Abstract
How temperate forests will respond to climate change is uncertain; projections range from severe decline to increased growth. We conducted field tests of sessile oak (Quercus petraea), a widespread keystone European forest tree species, including more than 150,000 trees sourced from 116 geographically diverse populations. The tests were planted on 23 field sites in six European countries, in order to expose them to a wide range of climates, including sites reflecting future warmer and drier climates. By assessing tree height and survival, our objectives were twofold: (1) to identify the source of differential population responses to climate (genetic differentiation due to past divergent climatic selection versus plastic responses to ongoing climate change), (2) to explore which climatic variables (temperature or precipitation) trigger the population responses. Tree growth and survival were modeled for contemporary climate and then projected using data from four regional climate models for years 2071-2100, using two greenhouse gas concentration trajectory scenarios each. Overall results indicated a moderate response of tree height and survival to climate variation, with changes in dryness (either annual or during the growing season) explaining the major part of the response. Whilst, on average, populations exhibited local adaptation, there was significant clinal population differentiation for height growth with winter temperature at the site of origin. The most moderate climate model (HIRHAM5-EC; rcp4.5) predicted minor decreases in height and survival, whilst the most extreme model (CCLM4-GEM2-ES; rcp8.5) predicted large decreases in survival and growth for southern and southeastern edge populations (Hungary and Turkey). Other non-marginal populations with continental climates were predicted to be severely and negatively affected (Bercé, France), while populations at the contemporary northern limit (colder and humid maritime regions; Denmark and Norway) will probably not show large changes in growth and survival in response to climate change.
Keywords: Climatic change, Climatic transfer distance, Mixed model, Quercus petraea, Survival, Tree growth
Introduction
Uncertainty about future CO2 emissions and the unknown capacity of forest ecosystems to adapt to warmer and drier climates make it difficult to anticipate their response to climatic change. Projections of possible responses include reduction in resilience (Huntingford et al., 2013; Tielbörger et al., 2014), shifts in species distributions (Delzon et al., 2013), forest diebacks (Allen et al., 2010; Choat et al., 2012) and serious biodiversity losses (Thuiller et al., 2011). The uncertainty of response is particularly acute for long lived tree species like oaks, because longevity may constrain adaptive evolutionary changes. Sessile oak (Quercus petraea [Matt.] Liebl.) is widely distributed across Europe, providing habitat for a diverse array of plants, animals, insects and fungi. Its human usage ranges from Early Neolithic acorn food consumption (Primavera & Fiorentino, 2013) and construction of wooden shelters and water wells (Tegel et al., 2012), to contemporary barrels for wine (Logan, 2005). Hence oaks are widely recognized as being of significant ecological, economic and cultural value (Hanewinkel et al., 2013).
Concerns about the vulnerability of oaks are due to a predicted adaptational lag in response to climatic change. In general terms the climate to which populations have adapted over long periods of time is shifting rapidly (Loarie et al., 2009). Populations are becoming dissociated from the local climatic conditions to which they have adapted (Davis & Shaw, 2001; Cheaib et al., 2012), and are likely to experience reductions in fitness. Due to the long lifespan of an oak tree, shifts in the genetic composition of populations are slow and opportunities for adaptation are limited. However, although their longevity may be a constraint, oaks exhibit a number of life history traits, such as prolific seed production and masting, high levels of genetic diversity, and extensive gene flow between populations (Kremer et al., 2012; Kremer, 2016) that can facilitate adaptation. As a result, predictions about future oak response to climate change are challenged by contrasting expectations. Current predictions are largely based on retrospective tree ring analyses over long time series (Tegel et al., 2012; Mérian et al., 2011; Lebourgeois et al., 2010) or on niche modeling and process-based models (Cheaib et al., 2012). Tree ring analyses have shortcomings, as they are limited to the very restricted set of traits that can be retrieved from tree ring signatures and ignore past mortality or competition effects. Niche or process-based models provide contrasting outcomes tightly linked to the assumptions of the model used, and usually ignore evolutionary processes that may enhance responsiveness to environmental change (Cheaib et al., 2012). In addition, niche models usually treat species as entities, neglecting the effect of divergent local adaptation among populations (but see Serra-Varela et al., 2015 for an approach that considers genetically-defined populations). However, oak populations have responded to climatic selection during historical global warming after the last glaciation (Petit et al., 2002a; Petit et al., 2002b), as evidenced by the high level of genetic differentiation for adaptive traits that is observed in common garden experiments (Kremer et al., 2002; Kremer et al., 2014). Quercus petraea shows clinal genetic variation along temperature gradients linked to altitude or latitude (Alberto et al. 2011; Vitasse et al., 2009). Therefore population-level variation must be taken into account when trying to predict the response of oaks to climate change.
In this study we explored, at a large geographic scale, the response of Quercus petraea populations to existing and predicted variations of climate. Our study was based on a continent-wide common garden experiment (provenance test), where geographic transfers of populations mimicked predicted temporal climatic change. Such transplantations of oak trees to planting sites different from their geographic origin offer the means to assess the potential of populations to adapt to climatic change. Our objectives were twofold, namely: (1) to identify the source of differential population responses to climate (genetic differentiation due to past divergent climatic selection versus plastic responses to ongoing climate change), and (2) to explore which climatic variables trigger these population responses.
Materials and Methods
We assessed the response of Quercus petraea populations to climate change by transplanting seedlings that originated from seeds collected in 116 stands across Europe (Table S1) into 23 field test sites (planting sites) (Table S2, Fig. 1). In this experiment, long-distance population transfers resulted in exposure to new climatic conditions (Figure S1), some of them similar to those predicted by climate change scenarios. Climatic data were obtained for both the test sites and the sites from which the populations originated, making it possible to estimate the “climate transfer distance”, defined as the difference between the “home” climate and the “destination” climate, as suggested by Mátyás (1994), and explained in detail by Leites et al. (2012a, 2012b). We recorded and analyzed two key adaptive traits, survival and growth, measured in the 23 test sites over the 20 years since establishment.
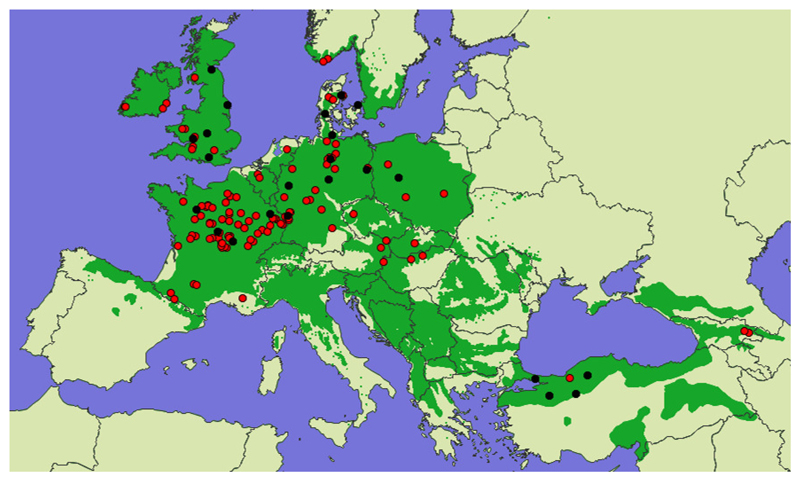
Fig. 1.
Location of source populations and test sites. Red symbols indicate the locations of the 116 Q. petraea populations from which seed were collected for field tests. Black symbols indicate the 23 field test sites. The contemporary distribution of natural and naturalized stands is shown in dark green (EUFORGEN, 2009).
Populations sampled and seed collection
Of the 116 natural Q. petraea populations sampled (Table S1), one subset, the Madsen collection (Madsen, 1990), comprised 14 populations that were planted at all testing sites. Subsequently, we refer to the set of mother trees from which the seeds were collected and represented by their seedlings in the experiments as the “population”, whereas the location of the population (the site at which the seeds were collected) is referred to as its “provenance”.
Field Tests
Seed lots were shared and transported to laboratories prior to sowing in different nurseries (one per institution hosting test sites). Seeds were sown in the spring after the harvesting of the acorns. After two years (exceptionally three) in the nursery, the seedlings were planted out at 23 sites (Table S2), distributed throughout the natural distribution of Q. petraea (Fig. 1).
Some test sites had climates similar to the site of origin for the planted seedlings, whereas other test sites differed in climate from the origin of the planted seedlings by as much as predicted by the end of the century. The design made it possible to estimate the contribution of climatic variation to the species response as a whole, but also to the population-specific response in terms of growth and survival.
The populations planted at each test site were laid out in standard experimental designs. Randomized complete block designs were used for each field site, with blocks and replicated plots to account for within-site variation. The field tests were managed independently by the various partners, according to their own management guidelines and the standard silvicultural practices of the country concerned. Differences in planting year were accounted for in the analysis, as described below.
Data collection and data input
Total height and survival were assessed repeatedly, at different ages, at each test site, with the frequency of measurement depending on local resources. We focused on these two major components of tree fitness and derived individual estimates for each tree and population at the reference age of 10 years after seed collection (about 7 to 8 years after planting in the field; see Appendix S1 for more details). The whole data set comprises measurements from 155 490 trees.
Contemporary climate data
Climate data for each provenance (seed origin), test site, and for more than 3800 regularly spaced points covering the current distribution of Q. petraea, were extracted from the WORLDCLIM dataset (Hijmans et al., 2005) downscaled to 1 km spatial resolution prior to point overlay as described in Zimmermann et al. (2009) and Dullinger et al. (2012), for the period defined as contemporary (1950-2000). Physiologically meaningful variables were constructed to represent the annual or seasonal balance between the thermal energy available for growth [DD5 = annual degree days > 5° C; GSDD5 = growing season (April to September) degree days 5° C] and the annual or seasonal available moisture (MAP = mean annual precipitation; GSP = growing season precipitation, April to September). We then calculated an annual dryness index and a growing season dryness index as suggested by Rehfeldt et al.(2014), and additional variables derived from monthly temperature and precipitation. The full list of climatic variables used, including their definition and units, are provided in Table S3.
