations: SPLCV+SPFMV, 5.5%; SPLCV+SPVG, 3.8%; SPFMV+SPVG, 10%; SPVG+SPLV, 0.7%; SPFMV+SPLV, 0.4%; and SPLCV+SPLV, 0.2%, respectively. The rate of single infection was SPLCV, 3.1%; SPVG, 4.4%; SPFMV, 2.7%; and SPLV, 0.2%, respectively (Fig. 1A).
The virus incidence of individual virus species was shown to be SPFMV, 71.5%; SPVG, 69.5%; SPLCV, 59.5%; and SPLV, 29.9%, regardless of multiple infection (Table 2).
Table 2.
Virus infection rates of sweet potato from germplasm and cultivars
| Detected virus | Germplasm* | Growing Cultivars** | ||
|---|---|---|---|---|
|
|
|
|||
| No. Virus detected samples | Rate (%) | No. Virus detected samples | Rate (%) | |
| SPFMV | 323 | 71.5 | 219 | 39.9 |
| SPLCV | 314 | 69.5 | 209 | 38.1 |
| SPVG | 269 | 59.5 | 152 | 27.7 |
| SPLV | 135 | 29.9 | 20 | 3.6 |
| Not infected | 73 | 16.2 | 208 | 37.9 |
Collected from “Bioenergy Crop Research Institute”.
Collected from “Yeojoo-si Agricultural Technology Center”.
SPFMV – Sweet potato feathery mottle virus, SPLCV – Sweet potato leaf curl virus, SPVG – Sweet potato virus G, SPLV – Sweet potato latent virus.
Virus infection status of growing cultivars in Korea
In 2012, a total of 549 sweet potato samples of 63 cultivars were collected and surveyed their virus infection status from the sweet potato growing greenhouse at the Yeoju-si Agricultural Technology Center. The virus infection status was tested for only four virus species – SPFMV, SPLCV, SPVG, and SPLV by RT-PCR. Among all of the tested samples, the virus incidence was 62.1% for multiple (34.2%) and single (29%) infection (Fig. 1B).
The incidence of quadruple virus (SPLCV+SPFMV+ SPVG+SPLV) infection was 2%, the triple virus incidence was 10.2% with the combinations of SPLCV+ SPFMV+SPVG, 9%, SPLCV+SPFMV+SPLV, 1% and SPFMV+SPVG+SPLV, 0.2%, respectively. In addition, the incidence of double infection was 22% with the combinations of SPLCV+SPFMV, 12%; SPFMV+SPVG, 6%; SPLCV+SPVG, 3%; and SPFMV+SPLV, 1%, respectively. The single infection status was shown to be SPFMV as 11%, SPLCV as 11%, and SPVG as 7% respectively (Fig. 1B). The virus infection rate for each virus species was SPFMV 39.9%, SPVG 38.1%, SPLCV 27.7%, and SPLV 3.6%, regardless of multiple infection (Table 2).
Among of surveyed cultivars, four cultivars – ‘AF’, ‘AW’, ‘CB’ and ‘CJ’ were not detected any tested viruses. Moreover, most of the surveyed cultivars were infected with viruses by more than 40% regardless of multiple or single infection. Some cultivars were 100% infected with SPFMV and SPLCV, respectively. Cultivar ‘AD’ was 100% infected with SPFMV from all tested samples. And other cultivars ‘AI’, ‘AM’, ‘AO’, ‘BR’, ‘BJ’ and ‘BS’ were 100% infected with SPLCV. All those 100% infected cultivars were included co-infected samples (Supplementary Table 2).
Virus infection status in growing fields from 2013 to 2014
From 2012 to 2014, sweet potatoes showing symptoms were collected from fields in Korea. The virus infection status was tested for only eight virus species which were already reported from Korea according to the results of virus incidence survey by Kwak et al (2014).
