Abstract
Identifying risk and protective factors associated with condomless sex among youth living with HIV is imperative for developing effective HIV prevention strategies. A cross-sectional sample of 1728 participants, 12–26 years of age, recruited from adolescent medicine clinics in 17 U.S. cities completed an audio-computer assisted self-interview with questions about their substance use, psychosocial factors, and attitudinal and behavioral factors. Guided by syndemics theory, a path analysis was used to assess the interrelations of these factors. Analyses of model fit statistics indicated statistically significant direct pathways between substance use, psychosocial factors, self-efficacy for risk-reduction, alternative risk-reduction attitudes and behaviors and condomless sex. The total indirect effect of self-efficacy for risk-reduction on condomless sex through alternative risk-reduction attitudes and behaviors was also significant. Multi-faceted, tailored interventions that address individual risk and protective factors and their combined synergistic effects are urgently needed to prevent condomless sex among this population.
Keywords: Youth living with HIV, HIV prevention, Structural equation modeling, Syndemics theory, Condomless sex
Introduction
In 2014, adolescents and young adults between the ages of 13 and 24 accounted for an estimated 22 % of all new HIV diagnoses [1]. Almost half (44 %) of youth living with HIV (YLWH) between the ages of 18 and 24 did not know that they were infected [2]. The majority of new HIV infections among youth in the U.S. are attributable to condomless anal or vaginal sex [3], although there are significant numbers of YLWH who were infected perinatally. In addition, YLWH engage in risky behaviors, such as condomless sex and substance use, at higher rates than uninfected youth in the U.S. [4]. Understanding and addressing risk and protective factors associated with condomless anal and vaginal sex among YLWH is critical for advancing primary and secondary prevention efforts.
Multiple risk and protective factors have been identified in the extant literature as being associated with condomless anal and vaginal sex among adolescents and young adults in the U.S. including substance use, emotional distress, and social support [5–7]. However, identified correlates of condomless anal and vaginal sex vary across different studies. For instance, several studies with perinatally-infected adolescents reported significant positive associations between substance use and condomless anal and vaginal sex [8–10], whereas other studies that included both perinatally- and behaviorally-infected youth have not found these same associations [11, 12]. Emotional distress has been positively associated with substance use and subsequent engagement in condomless anal and vaginal sex among YLWH [13, 14] but not consistently [11, 15]. In addition, higher levels of social support have been linked to lower levels of substance use, emotional distress, and condomless sex among YLWH [16, 17] but not always [18]. The use of different measures and divergent samples to assess the individual relationships between condomless sex and these particular risk factors may explain these discrepant findings [19].
Prior research has also found that higher levels of self-efficacy for risk-reduction or the belief in one’s own ability or confidence to use risk-reduction strategies such as condom use is associated with lower levels of substance use, emotional distress, and condomless sex among YLWH [12, 20]. Furthermore, it has been suggested that the use of alternative risk-reduction strategies such as serosorting, strategic positioning, or having an undetectable viral load in sexual situations may be associated with condomless anal and vaginal sex for YLWH but this relationship has yet to be confirmed by empirical evidence [21–25]. For instance, YLWH who believe that these particular alternative risk-reduction strategies are effective and who are confident in their ability to practice these strategies may choose to have condomless sex with a serodiscordant partner because that individual believes he or she is at lower risk of transmitting HIV [25]. What has yet to be fully understood, especially among YLWH, are the interrelations and their combined effects among all of these factors, which is critical to the development of tailored, effective secondary prevention interventions for this population [26].
Syndemics Theory
Syndemics theory posits that the co-occurrence and synergistic interaction of multiple adverse conditions or maladies within populations produce a stronger and more intense overall health outcome than if each of the conditions or maladies were experienced separately [27]. In his seminal work, Singer introduced the concept of syndemics to explain the overlapping and additive effects of substance abuse, violence and HIV among ethnic minority women. Moreover, he explained that poverty and marginalization increases the likelihood of one epidemic contributing to another epidemic, thereby magnifying the effect on a particular population [28, 29]. Over the past decade, researchers have increasingly characterized HIV epidemics among high-risk groups as a consequence of other prevalent problems, such as mental health disorders, substance use, and adverse social conditions (e.g., violence, low socioeconomic status) that interact with one other and contribute to HIV transmission [30–32].
Applying the syndemic framework to gay, bisexual and other men who have sex with men (GBMSM), Stall and colleagues argued that the experience of marginalization (e.g., heterosexism) plays a critical role in the development of syndemics for this highly stigmatized group [33]. Among a sample of 2881 adult GBMSM, Stall et al. found that the odds of engaging in unprotected anal intercourse and becoming infected with HIV increased significantly based upon the number of psychosocial problems (e.g., polysubstance use, depression, partner violence, and childhood sexual abuse) experienced [34]. Similarly, Mustanski and colleagues found with increasing psychosocial difficulties (e.g., binge drinking, street drug use, psychological distress, intimate partner violence, and sexual assault) that the odds of engaging in condomless anal sex, having multiple anal sex partners, and HIV-positive serostatus also increased among younger GBMSM [35]. Likewise, Halkitis et al. found a positive association between psychosocial burdens (e.g., drug use and mental health) and unprotected sexual behaviors among a sample of 199 GBMSM 50 years and older [36].
To date, the majority of research on syndemics has focused on GBMSM [37–42]. To our knowledge, no one else has applied syndemics theory to YLWH who suffer from similar maladies to GBMSM including adverse mental health outcomes, severe substance abuse problems, and lack of strong social support networks and who are also at high risk for transmitting HIV due to their engagement in condomless anal and vaginal sex [43].
The Current Study
The current study draws from a large, racially-diverse sample of YLWH receiving care at one of 17 adolescent medicine clinics (AMCs) across the U.S. The purpose of this study was to conduct a secondary data analysis using path analysis guided by syndemics theory to investigate multiple risk and protective factors associated with condomless anal and vaginal sex and to elucidate relationships among these factors identified earlier that could serve as potential intervention targets for YLWH in the U.S. The proposed theoretical model used in the current study is presented in Fig. 1. As the figure shows, the primary outcome variable is condomless anal and vaginal sex, as this is the primary mode of HIV transmission among adolescents and young adults [3]. Based upon existing published work identified earlier and potential gaps in the literature on syndemics [27–32], six additional factors (frequency of substance use, severity of substance use, emotional distress, social support, self-efficacy for risk-reduction, and alternative risk-reduction attitudes and behaviors) are hypothesized to operate synergistically to impact the extent to which YLWH engage in condomless anal and vaginal sex. These six factors are conceptualized as influencing condomless anal and vaginal sex while simultaneously impacting each other and being impacted by other factors in the model. It was also posited that both a proximal mediator (self-efficacy for risk-reduction) and a more distal mediator (alternative risk-reduction attitudes and behaviors) might account for the pathways through which the effects of frequency of substance use, severity of substance use, emotional distress, and social support relate to condomless anal and vaginal sex.