Climate change projection data
We extracted projected climatic variables for the decades 2071- 2100, from the European COrdinated Regional climate Downscaling EXperiment (EURO--CORDEX), a set of Regional Climate Models (RCMs) run consistently at 0.44° and 0.11° spatial resolution over Europe and summarized in Jacob et al. (2014). All RCMs were based on the Coupled Model Intercomparison Project Phase 5 (CMIP5) global simulations, developed for the Intergovernmental Panel on Climate Change (IPCC) Fifth Assessment Report (https://www.ipcc.ch/report/ar5/), the most recent version of climate data. For each RCM, we used two Representative Concentration Pathways (RCP) of greenhouse gas concentration trajectories: 4.5 watts/m2 and 8.5 watts/m2, a “moderate” and a “pessimistic” scenario, which we subsequently refer to as rcp4.5 and rcp8.5 respectively.
From the EURO-CORDEX-11 (0.11° spatial resolution) data archive, we used three different RCMs, that were fed with data from global runs of two General Circulation Models (GCM), resulting in the following four RCM/GCM combinations: (a) CCLM4 (Rockel et al., 2008) run by the CLMcom community and fed with Hadley GEM2-ES global data from the Hadley Centre of the United Kingdom Meteorological Office, abbreviated as “CCLM4-GEM2-ES”; (b) CCLM4 run by the same community and fed with CM5 global data from CNRM, the Centre National de Recherches Météorologiques – France, abbreviated as “CCLM4-CM5-CNRM”; (c) HIRHAM5 (Christensen et al., 2007) run by the Danish Meteorological Institute (DMI) and fed by EC-EARTH global data from the EC-Earth community, abbreviated as “HIRHAM5-EC”; (d) RACMO22E (Meijgaard et al., 2012) run by the Royal Netherlands Meteorological Institute (KNMI) and fed by EC-EARTH global data provided by the EC-Earth community, abbreviated as “RACMO22E-EC”. All data were statistically downscaled to the same 1 km spatial resolution as the current climate data (see above) using the change factor method (Diaz-Nieto & Wilby, 2005; Tabor & Williams, 2010) as described in Dullinger et al. (2012). The 1 km data were then sampled at all provenance and test sites, and at the 3800 points covering the distribution of Q. petraea.
As recommended by Knutti et al. (2010), we combined the projections by first calculating all predictions individually, and then averaging the predictions for each point in the dataset to an ensemble response by each RCP, in order to produce the maps for predictions of future growth and survival.
Statistical analysis: Climatic transfer distance response functions, according to a mixed model
For both traits (survival and tree height 10 years after seed collection) we used the approach described by Leites et al. (2012a, 2012b) to model plant response to climate at the species level. This was done by considering genetic differences between populations in a mixed effects model (SAS, 2004) which separated fixed and random effects.
The fixed effects express differences in population genetics due to climate, subdivided into three major components (Leites et al., 2012a, 2012b; Rehfeldt et al., 2003):
-
(i)
The effect of climate at the site of provenance. This term (called C) accounts for population genetic differentiation due to climate at the provenance site. The observed differences result from past climatic selection pressures that induced differential adaptation at the provenance site. Such differences are expressed as a growth potential and can be illustrated by the maximum value of the response function for the given population.
-
(ii)
The climatic transfer distance term [D = (climate at test site) – (climate at provenance); linear and quadratic], accounting for population differences (in height growth and survival) due to differences in climate between the provenance and the test sites. These differences are due to short-term plastic responses to climate change which can be viewed as a genotype by environment interaction, or plastic response of the population, which may comprise a genetic and environmental component. It can be illustrated by the shape of the response function curve.
-
(iii)
The D x C term accounting for interaction between climate transfer distances and the climate of the provenance site. Populations with similar transfer distances may respond differently, depending on the climate of the provenance. Such differences may also be due to the variation of plastic responses and genotype by environment interaction. The interaction term determines the placement of the curve along the x axis of the response function curve.
The full model is as follows:
| [1] |
where Yijkl = 10-yr-old tree survival rate per plot or individual tree height, corresponding to the lth tree for the jth population at the kth block nested in the ith test site.
β0 is the intercept.
Dij is the climatic transfer distance for the jth population at the the ith field test (= the difference between a climatic variable at the field test minus that at the provenance)
Cj is the value of a climate variable at the provenance of the jth population.
Dij*Cj is the interaction between the climatic transfer distance (for the jth population at the the ith field test), and the climate variable at the provenance (of the jth population).
Si = Site effect at the ith field test.
Pj = Population effect of the jth population due to factors other than climate.
Bk(Si) = Block effect at the kth block nested in the ith field test.
eikjl = error term.
Dij, Cj and Dij*Cj are fixed effects. Si, Pj and Bk(Si) are random effects (Leites et al., 2012a, 2012b).
With the random effects, we accounted for potential sources of variation not explained by the fixed effects and captured by the experimental design (test sites, populations, and blocks). Pj accounts for population variation generated by evolutionary drivers that were not captured by the C terms, such as selection due to other factors than climate, genetic drift, gene flow inbreeding. Thus, differences between populations were accounted for by the joint effects of population as a random effect and climate, through its three components as fixed effects. Planting site effects (Si ) accounted for all environmental effects contributing to site quality: soil fertility, texture, drainage, climate effects other than those accounted for by the climatic transfer distance fixed term (extreme events), and differences in silvicultural maintenance regimes (timing of weed control, etc.). Block nested in site accounted for within planting site variation (Leites et al., 2012a, 2012b).
We adopted this model because it accounts explicitly for climatic variation at the population level, producing a response function for each population driven mainly by the D and C terms. For the D terms, we used a quadratic function similar to that used for other species (Wang et al., 2010; Kapeller et al., 2012; Leites et al., 2012a, 2012b; Yang et al., 2015). In these earlier studies, when a population was planted over a wide range of climatic conditions, its response usually followed a “bell-shaped” curve. Thus, the D2 term places emphasis on the interaction between the population and the climate of the test sites. From a biological perspective, this interaction is shown by the response function of the population and is the expression of its plasticity - its ability to adjust to the new environment encountered at the test site. For the C terms, we used a linear response of provenance to climate. A large body of experimental results from common gardens with forest trees shows linear clinal variation of population responses to climatic or geographic gradients (Alberto et al., 2013 for a review; Morgenstern, 1996).
We used a screening procedure for selecting the climate variables for the linear and quadratic functions (Appendix S2). We found a single climate variable representing the seed source appropriate for expressing the clinal variation among populations. Inclusion of additional variables reduced the statistical quality of the models. In theory, while multivariate climate-transfer effects are intuitively appealing, they raise statistical complexity and become insurmountable constraints.
Exploration of transformed data
Overall variation in survival and height was broken down into different sources of variation across the whole dataset according to the mixed model. Due to the heterogeneous origins of the data, the analysis was conducted on raw data and then separately on transformed data (logarithmic for height, and arcsin (square root) for survival) to account for potential heteroscedasticity. Due to the unbalanced distribution of populations at test sites, the mixed model was also used on separate subsamples of populations, including the subsample comprising only populations present at all test sites (the Madsen collection). These different analyses (transformed vs. untransformed data, and different subsamples of populations) yielded similar results regarding the overall effects of the different terms of the mixed model, and the selection of climatic variables. Hence we only present here the results obtained from using the raw data and the complete sample of populations.
Response function for contrasting populations
The estimated parameters for the fixed terms of the model were used to predict height growth and survival for a wide hypothetical array of climate transfer distance values, for a sub-group of populations originating from contrasting provenances. Populations were chosen on the basis of contrasting values for the climate of provenance, their climate transfer distance, and their occurrence at a large number of testing sites (Appendix S3).
Predicted impact of climate change
The impact of climate change on a given population was predicted by estimating population growth and survival at the given provenance, using the response function derived from the mixed model. The independent variables accounting for climate transfer distance were the difference between the climate at the provenance in the future (average among regional models for either scenario rcp4.5 or rcp8.5), minus the contemporary climate. Thus, the model constructed from contemporary geographic climate differences was transposed, locally, according to projected changes in climate over time. Future responses were therefore estimated by taking as independent climatic variables those providing the best fit to the overall model of contemporary responses to geographic transfer.
Future height growth and survival responses were predicted for each of the selected contrasting populations for which individual transfer responses were constructed, as follows: Taking the local change in climate over time as the dependent variable (transfer distance), we used our model to predict the future response of contrasting populations, and for simplicity we illustrated the projected impacts with the two extreme model scenarios. These were HIRHAM5-EC with the rcp4.5 scenario (most moderate) and CCLM4-GEM2-ES with the rcp8.5 scenario (most extreme).
Finally, predictions of future response were systematically extended to the 3800 regularly spaced points covering the current distribution of Q. petraea, and to any point located on a 1 square km grid across Europe, restricted to the limits of the contemporary natural and naturalized species distribution (based on EUFORGEN 2009).
Results
Climatic sources of variation of survival and tree height
Survival
The climatic transfer distance of annual dryness index (ADI) was, by far, the most relevant variable as the fixed term (D term of the mixed model) for survival rate. The chosen model had a negative, significant ADI transfer distance quadratic term (D2), P = 0.0108 and the smallest Akaike Information Criterion (AIC) value (Table 1). Such a quadratic trend could be visualized by plotting mean survival per population per test site against annual dryness index (ADI) climatic transfer distance (Fig. 2a; notice that symbols indicate the country of the test site, not that of the seed source). Although the linear term corresponding to genetic differentiation due to climate (ADI) at the provenance level (C term of mixed model) was not significant for survival (P = 0.2922), when included in the model it yielded the best AIC value (for comparison with other models, see Table S4).
Table 1.