In 2013, a total of 813 samples were tested (Table 1). Among the tested samples, 82.0% of the samples were infected with viruses both of multiple infection (47.4%) and single infection (34.7%). The total incidence status of double, triple, quadruple, quintuple, sextuple and septuple infections were 28.0%, 10.2%, 6.8%, 2.1%, 0.1% and 0.1%, respectively (Table 3). Multiple virus infections were total 74 kinds of combinations within the 19 kinds of double species infection, the 23 kinds of triple, the 22 kinds of quadruple, the eight kinds of quintuple, the one kind of sextuple and the one kind of septuple species infection (Supplementary Table 3). The most abundant virus found in sweet potato fields in 2013 was SPSMV-1 at 58.3%, followed by SPLCV at 33.2%, SPFMV at 20.7%, SPV2 at 13.9%, SPVG at 13.5% and SPVC at 10.6%. SPCFV was not detected in 2013 from any of the samples we tested (Table 4). The most prevalent infection type was the single infection by SPSMV-1, with a rate of about 20% among all tested samples in 2013 (Supplementary Table 3).
Table 3.
Mixed infection type of eight species of sweet potato infecting viruses from sweet potato growing fields of Korea from 2012 to 2014
| Mixed infection type | 2012* | 2013 | 2014 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| No. Virus species combinations | No. Virus infected samples (Rate, %) | No. Virus species combinations | No. Virus infected samples (Rate, %) | No. Virus species combinations | No. Virus infected samples (Rate, %) | |
| Octuple | 1 | 1 (0.6) | Not detected | Not detected | ||
| Septuple | 2 | 2 (1.3) | 1 | 1 (0.1) | 2 | 3 (0.2) |
| Sextuple | 10 | 30 (19.5) | 1 | 1 (0.1) | 7 | 16 (1.3) |
| Quintuple | 9 | 50 (32.5) | 8 | 17 (2.1) | 19 | 51 (4.2) |
| Quadruple | 12 | 32 (20.8) | 22 | 55 (6.8) | 33 | 120 (9.8) |
| Triple | 8 | 19 (12.3) | 23 | 83 (10.2) | 38 | 208 (16.9) |
| Double | 7 | 13 (8.4) | 19 | 228 (28.0) | 22 | 251 (20. 4) |
| Single | 3 | 6 (3.9) | 7 | 284 (34.9) | 7 | 339 (27.6) |
| Not infected | - | 1 (0.6) | - | 146 (18.0) | - | 240 (19.5) |
|
| ||||||
| Total | 52 | 154 | 81 | 813 (100) | 127 | 1,228 (100) |
Survey results were obtained from the report of Kwak et al (2014).
Table 4.
Virus infection status of sweet potatoes from the growing fields of Korea from the 2012 to 2014
| Virus Speices | 2012* | 2013 | 2014 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| No. detected | Rate (%) | No. detected | Rate (%) | No. detected | Rate (%) | |
| SPFMV | 134 | 87.0 | 168 | 20.7 | 433 | 35.3 |
| SPLCV | 73 | 47.4 | 270 | 33.2 | 314 | 25.6 |
| SPVG | 89 | 57.8 | 110 | 13.5 | 229 | 18.7 |
| SPLV | 30 | 19.5 | 104 | 12.8 | 165 | 13.4 |
| SPV2 | 63 | 40.9 | 113 | 13.9 | 326 | 26.6 |
| SPVC | 131 | 85.0 | 86 | 10.6 | 375 | 30.5 |
| SPCFV | 47 | 30.5 | 0 | 0 | 31 | 2.5 |
| SPSMV-1 | 103 | 66.9 | 474 | 58.3 | 434 | 35.3 |
| Not detected | 1 | 0.6 | 146 | 18.0 | 240 | 19.5 |
Survey results were obtained from the report of Kwak et al (2014).
SPFMV – Sweet potato feathery mottle virus, SPLCV – Sweet potato leaf curl virus, SPVG – Sweet potato virus G, SPLV – Sweet potato latent virus, SPV2 – Sweet potato virus 2, SPVC – Sweet potato virus C, SPCFV – Sweet potato chlorotic fleck virus, SPSMV-1 – Sweet potato symptomless virus - 1.
In 2014, a total of 1,228 samples were tested. Among the tested samples, 80.5% of samples were infected, including multiple infection (52.8%) and single infection (27.6%). The total incidence status of double, triple, quadruple, quintuple, sextuple and septuple infections were 20.4%, 16.9%, 9.8%, 4.2%, 1.3% and 0.2%, respectively (Table 3). Multiple virus infections were total 120 kinds of virus species combinations within the 22 kinds of double species of infection, the 38 kinds of triple, the 33 kinds of quadruple, the 19 kinds of quintuple, the seven kinds of sextuple and the 2 kinds of septuple species infection (Supplementary Table 4). The most abundant virus in 2014 was SPSMV-1 at 35.3%, followed by SPFMV at 35.3%, SPVC at 30.5%, SPV2 at 26.6%, SPLCV at 25.6%, SPVG at 18.7%, SPLV at 13.4% and SPCFV at 2.5% (Table 4). The most prevalent infection type was a single infection of SPSMV-1, with a rate of about 12.9% among all tested samples (Supplementary Table 4).