Fig. 1.
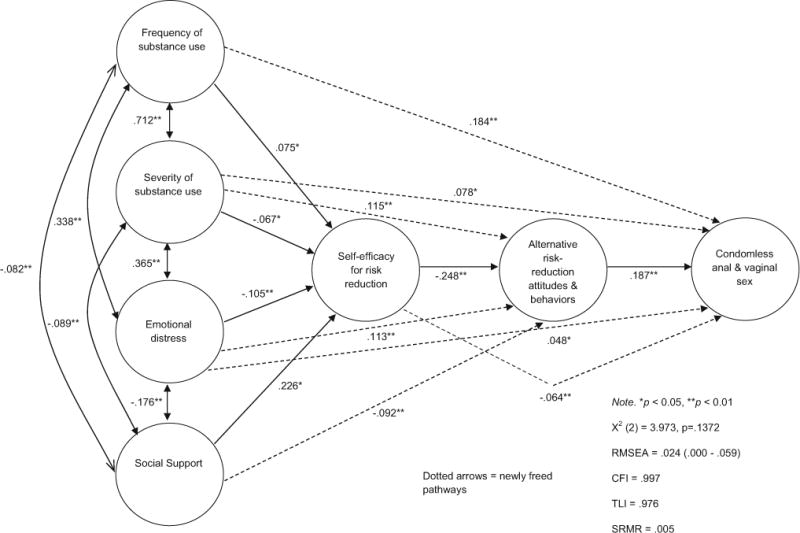
Path analysis: substance use, psychosocial factors, self-efficacy for risk-reduction, and alternative risk-reduction attitudes and behaviors predicting condomless sex among youth living with HIV in 17 U.S. cities (N = 1728)
Methods
Participants and Recruitment
From December 2009 through January 2012, participants were recruited from sites supported by the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN). These sites are located in 17 cities in the U.S. and Puerto Rico with established HIV epidemics. Research staff approached potential participants at one of their scheduled clinic visits. Inclusion criteria were being between the ages of 12 and 26 years, living with HIV and aware of one’s HIV serostatus, engaged in care at an ATN site, and able to understand written and/or spoken English or Spanish. All study materials (e.g., consent form, questions, flash cards) were made available in English and in Spanish at a fifth grade reading level. Materials were first translated from English into Spanish and then back-translated from Spanish into English using professional and lay translators to ensure consistency in meaning and content. Research staff members were available to participants who had any difficulties with reading in English or in Spanish. The study was approved by the Institutional Review Boards (IRBs) at each of the participating clinics and institutions of each of the protocol team members.
Study Procedures
After the screening process, all eligible youth were provided with an explanation of the study and were invited to participate. Trained clinical research staff members with experience working with YLWH then obtained signed informed consent or youth assent from those who agreed to participate. Within 2 weeks of providing informed consent or assent, participants completed an audio-computer assisted self-interview (ACASI) to assess current health status and behavioral risk factors followed by a 5 to 10-min debriefing interview. Flash cards with responses to each of the questions were provided to participants. The assessment and debriefing interview took between 45 and 90 min to complete. Participants were reimbursed a small incentive determined by the sites’ IRBs for their time and effort completing the assessment. Parents were not incentivized (e.g., provided with monetary compensation) in allowing their children to participate in the study. A total of 2225 youth enrolled in the study and completed the ACASI. For the current analyses, 1728 participants had complete data available for all of the constructs of interest. Missing data on the key variables (e.g., demographics, substance use, psychosocial factors, etc.…) were examined for potential differences between participants who had complete data and for those who did not have complete data. Effect size differences of the key variables tended to be in the small range (Cohen’s d = 0.2–0.3) suggesting that the two groups were not very different and that proceeding with the available data was warranted.
Measures
Existing valid and reliable measures were used to assess substance use (frequency and severity) and emotional distress among YLWH. Two different measures of substance use were included in the current study that differentiate between frequency of substance use and severity of substance use. Frequency of substance use does not necessarily suggest severity of use. Frequency of substance use may demonstrate a pattern of use, whereas severity of use may be indicative of a substance use disorder. For example, an adolescent or young adult might report a higher frequency of alcohol use over a specific time period but not necessarily meet diagnostic criteria for alcohol abuse or dependence. Other measures used in this study were created by ATN behavioral HIV scientists with extensive experience and background in the field to assess social support [44], self-efficacy for risk-reduction [45], and alternative risk-reduction attitudes and behaviors [46, 47] that were specifically developed for YLWH due to gaps in the literature on measures in these areas. Provided below is descriptive information on these measures followed by internal consistencies for each of the individual measures included in the study.
Demographic and HIV Questionnaire
Participants’ demographic characteristics and HIV-related factors were assessed including age, gender, race, ethnicity, sexual orientation, education, employment, mode of HIV infection, age at which participant learned they were HIV-positive, and HIV disclosure to anyone.
Frequency of Substance Use
An abridged 10-item version of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) was used to assess the frequency of substance use across a variety of drugs including tobacco products; alcoholic beverages; marijuana; cocaine or crack; amphetamines or stimulants; inhalants; sedatives or sleeping pills; hallucinogens, heroin, morphine, pain medication; and other drugs identified by the participant for the past 30 days and lifetime use only [48]. Sample items include: “In your life, which of the following substances have you ever used? (non-medical use only)” and “In the past 3 months, how often have you used the substances mentioned (first drug, second drug, etc.)”. Use of each specific drug was coded from zero to four, based on its use in the past 3 months, with no use coded as zero (0), used once or twice coded as one (1), used monthly coded as two (2), used weekly coded as three (3), and used daily or almost daily coded as four (4). A cumulative severity/frequency score was calculated for each participant for the ASSIST by summing the values across each of the 10 items to provide a measure of frequency of substance use with higher scores indicative of more severe/greater substance use.
Severity of Substance Use
The Car, Relax, Alone, Forget, Friends, and Trouble (CRAFFT) Substance Abuse Screening Test was used to assess the severity of substance use [49]. The CRAFFT is a 6-item screener with responses of “no” = 0 or “yes” = 1 designed to measure the probability of substance abuse/dependence diagnosis among adolescents and young adults in clinical settings. Sample items include: “Do you ever use alcohol or drugs to relax, feel better about yourself, or fit in?” and “Do you ever use alcohol or drugs while you are by yourself (alone)?” Scores of 2 or greater are suggestive of problem substance use, abuse or dependence. Internal consistency as measured by Cronbach’s coefficient alpha statistic for the 6-item CRAFFT scale with the current sample was .77.