Mixed model analysis of survival and tree height. Akaike Information Criterion (AIC; Akaike, 1973), estimated parameters, contribution to total variance (of random terms) and significance for the best full mixed model for each trait (10-year-old plants). Note that the climate variables are different for the two traits.
| Parameter or source of variation | Survival |
Tree height |
||||
|---|---|---|---|---|---|---|
| Fixed effects | Estimate | %¶ | P | Estimate | %¶ | P |
| AIC | -10352.5 | 1793112 | ||||
| Intercept | 1.02 | < 0.0001 | 260.7 | < 0.0001 | ||
| Climate at seed source: | ||||||
| Annual dryness index (ADI) | -2.57 | 0.2922 | ||||
| Mean temp. of the coldest month (MTCM) | 2.58 | 0.0006 | ||||
| Climate transfer distance: | ||||||
| Annual dryness index (ADI) | 2.36 | 0.5722 | ||||
| Growing season dryness index (GSDI) | -92.5 | 0. 3170 | ||||
| (Climate transfer distance)2 | -78.45 | 0.0108 | -4383.1 | <0.0001 | ||
| Clim. seed source x Clim. transfer dist. | -87.06 | 0.0530 | -58.7 | <0.0001 | ||
| Random effects | ||||||
| Site | 0.0219 | 56.6 | 0.0030 | 11552 | 59.4 | 0.0007 |
| Population | 0.0006 | 1.5 | <0.0001 | 272.9 | 1.4 | <0.0001 |
| Block (site) | 0.0055 | 14.2 | <0.0001 | 1802.9 | 9.3 | <0.0001 |
| Error | 0.0107 | 27.7 | <0.0001 | 5818.2 | 29.9 | <0.0001 |
Percent contribution to total variance (where 100 % is the sum of variances of all random terms).
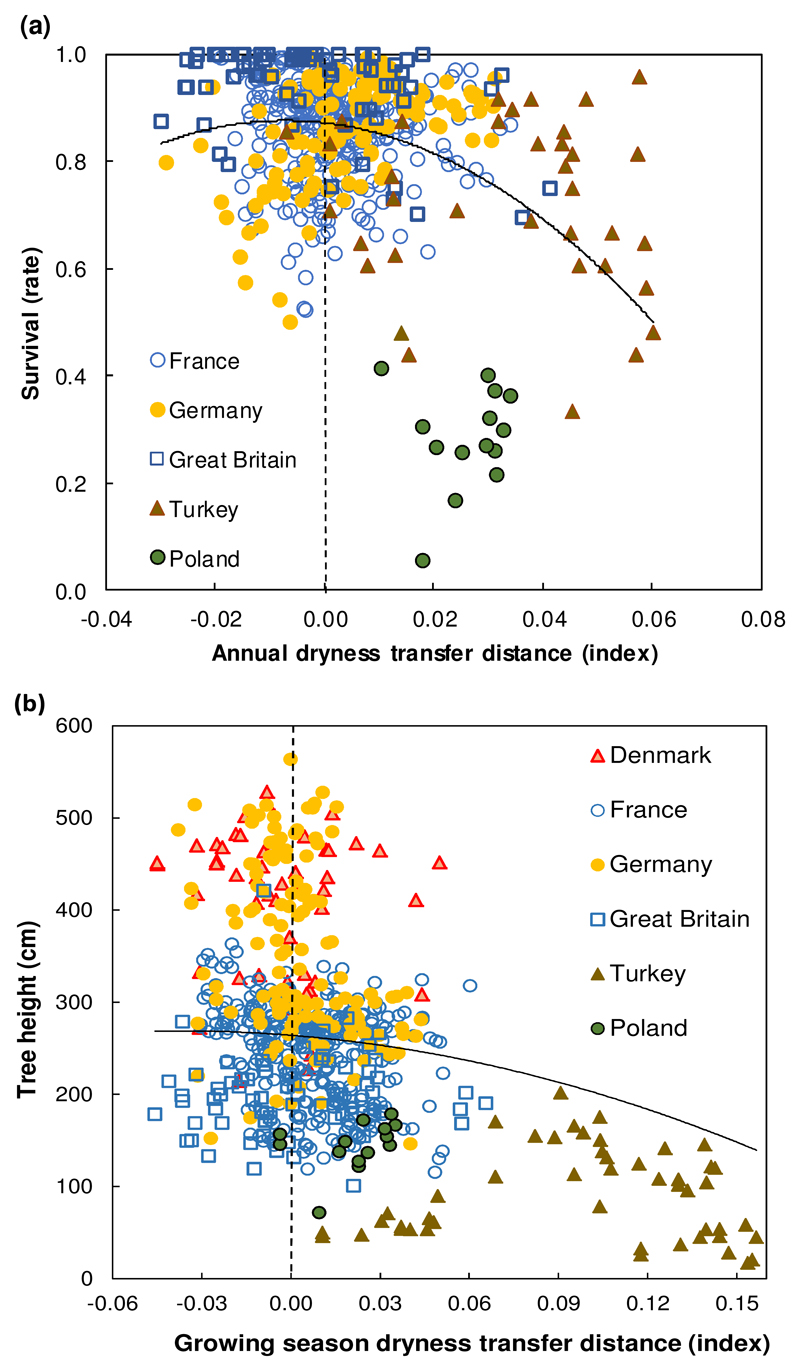
Fig. 2.
Observed response of survival (a) and tree height (b) to climatic transfer distance. Scatter plots, at 10-years-old, of population values by test site for: (a) survival rates against annual dryness index (ADI) transfer distance, and (b) tree height against growing season dryness index (GSDI) transfer distance. Symbols indicate the country of the test site, not that of the seed source. Larger positive values on the x axis indicate transfer to drier sites; negative values indicate transfer to wetter sites; a value of zero (vertical dashed line) indicates transfer to a test site with a climate similar to that of the site of provenance. Predicted values were estimated from the fixed terms of the best full model selected (as in Table 1; for survival, model uses ADI as a climatic variable for both transfer distance and for seed source (C term); for tree height, model uses mean temperature of the coldest month (MTCM) as a climatic variable for seed source). Note that model fit was made either with the rate of survival per plot or with individual tree height, not with the means per population per test site as this figure shows for clarity (see Material and Methods and Appendix S1 and S2).
Survival rate decreased when populations were transferred to warmer and drier sites (large positive climatic transfer values on x axis of Fig. 2a), or to colder and more moist sites (negative values on Fig. 2a). Meanwhile, survival rates were highest at sites with climates similar to those at the site from which the population originated (transfer distances close to 0; Fig. 2a). The shape of the response function indicating a decrease of survival towards warmer and drier sites was mostly driven by results at the test sites in Turkey (warmer and drier climates, see Fig. S1), and in Poland, where a severe summer drought occurred after the planting season.
All random terms (site, population and block nested in site) were highly significant (P ≤ 0.0030). It was remarkable that site, as a random term, contributed strongly to the total variance explained, accounting for 56.6 % of the total variance (of all random terms). Meanwhile, population (as a genetic effect other than the result of climate selection) only added a small contribution to the total variance explained (1.5 %). Block and residual terms accounted for 14.2 and 27.7 % of the total variance explained, respectively (Table 1).
Tree height
The growing season dryness index (GSDI) was, by far, the most relevant fixed term representing the climatic transfer distance (Table 1; for comparing with other climatic variables on the model selection process, see Table S5). Its transfer distance quadratic term was highly significant (P <0.0001, Table 1). Mean temperature of the coldest month (MTCM) was the best climate term explaining the climate of the seed source (C term of the mixed model), and was also highly significant (P = 0.0006; Table 1). The quadratic trend could be visualized by plotting mean tree height per population per test site against GSDI climatic transfer distance (Fig. 2b; notice that symbols indicate the country of the test site, not that of the seed source).
As for survival, tree heights were smaller when a population was transferred to warmer and drier sites (large positive climatic transfer values on x axis of Fig. 2b), or to colder and more moist sites (negative values on Fig. 2b). Height growth was again highest at sites with climates similar to those at the site from which the population originated (transfer distances close to 0; Fig. 2b).
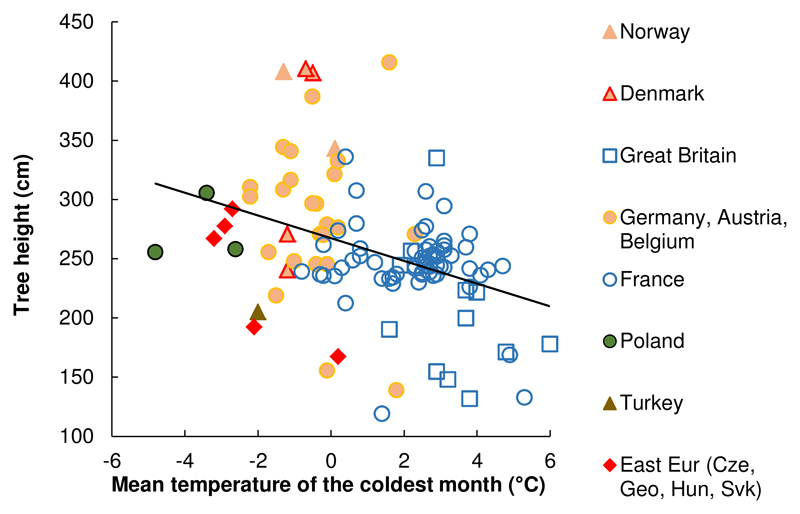
Correlations between tree height growth population means across sites against MTCM as seed source climate (analysis conducted for selection of candidate climatic variables for the C term of model 1) were significant and negative (Spearman’s coefficient r = -0.38, P < 0.0001), where tree height growth values were generally lower for populations originating from sites with a warmer mean temperature of the coldest month (Fig. 3; notice that colors indicate the country of the seed source, not the country of the test site). Such significant relationships were also found for the maximum temperatures in November, December, January and the mean temperature in November (Table S5).
Fig. 3.
Observed tree height (10-year-old trees) averaged by population, across test sites, and plotted against the mean temperature of the coldest months for the seed source (MTCM; r = - 0.38; P < 0.0001). Colors indicate the country of the seed source, not the country of the test site (for Eastern Europe: Cze = Czech Republic, Geo = Georgia, Hun = Hungary, Svk = Slovakia).