Changes of virus infection status year by year
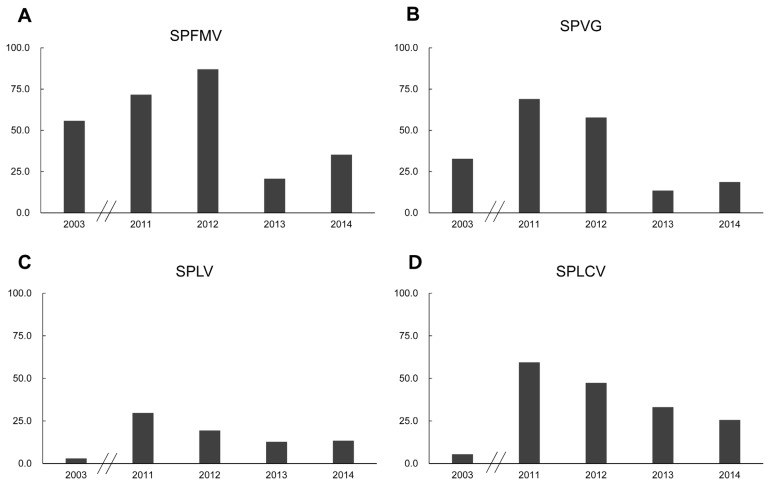
SPFMV infection was the most abundant from a formal survey in 2003 at 55.8%, which then increased to 71.6% from germplasm tested in 2011. The field survey results in 2012 showed an 87.0% incidence as the most abundant infected virus in the growing fields. In 2013, the SPFMV infection incidence decreased to 20.7%, and then incidence increased to 35.3% in 2014 (Fig. 2A).
Fig. 2.
Formally reported sweet potato-infecting virus infection rate of individual virus species by year. (A) SPFMV – Sweet potato feathery mottle virus. (B) SPV2– Sweet potato virus G. (C) SPLV – Sweet potato latent virus. (D) SPLCV – Sweet potato leaf curl virus. Results for 2003 were obtained from Kwak et al (2006).
The SPVG infection increased from 32.7% in 2003 to 68.9% in 2011. From the field survey results in 2012, SPVG showed a 57.8% of incidence, and then decreased to 13.5% in 2013. In 2014, the SPVG incidence was 18.7% (Fig. 2B).
The rate of SPLV infection represented the lowest incidence in 2003 as 3.0%. In 2011, the SPLV infection rate was revealed to be 29.7%. Results from the growing fields showed a decrease in the infection rate to 19.5% in 2012, 12.8% in 2013 and 13.4% in 2014 (Fig. 2C).
SPLCV infection significantly increased to 59.5% in 2011 from 5.5% in 2003. The survey results showed a continuous decrease from 2011 to 2014. In 2012, SPLCV infection was 47.4%, then 33.2% in 2013, and finally 25.6% in 2014 from a survey of the growing fields (Fig. 2D).