Emotional Distress
The global severity index (GSI) of the Brief Symptom Inventory (BSI) was used to assess emotional distress [50]. The BSI has been used with previous samples of YLWH [51, 52]. It includes nine primary subscales (Anxiety, Somatization, Obsessive Compulsive, Depression, Hostility, Phobic Anxiety, Paranoid, Psychoticism, and Interpersonal Sensitivity) and the GSI measures emotional distress. Participants rate their level of endorsement with the 53 items of this measure on a 4-point Likert-type scale (0 = “Not at all” to 4 = “Extremely”) with higher scores indicative of greater levels of psychological symptoms and emotional distress. Items include: “How much have you been distressed or bothered during the past 14 days, (including today) by:…faintness or dizziness; having to check and double-check what you do; feeling inferior to others; feeling no interest in things; feeling tense or keyed up; having urges to break or smash things; feeling uneasy in crowds, such as shopping or at a movie; others not giving you proper credit for your achievements; and the idea that something is wrong with your mind.” Alpha internal consistency estimates for BSI scores on each of the nine subscales ranged from .77 to .88 in the current study. In the final study analyses, the composite GSI score was used, which is calculated from the mean of the nine individual subscale mean scores.
Social Support
Social support (SS) was assessed with a 6-item measure that was developed by ATN-affiliated scientists [44]. Sample items include: “There are people in my life that are supportive about…: using condoms; avoiding drug use; taking HIV medications.” Participants rate their level of agreement with items on a 5-point Likert-type scale (1 = “Strongly disagree” to 5 = “Strongly agree”) with higher scores indicative of greater levels of social support. The dimensional characteristics of the 6-item SS scale were examined using principal component analysis. Both a parallel analysis procedure [53, 54] and the Scree test [55] were employed to aid in the interpretation of the underlying number of components to retain, and both procedures suggested the best solution was to retain a single component. All six items had very good component loadings (range: .63 to .78), with an averaged component loading for the six items of .73. Cronbach’s alpha for the current study was .82.
Self-Efficacy for Risk-Reduction
Participants were asked to evaluate four different scenarios about having sexual intercourse with new, current, or past partners to assess self-efficacy for risk-reduction (SERR) using an instrument developed by ATN-affiliated scientists who are experts in the field of adolescent behavioral HIV research [45]. Scenario topics include: (1) sex while under the influence of alcohol; (2) sex because of feeling lonely; (3) sex with an ex-partner; and (4) unprotected sex with long-term partner as they were frequently identified by YLWH in early pilot work and may be particularly salient for YLWH who are in similar situations where discussing safer sex activities or risk-reduction strategies with sexual partners becomes important. Because a substantial proportion of participants did not respond to the three items of scenario 3, sex with an ex-partner, this scenario was not included in the current analyses. The final SERR consisted of the mean of the items scores for the remaining eight items that assessed scenarios 1, 2, and 4. Sample items include: “How confident are you that you could make an effective decision of whether to tell this person you are HIV positive in this situation?” and “How confident are you that you could bring up the need to practice safer sex in this situation?” Participants rated items for each scenario on a 10-point Likert-type scale (0 = “Cannot do at all” to 10 = “Certain can do”) with higher scores indicative of greater levels of self-efficacy for risk-reduction or having greater confidence in one’s ability to engage in risk-reduction strategies.
The dimensional characteristics of the 8-item version of this measure were examined using principal component analysis. Based on the parallel analysis procedure and the Scree Test, both a one and a two component solution were suggested as possible dimensional solutions, and both component loading patterns were examined. Both solutions presented viable loading patterns, but the one component solution used all eight items and measured a broader construct of self-efficacy for risk reduction and so it was chosen to be used in the final analyses. All eight items were found to have good component loadings (range: .47 to .77), with an averaged component loading for the eight items of .65. Cronbach’s alpha scores for the 8-item measure in the current study was .80.
Alternative Risk-Reduction Attitudes and Behaviors
Participants were asked to assess their alternative risk-reduction attitudes and behaviors (AR-RAB) including serosorting, strategic positioning, and viral load using an 11-item measure developed by ATN-affiliated scientists [46, 47]. Sample items include: “If my viral load is low or undetectable I am less likely to infect another person with HIV if I have unprotected sex (sex without a condom or barrier)” and “I practice safer sex (sex with a condom or barrier) less often now because new medical treatments for HIV/AIDS have come along.” Participants rated their level of agreement with items on a 4-point Likert-type scale (1 = “Strongly disagree” to 4 = “Strongly agree”) with higher scores indicative of higher levels of alternative risk-reduction attitudes and behaviors.
The dimensional characteristics of this 11-item measure were examined using principal component analysis. Both the parallel analysis procedure and the Scree Test suggested the best solution was to retain a single component. Upon direct examination of the one component item loading pattern, there were two items that had low component loadings (<.40) and were accordingly removed from the final solution. The nine retained items were found to have acceptable component loadings (range: .41 to .65), with an averaged component loading for the nine items of .52. Thus, a shortened 9-item version of this instrument and a mean item score was used in the final analyses. Cronbach’s alpha in the current study was .66.
Condomless Sex
Participants were asked to indicate the number of unprotected acts of anal and vaginal sex they had engaged in during the past 90 days.
Statistical Analyses
All descriptive and bivariate analyses were conducted using IBM SPSS Statistics for Windows, Release 20.0.0 (© IBM Corp., 2011, Armonk, NY, www.ibm.com). First, descriptive analyses were conducted to examine participant demographic characteristics. Second, bivariate analyses were performed to examine posited relationships between specific factors as noted below. For our path analysis using a structural equation modeling framework, an exploratory theoretical model was developed as a third step that incorporated constructs that were predicted to explain risky sexual behaviors based upon the extant literature (see Fig. 1). The internal consistency of constructs using the coefficient alpha statistic was also calculated [56].
Significant bivariate relationships among the set of factors measuring the theoretical constructs hypothesized in the complex mediational chain of direct and indirect causes of effects on the outcome were examined. A natural log transformation was applied to the outcome variable (number of acts of condomless anal and vaginal sex in the past 90 days) for use in all analyses to correct for variable skewness. Then, bivariate relationships were examined between the independent factors (frequency of substance use, severity of substance use, emotional distress, social support, self-efficacy for risk-reduction, and alternative risk-reduction attitudes and behaviors) and the outcome. Next, the bivariate relationship of the outcome and the proximal mediator variable (self-efficacy for risk-reduction) was examined, which was theorized to mediate the effects of the independent factors on the outcome through a more distal mediator (alternative risk-reduction attitudes and behaviors), and the bivariate relationship between the outcome variable and the distal mediator was also examined.