In the chosen model (and almost all the competing models), all random terms (site, population and block nested in site) were highly significant (P ≤ 0.0007). As with survival, the site random term contributed strongly to the total variance explained for tree height (as proportion of the sum of variances of all random terms): 59.4 % of the total variance (Table 1). This large contribution of site as a random term to the total variance explained was consistent with the considerable variability of tree height across sites and populations with a transfer distance close to zero (Fig. 2b). The population as a random term contributed only 1.4 % of the variation explained (similar to that for survival; Table 1). The contributions of block and residual to the total variance explained were 9.3 % and 29.9 %, respectively.
Predicted response curves to climatic transfer distances and climatic change impacts
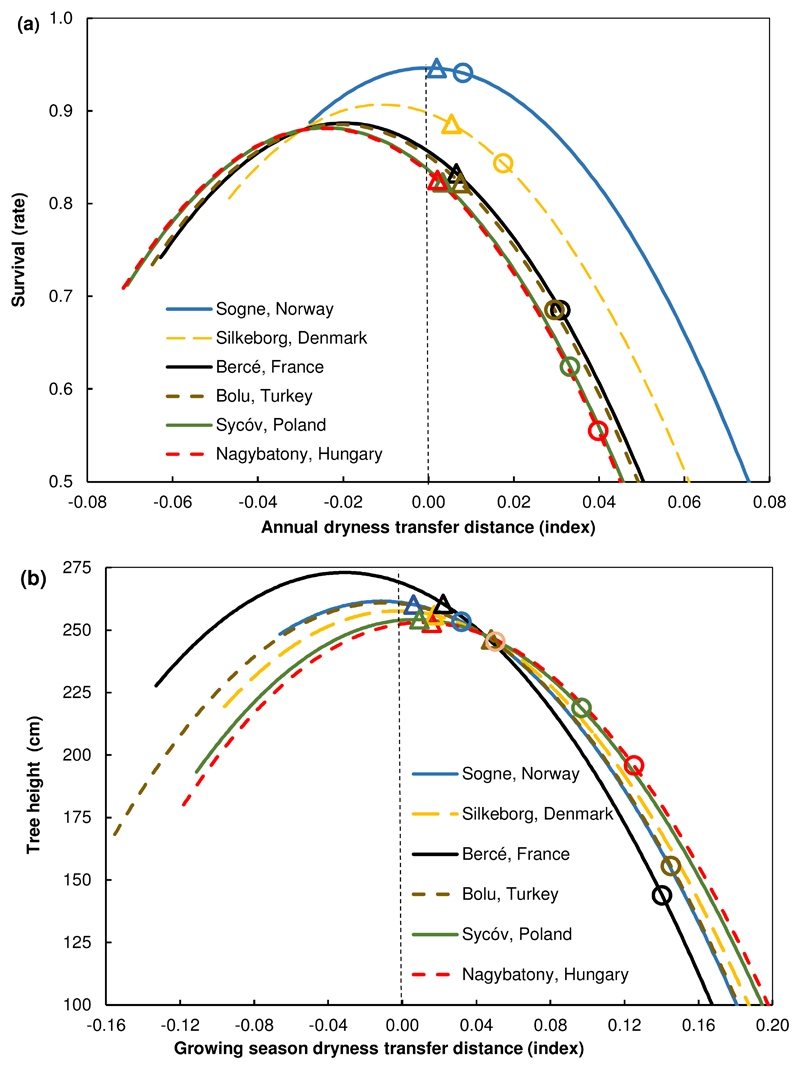
We selected a subset of six populations originating from contrasting home climates to illustrate the variation in population response (Appendix S3). The estimated population response functions of survival (based solely on fixed effects), suggest that populations originating from sites with moderate maritime climates (Silkeborg, Denmark), or with humid and colder sites (Sogne, Norway), are located in habitats with near-optimal climatic conditions (maximum value of the predicted survival response curve close to the zero value for climatic transfer distance; in other words, the population occupies a site with a climate that allows the full expression of the population growth potential; Fig. 4a). For Sogne, the maximum growth value was at a positive annual dryness index transfer distance value, indicating that it might have its optimum on a slightly warmer and drier site than the current sites (slightly to the right of the zero value for transfer distance; Fig. 4a). Meanwhile, populations originating from drier, continental sites, with warmer, drier summers (Bercé, France; Bolu, Turkey; Sycóv, Poland or the most extreme continental Nagybatony, Hungary), inhabit suboptimal climates (the optimum sites for these populations would be colder and more humid sites than the site they currently occupy, to the left of the zero value for climatic transfer distance; Fig. 4a).
Fig. 4.
Climatic transfer distance predicted response functions of (a) survival and (b) tree height for six contrasting populations. Predicted response functions for 10-year-old trees of: (a) survival plotted against annual dryness index transfer distance and (b) tree height plotted against growing season dryness index transfer distance. Point symbols (on the predicted response curves) indicate the predicted survival or tree height for the population at its site of origin, following a climatic transfer distance due to climatic change predicted for the years 2071-2100, as follows: triangles (Δ) under the HIRHAM5-EC with rcp4.5 (a moderate model-scenario); circles (○) under the CCLM4-GEM2-ES with the rcp8.5 (the most extreme model-scenario). Populations originated from contrasting environments: Sogne, Norway (extreme maritime climate); Silkeborg, Denmark (maritime); Bolu, Turkey (continental, but high elevation); Bercé, France, (continental climate), and Nagybatony, Hungary, Sycóv, Poland (extreme continental climate). The vertical dashed line indicates the climate of the population when growing as local (climate transfer distance = 0; in other words, under contemporary climate). Predictions were made using the parameters estimated for the fixed effect terms of the full mixed model chosen (as in Table 1).
We found less differentiation between populations for tree height than for survival, as indicated by the close or overlapping predicted response function curves for contrasting populations (Fig. 4b). Almost all populations seemed to be growing in conditions close to their optimum at the site of origin, and there was little differentiation between them. Only the population from Bercé, France, was identified as having an optimum at colder, wetter sites (left of the zero value for climatic transfer distance) than the site of origin. This finding was consistent with the survival response transfer function.
Response functions to future climates
The temperature/moisture balance represented by the ADI or GSDI values will change with projected changes in climate, shifting the present locations of Q. petraea populations to drier conditions in the future (higher positive values of ADI on Fig. 4a and of GSDI on Fig. 4b).
Climatic change would have a very moderate, nearly negligible effect under the moderate rcp4.5 scenario with HIRHAM5-EC (triangles on Fig. 4). However, there would be large and differential effects on the populations studied under the extreme rcp8.5 scenario with CCLM4-GEM2-ES (circles on Fig. 4). Generally, survival decreased more strongly for populations already growing in drier, suboptimal environments, like Bolu, Turkey and Bercé, France. The reduction in survival (compared to the predicted rate for local populations, estimated as the intersection of dashed line –zero transfer distance- to response curve functions; Fig. 4a), would be -16.5 % and -17.1 % for Bolu and Bercé, respectively. However, reduction in survival would be even more severe for Sycóv, Poland (-21.1 %) and for the most continental site, i.e. Nagybatony, Hungary (-27.7 %; Fig. 4a).
By contrast, populations from sites with highly maritime climates, such as Sogne, Norway, or Silkeborg, Denmark, would be affected only marginally by either model scenario. For example, survival would not be reduced in Sogne (change of 0.0 %) under the moderate rcp4.5 scenario with HIRHAM5-EC, and negligibly (-0.06 %) under the rcp8.5 scenario with CCLM4-GEM2-ES. Similarly, Silkeborg would suffer only -1.1 % and -5.4 % reduction in survival under these scenarios, when compared to contemporary predicted survival (Fig. 4a).
A similar impact was predicted for tree height (Fig. 4b). A very moderate reduction in tree height was foreseen under the rcp4.5 scenario with HIRHAM5-EC (about – 2 %; triangles on Fig. 4b). In contrast, under the rcp8.5 scenario with CCLM4-GEM2-ES (circles on Fig. 4b), large and differential effects on the populations would be expected. Growth reduction would be up to -13.9 % for Sycóv, -22.5 % for Nagybatony, and the largest growth reduction would be for Bolu (-40.3 %) and Bercé (-46.5 %; Fig. 4b).
Again in sharp contrast, tree height of Sogne or Silkeborg, from maritime climates, would be reduced by only 3.0 % and 4.7 %, respectively, under the extreme rcp8.5 scenario with CCLM4-GEM2-ES (Fig. 4b).