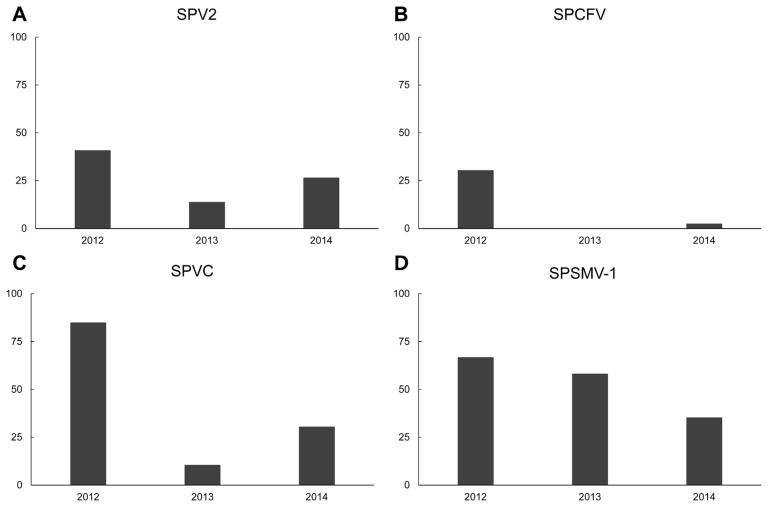
Newly reported sweet potato viruses - SPVC, SPV2, SPCFV and SPSMV-1 - were also surveyed from 2012 to 2014 from growing fields. The SPV2 infection rate was 40.9% in 2012. In 2013, the infection rate was decreased to 13.9%, then increased to 26.6% in 2014 (Fig. 3A). The SPCFV infection rate was 30.5% in 2012; however, there was no samples that were infected with SPCFV in 2013. SPCFV was detected in 2.5% of the samples in 2014 (Fig. 3B). SPVC infection was 85% in 2012, the second most abundant virus among collected samples. In 2013, the infection rate decreased to 10.6%, then increased to 30.5% in 2014 (Fig. 3C).
Fig. 3.
Newly surveyed and reported sweet potato virus infection rate of individual virus species by year. (A) SPV2 – Sweet potato virus 2. (B) SPCFV – Sweet potato chlorotic fleck virus. (C) SPVC – Sweet potato virus C. (D) SPSMV-1 – Sweet potato symptomless virus-1.
SPSMV-1 had a 66.9% infection ratio in 2012. However, in 2013, SPSMV-1 was the most abundant virus found in multiple and single infections from all tested samples as 58.3% infection status. In 2014, the infection rate was decreased to 35.3% (Fig. 3D).
Discussion
Sweet potato is propagated by mostly vegetative means in growing fields. Moreover, production of sweet potato can be affected by virus infection. Therefore, the virus infection status of germplasm and cultivars grown in the field represents very important information to growing farmers. In this study, a nationwide survey was performed to investigate the current incidence of viral diseases in Korean sweet potatoes for germplasm and growing fields during 2011 to 2014. Previous studies reported SPFMV, SPLV, SPVG, and SPLCV infection of sweet potato samples from growing fields in 2003 (Kwak et al., 2006). Newly detected viruses (SPVC, SPV2, SPCFV and SPSMV-1) were also reported from surveyed samples taken in 2012 in Korea.
For sweet potatoes from the germplasm of Korea, a total of 83.8% of the tested samples were infected with viruses in 2011. Commercial cultivars used to supply growing fields were infected with viruses at a rate of 62.1% in 2012.
Among surveyed viruses, five Potyvirus (family Potyviridae) species (SPFMV, SPVC, SPVG, SPV2 and SPLV) showed significant changes in virus infection status. All of those viruses decreased between 2012 and 2013. SPFMV showed the highest infection rate at 87.0% in 2012; however, the infection rate dropped to 20.7% in 2013, and then increased to 35.3% in 2014. The infection rate of SPVG showed a similar tendency. The SPVG infection rate was 57.8% in 2012, and then dropped to 13.5% in 2013, just like the SPFMV infection rate in 2013. It also increased slightly to 18.6% in 2014. The SPLV infection rate was 19.5% in 2012, but then also dropped to 12.8% in 2013, similar to other Potyviruses. Likewise, the SPLV infection rate increased slightly to 13.4% in 2014. SPVC was first reported in 2012; however, the infection rate was the second highest in 2012 at 85.0%. Nonetheless, the SPVC infection rate dropped to 20.7% in 2013, and then increased to 35.3% in 2014, along with other surveyed potyviruses (Fig. 2A, 2B, 2C, 3A, 3C, Table 4).
SPCFV (a member of the genus Carlavirus in the family Betaflexiviridae) was reported first and surveyed at a 30.5% infection rate in 2012 in Korea. Like all surveyed potyviruses in this study, the SPCFV infection was significantly decreased in 2013. SPCFV was not detected in any collected samples in 2013. SPCFV was then detected again in 2014 at 2.5% (Fig. 3B, Table 4).
The decline in the virus infection rate could be the result of using a virus-clean stock. Gibson et al. (2014) reviewed a method of counterbalancing virus infection using healthy parents as propagation material in a developing country. After the selection of symptomless parents as propagation material, the virus incidence was maintained at a low level for a few years. Although the propagation material came from a virus-clean stock, the virus infection rate began to increase after planting in the field year after year. All surveyed RNA viruses in this study showed a similar tendency of repeated cycles of degeneration and replacement.
