Abstract
Background
Extreme ambient temperatures are linked to cardiac events in the general population, but this relationship is unclear among pregnant women. We estimated the associations and attributable risk between ambient temperature and the risk of cardiovascular event at labor/delivery, and investigated whether these associations vary by maternal race/ethnicity.
Methods
We identified 680 women with singleton deliveries affected by cardiovascular events across 12 US sites (2002–2008). Average daily temperature during the week before, delivery day, and each of the seven days before delivery was estimated for each woman. In a case-crossover analysis, exposures during these hazard periods were compared to two control periods before and after delivery using conditional logistic regression adjusted for other environmental factors.
Results
During the cold season (October-April), 1°C lower during the week prior to delivery was associated with a 4% (95% CI: 1–7%) increased risk of having a labor/delivery affected by cardiovascular events including cardiac arrest and stroke. During the warm season (May–September), 1°C higher during the week prior was associated with a 7% (95% CI: 3–12%) increased risk. These risks translated to 13.4 and 23.9 excess events per 100 000 singleton deliveries during the cold and warm season, respectively. During the warm season, the risks were more pronounced on days closer to delivery and Black women appeared to be more susceptible to the same temperature increase.
Conclusion
Small changes in temperature appear to affect the risk of having cardiovascular events at labor/delivery. Black women had a differentially higher warm season risk. These findings merit further investigation.
Keywords: Temperature, cardiovascular events, labor and delivery, climate change, pregnancy
Background
Cardiovascular events are a leading cause of maternal morbidity and mortality. They are responsible for approximately 15% of all pregnancy-related deaths (Creanga et al., 2015). This proportion has increased in the US from approximately 3% during the years 1987–1990 and has a consistent racial/ethnic disparity over time, with Black women experiencing significantly higher risk (CDC, 2016; Creanga et al., 2015). One factor potentially contributing to this upward trend is the shift in population-level cardiovascular risk factors including maternal age, obesity, and gestational complications (Ferrara, 2007; Lu et al., 2001). Although the incidence of cardiovascular events during labor and delivery is relatively low at 0.3% (Männistö et al., 2015), in addition to being potentially life-threatening, these events have long-term implications for affected women and their families (Sliwa and Bohm, 2014). Consequently, it is critical to identify and understand potentially modifiable risk factors. Our group has published some early evidence on the potential acute association between ambient air pollution and the risk of cardiovascular events at labor and delivery (Männistö et al., 2015), but other environment risk factors are largely unknown.
Concurrent with the increase in pregnancy-related cardiovascular events is the steady increase in ambient temperature associated with climate change (NOAA, 2011). Studies have linked extreme ambient temperatures with acute cardiovascular risk in the general population as well as in susceptible subgroups including those with lower socioeconomic status, the elderly, and those with other comorbid conditions (Medina-Ramon and Schwartz, 2007; Wang et al., 2016; Yang et al., 2015). A role for temperature in the pathogenesis of cardiovascular risks is plausible given the oxidative stress and systemic inflammation induced shortly after exposure (Cai et al., 2016; Cheng et al., 2015; Halonen et al., 2010; Hong et al., 2012; Quindry et al., 2013). Since pregnancy itself increases the cardiovascular burden (Ouzounian and Elkayam, 2012), the role of extreme ambient temperature exposure on cardiovascular burden during pregnancy merits attention. To date, no studies have investigated the relationship between ambient temperature and cardiovascular risk among pregnant women.
The aims of this paper were first to examine the acute associations between ambient temperature and the risk of having a labor/delivery affected by cardiovascular events in the US. Second, given the significant racial/ethnic disparity in the risk of pregnancy-related cardiovascular mortality/morbidity (CDC, 2016; Creanga et al., 2015) and evidence of differential temperature health effects by racial/ethnic group (Basu et al., 2012), we sought to investigate whether these associations differ by race/ethnicity. Third, we calculated the excess number of cases potentially attributable to temperature changes in the US.
Materials and Methods
Data and study population
The Air Quality and Reproductive Health (AQRH) study estimated environmental exposures in order to evaluate the association of ambient environmental risk factors with reproductive and perinatal outcomes in ongoing studies at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Chen et al., 2014). In 2013, the AQRH study linked meteorological data estimated from the Weather Research and Forecasting (WRF) model v3.2.1 to the participants in the Consortium on Safe Labor (CSL) Study. CSL was an observational cohort study with 228,438 deliveries (233,736 newborns) at ≥23 weeks of gestation from 12 clinical centers (19 hospitals and 15 hospital referral regions) across the US from 2002–2008 (Zhang et al., 2010) (eFigure 1). Data on maternal demographics; lifestyle; medical history; labor and delivery, obstetric, and neonatal outcomes were obtained from electronic delivery records and discharge summaries. Initially, 686 (0.3%) singleton pregnancies complicated by cardiovascular events at labor/delivery were eligible. Six women had cardiovascular events in more than one pregnancy during the study period, so only their first pregnancy was included, leaving 680 pregnancies in the final analyses. The study was approved by the institutional review boards of all participating institutions. Informed consent was not required since data were anonymized.