A formal path analysis using a structural equation modeling framework was conducted to test the initial theoretical model that examined the joint relationships of all the individual constructs on the outcome variable. Finally, exploratory model adjustments were performed to refine and better explain the complex relationships among the model constructs. The full model-based analyses via path analysis were conducted using the SEM software package Mplus, Version 7.0 [57]. The quality of the models were judged using the minimum fit function Chi square statistic, the comparative fit index (CFI) [58], the non-normed or Tucker-Lewis index (TLI) [59], and the standardized root mean square residual (SRMR) [60]. Evidence for mediation was determined if the indirect effect from the individual construct through the mediator to the outcome was statistically significant.
Results
Participant Characteristics
The mean age of the sample was 20.41 years (SD = 2.63). The majority of participants self-identified as male (66 %), non-Hispanic/Latino (79.4 %) and African American/Black (66.2 %). Relatively equal number of participants self-identified as either heterosexual/straight (42.1 %) or gay (41.5 %). The majority (32.2 %) reported having less than a high school education and more than half (64.6 %) were currently unemployed. Thirty-eight percent reported that they found out about their HIV-positive status at 19 years of age or older. Approximately 73 % indicated they had been behaviorally-infected with HIV, and 83.3 % had disclosed their HIV serostatus to someone else. Table 1 provides participant demographic and HIV characteristics.
Table 1.
Means, standard deviations, and frequencies of demographic characteristics and HIV factors (N = 1728)
| Characteristic | N | Mean (SD) or count (%) |
|---|---|---|
| Age | 1726 | 20.41 (2.63) |
| Gender | 1727 | |
| Male | 1140 (66.0 %) | |
| Female | 552 (32.0 %) | |
| Transgender | 35 (2.0 %) | |
| Ethnicity | 1726 | |
| Non-hispanic/latino | 1371 (79.4 %) | |
| Hispanic/latino | 355 (20.6 %) | |
| Race | 1719 | |
| Black/African American | 1138 (66.2 %) | |
| White | 239 (13.9 %) | |
| Asian/Pacific Islander | 19 (1.1 %) | |
| Native American/alaskan native | 15 (0.9 %) | |
| Multiracial | 199 (11.6 %) | |
| Other | 109 (6.3 %) | |
| Sexual orientation | 1724 | |
| Heterosexual/straight | 725 (42.1 %) | |
| Gay | 715 (41.5 %) | |
| Lesbian | 18 (1.0 %) | |
| Queer | 8 (0.5 %) | |
| Bisexual | 216 (12.5 %) | |
| Questioning | 30 (1.7 %) | |
| Other | 12 (0.7 %) | |
| Educational level | 1723 | |
| < High school | 555 (32.2 %) | |
| High school graduate | 470 (27.3 %) | |
| > High school | 698 (40.5 %) | |
| Employment status | 1724 | |
| Not employed | 1113 (64.6 %) | |
| Employed | 611 (35.4 %) | |
| Mode of HIV infection | 1710 | |
| Behavioral | 1262 (73.8 %) | |
| Perinatal | 448 (26.2 %) | |
| Age when found out HIV+ | 1711 | |
| 12 or younger | 337 (19.7 %) | |
| 13–18 | 653 (38.2 %) | |
| 19 or older | 721 (42.1 %) | |
| HIV disclosure to anyone | 1727 | |
| Yes | 1439 (83.3 %) | |
| No | 288 (16.7 %) |
Means, Standard Deviations, and Bivariate Relationships for Constructs of Interest
Table 2 presents scale means and standard deviations for all constructs of interest. Bivariate Pearson r correlations were examined between our proposed outcome variable, condomless anal and vaginal sex, and the four independent predictor factors (frequency of substance use, severity of substance use, emotional distress, and social support). Significant relationships were found for each bivariate correlation, with condomless anal and vaginal sex significantly correlated with frequency of substance use (r = .288, p < .001), severity of substance use (r = .265, p < .001), emotional distress (r = .187, p < .001), and social support (r = −.078, p = .001). In addition, condomless anal and vaginal sex was significantly correlated with the proximal mediator, self-efficacy for risk-reduction (r = −.137, p < .001), and was also significantly correlated with the distal mediator, alternative risk-reduction attitudes and behaviors (r = .260, p < .001).
Table 2.
Scale means and standard deviations for all factors of interest (N = 1728)
| Scale | Mean (SD) |
|---|---|
| ASSIST | 7.82 (6.16) |
| CRAFFT | 2.26 (1.88) |
| GSI | 0.94 (0.79) |
| SS | 4.28 (0.76) |
| SERR | 7.79 (1.84) |
| AR-RAB | 1.99 (0.53) |
|
| |
| Outcome variable | Geometric mean (95% CI) |
|
| |
| CS | 1.19 (1.07, 1.33) |
ASSIST alcohol, smoking and substance involvement screening test, CRAFFT car, relax, alone, forget, friends, and trouble, GSI global severity index, SS social support, SERR self-efficacy for risk-reduction, AR-RAB risk-reduction attitudes and behaviors, CS condomless sex; geometric mean and 95 % confidence intervals (CI) was calculated for CS
Path Analysis Using a Structural Equation Modeling Framework
Based on modification indices contained within the Mplus output, it was noted that allowing additional path parameters to be freely estimated in the original model would provide improvement of the overall model fit. Consequently, a sequenced series of exploratory model adjustments were conducted in which seven additional model parameters were ultimately allowed to be freely estimated. This procedure was commenced by freeing the path that would provide the largest gain in model fit, using the Chi square difference test to determine if the improvement in fit was statistically significant, and if it was significant, to then re-evaluate the resulting overall model Chi square and the other measures of model fit to determine if freeing an additional path would significantly improve the model fit. Each of the seven additional adjusted models provided a significant improvement in model fit.
