Abstract
Understanding pathways of risk following a natural disaster may help create next-generation targeted interventions. The current study examined if a biomarker of cognitive-affective response (pupil dilation) could identify which individuals are at greatest risk for depression following disaster-related stress. Fifty-one women completed a computer-based task assessing pupillary response to facial expressions of emotion and reported their depressive symptoms before the 2011 Binghamton flood. Following the flood, women were assessed for objective levels of flood-related stress and again reported their depressive symptoms. Supporting the proposed diathesis-stress model, decreased pupil dilation to emotional expressions predicted a significant increase in post-flood depressive symptoms, but only among women who experienced higher levels of flood-related stress. Findings suggest that reduced cognitive-affective response to emotional stimuli (measured via pupillary response) can increase risk for depression in the context of high levels of objective life stress.
Keywords: Depression, natural disaster, pupillometry, stress, affective neuroscience
Natural disasters have devastating effects on communities, and affected residents often experience a cascade of negative life events including damage or loss of housing, financial strain, and disruptions to school and work. For example, during the 2011 Binghamton flood, remnants of Tropical Storm Lee hit the greater Binghamton, New York area leading to record-high floodwaters that caused the evacuation of 22,000 residents, an estimated $513 million in property damages, and the temporary shut-down of local schools and businesses (Micale, 2012). President Barack Obama declared the region a major disaster area, and the federal government responded with financial aid and National Guard assistance (Micale, 2012). Analogous to research examining general life stress and depression, studies examining the effects of natural disasters similar to the 2011 Binghamton flood have found that disaster-related stress predicts significant increases in depressive symptoms and disorders (for a review, see Bonanno, Brewin, Kaniasty, & La Greca, 2010).
Despite the robust link between stress and depression, research has also shown that only 20 to 25% of individuals experiencing major stressful life events develop depression (van Praag, de Koet, & van Os, 2004). This is consistent with diathesis-stress models, which suggest that not all individuals are equally susceptible to stress and propose that individuals with certain vulnerabilities are more likely to experience depression following stressful life events (e.g, Ingram & Price, 2010). Therefore, examining individual differences in response to stressful life events may provide insights into mechanisms underlying risk and resilience. For example, there has been considerable interest in identifying stable, individual risk factors that may predict adverse outcomes such as depression following stressful life events, and there is evidence that this approach may be particularly helpful in the identification of individuals at greatest risk following natural disasters (e.g., Felton, Cole, & Martin, 2013; Kopala-Sibley et al., 2016). Given the profound impact of natural disasters on community mental health, identifying risk factors that may help to predict which individuals are at greatest risk for depression following disaster-related stress may help to guide the development of next-stage personalized and mechanistically informed prevention and intervention efforts.
The way an individual responds to emotional information may be a particularly important predictor of how they will respond to a natural disaster. Natural disasters evoke myriad emotions, many relating to themes of loss and sadness, and an individual’s response to emotional information may contribute greatly to psychological outcomes following the disaster. Therefore, individual differences in cognitive-affective response to emotional information may help to identify those at greatest risk of depression symptom increases following a natural disaster. For example, research has shown that expressive suppression, an emotional response that dampens the outward expression of emotion, is linked to greater stress-related symptoms (Moore, Zoellner, & Mollenholt, 2008) whereas emotion regulation techniques, such as cognitive reappraisal, reduce the risk of depression following high levels of stress (Troy, Wilhelm, Shallcross, & Mauss, 2010). However, there is concern that the self-report methods used in previous research may limit the predictive validity of these measures as previous research has shown that moment-to-moment measures of cognitive-affective response are more closely linked to psychopathology than self-report measures (e.g., Shiffmann, Stone, & Hufford, 2008). Therefore, additional research is needed that uses more ecologically-valid measures to examine cognitive-affective response as a diathesis for depression.