Compared to sweet potato-infecting RNA viruses, the incidence of SPLCV in Korea increased markedly from 5.5% in 2003 to 59.5% in 2011 (Kwak et al., 2006, 2014). The SPLCV infection rate was 47.4% in 2012. It then decreased continuously year after year to 33.2% in 2013 and 25.6% in 2014. In addition, the other Geminivirus (genus Mastrevirus) – SPSMV-1 – was first reported in 2012 with a 66.9% infection rate. As with SPLCV, SPSMV-1 showed a decreasing incidence year after year of 58.3% in 2013, and then 35.3% in 2014. Unlike RNA viruses, DNA viruses showed a continuous decrease (Fig. 2D, 3D).
Compared to RNA viruses, sweet potato-infecting geminiviruses were not markedly decreased in 2013. Virus-free stock preparation of sweet potato was mostly derived by tissue culture of virus-cleaned meristems. This process effectively removed RNA viruses from sweet potato propagation materials (Wang and Valkonen, 2008). In contrast to RNA viruses, DNA viruses – in the case of SPLCV – were eliminated by about 50% through meristem tissue culture (Arkorful et al., 2015; Cheong et al., 2010). Therefore, the DNA virus infection rate was not drastically decreased between 2012 and 2013. Unlike the RNA virus infection rate, in 2014, the DNA virus infection rate decreased. Previous studies showed degeneration of RNA virus infection over five generations (Adikini et al., 2015; Gibson et al., 2014). However, there were no previous studies about the reversion of sweet potato-infecting DNA viruses. The present survey results could suggest that SPLCV and SPSMV-1 might be degenerated as well in sweet potato growing fields of Korea.
While the infection rate of each virus species showed a tendency to decline, the virus infection status showed more varied combinations in 2013 and 2014. In 2012, most of the samples were mixed infections with at least two of the eight viruses represented as 49 different combinations with a 95.5% infection rate (Table 3). The most prevalent infection type (32.5%) was a quintuple infection with SPFMV, SPVC, and three other viruses in 2012. However, in 2013, a single infection was the most prevalent at 34.93%. Nevertheless, due to the high rate of single infection, mixed infection combinations were more varied than the survey results in 2012 as 74 kinds of mixed species combinations. As in the results from 2013, the most prevalent virus infection was single infection at 27.61%, with the highest infection rate seen with SPSMV-1 (12.9%). Compared to 2013, the infection combinations were more varied in 2014 with 120 combinations of mixed infection (Table 3).
The synergy of virus co-infection in sweet potato was reported in several studies. Most of the synergies were related to SPCSV (genus Crinivirus) with other viruses. Fortunately, even though SPCSV was previously reported in Korea, it was not detected in any of the collected samples during the survey (data not shown). However, SPVC, SPV2, SPCFV and SPSMV-1 were newly reported in 2012 (Kwak et al., 2014). Those viruses might have been introduced from other countries between 2003 and 2012. The distribution of SPCFV converged in Southern and Central America including Peru, Bolivia, Columbia, Panama, Cuba and Brazil (Aritua et al., 2009). SPSMV-1 was first reported in sweet potato germplasm from the CIP (International Potato Center) in Peru (Kreuze et al., 2009). For that reason, those viruses were introduced to Korea when the planting materials were imported. Subsequently, even the present survey could not find SPCSV infection, raising the possibility that SPCSV could be introduced to Korea again.
In conclusion, the virus infection status of sweet potato varied throughout the course of the survey. While both a decline and increase in the virus infection rate was observed, the reason was not clearly identified. Moreover, newly introduced viruses were reported during the course of the survey. Therefore, the sweet potato virus infection status of growing fields and germplasm must be monitored closely in the future. Moreover, further studies should be conducted on the relationship between planting materials and growing fields with regard to sweet potato virus infection.
Supplementary Information
Acknowledgments
This research was supported by grants (110034-05-4-HD110) from Agricultural Biotechnology Development Program, Ministry of Agriculture, Food and Rural Affairs of Republic of Korea and supported by grants (PJ01125502) from Rural Development Administration (RDA).