The results of the adjusted theoretical model are presented in Fig. 1. As noted in this figure, there are seven newly freed pathways that are significant. Three of the newly freed pathways, frequency of substance use to condomless anal and vaginal sex, severity of substance use to condomless anal and vaginal sex, and emotional distress to condomless anal and vaginal sex, represent direct pathways to condomless anal and vaginal sex indicating that the effects of these constructs were not fully mediated by self-efficacy for risk-reduction or alternative risk-reduction attitudes and behaviors. It is important to note that both frequency of substance use and severity of substance use are separately and significantly related to condomless anal and vaginal sex after controlling for all other variables in the model supporting their unique contribution to the final model. Three of the newly freed pathways, severity of substance use to alternative risk-reduction attitudes and behaviors, emotional distress to alternative risk-reduction attitudes and behaviors and social support to alternative risk-reduction attitudes and behaviors, represent additional direct pathways to alternative risk-reduction attitudes and behaviors. One newly freed path, from self-efficacy for risk-reduction to condomless anal and vaginal sex, represents an additional direct path from self-efficacy for risk-reduction to condomless anal and vaginal sex. All previous direct pathways from frequency of substance use, severity of substance use, emotional distress, and social support to self-efficacy for risk-reduction remained significant. Furthermore, although some path values changed because they were adjusted in the final model, including the path from self-efficacy for risk-reduction to alternative risk-reduction attitudes and behaviors, and the path from alternative risk-reduction attitudes and behaviors to condomless anal and vaginal sex, were significant. In addition, all indirect pathways were also significant or marginally significant, which include: (a) the path (p = .030) from frequency of substance use through self-efficacy for risk-reduction and alternative risk-reduction attitudes and behaviors to condomless anal and vaginal sex; (b) the path (p = .051) from severity of substance use through self-efficacy for risk-reduction and alternative risk-reduction attitudes and behaviors to condomless anal and vaginal sex; (c) the path (p < .001) from emotional distress through self-efficacy for risk-reduction and alternative risk-reduction attitudes and behaviors to condomless anal and vaginal sex; and (d) the path (p < .001) from social support through self-efficacy for risk reduction and alternative risk-reduction attitudes and behaviors to condomless anal and vaginal sex. Moreover, there were three new indirect pathways that were significant: (a) the path (p < .001) from severity of substance use to alternative risk-reduction attitudes and behaviors to condomless anal and vaginal sex; (b) the path (p < .001) from emotional distress to alternative risk-reduction attitudes and behaviors to condomless anal and vaginal sex; and (c) the path (p < .001) from social support to alternative risk-reduction attitudes and behaviors to condomless anal and vaginal sex. Finally, the model now fit very well, with the Chi square measure of model fit no longer being significant (X2 (2) = 3.97, p = .137), and the other measures of model fit also displaying very good fit (CFI = .997; TLI = .976; SRMR = .005).
Discussion
To our knowledge, this is one of the first studies to use path analysis using a structural equation modeling framework to explore multiple risk and protective factors associated with condomless anal and vaginal sex among a large, racially-diverse sample of YLWH in 17 cities throughout the U.S. This study is novel in that it uses syndemics theory to examine the interrelationships among substance use (frequency and severity), psychosocial factors (social support and emotional distress), self-efficacy for risk-reduction, alternative risk-reduction attitudes and behaviors, and condomless anal and vaginal sex as an initial step towards developing targeted preventive interventions for YLWH [27–32]. By shedding light on how some of these key factors work synergistically, our findings have important implications for intervention development.
Results from the current study depict a complex picture of factors that contribute to condomless anal and vaginal sex among YLWH. Consistent with other studies [8–10, 13], and with syndemics theory [28, 29], significant positive associations were found between frequency of substance use, severity of substance use, emotional distress, alternative risk-reduction attitudes and behaviors and condomless anal and vaginal sex. Positive associations were found among frequency of substance use, severity of substance use, and emotional distress, which is in line with prior research [61]. Also, as expected, significant negative associations were found between emotional distress and social support, emotional distress and self-efficacy for risk-reduction, and severity of substance use and self-efficacy for risk-reduction [12].
The unique contributions of our study lie in the use of path analysis to characterize the multiple associations among all of the factors in the model, and their influence on condomless anal and vaginal sex. Notably, some significant relationships within the model did not occur in the expected direction. For instance, a significant positive association was found between frequency of substance use and self-efficacy for risk-reduction as well as between severity of substance use and alternative risk-reduction attitudes and behaviors. Although surprising, it is quite possible that the impaired judgment resulting from substance use, may lead those youth who use substances more frequently to feel more confident in their ability to use these alternative risk-reduction strategies. As they engage in more severe substance use, this impaired judgment regarding their ability to engage in alternative risk-reduction strategies, might increase their positive attitudes towards risk-reduction. For example, YLWH who are under the influence of alcohol or other drugs may feel more socially lubricated and consequently are overly optimistic about their capacity for reducing risk.
It was also surprising that there was a positive association between emotional distress and alternative risk-reduction attitudes and behaviors. It may be that YLWH who are experiencing high levels of emotional distress may have more favorable attitudes and behaviors toward risk-reduction because the thought of engaging in these alternative strategies may help to reduce some of their distress. For instance, for those YLWH who are potentially concerned about transmitting HIV to their sexual partners, having more favorable attitudes and behaviors toward risk-reduction could help to appease this particular concern.
Interestingly, social support did not directly predict condomless anal and vaginal sex; rather, social support was fully mediated indirectly through self-efficacy for risk-reduction and directly through alternative risk-reduction attitudes and behaviors. This finding supports our interpretation that the availability of prevention alternatives to condom use (e.g., strategic positioning, serosorting, and undetectable viral load) may be influencing the relationship between social support, self-efficacy, and condomless sex. That is, YLWH who experience social support feel more confident in their own ability to use risk-reduction strategies effectively but social support also negatively influences their attitudes and behaviors toward risk-reduction. This divergent effect may not intuitively make sense but it is possible that an individual could have high self-efficacy in practicing risk-reduction, but not actually engage in those behaviors or feel positive towards engaging these behaviors. For instance, in our intervention work, we might be able to teach YLWH to have confidence in their ability to use condoms but that confidence might not translate to actually using condoms during a sexual encounter.
It is also interesting to note that severity of substance use and emotional distress were the only two factors that directly predicted condomless anal and vaginal sex and alternative risk-reduction attitudes and behaviors and were mediated indirectly through self-efficacy for risk-reduction. Substance use and emotional distress have been found in prior research to contribute to engagement in high-risk sexual behavior among YLWH [12, 13]. This suggests that future health intervention development for YLWH in the U.S. needs to address how substance use severity and emotional distress may be directly and indirectly mediated through condomless anal and vaginal sex. Thus, assisting these youth in developing effective risk-reduction strategies may be only one component of a prevention intervention; it may be equally as important to help them develop effective tools through psychological interventions (e.g., substance abuse counseling) to better cope with and/or ameliorate their emotional distress or the severity of their substance use in order to prevent condomless anal and vaginal sex.