Pupillary response to emotional stimuli may be one such promising marker of cognitive-affective response. Pupillary responses provide a peripheral measure of cognitive-affective responding as the pupil dilates in response to emotional information and is innervated by brain regions involved in cognitive and emotional processing such as the dorsolateral prefrontal cortex and anterior cingulate cortex (Murphy, O’Connell, O’Sullivan, Robertson, & Balsters, 2014; Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003). Previous research has also indicated that pupil dilation may reflect regulatory control of cognitive-affective response. For example, in healthy individuals, cognitive reappraisal is linked to a relative increase in pupillary response to negative stimuli, which is in turn related to decreased activation in the amygdala and insula (Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007; Urry et al., 2006). In contrast, a relative decrease in pupil dilation to an emotional stimulus may occur when an individual employs an emotional response strategy such as suppression, a hypothesis supported by research showing that decreased pupil dilation is associated with reduced brain activity integral to successful emotion regulation, such as dorsolateral prefrontal (Siegle et al., 2003) and medial frontal (Urry et al., 2006) activation. Additionally, a decrease in pupil dilation is observed when emotion regulation resources are overwhelmed (Granholm, Asarnow, Sarkin, & Dykes, 1996; Granholm, Morris, Sarkin, Asarnow, & Jeste, 1997; Minassian, Granholm, Verney, & Perry, 2004) and the individual displays blunted cognitive-affective processing of the task at hand. Notably, there is evidence that decreased pupillary response to negative information is a risk factor for depression. For example, children with current major depressive disorder (MDD) exhibit decreased sustained pupillary response to negative emotional words (Silk et al., 2007) and adults with remitted MDD display decreased pupillary response to negative stimuli following a negative mood induction (Steidtmann, Ingram, & Siegle, 2010). Taken together, these findings suggest that decreased pupillary response may reflect blunted cognitive-affective response to emotional stimuli, which in turn increases risk for depression. However, no studies to date have examined whether pupillary response to emotional stimuli actually predicts prospective changes in depressive symptoms in adults.
The goal of this study, therefore, was to address this gap in the literature. We hypothesized that decreased pupillary response to emotional stimuli would predict prospective changes in depressive symptoms and that this effect would be particularly pronounced for those experiencing high levels of stress. Specifically, individuals who exhibit decreased pupillary response to negative stimuli may be more likely to experience blunted cognitive-affective response or “shut down” during stressful life events and thus not respond to salient and necessary cues following the event, placing them at greater risk for depression. We tested this hypothesis by examining whether levels of objective life stress related to the Binghamton Flood would moderate the link between pupillary response to emotional stimuli and prospective changes in depressive symptoms. We focused on women in this study as they have twice the risk for depression as men (Kessler, McGonagle, Swartz, Blazer, & Nelson, 1993) and women, relative to men, are at increased risk for depression following increases in life stress (Hankin & Abramson, 2001). Based on prior research demonstrating the specificity of pupillary response to negative stimuli in depression (Silk et al., 2007; Steidtmann et al., 2010), we hypothesized that pupillary response to negative, but not positive, stimuli would predict greater post-flood depressive symptoms among women experiencing higher levels of flood-related stress.
Method
Participants
Participants in this study were 51 women recruited from the community as part of a larger multi-wave longitudinal study of the intergenerational transmission of depression. Women included in the current study were living in the greater Binghamton area at the time of the 2011 flood, reported a life event indicating that they or their child had been impacted by the flood to some extent, and completed study appointments both prior to and following the flood. On average, participants completed pre-flood assessment 100.61 days (SD = 79.47) before the flood and post-flood assessments 113.20 days (SD = 74.78) after. To participate in the study, women were either required to have a lifetime history of MDD (n = 20) or no lifetime diagnosis of any DSM-IV mood disorders (n = 31). Exclusion criteria for the study included symptoms of schizophrenia, organic mental disorder, alcohol or substance dependence within the last six months, or history of bipolar disorder. The average age of women in our sample was 40.75 years (SD = 6.42, Range = 29 – 55), 65% were currently married, and 90% were Caucasian. The median annual family income was $55,001 to $60,000, and 49% of women had a bachelor’s degree or higher.