Mapping population response functions to future climates
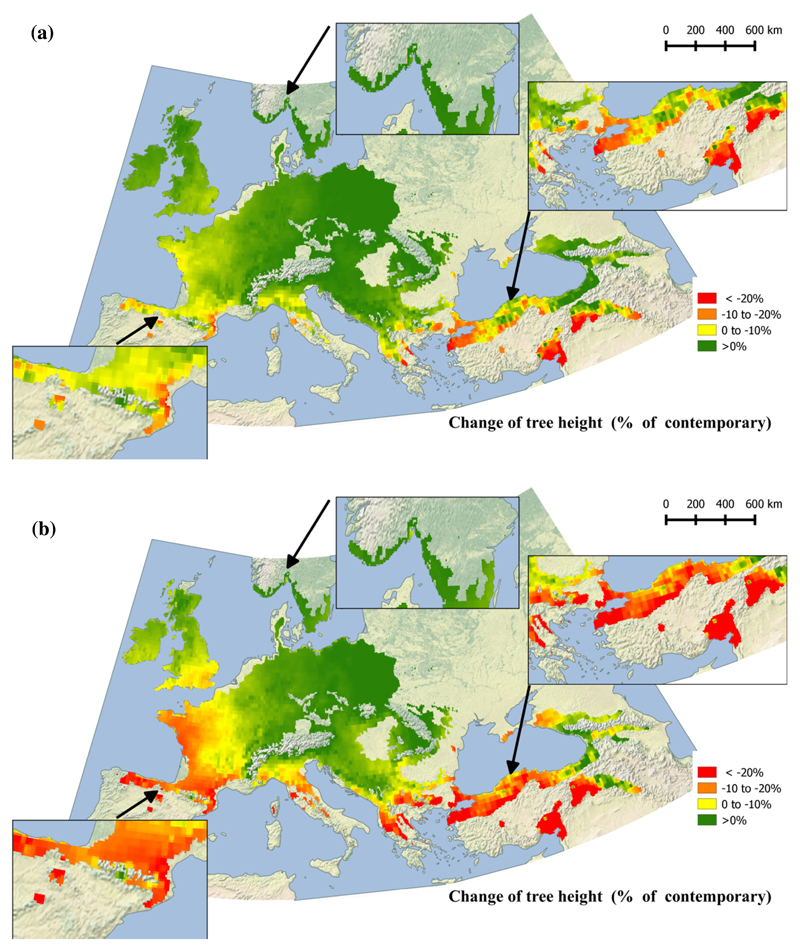
The regional divergence in the likely impact of climatic change across Europe became more evident when mapping predicted growth across the European continent for the years 2071-2100 (based on the fixed terms of the mixed model; Fig. 5 is the future change in growth relative to the contemporary growth, the latter is displayed in Figure S5). Quercus petraea populations occurring close to the southern margin of the species’ distribution will likely decrease in growth, and some populations will probably become extinct as a result. This is likely for populations in Northern Spain, Central and Southern France, South-Eastern Serbia, Western Hungary, Eastern Georgia and Northwestern Turkey. By contrast, populations growing close to the northern limit of the distribution and currently exhibiting marginal growth, such as those in Northeastern Poland, Scotland, Southern Norway and Sweden, will likely grow faster. These effects would be much larger under the more pessimistic rcp8.5 scenarios (Fig. 5b) than under the moderate rcp4.5 scenarios (Fig. 5a).
Fig. 5.
Projected growth anomaly for years 2071-2100 in comparison to contemporary, averaging across four models by RCP scenarios: (a) moderate, rcp4.5, and (b) pessimistic, rcp8.5. Predicted Q. petraea height growth anomaly expressed as change in comparison to contemporary. Predictions are based on fixed-term parameters from the fitted mixed model (growing season dryness index as climate transfer distance and mean temperature of the coldest month as seed source climate).
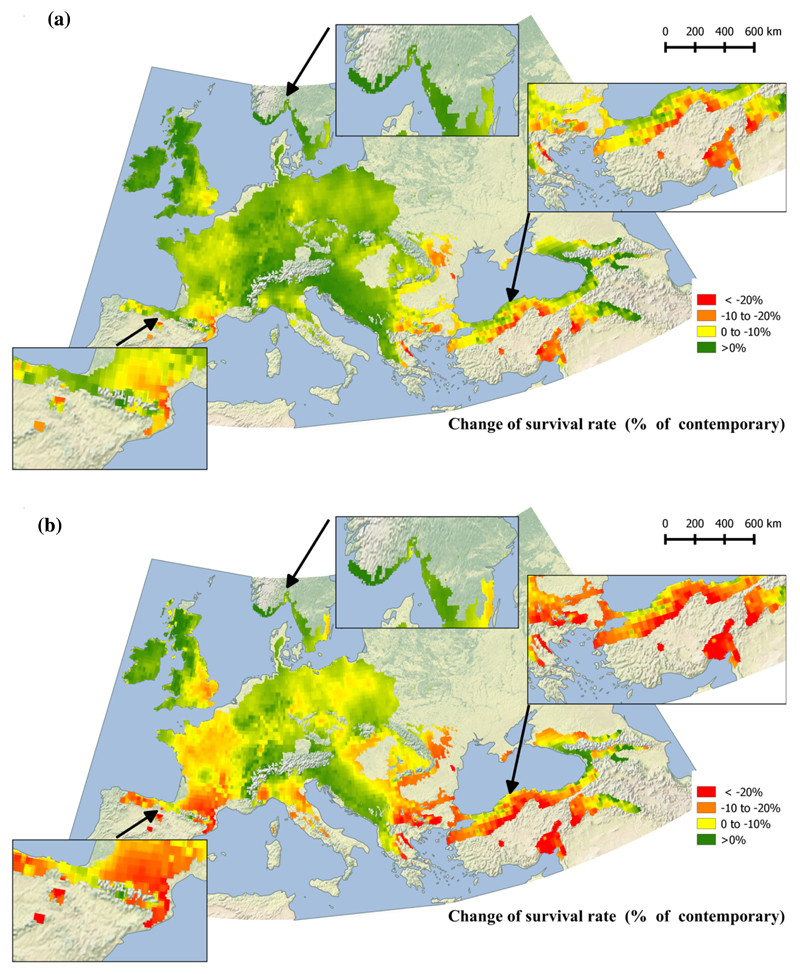
The same trend is projected for survival: a significant decrease is expected at the southern and southeastern limits of the distribution, and an improvement in survival is expected at the northern and northwestern limits (Fig. 6) compared to contemporary survival (Figure S6).
Fig. 6.
Projected survival anomaly for years 2071-2100 in comparison to contemporary, averaging across four models by RCP scenarios: (a) moderate, rcp4.5, and (b) pessimistic, rcp8.5. Predicted Q. petraea survival (%) anomaly, expressed as change in comparison to contemporary. Predictions are based on fixed-term parameters from the fitted mixed model (annual dryness index as climatic variable for transfer distance and for seed source).
Discussion
Moderate response of oak populations to climate variation
Overall the response of Quercus petraea populations to climatic variation, represented in our mixed model by climate at seed source, climatic transfer distance and their interaction, was significant but moderate, as illustrated by the rather flat curve of the overall response of survival and height to climate transfer distance (Fig.2 and Fig.3). Interestingly these results are consistent with longitudinal data from Quercus tree rings, where stem annual increments were examined against monthly temperatures and precipitation. In a recent study (Härdtle et al., 2013), 21 to 24% of tree ring variation in central Europe was explained by climate. Dendroclimatological monitoring highlighted the rather low climatic signature in the variation of oak tree rings in comparison to other species (Mérian et al 2011; Lebourgeois, 2010; Barsoum, 2015). Some authors even coined these trends as “the loss of sensitivity” (D’Arrigo et al.,2007; Wilson et al., 2012). Finally tree ring analyses also revealed a rather unstable climate-growth relationship in Quercus in comparison to other species. When detected, these relationships tended to be variable across years or within years, contributing to a dilution of the overall climatic signal (Merian et al., 2011; Barsoum et al., 2015).
In sharp contrast, the effect of test site (as a random effect) explained 57 % and 59 % of the total variance explained due to random terms, for survival and height respectively (P ≤ 0.003; Table 1), indicating that both are largely independent of the climatic factors tested as fixed terms. In our model, site effect includes very diverse sources of variation, including local site conditions (soil and competition with other plants), silvicultural practices or management procedures etc. These diverse contributions are not individually captured by our model since we focused on climatic sources of variation, so they are subsumed into the single component “site”. However these sources of variation correspond to the “real world” as each country and forest management has its own management practices, and local ecological conditions are specific to the sites. Again these figures are in line with the results obtained from studies of annual rings. As mentioned by Barsoum et al. (2015) “local factors may have a greater influence on all growth than the wider regional climate, with stand management being a likely cause of observed variation.” An illustration of the combined moderate response to climate and large site variation is summarized in Fig. 2b for growth. The overall response curve is nearly flat, while large deviations due to site variation are present for a given ecological transfer distance. These two results are markedly different from those reported in previous studies using similar approaches although with different species (mostly conifers). In particular, the variability of tree height at sites with a climate similar to that at the site of provenance (when climatic transfer distance close to zero) was found to be considerable. Population means per site were between 46 and 515 cm for a narrow range of GSDI transfer distance values (-0.02 to 0.02; Fig. 2b), while at GSDI transfer distances of 0.06, height growth decreased only slightly.
Overall our results indicate a moderate response to climate variation of growth rate and, to a lesser extent, survival, and considerable plasticity in response to planting site effects, including extreme climatic events not accounted explicitly by the model. Moderate phenotypic responses would undoubtedly be a major advantage in long-lived tree species remaining in place for several centuries, during which time they must be able to cope with the variability of the climate (Kremer et al., 2010), while plasticity regarding local conditions may allow newly produced seedlings to colonize environmentally diverse sites. Quercus petraea is probably efficient at exploiting the full benefit of favorable soil conditions, and simply survives and grows less well under unfavorable soil conditions.
Genetic population divergence along winter temperatures
Our results reveal that the overall response of height growth and survival includes a population specific component with three elements. The first reflects past climatic selection pressures that drove genetic differentiation among populations. The second corresponds to the quadratic response of the population to the climatic transfer distance. The third combines the remaining sources of variation including divergent selection due to soil characteristics, genetic drift etc. Of these three elements, the first and second were highly significant. For example the effect of MTCM at site of origin on tree growth suggests that cold winter temperatures have acted as a selective force, promoting genetic differentiation between populations. Continuous clinal variation in height growth in response to winter temperatures has not been reported previously in oak provenance tests, whilst there are scarce indications of higher growth of northern populations of Q. petraea exposed to higher winter temperatures (Jensen, 2000). This lack of evidence is mainly due to the lack of trials with rangewide sampling of oak populations. While the establishment of provenance tests has been common practice in forest genetics, including for oaks (Kleinschmit, 1993; Kleinschmit & Svolba, 1994), most have only sampled at a regional scale (Jensen, 2000; Jensen & Hansen, 2008; Baliuckas & Pliura, 2003; Deans & Harvey, 1996; Liesebach & Stephan, 2000; Burianek et al., 2011; Sinclair et al., 2016) or were assessed only during the juvenile stage (Liepe, 1993; Maurer et al., 2000; Alberto et al., 2011), thus limiting the potential to identify rangewide climatic clines. Partly due to their incomplete sampling of the natural distribution such trials failed to detect a genetic gradient in variation in growth. Our results are therefore the first to show a negative, genetically-determined relationship between height growth and winter temperatures. The negative correlation between MTCM of seed source and height suggests that high temperatures are more limiting for tree growth than lower temperatures. At first sight, this appears counterintuitive, but it may in fact represent an adaptation to warmer sites, at which a lower growth rate may be seen as a means to avoid drought stress during warm, dry summers. One hypothesis is that warm winters delay leaf unfolding due to a lack of chilling (delay bud dormancy release, see Dantec et al., 2014), reduce the growing season length and therefore impact on annual growth. However, warm winters are likely to be correlated with warm, dry summers and so the relationship with MTCM probably reflects the overall yearly temperatures rather than a warm winter effect per se.