In light of the complex pattern of associations among factors identified here, the current study points to the need for multi-faceted, tailored interventions that address individual factors (e.g., frequency of substance use, severity of substance use, emotional distress, social support, self-efficacy for risk reduction, and alternative risk-reduction attitudes and behaviors) and the combination of these factors impact in order to prevent condomless anal and vaginal sex among YLWH in the U.S. These results are especially important given the rising number of adolescents and young adults who are becoming infected with HIV at astounding rates each year [1]. However, individual factors are only one piece of the puzzle to preventing condomless anal and vaginal sex. Socio-cultural and structural factors also need to be addressed in order to eliminate new HIV transmissions among young people living in the U.S.
In addition, the current study points to the critical need of developing a better algorithm for measuring risk that incorporates current and emerging prevention strategies, such as having an undetectable viral load, serosorting, and strategic positioning. Health interventions need to incorporate the full complement of prevention approaches and operational definitions of the mediating and moderating factors that reflect the current state of prevention strategies. Given the complex relationships influencing risk, which is consistent with syndemics theory, interventions must also be flexible and adaptable so they can be maximally relevant to the recipients.
Limitations
This study has some limitations. First, this was a clinicbased convenience sample of YLWH who were currently engaged in care in the U.S. Youth who are not engaged in care or undiagnosed with HIV may have different patterns of behavior than the young people in this study. Thus, these results may not generalize to other populations of YLWH, especially those living outside of the U.S. Second, self-report measures were used to assess the factors of interest, which may be subject to bias. However, ACASI was used to encourage participants to answer questions honestly and carefully to minimize any possibility of social desirability bias or errors. Third, the outcome variable was limited to the number of unprotected acts of anal and vaginal sex participants had engaged in during the past 90 days because that is how the question was asked on the assessment. Consequently, examining specific sexual acts (e.g., anal or vaginal sex) by different groups (e.g., GBMSM or heterosexual females) was not possible. Fourth, we did not have access to clinical data on how long participants were in HIV treatment or on antiretroviral therapy. Finally, our data were cross-sectional, which limits our ability to examine directionality of the relationships under investigation in the current study.
Despite these limitations, the present study includes a large, national sample of racially-diverse YLHW that is geographically representative of the HIV epidemic in the U.S. It also represents one of the first studies to use a theoretically-grounded model using path analysis to explain and visually depict the complex and synergistic interrelations of substance use, psychosocial factors, self-efficacy for risk-reduction, alternative risk-reduction attitudes and behaviors, and condomless anal and vaginal sex among YLWH in the U.S.
Conclusions
Further research is necessary to understand the multiple risk and protective factors associated with condomless anal and vaginal sex among YLWH in the U.S. Findings from this study examined through a syndemics theoretical lens suggest that multiple factors working together need to be taken into consideration when designing health interventions to prevent condomless anal and vaginal sex between YLWH and their sexual partners. Future research is needed to test the final model among specific high-risk target populations such as young GBMSM, racial and ethnic minorities, and behaviorally-infected youth to determine any potential differences in our model among these groups.
Acknowledgments
Clinics were located in the following locations: Los Angeles, California; San Francisco, California; Washington, DC; Baltimore, Maryland; Boston, Massachusetts; Chicago, Illinois; Philadelphia, Pennsylvania; New York City (Bronx and Manhattan), New York; San Juan, Puerto Rico; New Orleans, Louisiana; Memphis, Tennessee; Miami, Florida; Tampa, Florida; Ft. Lauderdale, Florida; Detroit, Michigan; Denver, Colorado; and Houston, Texas. This research was supported by The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health [U01HD040533 and U01HD040474] through the National Institute of Child Health and Human Development (Kapogiannis, Lee), with supplemental funding from the National Institutes on Drug Abuse (Davenny, Kahana) and Mental Health (Brouwers, Allison). Support was also provided to the first author by the Providence/Boston Center for AIDS Research (P30AI042853, PI: Cu-Uvin). The study was scientifically reviewed by the ATN’s Behavioral Leadership Group. Network, scientific and logistical support was provided by the ATN Coordinating Center (Wilson, Partlow) at the University of Alabama at Birmingham. Network operations and data management support was provided by the ATN Data and Operations center at Westat, Inc. (Korelitz, Driver). We acknowledge the contribution of the investigators and staff at the following sites that participated in this study: University of South Florida, Tampa (Emmanuel, Lujan-Zilbermann, Julian), Children’s Hospital of Los Angeles (Belzer, Flores, Tucker), Children’s National Medical Center (D’Angelo, Hagler, Trexler), Children’s Hospital of Philadelphia (Douglas, Tanney, DiBenedetto), John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center (Martinez, Bojan, Jackson), University of Puerto Rico (Febo, Ayala-Flores, Fuentes-Gomez), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos), Mount Sinai Medical Center (Steever, Geiger), University of California-San Francisco (Moscicki, Auerswald, Irish), Tulane University Health Sciences Center (Abdalian, Kozina, Baker), University of Maryland (Peralta, Gorle), University of Miami School of Medicine (Friedman, Maturo, Major-Wilson), Children’s Diagnostic and Treatment Center (Puga, Leonard, Inman), St. Jude’s Children’s Research Hospital (Flynn, Dillard), Children’s Memorial Hospital (Garofalo, Brennan, Flanagan), Baylor College of Medicine (Paul, Calles, Cooper), Wayne State University (Secord, Cromer, Green-Jones), Johns Hopkins University School of Medicine (Agwu, Anderson, Park), The Fenway Institute—Boston (Mayer, George, Dormitzer), and University of Colorado, Denver (Reirden, Hahn, Witte). We are greatly appreciative to the members of the local youth Community Advisory Boards for their guidance and insights, and are especially grateful to all of the adolescents and young adults who participated in this study.
Footnotes
Conflicts of Interest The authors declare no conflicts of interest.
Research Involving Human Participants and/or Animals All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Centers for Disease Control and Prevention (CDC) HIV surveillance report. 2014;26 http://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-us.pdf. Published November 2015. Accessed 30 June 2016. [Google Scholar]
- 2.CDC. HIV among youth. http://www.cdc.gov/hiv/risk/age/youth/index.html?s_cid=tw_std0141316. Accessed 30 June 2016.
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 30 June 2016.