References
- Adikini S, Mukasa SB, Mwanga R, Gibson RW. Sweet potato cultivar degeneration rate under high and low sweet potato virus disease pressure zones in Uganda. Can J Plant Pathol. 2015;37:136–147. doi: 10.1080/07060661.2015.1004111. [DOI] [Google Scholar]
- Aritua V, Barg E, Adipala E, Gibson RW, Lesemann DE, Vetten HJ. Host range, purification, and genetic variability in Sweet potato chlorotic fleck virus. Plant Dis. 2009;93:87–93. doi: 10.1094/PDIS-93-1-0087. [DOI] [PubMed] [Google Scholar]
- Arkorful E, Appiah AS, Dzahini-Obiatey H. Screening for sweet potato (Ipomoea Batatas L.) leaf curl virus (SPLCV) and its elimination using thermotherapy-meristem tip culture technique. J Agric Sci. 2015;10:1–9. [Google Scholar]
- Ateka EM, Barg E, Njeru RW, Thompson G, Vetten HJ. Biological and molecular variability among geographically diverse isolates of Sweet potato virus 2. Arch Virol. 2007;152:479–488. doi: 10.1007/s00705-006-0879-8. [DOI] [PubMed] [Google Scholar]
- Banks GK, Bedford ID, Beitia FJ, Rodriguez-Cerezo E, Markham PG. A novel geminivirus of Ipomoea Indica (Convolvulacae) from Southern Spain. Plant Dis. 1999;83:486. doi: 10.1094/PDIS.1999.83.5.486B. [DOI] [PubMed] [Google Scholar]
- Cheong EJ, Suzanne H, Sarbagh S, Ruhui L. Development of a reliable technique to eliminate Sweet potato leaf curl virus through meristem tip culture combined with therapy of infected ipomoea species. Korean J Plant Resour. 2010;23:233–241. [Google Scholar]
- Choi E, Lee G, Park J, Lee TK, Choi HS, Lee S. Molecular characterization and an infectious clone construction of Sweet potato leaf curl virus (SPLCV) isolated from Korea. Acta Virol. 2012;56:187–198. doi: 10.4149/av_2012_03_187. [DOI] [PubMed] [Google Scholar]
- Clark CA, Hoy MW. Effects of common viruses on yield and quality of beauregard sweetpotato in Louisiana. Plant Dis. 2006;90:83–88. doi: 10.1094/PD-90-0083. [DOI] [PubMed] [Google Scholar]
- Clark CA, Davis JA, Abad JA, Cuellar WJ, Fuentes S, Kreuze JF, Gibson RW, Mukasa SB, Tugume AK, Tairo FD, Valkonen JPT. Sweetpotato viruses: 15 years of progress on understanding and managing complex diseases. Plant Dis. 2012;96:168–185. doi: 10.1094/PDIS-07-11-0550. [DOI] [PubMed] [Google Scholar]
- Colinet D, Kummert J. Identification of a sweet potato feathery mottle virus isolate from China (SPFMV-CH) by the polymerase chain reaction with degenerate primers. J Virol Methods. 1993;45:149–159. doi: 10.1016/0166-0934(93)90099-D. [DOI] [PubMed] [Google Scholar]
- Fauquet CM, Stanley J. Geminivirus classification and nomenclature: progress and problems. Ann Appl Biol. 2003;142:165–189. doi: 10.1111/j.1744-7348.2003.tb00241.x. [DOI] [Google Scholar]
- Feng G, Yifu G, Pinbo Z. Production and deployment of virus-free sweetpotato in China. Crop Prot. 2000;19:105–111. doi: 10.1016/S0261-2194(99)00085-X. [DOI] [Google Scholar]
- Fuentes S, Salazar LF. First report of Sweet potato leaf curl virus in Peru. Plant Dis. 2003;87:98. doi: 10.1094/PDIS.2003.87.1.98C. [DOI] [PubMed] [Google Scholar]
- Gibson RW, Mpembe I, Alicai T, Carey EE, Mwanga ROM, Seal SE, Vetten HJ. Symptoms, aetiology and serological analysis of sweet potato virus disease in Uganda. Plant Pathol. 1998;47:95–102. doi: 10.1046/j.1365-3059.1998.00196.x. [DOI] [Google Scholar]