Differential population response to climate
Population growth and survival decreased in response to change towards drier conditions. Height and survival rates were greatest at sites with climates similar to that at the site of population origin. Dryness index (ratio of temperatures above 5°C -corresponding to heat above the no-growth threshold- to the availability of moisture), either throughout the year for survival or during the growing season for tree growth, were responsible for shaping the shift in population characteristics due to the climatic transfer distance. As mentioned above, for survival and growth the model fit was probably limited by a lack of test sites that were substantially colder and more humid than any of the sites of origin. The general pattern of the fitted population response curve supported the hypothesis of local adaptation for most populations, assuming that growth and survival were tightly correlated with fitness. This pattern corresponded to trends generally observed in other case studies, where performance in adaptive traits decreased with increasing distance (both for temperature and humidity) from the climate of the site of the origin (Rehfeldt et al., 1999; Rehfeldt et al., 2002; Rehfeldt et al., 2003; Tchebakova et al., 2005). Our finding that growth and survival were affected by differences in drought levels was consistent with results of dendrochronological studies, in which spring precipitation and summer temperature were shown to be correlated with Q. petraea tree ring growth over the last 2500 years (Büntgen et al., 2011). Similarly, high temperatures in March and water stress in May to July appear to be the main factors limiting radial growth (Mérian et al., 2011).
Our results show also population genetic differentiation in the shape of the response function along the climatic transfer distance (Fig. 4), and these differed between survival and growth. For example, populations originating from the Northern part of the distribution (Silkeborg, Denmark or Sogne, Norway) were located in habitats with near-optimal climates, whereas other populations were currently located in climates warmer than their optimum. For height, population differentiation was less pronounced. This finding contrasts with those for conifer species, where northern populations are thought to occupy suboptimal conditions (usually colder than their optimum). For instance, this has been shown for Picea glauca in Quebec (Li et al.,1997; Beaulieu & Rainville 2005) or Larix spp. and Pinus sylvestris in Siberia (Tchebakova et al., 2005).
Predicted response curves to climatic transfer distances and climate change impacts
For all populations, survival and height were projected to decrease following transfer to much warmer and drier sites (toward positive values of ADI or GSDI transfer distances).
When the climatic transfer distances were viewed as contemporary populations experiencing future climates, it was evident that adaptation to climate change would be possible for populations currently living in habitats slightly colder than their optimum (Beaulieu & Rainville 2005). In our case, these populations were situated in Denmark and Norway. Unfortunately, populations already growing under warmer and drier climates than their optimum, will suffer the most from climatic change impacts, for instance those from Bercé (France), Bolu (Turkey) and Nagybatony (Hungary). A similar decoupling has being reported for southeastern Larix spp. populations (Tchebakova et al.,2005).
Climate change impacts are projected to be only moderate under the ensemble of models using the rcp4.5 scenario. However, under the rcp8.5 scenario, the impacts are much more severe (Fig. 5 and 6). At present, it seems that the rcp8.5 is the more likely scenario, given that emissions continue to grow according to “business as usual” (Hansen et al., 2012). If this trend continues, then the climate transfer distances would increase and will be even higher than those tested in our experiment. This would result in an even more severe decrease in survival and growth in many regions, particularly if the transfer distances exceed the adaptation threshold of 0.06 units of growing season dryness index (Fig. 2b). There is already evidence suggesting that Q. petraea populations are declining at the xeric limit of its distribution in the White Carpathian wooded grasslands (Czech Republic). This is specifically observed at sites with high levels of insolation, at which severe droughts have occurred during the summers over the past 30 years (Dolezal et al., 2010). Similar observations were recently made for beech (Horváth & Mátyás, 2016).
Quercus petraea forest decline at the southern and southeastern limits could be moderated if trees were able to respond to elevated CO2 levels with mitigating mechanisms such as enhanced water use efficiency (Picon et al., 1996; Battipaglia et al., 2013; Keenan et al. 2013; AbdElgawad et al. 2016). However, much more analysis of oak responses to elevated CO2 is required before conclusions can be drawn.
Limitations of this study and management implications
While international multisite provenance tests are invaluable for analyzing population responses to climate variation, we caution against overinterpretation of our results concerning Q. petraea. There are a number of limitations to be considered before making general conclusions about the adaptation of oak to ongoing climate change, as follows:.
-
(a)
First of all, this study is based on evaluation of juvenile growth and survival. In most European countries rotation age in Quercus petraea is more than 150 years, and there is no current information on the juvenile-mature correlation for growth in oaks. Furthermore, early evaluation may be more impacted by stochastic or deterministic variation caused by transplantation, maternal effects and competition with weeds. Assembling later stage assessments is necessary but will be a challenging task dependent on the resources available in the different countries and institutions hosting the experiments.
-
(b)
Secondly, there may be ascertainment bias due to the sampling of populations and the selection of test sites (Figure S1). Most of the test sites were established in the “optimal” region of the climatic envelope of the species and contain a limited number of provenances from the ecological margins of the species. To what extent this sampling scheme may have shaped the transfer functions is unknown but we would anticipate that the central part of transfer function would be less affected, while the predominant effects of plasticity would also remain.
-
(c)
The third limitation is the unbalanced design of the experiment. Although populations of the Madsen core collection were planted in all sites, the overall experimental design was unbalanced in population representation. The mixed model accommodated this to some extent as shown by the congruent results obtained with the separate analysis conducted on the subset of common populations. The congruence of the two analyses is most likely due to the representative sampling of the core population across the ecological range of the species. However inferences about response functions of provenances tested in a limited number of sites should be treated with caution.
-
(d)
Finally the choice of years for recording climate data may also be a source of bias. We did not use the climatic record over the period when the trees were growing (1989-1999), but rather the climate data for an extended reference period (1950-1990). We wanted to capture the full extent of climatic variation that populations have experienced and adapted to; data from an extended period will better account for this. However, whilst more appropriate for assessing population differentiation, this choice might underestimate the climate transfer distance, especially if extreme climate events occurred between 1989 and 1999. Given that the growing period considered (1989 to 1999) was included in the reference period, the underestimation should be minimal.
Despite these limitations, data from replicated provenance tests are essential to guide adaptive management strategies. Our results clearly show that the climate effects that have shaped population differentiation in the past, and the climate change simulated by the spatial transfer of populations, account for a statistically significant yet moderate part of the variation of survival and height in Q. petraea. Given the experimental constraints and limitations, we suspect that these sources of variation have been underestimated in this study. This is why strong population and regional site effects were observed when projecting the survival and height growth under future climatic change scenarios. Climate change under the more extreme rcp8.5 scenario is expected to severely reduce height growth and survival at the southern and southeastern margins of the contemporary distribution, particularly in Turkey and Hungary. In contrast, the projected changes are minimal for the northern margins of oak distribution in Europe. Our results therefore capture the variation in risk exposure of Q. petraea across Europe.
This experiment can also be seen as a test of the concept of assisted migration, which proposes deliberate displacement of populations and in some cases species, based on climate change predictions. Assisted migration consists of transplanting populations to new locations with climates to which they are best adapted. In general, these locations differ (northern and at higher elevations) from those in which they currently grow (Aitken et al., 2008; Mátyás et al., 2010; Gray & Hamann, 2011; Fettig et al., 2013; Castellanos-Acuña et al., 2015).
Finally, this study highlighted the importance of local site factors and the plastic response of oak to site variation. Changes in local silvicultural practice, which have yet to be developed and explored, should also receive major attention for adaptive management to face climatic change.
Supplementary Material
Acknowledgments
A full documentation of the provenances is available from a publicly accessible database (https://w3.pierroton.inra.fr:8006/materials ). Funding was provided by the European Union (MOTIVE grant 226544, and FORGER grant 289119, ERC TREEPEACE grant 339728) and the French Office National des Forêts; JBL was additionally supported by LabEx COTE. CSR obtained a sabbatical fellowship from the Mexican Council of Science and Technology (CONACyT, grant 232838) and Universidad Michoacana de San Nicolas de Hidalgo. This work would not have been possible without the organization, guidance and legacy of Søren F. Madsen (deceased), from the Danish Forest and Landscape Research Institute, Hoersholm, Denmark. We thank the personnel of the institutions that contributed to the collection of seeds, the raising of the seedlings in the nursery, the long standing field test maintenance, and the data acquisition. We acknowledge the assistance and help of: the staff of the Office National des Forêts (ONF); Patrick Reynet and Jean Marc Louvet at INRA Cestas, France; Jochen Kleinschmit and Josef Svolba, Niedersächsische Forstliche Versuchsanstalt, Abteilung Forstpflanzenzüchtung, Staufenberg, Escherode, Germany; Klaus Liepe and Armin O. König, Thünen-Institut für Forstgenetik, Grosshansdorf, Germany; Alwin Janssen, Nordwestdeutsche Forstliche Versuchsanstalt, Abteilung Waldgenressourcen, Hann-Münden, Germany; Henryk Fober, Instytut Dendrologii PAN, Kórnik, Poland; Alan M. Fletcher and Edward P. Cundall, Northern Research Station, Scotland; John Douglas Deans, Centre for Ecology & Hydrology, Bush Estate, Penicuik, Midlothian, United Kingdom; M. Kizmaz, Forest Research Institute, Ankara, Turkey; Bart de Cuyper and Alphonse Nanson, Station de Recherches Forestières et Hydrobiologiques, Groenendaal-Hoeilaart, Belgium; Lásló Széll, Forest Research Institute Botanikus kert, Sárvár, Hungary. We thank for seed supply to: Tore Skrøppa, Norwegian Forest Research Institute, Ås, Norway and Finnvid Prescher, Södra Fröstation, Lagan, Sweden. Valuable guidance for the mixed model analysis was provided by Gerald E. Rehfelt, USDA-Forest Service at Moscow, Idaho; Laura Leites, School of Forest Resources, Pennsylvania State University; Tongli Wang and Sally Aitken, Centre for Forest Conservation Genetics and Department of Forest Sciences and University of British Columbia, Canada. We appreciate valuable comments that significantly helped to improve the manuscript from: Arndt Hampte, INRA Cestas, France and three anonymous reviewers.