- 4.Hein K, Dell R, Futterman D, Rotheram-Borus MJ, Shaffer N. Comparison of HIV + and HIV − adolescents: risk factors and psychosocial determinants. Pediatrics. 1995;95(1):96–104. [PubMed] [Google Scholar]
- 5.Graves KL, Leigh BC. The relationship of substance use to sexual activity among young adults in the United States. Fam Plan Perspect. 1995;27(1):18–22. [PubMed] [Google Scholar]
- 6.Lehrer JA, Shrier LA, Gortmaker S, Buka S. Depressive symptoms as a longitudinal predictor of sexual risk behaviors among US middle and high school students. Pediatrics. 2006;118(1):189–200. doi: 10.1542/peds.2005-1320. [DOI] [PubMed] [Google Scholar]
- 7.Basen-Engquist K. Psychosocial predictors of “safer sex” behaviors in young adults. AIDS Educ Prev. 1992;4(2):120–34. [PubMed] [Google Scholar]
- 8.Elkington KS, Bauermeister JA, Santamaria EK, Dolezal C, Mellins CA. Substance use and the development of sexual risk behaviors in youth perinatally exposed to HIV. J Pediatr Psychol. 2015;40(4):442–54. doi: 10.1093/jpepsy/jsu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkington KS, Bauermeister JA, Brackis-Cott E, Dolezal C, Mellins CA. Substance use and sexual risk behaviors in perinatally human immunodeficiency virus-exposed youth: roles of caregivers, peers and HIV status. J Adolesc Health. 2009;45(2):133–41. doi: 10.1016/j.jadohealth.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassiopoulos K, Moscicki AB, Mellins C, et al. Sexual risk behavior among youth with perinatal HIV infection in the United States: predictors and implications for intervention development. Clin Infect Dis. 2013;56(2):283–90. doi: 10.1093/cid/cis816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naar-King S, Wright K, Parsons JT, Frey M, Templin T, Ondersma S. Transtheoretical model and condom use in HIV-positive youths. Health Psychol. 2006;25(5):648–52. doi: 10.1037/0278-6133.25.5.648. [DOI] [PubMed] [Google Scholar]
- 12.Outlaw AY, Naar-King S, Janisse H, Parsons JT, Adolescent Trials Network for HIV/AIDS Interventions Predictors of condom use in a multisite study of high-risk youth living with HIV. AIDS Educ Prev. 2010;22(1):1–14. doi: 10.1521/aeap.2010.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Outlaw AY, Phillips G, 2nd, Hightow-Weidman LB, et al. Age of MSM sexual debut and risk factors: results from a multisite study of racial/ethnic minority YMSM living with HIV. AIDS Patient Care STDS. 2011;25(Suppl 1):S23–9. doi: 10.1089/apc.2011.9879. [DOI] [PubMed] [Google Scholar]
- 14.Stein JA, Rotheram-Borus MJ, Swendeman D, Milburn NG. Predictors of sexual transmission risk behaviors among HIV-positive young men. AIDS Care. 2005;17(4):433–42. doi: 10.1080/09540120412331291724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotheram-Borus MJ, Murphy DA, Swendeman D, et al. Substance use and its relationship to depression, anxiety, and isolation among youth living with HIV. Int J Behav Med. 1999;6(4):293–311. doi: 10.1207/s15327558ijbm0604_1. [DOI] [PubMed] [Google Scholar]
- 16.Murphy DA, Durako SJ, Moscicki AB, Vermund SH, et al. No change in health risk behaviors over time among HIV infected adolescents in care: role of psychological distress. J Adolesc Health. 2001;29(3 Suppl):57–63. doi: 10.1016/s1054-139x(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 17.Stiffman AR, Doré P, Earls F, Cunningham R. The influence of mental health problems on AIDS-related risk behaviors in young adults. J Nerv Ment Dis. 1992;180(5):314–20. doi: 10.1097/00005053-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Hensel DJ, Stupiansky NW, Orr DP, Fortenberry JD. Event-level marijuana use, alcohol use, and condom use among adolescent women. Sex Transm Dis. 2011;38(3):239–43. doi: 10.1097/OLQ.0b013e3181f422ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: II. Accuracy of self-reports. Ann Behav Med. 2003;26(2):104–23. doi: 10.1207/s15324796abm2602_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiener LS, Battles HB, Wood LV. A longitudinal study of adolescents with perinatally or transfusion acquired HIV infection: sexual knowledge, risk reduction self-efficacy and sexual behavior. AIDS Behav. 2007;11(3):471–8. doi: 10.1007/s10461-006-9162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice E, Batterham P, Rotheram-Borus MJ. Unprotected sex among youth living with HIV before and after the advent of highly active antiretroviral therapy. Perspect Sex Reprod Health. 2006;38(3):162–7. doi: 10.1363/psrh.38.162.06. [DOI] [PubMed] [Google Scholar]
- 22.Crosby RA, Mena L, Geter A. Favourable attitudes towards serosorting are associated with overall less frequent condom use among young Black men having sex men. Sex Health. 2015 doi: 10.1071/SH15156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lightfoot M, Song J, Rotheram-Borus MJ, Newman P. The influence of partner type and risk status on the sexual behavior of young men who have sex with men living with HIV/AIDS. J Acquir Immune Defic Syndr. 2005;38(1):61–8. doi: 10.1097/00126334-200501010-00012. [DOI] [PubMed] [Google Scholar]
- 24.Phillips G, 2nd, Outlaw AY, Hightow-Weidman LB, Jones KC, Wohl AR, Futterman D, Skinner JA, Fields S, Hidalgo J, YMSM of Color SPNS Initiative Study Group Sexual behaviors of racial/ethnic minority young men who have sex with men. AIDS Patient Care STDS. 2011;(Suppl 1):S47–53. doi: 10.1089/apc.2011.9876. [DOI] [PubMed] [Google Scholar]
- 25.Bruce D, Harper GW, Suleta K, Adolescent Medicine Trials Network for HIV/AIDS Interventions Sexual risk behavior and risk reduction beliefs among HIV-positive young men who have sex with men. AIDS Behav. 2013;17(4):1515–23. doi: 10.1007/s10461-012-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eaton LA, Kalichman SC, O’Donnell DA, Karchner WD. A strategy for selecting sexual partners believed to pose little/no risks for HIV: serosorting and its implications for HIV transmission. AIDS Care. 2009;21(1):1279–88. doi: 10.1080/09540120902803208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer MC, Erickson PI, Badiane L, et al. Syndemics, sex and the city: understanding sexually transmitted diseases in social and cultural context. Soc Sci Med. 2006;63(8):2010–21. doi: 10.1016/j.socscimed.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer M. AIDS and the health crisis of the US urban poor: the perspective of critical medical anthropology. Soc Sci Med. 1994;39(7):931–48. doi: 10.1016/0277-9536(94)90205-4. [DOI] [PubMed] [Google Scholar]
- 29.Singer M. A dose of drugs, a touch of violence, a case of AIDS: conceptualizing the SAVA syndemic. Free Inq Creat Sociol. 1996;24(2):99–110. [Google Scholar]
- 30.Walkup J, Blank MB, Gonzalez JS, et al. The impact of mental health and substance abuse factors on HIV prevention and treatment. J Acquir Immune Defic Syndr. 2008;47(Suppl 1):S15–9. doi: 10.1097/QAI.0b013e3181605b26. [DOI] [PubMed] [Google Scholar]