Measures
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1994) was used to assess for lifetime history of DSM-IV Axis I disorders. The SCID-I is a widely used diagnostic interview with well-established psychometric properties (Lobbestael, Leurgans, & Arntz, 2011; Zanarini & Frankenburg, 2001).
Women’s symptoms of depression during the pre- and post-flood appointments were assessed using the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996), a 21-item questionnaire that assesses the severity of current depressive symptoms in the past two weeks. The measure has demonstrated good internal consistency and validity in previous research (Dozois, Dobson, & Ahnberg, 1998) and exhibited excellent internal consistency in the current sample (Pre BDI-II: α = .93, M = 6.78, SD = 8.09; Post BDI-II: α = .94, M = 6.94, SD = 8.48).
Women’s levels of episodic flood-related stress were assessed during the post-flood appointment using the semi-structured UCLA Life Stress Interview (LSI; Hammen, 1991). During the UCLA LSI, women were interviewed about the occurrence of acute events related to the flood. Any flood-related events reported were then probed to elicit information about the event including its timing, duration, and associated circumstances. Objective information was obtained about each reported episodic stressor and presented to a team of coders who were blind to any subjective experiences the women may have reported during the interview. The coders assigned each episode of stress a negative impact stress score on a scale of 1 to 5, where a score of 1 was indicative of no stress and a score of 5 indicated that the reported episode was characterized by severe stress impact. To avoid inflating episodic stress scores by inclusion of events rated as having no impact (score = 1), we recoded the objective impact scores from a scale of 1–5 to a scale of 0–4 and then computed a total objective episodic stress score for flood-related events (M = 0.71, SD = 1.01).
Pupil dilation was obtained in a morphed faces task (cf. Burkhouse, Gibb, & Siegle, 2014), which was administered during a pre-flood assessment. Stimuli for this task were drawn from a stimulus set of full-color pictures of actors taken from a standardized stimulus set (Matsumoto & Ekman, 1988) displaying a variety of emotions (e.g., sad, happy, angry, neutral). The stimuli consisted of emotional and neutral photographs from each actor, morphed to form a continuum of 10% increments between the two photographs, similar to previous creations of morphed emotional facial expressions (Young, Perrett, Calder, Sprengelmeyer, & Ekman, 2002). Each emotion is represented by 4 continua (2 male and 2 female actors), for a total of 12 continua. Eleven morphed images were used from each continuum, representing 10% increments of the two emotions ranging from 100% neutral (0% target emotion) to 100% target emotion (e.g., 90% Neutral, 10% Sad; 80% Neutral, 20% Sad; and so on). The pictures, measuring 8.0(w) × 6.5(h) inches, were presented, one at a time, in random order in 2 blocks and the participant was instructed to indicate which emotion was being presented (sad, happy, angry, neutral) by pressing a corresponding button on a keypad. The image remained on the screen until the participant made a response. Participants completed 264 trials. Consistent with previous research (e.g., Burkhouse et al., 2014, 2015), responses were binned into three separate morph conditions for analyses: low (0%, 10%, 20% and 30%), medium (40%, 50%, 60% and 70%) and high (80%, 90% and 100%).
During the morphed faces task, pupil size was recorded using Tobii T60XL eye trackers, which took measurements every 16.7ms (60 Hz) for 3s following the onset of each facial stimulus. Data were cleaned using Siegle et al.’s, (2008) standard procedures. Trials comprised of over 50% blinks were removed from consideration. Following standard procedures, linear interpolation was used to replaced blinks throughout the data set and the data were smoothed using a 10-point weighted average filter. The average pupil diameter over the 333 ms preceding the onset of the stimulus was subtracted from pupil diameter after stimulus onset to produce stimulus-related pupil dilation. Peak stimulus-related pupil dilation was calculated by taking the maximum pupil response on average across all trials for each emotion (angry, happy, and sad). The average pupillary responses to each emotion type across each morph level for all the participants is visually depicted in Figure S1 in the Supplemental Material available online.