Study design
We used a case-crossover design to assess the risk of a cardiovascular event during a short “hazard period” after exposure to the hypothesized trigger—ambient temperature. The unique feature of the case-crossover design is that each woman serves as her own control. This self-matching feature allows complete control for non-time-varying potential confounders such as genetic factors, consistent characteristics or behavior (e.g., obesity or smoking), or any underlying cardiac susceptibility either known or unknown. This approach essentially holds all time invariant characteristics about a woman and her delivery constant except for the varying temperature or other environmental exposures of interest. As a result, any difference in risk detected after controlling for other varying environmental exposures (e.g., humidity and air pollution) can reasonably be attributed to ambient temperature.
Exposure assessment
Since the CSL data were anonymized, we used the 15 distinct hospital referral regions as a proxy for maternal residence and local mobility associated with daily activities (e.g., work, errands). Hourly temperature and humidity were obtained from the WRF model and averaged across each hospital referral region. WRF is a state-of-the-art weather prediction system, designed and utilized by many leading research, governmental and academic entities for atmospheric research and forecasting. Its modeling approach and performance have been reported elsewhere (Zhang et al., 2014a).
To assess acute exposure, we first calculated average daily temperature for the following hazard periods: the week prior to delivery, the day of delivery (lag0), and each of the seven days before delivery (lag1-lag7). The week prior to delivery and the days comprising the week prior to delivery were chosen as the hazard periods given evidence suggesting acute cardiovascular effects within seven days in the non-pregnant population (Dahlquist et al., 2016; Wang et al., 2016; Wichmann et al., 2013; Zheng et al., 2016). Temperature during this hazard period was then compared to two control periods: the second week after delivery and the week two full gestational weeks before delivery. A post-event control period may seem counterintuitive because the person is considered no longer at risk after the event. However, studies have shown that this bidirectional control selection method is preferred over the unidirectional method (i.e., selecting control periods only before the event), which is sensitive to time trend bias (Bateson and Schwartz, 1999; Navidi, 1998). This is especially true in studies where exposure has a high seasonal trend and is exogenous to the population under study. We also chose control periods to be relatively close to delivery, so that the seasonal variation in temperature was minimized.
We also obtained concentrations of particulate matter with diameter <2.5 microns (PM2.5) and ozone (O3) during the same exposure windows using modified Community Multiscale Air Quality models (CMAQ). CMAQ models estimated air pollution levels based on inputs from several sources: local emissions obtained from the National Emission Inventories, local weather obtained from the WRF, and photochemical properties of pollutants (Chen et al., 2014). Air pollutant concentrations were also corrected for measurement errors between modeled and observed levels at local air monitors using inverse distance weighting (Chen et al., 2014).
Outcome assessment
Our primary outcome of interest was labor/delivery affected by any cardiovascular event consisting of ischemic heart disease, stroke, heart failure, cardiac arrest/failure, and other or unspecified cardiovascular events. Throughout the study time period, these outcomes were identified from labor/delivery discharge summaries using International Classification of Disease, 9th version (ICD-9) codes (eTable 1).
Statistical analysis
For each woman, we compared a hazard period (lag0, lag1-lag7, or week’s average) to the control periods before and after the event. Recognizing that the stress of labor/delivery is a proximal factor increasing risk, this approach modeled the joint event (labor/delivery with cardiovascular event), and tested whether the women with the joint event had exposure to higher or lower temperature during the hazard period compared to the control periods while holding everything else about their delivery, time-invariant characteristics, and other environmental exposures constant. Since temperature has strong seasonal variation, we stratified our analyses by delivery season: cold (October-April) and warm (May-September). We expected the temperature difference between the case and control periods were small, and a quadratic term for temperature was not statistically significant, so we used temperature as a continuous variable to retain information on risk associated with small temperature changes. Conditional logistic regression models calculated the odds ratio (OR) and 95% confidence interval (CI) for 1°C lower in temperature in the cold season and 1°C higher in temperature in the warm season. We adjusted our analyses for relative humidity, PM2.5, and O3 during the same exposure window. To assess race-specific risks, we included an interaction term between race and temperature.Of note, the race-specific analyses only included three groups (Non-Hispanic White, Non-Hispanic Black, and Hispanic) due to sample size constraints in the other groups but the overall analyses included all participants.