References
- AbdElgawad H, Zinta G, Beemster GTS, Janssens IA, Asard H. Future climate CO2 levels mitigate stress impact on plants: increased defense or decreased challenge? Frontiers in Plant Science. 2016;7:1–7. doi: 10.3389/fpls.2016.00556. article 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evolutionary Applications. 2008;1:95–111. doi: 10.1111/j.1752-4571.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. Information theory and an extension of the maximum likelihood principle. Proceedings of the Second International Symposium on Information Theory. 1973:267–281. [Google Scholar]
- Alberto F, Bouffier L, Louvet J-M, et al. Adaptive responses for seed and leaf phenology in natural populations of sessile oak along an altitudinal gradient. Journal of Evolutionary Biology. 2011;24:1442–1454. doi: 10.1111/j.1420-9101.2011.02277.x. [DOI] [PubMed] [Google Scholar]
- Alberto F, Aitken S, Alia R, et al. Potential for evolutionary response to climate change - evidence from tree populations. Global Change Biology. 2013;19:1645–1661. doi: 10.1111/gcb.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management. 2010;259(4):660–684. [Google Scholar]
- Baliuckas V, Pliura A. Genetic variation and phenotypic plasticity of Quercus robur L. populations and open pollinated families in Lithuania. Scand J For Res. 2003;18:305–319. [Google Scholar]
- Barsoum N, Eaton EL, Levanic T, Pargade J, Bonnart X, Morison JIL. Climatic drivers of oak growth over the past one hundred years in mixed and monoculture stands in southern England and northern France. Eur J Forest Res. 2015;134:33–51. [Google Scholar]
- Battipaglia G, Saurer M, Cherubini P, Calfapietra C, McCarthy HR, Norby RJ, Cotrufo MF. Elevated CO2 increases tree-level intrinsic water use efficiency: insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytologist. 2013;197:544–554. doi: 10.1111/nph.12044. [DOI] [PubMed] [Google Scholar]
- Beaulieu J, Rainville A. Adaptation to climate change: Genetic variation is both a short- and a long-term solution. Forest Chronicle. 2005;81:704–709. [Google Scholar]
- Büntgen U, Tegel W, Nicolussi K, et al. 2500 years of European climate variability and human susceptibility. Science. 2011;331:578–582. doi: 10.1126/science.1197175. [DOI] [PubMed] [Google Scholar]
- Burianek V, Benedikova M, Kyselakova J. Evaluation of twenty years old pedunculate and sessile oak provenance trial. Journal of Forest Science. 2011;57:153–169. [Google Scholar]
- Castellanos-Acuña D, Lindig-Cisneros RA, Sáenz-Romero C. Altitudinal assisted migration of Mexican pines as an adaptation to climate change. Ecosphere. 2015;6:1–16. Article 2. [Google Scholar]
- Cheaib A, Badeau V, Boe J, et al. Climate change impacts on tree ranges: model inter-comparison facilitates understanding and quantification of uncertainty. Ecology Letters. 2012;15:533–544. doi: 10.1111/j.1461-0248.2012.01764.x. [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Brodribb TJ, et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- Christensen OB, Drews M, Christensen JH, Dethloff K, Ketelsen K, Hebestadt I, Rinke A. The HIRHAM Regional Climate Model version 5. Danish Meteorological Institute Technical Report 06-17, Danish Meteorological Institute; Copenhagen: 2007. [Google Scholar]
- Dantec CF, Vitasse Y, Bonhomme M, Louvet JM, Kremer A, Delzon S. Chilling and heat requirements for leaf unfolding in European beech and sessile oak populations at the southern limit of their distribution range. International Journal of Biometeorology. 2014;58:1853–1864. doi: 10.1007/s00484-014-0787-7. [DOI] [PubMed] [Google Scholar]
- D’Arrigo R, Wilson R, Liepert B, Cherubini P. On the‘Divergence Problem’ in northern forests: a review of the tree ring evidence and possible causes. Glob Planet Change. 2007;60:289–305. [Google Scholar]
- Davis MB, Shaw RG. Range shifts and adaptive responses to quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. [DOI] [PubMed] [Google Scholar]
- Deans JD, Harvey FJ. Frost hardiness of 16 European provenances of sessile oak growing in Scotland. Forestry. 1996;69:5–11. [Google Scholar]
- Delzon S, Urli M, Samalens JC, et al. Field evidence of colonisation by Holm oak, at the northern margin of its distribution range, during the Anthropocene period. PloS One. 2013;8:e80443. doi: 10.1371/journal.pone.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Nieto J, Wilby RL. A comparison of statistical downscaling and climate change factor methods: impacts on low flows in the river Thanes, United Kingdom. Climate Change. 2005;69:245–268. [Google Scholar]
- Dolezal J, Mazurek P, Klimesova J. Oak decline in southern Moravia: the association between climate change and early and late wood formation in oaks. Preslia. 2010;82:289–306. [Google Scholar]
- Dullinger S, Gattringer A, Thuiller W, et al. Extinction debt of high-mountain plants under twenty-first-century climate change. Nature Climate Change. 2012;2:619–622. [Google Scholar]
- EUFORGEN (European Forests Genetic Resource Program) Distribution Map of Sessile Oak (Quercus petraea) 2009 http://www.euforgen.org/fileadmin/templates/euforgen.org/upload/Documents/Maps/PDF/Quercus_petraea.pdf.
- Fettig CJ, Reid ML, Bentz BJ, Sevanto S, Spittlehouse DL, Wang T. Changing climates, changing forests: A Western North American perspective. Journal of Forestry. 2013;111:214–228. [Google Scholar]
- Gray LK, Hamann A. Strategies for reforestation under uncertain future climates: guidelines for Alberta, Canada. PLoS One. 2011;6:e22977. doi: 10.1371/journal.pone.0022977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanewinkel M, Cullmann DA, Schelhaas MJ, Nabuurs GJ, Zimmermann NE. Climate change may cause severe loss in the economic value of European forest land. Nature Climatic Change. 2013;3:203–207. [Google Scholar]
- Hansen J, Sato M, Ruedy R. Perception of climate change. Proceedings of Natural Academy of Sciences, USA. 2012;109:E2415–E2423. doi: 10.1073/pnas.1205276109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härdtle W, Niemeyer T, Assmann T, Aulinger A, Fichtner A, Lang A, Leuschner C, Neuwirth B, Pfister L, Quante M, Ries C, et al. Climatic responses of tree-ring width and d13C signatures of sessile oak (Quercus petraea Liebl.) on soils with contrasting water supply. Plant Ecol. 2013;214:1147–1156. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very height resolution interpolated surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Horváth A, Mátyás CS. The decline of vitality caused by increasing drought in a Beech provenance trial predicted by juvenile growth. South-East European Forestry. 2016;7:1–8. [Google Scholar]
- Huntingford C, Zelazowski P, Galbraith D, et al. Simulated resilience of tropical rainforests to CO2-induced climate change. Nature Geoscience. 2013;6:268–273. [Google Scholar]
- Jacob D, Petersen J, Eggert B, et al. EURO-CORDEX: new high-resolution climate change projections for European impact research. Regional Environmental Change. 2014;14:563–578. [Google Scholar]
- Jensen JS. Provenance variation in phenotypic traits in Quercus robur and Quercus petraea in danish provenance trails. Scand J For Res. 2000;15:297–308. [Google Scholar]
- Jensen JS, Hansen JK. Geographic variation in phenology of Quercus petraea (Matt.)Liebl. And Quercus robur L. oak grown in a grennhouse. Scand J For Res. 2008;23:179–188. [Google Scholar]
- Kapeller S, Lexer MJ, Geburek T, Hiebl J, Schueler S. Intraspecific variation in climate response of Norway spruce in the eastern Alpine range: Selecting appropriate provenances for future climate. Forest Ecology and Management. 2012;271:46–57. [Google Scholar]
- Keenan TF, Hollinger DY, Bohrer G, Dragoni D, Munger JW, Schmid HP, Richardson AD. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature. 2013;499:324–327. doi: 10.1038/nature12291. [DOI] [PubMed] [Google Scholar]
- Kleinschmit J. Intraspecific variation of growth and adaptive traits in European oak species. Annales des Sciences Forestieres. 1993;50:166s–185s. [Google Scholar]
- Kleinschmit J, Svolba J. Intraspecific variation of growth and stem form in Quercus robur and Quercus petraea. In: Kremer A, Muhs HJ, editors. Inter- and Intra-specific Variation in European Oaks: Evolutionary Implications and Practical Consequences. Science Research Development European Commission; Brussels: 1994. pp. 217–238. [Google Scholar]
- Knutti R, Furrer R, Tebaldi C, Cermak J, Meehl GA. Challenges in combining projections from multiple climate models. Journal of Climate. 2010;23:2739–2758. [Google Scholar]
- Kremer A. Microevolution of European temperate oaks in response to environmental changes. Compte Rendus Biologies. 2016 doi: 10.1016/j.crvi.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Kremer A, Le Corre V, Petit RJ, Ducousso A. Historical and contemporary dynamics of adaptive differentiation in European oaks. In: DeWoody J, Bickham JW, Michler CH, et al., editors. Molecular Approaches in Natural Resource Conservation and Management. Cambridge University Press; Cambridge: 2010. pp. 101–122. [Google Scholar]