Procedure
Potential participants were recruited from the community through a variety of means (e.g., television, newspaper and bus ads, flyers). Participants responding to the recruitment advertisements were initially screened over the phone to determine potential eligibility. Upon arrival at the laboratory, participants were asked to provide informed consent. Next, a research assistant administered the SCID-I and then participants completed the morphed faces task and questionnaires. Following this baseline assessment, participants completed follow-up appointments every 6 months for 2 years, during which they completed questionnaires. The current report focuses on the assessments directly before and directly after the 2011 Binghamton flood. All study procedures were approved by the University’s Institutional Review Board.
Results
An initial inspection of the data revealed several variables that exhibited significant skew (z > 3.29; cf. Tabachnick & Fidell, 2007). These variables (pre- and post-flood BDI-II scores, flood-related stress scores) were square-root transformed prior to further analysis to satisfy assumptions of normality. Next, we used a generalized linear mixed model to examine predictors of depressive symptoms following the 2011 Binghamton flood. In these analyses, women’s post-flood depression scores were entered as the dependent variable. Flood-related stress scores and mean peak pupillary response scores for each emotion at each morph level per subject were added as predictor variables, with Morph (high, medium, low) and Emotion (angry, happy, sad) as repeated measures. MDD history, pre-flood depression scores, and the number of days from the flood to the post-flood assessment were entered as covariates in all analyses. Results of these analyses are presented in Table 1.1
Table 1.
Results of the Generalized Linear Mixed Model analyses predicting prospective changes in women’s depressive symptoms as a function of pupil dilation and flood-related stress.
| F
|
|
|---|---|
| Emotion | 0.12 |
| Morph | 0.19 |
| Peak Pupil | 0.09 |
| Flood Stress | 10.77** |
| MDD Life | 20.92*** |
| Pre BDI-II | 324.15*** |
| Days After Flood | 26.32*** |
| Emotion × Morph | 0.24 |
| Emotion × Peak Pupil | 0.13 |
| Emotion × Flood Stress | 0.89 |
| Morph × Peak Pupil | 0.08 |
| Morph × Flood Stress | 0.14 |
| Peak Pupil × Flood Stress | 7.16** |
| Emotion × Morph × Peak Pupil | 0.22 |
| Emotion × Morph × Flood Stress | 0.19 |
| Emotion × Peak Pupil × Flood Stress | 0.48 |
| Morph × Peak Pupil × Flood Stress | 0.07 |
| Emotion × Morph × Peak Pupil × Flood Stress | 0.20 |
Note. Peak Pupil = average peak pupil dilation to facial displays of emotion. MDD Life = women’s lifetime history of major depressive disorder (yes, no).
p < .01.
p < .001.
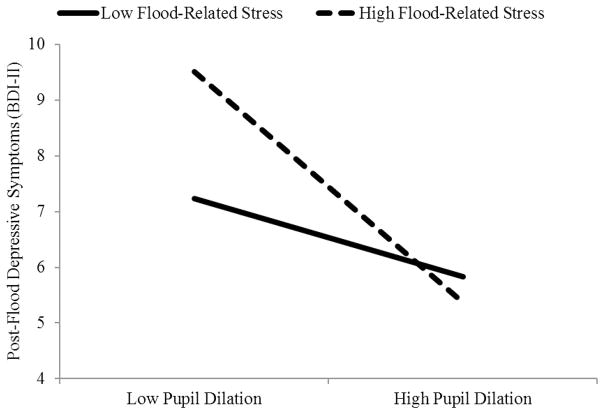
As seen in the table, there was a significant Peak Pupil × Stress interaction that was notably not moderated by Emotion or Morph. To determine the form of the Pupil × Stress interaction, we examined the interaction following the guidelines of Aiken and West (1991) by solving the regression equation for values 1 SD above and below the mean for peak pupil dilation (collapsing across morph levels and emotion type) and flood-related stress (Aiken & West, 1991; see Figure 1). We found that, among women who experienced higher levels of flood-related stress (+1 SD), decreased peak pupil dilation to emotional facial expressions predicted a significant increase in post-flood depressive symptoms, t(44) = −2.31, p = .03, rpartial = −.33. In contrast, among women who experienced lower levels of flood-related stress (−1 SD), peak pupil dilation to emotional facial expressions did not significantly predict changes in depressive symptoms, t(44) = −0.25, p = .80, rpartial = −.04.