- Gibson RW, Wasswa P, Tufan HA. The ability of cultivars of sweetpotato in East Africa to ‘revert’ from Sweet potato feathery mottle virus infection. Virus Res. 2014;186:130–134. doi: 10.1016/j.virusres.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Gutierrez DL, Fuentes S, Salazar LF. Sweetpotato virus disease (SPVD): distribution, incidence, and effect on sweetpotato yield in Peru. Plant Dis. 2003;87:297–302. doi: 10.1094/PDIS.2003.87.3.297. [DOI] [PubMed] [Google Scholar]
- Karyeija RF, Kreuze JF, Gibson RW, Valkonen JPT. Synergistic interactions of a potyvirus and a phloem-limited crinivirus in sweet potato plants. Virology. 2000;269:26–36. doi: 10.1006/viro.1999.0169. [DOI] [PubMed] [Google Scholar]
- Karyeija RF, Kreuze JF, Gibson RW, Valkonen JPT. Variability of sweetpotato feathery mottle virus in Africa. Afr Crop Sci J. 2001;9:293–300. doi: 10.4314/acsj.v9i1.27651. [DOI] [Google Scholar]
- Kil EJ, Kim J, Byun HS, Kwak HR, Kim MK, Choi HS, Chung MN, Lee S. First report of Sweet potato golden vein associated virus infecting sweet potato in Korea. Plant Dis. 2014;98:1163. doi: 10.1094/PDIS-02-14-0123-PDN. [DOI] [PubMed] [Google Scholar]
- Kim J, Kil EJ, Kim S, Seo H, Byun HS, Park J, Chung MN, Kwak HR, Kim MK, Kim CS, Yang JW, Lee KY, Choi HS, Lee S. Seed transmission of Sweet potato leaf curl virus in sweet potato (Ipomoea batatas) Plant Pathol. 2015;64:1284–1291. doi: 10.1111/ppa.12366. [DOI] [Google Scholar]
- Kreuze JF, Perez A, Untiveros M, Quispe D, Fuentes S, Barker I, Simon S. Complete viral genome sequence and discovery of novel viruses by deep sequencing of small RNAs: a generic method for diagnosis, discovery and sequencing of viruses. Virology. 2009;388:1–7. doi: 10.1016/j.virol.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Kwak HR, Kim J, Kim MK, Jung MN, Seo J-K, Jung M-N, Kim JS, Lee S, Choi HS. Molecular characterization of five potyviruses infecting Korean sweet potatoes based on analyses of complete genome sequences. Plant Pathol J. 2015;31:388–401. doi: 10.5423/PPJ.OA.04.2015.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HR, Kim MK, Shin JC, Lee YJ, Seo JK, Lee HU, Jung MN, Kim SH, Choi HS. The current incidence of viral disease in Korean sweet potatoes and development of multiplex rt-PCR assays for simultaneous detection of eight sweet potato viruses. Plant Pathol J. 2014;30:416–424. doi: 10.5423/PPJ.OA.04.2014.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HR, Kim MK, Chung MN, Lee SH, Park JW, Kim KH, Choi HS. Virus disease incidences of sweet potatoes in Korea. Plant Pathol J. 2006;22:239–247. doi: 10.5423/PPJ.2006.22.3.239. [DOI] [Google Scholar]
- Kwak HR, Kim MK, Jung MN, Lee SH, Park JW, Kim KH, Ko SJ, Choi HS. Genetic diversity of sweet potato feathery mottle virus from sweet potatoes in Korea. Plant Pathol J. 2007;23:13–21. doi: 10.5423/PPJ.2007.23.1.013. [DOI] [Google Scholar]
- Li F, Xu D, Abad J, Li R. Phylogenetic relationships of closely related potyviruses infecting sweet potato determined by genomic characterization of Sweet potato virus G and Sweet potato virus 2. Virus Genes. 2012;45:118–125. doi: 10.1007/s11262-012-0749-2. [DOI] [PubMed] [Google Scholar]
- Loebenstein G, Thottappilly G. The sweetpotato. Springer Science & Business Media; 2009. [DOI] [Google Scholar]
- Lotrakul P, Valverde RA. Cloning of a DNA-A-like genomic component of Sweet potato leaf curl virus: nucleotide sequence and phylogenetic relationships. Mol Plant Pathol. 1999 On-line No.0206LOTRAKUL. [Google Scholar]