- 31.DiStefano AS, Cayetano RT. Health care and social service providers’ observations on the intersection of HIV/AIDS and violence among their clients and patients. Qual Health Res. 2011;21(7):884–99. doi: 10.1177/1049732311403501. [DOI] [PubMed] [Google Scholar]
- 32.Robinson AC, Knowlton AR, Gielen AC, Gallo JJ. Substance use, mental illness, and familial conflict non-negotiation among HIV-positive African-Americans: latent class regression and a new syndemic framework. J Behav Med. 2016;39(1):1–12. doi: 10.1007/s10865-015-9670-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stall R, Friedman M, Catania J. Interacting epidemics and gay men’s health: a theory of syndemic production among urban gay men. In: Wolitski RJ, Stall R, Valdiserri RO, editors. Unequal opportunity: Health disparities affecting gay and bisexual men in the United States. Oxford; Oxford University Press; 2008. [Google Scholar]
- 34.Stall R, Mills TC, Williamson J, et al. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. Am J Public Health. 2003;93(6):939–42. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mustanski B, Garofalo R, Herrick A, Donenberg G. Psychosocial health problems increase risk for HIV among urban young men who have sex with men: preliminary evidence of a syndemic in need of attention. Ann Behav Med. 2007;34(1):37–45. doi: 10.1080/08836610701495268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halkitis PN, Kupprat SA, Hampton MB, et al. Evidence for a syndemic in aging HIV-positive gay, bisexual, and other MSM: implications for a holistic approach to prevention and healthcare. Nat Resour Model. 2012;36(2):365–86. doi: 10.1111/napa.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halkitis PN. Reframing HIV prevention for gay men in the United States. Am Psychol. 2010;65(8):752–63. doi: 10.1037/0003-066X.65.8.752. [DOI] [PubMed] [Google Scholar]
- 38.Halkitis PN, Moeller RW, Siconolfi DE, Storholm ED, Solomon TM, Bub KL. Measurement model exploring a syndemic in emerging adult gay and bisexual men. AIDS Behav. 2013;17(2):662–73. doi: 10.1007/s10461-012-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starks TJ, Millar BM, Eggleston JJ, Parsons JT. Syndemic factors associated with HIV risk for gay and bisexual men: comparing latent class and latent factor modeling. AIDS Behav. 2014;18(11):2075–9. doi: 10.1007/s10461-014-0841-9. [DOI] [PubMed] [Google Scholar]
- 40.Klein H. Using a syndemics theory approach to study HIV risk taking in a population of men who use the internet to find partners for unprotected sex. Am J Mens Health. 2011;5(6):466–76. doi: 10.1177/1557988311398472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrick A, Stall R, Egan J, Schrager S, Kipke M. Pathways towards risk: syndemic conditions mediate the effect of adversity on HIV risk behaviors among young men who have sex with men (YMSM) J Urban Health. 2014;91(5):969–82. doi: 10.1007/s11524-014-9896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mimiaga MJ, OCleirigh C, Biello KB, et al. The effect of psychosocial syndemic production on 4-year HIV incidence and risk behavior in a large cohort of sexually active men who have sex with men. J Acquir Immune Defic Syndr. 2015;68(3):329–36. doi: 10.1097/QAI.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai AC, Burns BF. Syndemics of psychosocial problems and HIV risk: a systematic review of empirical tests of the disease interaction concept. Soc Sci Med. 2015;139:26–35. doi: 10.1016/j.socscimed.2015.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macdonell KE, Naar-King S, Murphy DA, et al. Predictors of medication adherence in high risk youth of color living with HIV. J Pediatr Psychol. 2010;35(6):593–601. doi: 10.1093/jpepsy/jsp080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adolescent Trials Network (ATN) 106 Protocol. Network-wide assessment of current health status and behavioral risk factors: an expanded study for new sites in ATN III. Unpublished document. [Google Scholar]
- 46.Parsons JT, Rosof E, Mustanski B. Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. J Health Psychol. 2007;12(2):357–70. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsons JT, Rosof E, Punzalan JC, Di Maria L. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance use among HIV-positive men and women: results of a pilot project. AIDS Patient Care STDS. 2005;19(1):31–9. doi: 10.1089/apc.2005.19.31. [DOI] [PubMed] [Google Scholar]
- 48.WHO Assist Working Group. The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 49.Knight JR, Sherritt L, Shrier LA, Harris SK, Chang G. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156(6):607–14. doi: 10.1001/archpedi.156.6.607. [DOI] [PubMed] [Google Scholar]
- 50.Derogatis L, Spencer M. The brief symptom inventory (BSI): administration, scoring, and procedures manual-1. Baltimore, MD: Johns Hopkins University School of Medicine, Clinical Psychometrics Research Unit; 1982. [Google Scholar]
- 51.Brown LK, Whiteley L, Harper GW, Nichols S, Nieves A. Psychological symptoms among 2032 youth living with HIV: a multisite study. AIDS Patient Care STDS. 2015;29(4):212–9. doi: 10.1089/apc.2014.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lam PK, Naar-King S, Wright K. Social support and disclosure as predictors of mental health in HIV-positive youth. AIDS Patient Care STDS. 2007;21(1):20–9. doi: 10.1089/apc.2006.005. [DOI] [PubMed] [Google Scholar]
- 53.Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30(20):179–85. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- 54.O’Connor BP. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer’s MAP test. Behav Res Methods Instrum Comput. 2000;32(3):396–402. doi: 10.3758/bf03200807. [DOI] [PubMed] [Google Scholar]
- 55.Cattell RB. The scree test for the number of factors. Multivar Behav Res. 1966;1(2):245–76. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- 56.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. [Google Scholar]
- 57.Muthén LK, Muthén BO. Mplus User’s Guide. 7. Vol. 2012 Los Angeles, CA: 1998–2013. [Google Scholar]
- 58.Bentler PM. Comparative fit indexes in structural equation models. Psychol Bull. 1990;107(2):238–46. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 59.Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38(1):1–10. [Google Scholar]
- 60.Bentler PM. EQS: a structural equations program. Los Angeles, CA: BMDP Statistical Software; 1989. [Google Scholar]
- 61.Nugent NR, Brown LK, Belzer M, Harper GW, et al. Youth living with HIV and problem substance use: elevated distress is associated with nonadherence and sexual risk. J Int Assoc Physicians AIDS Care (Chic) 2010;9(2):113–5. doi: 10.1177/1545109709357472. [DOI] [PMC free article] [PubMed] [Google Scholar]