Figure 1.
Interaction between flood-related stress and peak pupil dilation to facial displays of emotion predicting depressive symptoms following the flood.
Note. BDI-II = Beck Depression Inventory-II. To facilitate comparisons with other studies, this figure was created based on untransformed post-flood BDI-II scores.
Exploratory analyses indicated that women’s MDD history did not moderate any of the presented findings (lowest p = .26). Given potential concerns that the effects of flood-related stress may be confounded with women’s family income, we should note that our significant flood × pupil interaction and subsequent main effect of pupil dilation among women with higher levels of flood-related stress were maintained even after including family income as a covariate in our analyses (all ps < .05).
Discussion
The aim of the current study was to examine if decreased pupillary response to facial displays of emotion, a physiological marker of cognitive-affective responding, would predict prospective changes in depressive symptoms and whether this relation would be stronger among individuals exposed to high levels of disaster-related stress. Our findings indicated that decreased pupil dilation to emotional facial expressions predicted a significant increase in post-flood depressive symptoms only among women who experienced higher levels of flood-related stress. Further, these findings were maintained after statistically controlling for the influence of women’s family income and MDD history providing some evidence that our results were not confounded by women’s socioeconomic status or lifetime history of depression.
Previous research using pupillometry has shown that decreased pupillary response to negative information is related to decreased activity in dorsolateral prefrontal regions (Siegle, Steinhauer, Friedman, Thompson, & Thase, 2011), which is associated with decreased ability to effectively regulate emotions and reduced executive control (for a review, see Etkin, Buchel, & Gross, 2015). Following a natural disaster, it may be that individuals exhibiting decreased pupillary response to emotional stimuli respond to high levels of stress in a maladaptive manner given their deficits in emotion regulation, thereby increasing their risk for subsequent depression. For example, it may be that these individuals “shut-down” and stop responding to the necessary cues in their environment that prompt them to act or respond in an effective manner to the stressor at hand. In this case, the consequences of the stressor continue to grow, thus increasing the individual’s depression and further propagating this cycle.
Notably, our findings were not specific to negative facial expressions, which is inconsistent with some previous research (i.e., Silk et al., 2007; Steidtmann et al., 2010). However, our findings are consistent with the Emotion Context Insensitivity (ECI) theory of depression, which posits that depressed and at-risk individuals display diminished emotional and physiological reactivity to both positive and negative stimuli (Rottenberg, Gross, & Gotlib, 2005). Rottenberg and colleagues propose that blunted reactivity across positive and negative emotional contexts is driven by symptoms of withdrawal and anhedonia that are characteristic of current major depressive disorder. Supporting the ECI theory, a meta-analysis found that currently depressed individuals exhibited blunted response to both positive and negative stimuli (i.e., images and films) across self-report, behavioral, and physiological measures (Bylsma, Morris, & Rottenberg, 2008). Similarly, there are several studies examining cognitive-affective responding in currently depressed individuals using the late positive potential (LPP), a bilateral and posterior slow wave event-related potential (ERP) with amplitudes that increase or decrease in relation to sustained engagement with an emotional stimulus (for a review, see Proudfit, Bress, Foti, Kujawa, & Klein, 2015), which have shown that individuals with current MDD, compared to those with no history of depression, exhibit a reduced LPP to unpleasant and pleasant pictures or words (Blackburn, Roxborough, Muir, Glabus, & Blackwood, 1990; Weinberg, Perlman, Kotov, & Hajcak, 2016). Taken together with the results of the current study, it appears that reduced cognitive-affective response to both positive and negative stimuli may be a risk factor for depression, especially in the context of higher levels of life stress.