- Lotrakul P, Valverde RA, Clark CA, Sim J, De La Torre R. Detection of a geminivirus infecting sweet potato in the United States. Plant Dis. 1998;82:1253–1257. doi: 10.1094/PDIS.1998.82.11.1253. [DOI] [PubMed] [Google Scholar]
- Lotrakul P, Valverde RA, Clark CA, Hurtt S, Hoy MW. Sweetpotato leaf curl virus and related geminiviruses in sweetpotato. Acta Hortic. 2002;583:135–141. doi: 10.17660/ActaHortic.2002.583.15. [DOI] [Google Scholar]
- Lozano G, Trenado HP, Valverde RA, Navas-Castillo J. Novel begomovirus species of recombinant nature in sweet potato (Ipomoea batatas) and Ipomoea indica: taxonomic and phylogenetic implications. J Gen Virol. 2009;90:2550–2562. doi: 10.1099/vir.0.012542-0. [DOI] [PubMed] [Google Scholar]
- Moyer JW, Salazar LF. Viruses and virus-like diseases of sweet potato. Plant Dis. 1989;73:451–455. doi: 10.1094/PD-73-0451. [DOI] [Google Scholar]
- Mukasa SB, Rubaihayo PR, Valkonen JPT. Interactions between a crinivirus, an ipomovirus and a potyvirus in coinfected sweetpotato plants. Plant Pathol. 2006;55:458–467. doi: 10.1111/j.1365-3059.2006.01350.x. [DOI] [Google Scholar]
- Paprotka T, Boiteux LS, Fonseca MEN, Resende RO, Jeske H, Faria JC, Ribeiro SG. Genomic diversity of sweet potato geminiviruses in a Brazilian germplasm bank. Virus Res. 2010;149:224–233. doi: 10.1016/j.virusres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Park J, Kim S, Choi E, Kwak HR, Kim MK, Lee KY, Choi HS, Lee S. Molecular characterization of Sweet potato leaf curl virus (SPLCV) isolates from Korea: phylogenetic relationship and recombination analysis. Acta Virol. 2011;55:327–335. doi: 10.4149/av_2011_04_327. [DOI] [PubMed] [Google Scholar]
- Rodríguez P, Bejerman PEN, Luque AV, Feo LD. Complete nucleotide sequence of an Argentinean isolate of sweet potato virus G. Virus Genes. 2012;45:593–595. doi: 10.1007/s11262-012-0784-z. [DOI] [PubMed] [Google Scholar]
- Tairo F, Mukasa SB, Jones RAC, Kullya A, Rubaihayo PR, Valkonen JPT. Unravelling the genetic diversity of the three main viruses involved in Sweet Potato Virus Disease (SPVD), and its practical implications. Mol Plant Pathol. 2005;6:199–211. doi: 10.1111/j.1364-3703.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Untiveros M, Fuentes S, Kreuze J. Molecular variability of Sweet potato feathery mottle virus and other potyviruses infecting sweet potato in Peru. Arch Virol. 2008;153:473–483. doi: 10.1007/s00705-007-0019-0. [DOI] [PubMed] [Google Scholar]
- Untiveros M, Fuentes S, Salazar LF. Synergistic interaction of Sweet potato chlorotic stunt virus (Crinivirus) with Carla-, Cucumo-, Ipomo-, and Potyviruses infecting sweet potato. Plant Dis. 2007;91:669–676. doi: 10.1094/PDIS-91-6-0669. [DOI] [PubMed] [Google Scholar]
- Wang M, Abad J, Fuentes S, Li R. Complete genome sequence of the original Taiwanese isolate of sweet potato latent virus and its relationship to other potyviruses infecting sweet potato. Arch Virol. 2013;158:2189–2192. doi: 10.1007/s00705-013-1705-8. [DOI] [PubMed] [Google Scholar]
- Wang QC, Valkonen JPT. Elimination of two viruses which interact synergistically from sweetpotato by shoot tip culture and cryotherapy. J Virol Methods. 2008;154:135–145. doi: 10.1016/j.jviromet.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Yun WS, Lee YH, Kim KH. First report of Sweet potato latent virus and Sweet potato chlorotic stunt virus isolated from sweet potato in Korea. Plant Pathol J. 2002;18:126–129. doi: 10.5423/PPJ.2002.18.3.126. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.