- Kremer A, Kleinschmit J, Cottrell J, et al. Is there a correlation between chloroplastic and nuclear divergence, or what are the roles of history and selection on genetic diversity in European oaks? Forest Ecology and Management. 2002b;156:75–87. [Google Scholar]
- Kremer A, Ronce O, Robledo-Arnuncio JJ, et al. Long-distance gene flow and adaptation of forest trees to rapid climate change. Ecology Letters. 2012;15:378–392. doi: 10.1111/j.1461-0248.2012.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer A, Potts BM, Delzon S. Genetic divergence in forest trees: understanding the consequences of climate change. Functional Ecology. 2014;28:22–36. [Google Scholar]
- Lebourgeois F, Rathgeber CKB, Mérian P, Ulrich E. Sensitivity of French temperate forests to climate variability and extreme events: example of the French Network RENECOFOR. In: Astrade L, Miramont C, editors. Panorama de la Dendrochronologie en France. Laboratoire EDYTEM, Université de Savoie /CNRS; Le Bourget-du-Lac, France: 2010. pp. 19–26. Collection Edytem 11, Proceedings, Dignes-les-Bains, Alpes de Haute Provence, 9-10 October 2009. [Google Scholar]
- Leites LP, Rehfeldt GE, Robinson AP, Crookston NL, Jaquish BC. Possibilities and limitations of using historic provenance tests to infer forest species growth responses to climate change. Natural Resources Modeling. 2012a;25:409–433. [Google Scholar]
- Leites LP, Robinson AP, Rehfeldt GE, Marshall JD, Crookston NL. Height growth response to climatic changes differs among populations of Douglas-fir: a novel analysis of historic data. Ecological Applications. 2012b;22:154–165. doi: 10.1890/11-0150.1. [DOI] [PubMed] [Google Scholar]
- Liepe K. Growth chamber trial on frost hardiness and field trial on flushing of sessile oak (Quercus petraea Liebl.) Ann For Sci. 1993;50:208–214. [Google Scholar]
- Liesebach M, Stephan BR. Development of provenances of Quercus petraea and Quercus robur from acrons to six year old plants in relations to species specific traits. Glas Šum Pokuse. 2000;37:413–423. [Google Scholar]
- Li P, Beaulieu J, Bousquet J. Genetic structure and patterns of genetic variation among populations in eastern white spruce (Picea glauca) Can J For Res. 1997;27:189–198. [Google Scholar]
- Loarie SR, Duffy PB, Hamilton H, et al. The velocity of climate change. Nature. 2009;462:1052–U111. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- Logan WB. Oak, the Frame of Civilization. Northon & Company; New York: 2005. [Google Scholar]
- Madsen SF. International Provenance Trial with Quercus petraea. The 1989-Series of Provenance Experiments with Quercus petraea (Matt.) Liebl. Description of Seed Stands and Seed Collection. Danish Forest Experiment Station; Denmark: 1990. [Google Scholar]
- Marris E. Planting the forest of the future. Nature. 2009;459:906–908. doi: 10.1038/459906a. [DOI] [PubMed] [Google Scholar]
- Mátyás C. Modeling climate change effects with provenance tests data. Tree Physiology. 1994;14:797–804. doi: 10.1093/treephys/14.7-8-9.797. [DOI] [PubMed] [Google Scholar]
- Mátyás C, Berki I, Czúcz GB, Móricz N, Rasztovits E. Future of beech in Southern Europe from the perspective of evolutionary ecology. Acta Silvatica & Lignaria Hungarica. 2010;6:91–110. [Google Scholar]
- Maurer WD, Tabel U, König AO, Stephan BR, Müller-Starck G. Provenance trials on Quercus robur L. and Quercus petraea (Matt.)Liebl. in Rhineland-palatinate (Germany): preliminary results of phenotypic and genetic surveys. Glas Šum Pokuse. 2000;37:329–345. [Google Scholar]
- Meijgaard Ev, Van Ulft LH, Lenderink G, de Roode SR, Wipfler L, Boers R, Timmermans RMA. Refinement and application of a regional atmospheric model for climate scenario calculations of Western Europe. Royal Netherlands Meteorological Institute; Copenhagen: 2012. KvR 054/12. [Google Scholar]
- Mérian P, Bontemps JD, Bergès L, Lebourgeois F. Spatial variation and temporal instability in climate-growth relationships of sessile oak (Quercus petraea [Matt.] Liebl.) under temperate conditions. Plant Ecology. 2011;212:1855–1871. [Google Scholar]
- Morgenstern EK. Geographic Variation in Forest Trees. University of British Columbia Press; Vancouver, Canada: 1996. p. 209. [Google Scholar]
- Petit RJ, Brewer S, Bordács S, et al. Identification of refugia and postglacial colonization routes of European white oaks based on chloroplast DNA and fossil pollen evidence. Forest Ecology and Management. 2002a;156:49–74. [Google Scholar]
- Petit RJ, Csaikl UM, Bordács S, et al. Chloroplast DNA variation in European white oaks: phylogeography and patterns of diversity based on data from over 2,600 populations. Forest Ecology and Management. 2002b;156:5–26. [Google Scholar]
- Picon C, Guehl JM, Aussenac G. Growth dynamics, transpiration and water-use efficiency in Quercus robur plants submitted to elevated CO2 and drought. Annales des Sciences Forestières. 1996;53:431–446. [Google Scholar]
- Primavera M, Fiorentino G. Acorn gatherers; fruit storage and processing in south-east Italy during the bronze age. Origini: Preistoria e protostoria delle civiltà antiche. 2013;35:211–228. [Google Scholar]
- Rehfeldt GE, Tchebakova NM, Barnhardt LK. Efficacy of climate transfer functions: introduction of Eurasian populations of Larix into Alberta. Canadian Journal of Forest Research. 1999;29:1660–1668. [Google Scholar]
- Rehfeldt GE, Tchebakova NM, Parfenova YI, Wykoff WR, Kouzmina NA, Milyutin LI. Intraspecific responses to climate in Pinus sylvestris. Global Change Biology. 2002;8:912–929. [Google Scholar]
- Rehfeldt GE, Tchebakova NM, Milyutin LI, Parfenova YI, Wykoff WR, Kouzmina NA, et al. Assessing population responses to climate in Pinus sylvestris and Larix spp. of Eurasia with climate-transfer models. Eurasian Journal of Forest Research. 2003;6:83–98. [Google Scholar]
- Rehfeldt GE, Jaquish BC, López-Upton J, Sáenz-Romero C, StClair JB, Leites LP, Joyce DG. Comparative genetic responses to climate for the varieties of Pinus ponderosa and Pseudotsuga menziesii: Realized climate niches. Forest Ecology and Management. 2014;324:126–137. [Google Scholar]
- Rockel B, Will A, Hense A. Special issue regional climate modeling with COSMO-CLM (CCLM) Meteorologische Zeitschrift. 2008;17:347–348. [Google Scholar]
- SAS Institute Inc. SAS/STAT Computer Software. Release 9.1. 3rd Edition. SAS Institute Inc Cary N.C; USA: 2004. [Google Scholar]
- Serra-Varela MJ, Grivet D, Vincenot L, Broennimann O, Gonzalo-Jiménez J, Zimmermann NE. Does phylogeographic structure relate to climatic niche divergence? A test using maritime pine (Pinus pinaster Ait.) Global Ecology and Biogeography. 2015;24:1302–1313. [Google Scholar]
- Sinclair FH, Stone GN, Nicholls JA, Cavers S, Gibbs M, Butterill G, Wagner S, Ducousso A, Gerber S, Petit R, Kremer A, et al. Impacts of local adaptation of forest trees on associations with herbivorous insects: implications for adaptive forest management. Evolutionary Applications. 2016;8:972–987. doi: 10.1111/eva.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor K, Williams JW. Globally downscaled climate projections for assessing the conservation impacts of climate change. Ecological Applications. 2010;20:554–565. doi: 10.1890/09-0173.1. [DOI] [PubMed] [Google Scholar]
- Tchebakova NM, Rehfeldt GE, Parfenova EI. Impacts of climate change on the distribution of Larix spp. and Ledeb. and Pinus sylvestris and their climatypes in Siberia. Mitigation and Adaptation Strategies for Global Change. 2005;11:861–882. [Google Scholar]
- Tegel W, Elburg R, Hakelberg D, Stäulbe H, Büntgen U. Early Neolithic water wells reveal the world’s oldest wood architechture. PlosOne. 2012;7:e51374. doi: 10.1371/journal.pone.0051374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W, Lavergne S, Roquet C, Boulangeat I, Lafourcade B, Araujo MB. Consequences of climate change on the tree of life in Europe. Nature. 2011;470:531–534. doi: 10.1038/nature09705. [DOI] [PubMed] [Google Scholar]
- Tielbörger K, Bilton MC, Metz J, et al. Middle-Eastern plant communities tolerate 9 years of drought in a multi-site climate manipulation experiment. Nature Communications. 2014;5:1–9. doi: 10.1038/ncomms6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitasse Y, Delzon S, Bresson CC, Michalet R, Kremer A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Canadian Journal of Forest Research. 2009;39:1259–2009. [Google Scholar]
- Wang T, O'Neill GA, Aitken SN. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecological Applications. 2010;20:153–163. doi: 10.1890/08-2257.1. [DOI] [PubMed] [Google Scholar]
- Wilson R, Miles D, Loader NJ, Melvin T, Cunningham L, Cooper R, Briffa K. A millennial long March–July precipitation reconstruction for southern-central England. Clim Dyn. 2012 doi: 10.1007/s00382-012-1318-z. [DOI] [Google Scholar]
- Yang J, Pedlar JH, McKenney DW, Weersink A. The development of universal response functions to facilitate climate smart regeneration of black spruce and white pine in Ontario, Canada. Forest Ecology and Management. 2015;339:34–43. [Google Scholar]
- Zimmermann NE, Yoccoz NG, Edwards TC, Jr, et al. Climatic extremes improve predictions of spatial patterns of tree species. Proceedings of Natural Academy of Sciences, USA. 2009;106:19723–19728. doi: 10.1073/pnas.0901643106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.