Importantly, these findings suggest that interventions designed to target deficits in cognitive-affective responding may be effective for prevention and intervention programs for depression following natural disasters. For example, Siegle and colleagues (2011) found that individuals diagnosed with MDD who displayed higher initial depression severity and decreased sustained pupillary response to negative words were more likely to achieve remission following sixteen sessions of Cognitive Therapy (CT), suggesting that CT may have particular utility in targeting and improving the emotion regulation and executive control deficits associated with decreased pupillary response (Siegle et al., 2011). In light of the current findings, it is certainly plausible that individuals displaying decreased pupillary response to emotional stimuli and relatively higher levels of disaster-related stress may be good candidates for CT to alleviate their depression. Future research is therefore needed to determine if pupillometry could predict response to prevention and intervention efforts following natural disasters.
The current study displayed several strengths, such as examining response to a natural disaster and thus providing an opportunity for a “natural experiment” that capitalized on the objectivity of a stressor occurring outside a person’s control. Additionally, the current study examined pupillary response to emotional faces as a biomarker of cognitive-affective responding to improve upon previous research that utilized self-report measures. However, there were limitations that highlight areas for future research. First, the study focused on women and future research is needed to determine the generalizability of the results to men. Second, the study focused only on depressive symptoms, and, due to the small sample size, was unable to examine depressive diagnoses following the flood. Finally, given that pupillometry is a measure of peripheral physiology, our findings cannot point to the precise neural mechanisms underlying the link between cognitive-affective response to emotional stimuli, stress, and depression.
In summary, the current study is the first to examine how pupillary response to emotional stimuli may interact with life stress to predict prospective depression. Importantly, this study adds to the growing body of research supporting the role of cognitive-affective response in diathesis-stress models of depression and complements previous studies that indicate pupillary response can predict risk for future depression (Burkhouse et al., 2015) and treatment response for depression (Siegle et al., 2011). Of particular interest, pupillometry is an inexpensive, noninvasive assessment and could be easily implemented in hospitals and outpatient settings. If replicated and extended, the current findings may further our understanding of how cognitive-affective response plays a role in stress and depression and also aid clinicians in identifying those most at risk following a natural disaster.
Supplementary Material
Acknowledgments
This project was supported by National Institute of Child Health and Human Development grant HD057066 and National Institute of Mental Health grant MH098060 awarded to B. E. Gibb. We would like to thank Ashley Johnson, Lindsey Stone, Andrea Hanley, Michael Van Wie, Devra Alper, Cope Feurer, Eric Funk, and Effua Sosoo for their help in conducting assessments for this project.
Footnotes
Analyses examining the link between pupillary response and pre-flood depressive symptoms can be found in the Supplementary Material available online.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Beck A, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio, TX, 78204–2498: 1996. [Google Scholar]
- Blackburn IM, Roxborough HM, Muir WJ, Glabus M, Blackwood DH. Perceptual and physiological dysfunction in depression. Psychological Medicine. 1990;20:95–103. doi: 10.1017/s003329170001326x. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Brewin CR, Kaniasty K, La Greca AM. Weighing the costs of disaster consequences, risks, and resilience in individuals, families, and communities. Psychological Science in the Public Interest. 2010;11:1–49. doi: 10.1177/1529100610387086. [DOI] [PubMed] [Google Scholar]
- Burkhouse KL, Gibb BE, Siegle GJ. Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. Journal of Child Psychology and Psychiatry. 2014;55:1009–1016. doi: 10.1111/jcpp.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Siegle GJ, Woody ML, Kudinova AY, Gibb BE. Pupillary reactivity to sad stimuli as a biomarker of depression risk U: Evidence from a prospective study of children. Journal of Abnormal Psychology. 2015;124:498–506. doi: 10.1037/abn0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological Assessment. 1998;10:83–89. [Google Scholar]
- Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nature Reviews Neuroscience. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Felton JW, Cole DA, Martin NC. Effects of rumination on child and adolescent depressive reactions to a natural disaster: The 2010 Nashville flood. Journal of Abnormal Psychology. 2013;122:64–73. doi: 10.1037/a0029303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for Axis I DSM-IV disorders. 1994 doi: 10.1001/archpsyc.1992.01820080032005. Patient Edition (SCID-I/P) [DOI] [PubMed] [Google Scholar]
- Granholm E, Asarnow RF, Sarkin AJ, Dykes KL. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33:457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris SK, Sarkin AJ, Asarnow RF, Jeste DV. Pupillary responses index overload of working memory resources in schizophrenia. Journal of Abnormal Psychology. 1997;106:458–467. doi: 10.1037//0021-843x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Hammen C. The generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive-vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Price MJ. Understanding psychopathology: The role of vulnerability. In: Ingram RE, Price JM, editors. Vulnerability to Psychopathology: Risk Across the Lifespan. 2. New York, NY: Guilford Press; 2010. pp. 3–17. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle Ka, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Kotov R, Bromet EJ, Carlson GA, Danzig AP, Black SR, Klein DN. Personality diatheses and Hurricane Sandy: Effects on post-disaster depression. Psychological Medicine. 2016;46:865–875. doi: 10.1017/S0033291715002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobbestael J, Leurgans M, Arntz A. Interrater reliability of the Structured Clinical Interview for DSM-IV Axis 1 Disorders (SCID-I) and Axis II Disorders (SCID-II) Clinical Psychology and Psychotherapy. 2011;18:75–79. doi: 10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. Japanese and Caucasian facial expressions of emotion. San Francisco, CA: San Francisco State University; 1988. [Google Scholar]
- Micale J. Tier flood damage estimate: $1 billion. Press & Sun-Bulletin; 2012. Feb 1, [Google Scholar]
- Minassian A, Granholm E, Verney S, Perry W. Pupillary dilation to simple vs. complex tasks and its relationship to disturbance in schizophrenia patients. International Journal of Psychophysiology. 2004;52:53–62. doi: 10.1016/j.ijpsycho.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Moore SA, Zoellner LA, Mollenholt N. Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behaviour Research and Therapy. 2008;46:993–1000. doi: 10.1016/j.brat.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping. 2014;35:4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH, Bress JN, Foti D, Kujawa A, Klein DN. Depression and Event-related Potentials: Emotional disengagement and reward insensitivity. Current Opinion in Psychology. 2015;4:110–113. doi: 10.1016/j.copsyc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Shiffmann S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ichikawa N, Steinhauer SR. Blink before and after you think: Blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology. 2008;45:679–687. doi: 10.1111/j.1469-8986.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: Utility and neural correlates. Biological Psychiatry. 2011;69:726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. NeuroImage. 2003;20:114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Birmaher B, Axelson D, … Siegle GJ. Pupillary reactivity to emotional information in child and adolescent depression: Links to clinical and ecological measures. American Journal of Psychiatry. 2007;164:1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidtmann D, Ingram RE, Siegle GJ. Pupil response to negative emotional information in individuals at risk for depression. Cognition & Emotion. 2010;24:480–496. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5. Needham Height, MA: Allyn & Bacon; 2007. [Google Scholar]
- Troy AS, Wilhelm FH, Shallcross AJ, Mauss IB. Seeing the silver lining: Cognitive reappraisal ability moderates the relationship between stress and depressive symptoms. Emotion. 2010;10:783–795. doi: 10.1037/a0020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, … Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag HM, de Koet ER, van Os J. Stress, the brain, and depression. Cambridge, England: Cambridge University Press; 2004. [Google Scholar]
- Weinberg A, Perlman G, Kotov R, Hajcak G. Depression and reduced neural response to emotional images: Distinction from anxiety, and importance of symptom dimensions and age of onset. Journal of Abnormal Psychology. 2016;125:26–39. doi: 10.1037/abn0000118. [DOI] [PubMed] [Google Scholar]
- Young A, Perrett D, Calder A, Sprengelmeyer R, Ekman P. Facial Expressions of Emotion - Stimuli and Tests (FEEST) Bury St. Edmunds, England: Thames Valley Test; 2002. [Google Scholar]
- Zanarini MC, Frankenburg FR. Attainment and maintenance of reliability of Axis I and Axis II disorders over the course of a longitudinal study. Comprehensive Psychiatry. 2001;42:369–374. doi: 10.1053/comp.2001.24556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.