Attributable risk
We calculated the attributable risk (AR) to estimate the race- and season-specific number of excess cardiovascular cases per 100 000 deliveries associated with 1°C lower in temperature in the cold season and 1°C higher in temperature in the warm season using the following formula:
where Ie stands for the race- and season-specific incidence among the exposed (i.e., risk associated with 1°C change) and Iu stands for race- and season-specific background incidence calculated from our study. Ie was calculated as Iu times the corresponding OR (which approximates relative risk since cardiovascular events are rare) for a 1°C change in temperature. All analyses were performed using SAS version 9.4 (Cary, NC).
Sensitivity analysis
In addition to the main analyses, we repeated our analyses restricting the cold season to November-February, and warm season to May-August to ensure robustness of our findings.
Results
The analysis included 680 (0.3% of the original cohort) women with singleton deliveries complicated by any cardiovascular event at labor/delivery. Cardiac arrests were the most common, followed by unspecified events and heart failure. This trend was generally consistent across race (Table 1). Table 2 describes the temperature distribution for the week preceding delivery by season; site-specific temperature distributions are also provided in eTable 2. eTable 3 presents the corresponding correlation coefficients between temperature and its covariates.
Table 1.
Characteristics of study participants (n=680).
| Cardiovascular (CV) Events, No. (%)a | White | Black | Hispanic | Asian/Pacific Islander | Missing/Unknown |
|---|---|---|---|---|---|
| 297 (43.7%) | 204 (30.0%) | 122 (17.9%) | 23 (3.4%) | 34 (5.0%) | |
| Acute Myocardial Infarction | 47 (15.8) | 3 (1.5) | 7 (5.7) | 2 (8.7) | 5 (14.7) |
| Stroke | 35 (11.8) | 18 (8.8) | 13 (10.7) | 2 (8.7) | 2 (5.9) |
| Heart Failure | 33 (11.1) | 32 (15.7) | 12 (9.8) | 4 (17.4) | 7 (20.6) |
| Cardiac Arrest | 127 (42.8) | 105 (51.5) | 67 (54.9) | 8 (34.8) | 12 (35.3) |
| Unspecified CV events | 57 (19.2) | 47 (23.0) | 24 (19.7) | 7 (30.4) | 8 (23.5) |
| Maternal Age, mean (SD), year | 29.6 (6.2) | 28.6 (7.4) | 28.6 (7.1) | 30.5 (5.5) | 27.0 (6.3) |
| Pre-Pregnancy BMI, mean (SD), kg/m2 | 26.3 (5.9) | 28.8 (7.3) | 26.8 (5.7) | 25.1 (4.8) | 25.1 (5.1) |
| Gestational Age, mean(SD), weeks | 37.2 (3.2) | 36.8 (4.0) | 36.8 (4.2) | 37.9 (4.1) | 35.8 (5.2) |
| Insurance, No. (%) | |||||
| Private | 207 (69.7) | 55 (27.0) | 31 (25.4) | 11 (47.8) | 10 (29.4) |
| Public | 74 (24.9) | 133 (65.2) | 75 (61.5) | 9 (39.1) | 17 (50.0) |
| Other | 3 (1.0) | 2 (1.0) | 1 (0.8) | 1 (4.4) | 2 (5.9) |
| Unknown | 13 (4.4) | 14 (6.9) | 15 (12.3) | 2 (8.7) | 5 (14.7) |
| Smoking during pregnancy, No. (%) | 25 (8.4) | 20 (9.8) | 4 (3.3) | 3 (13.0) | 0 (0.0) |
| Drinking during pregnancy, No. (%) | 11 (3.7) | 7 (3.4) | 2 (1.6) | 2 (8.7) | 0 (0.0) |
| Parity, No. (%) | |||||
| 0 | 126 (42.2) | 75 (36.8) | 52 (42.6) | 14 (60.9) | 18 (52.9) |
| 1 | 76 (25.6) | 61 (29.9) | 29 (23.8) | 4 (17.4) | 9 (26.5) |
| ≥2 | 95 (32.0) | 68 (33.3) | 41 (33.6) | 5 (21.7) | 7 (20.6) |
| Hypertensive disorders of pregnancy, No. (%) | |||||
| Yes | 40 (19.3) | 31 (20.4) | 14 (13.2) | 3 (16.7) | 2 (6.5) |
| No | 167 (80.7) | 121 (79.6) | 92 (86.8) | 15 (83.3) | 29 (93.6) |
| Gestational Diabetes Mellitus, No. (%) | |||||
| Yes | 18 (6.1) | 16 (7.8) | 11 (9.0) | 1 (4.4) | 0 (0.0) |
| No | 279 (93.4) | 188 (92.2) | 111 (91.0) | 22 (95.7) | 34 (100.0) |
| Season of delivery, No. (%) | |||||
| Cold (October-April) | 157 (52.9) | 118 (57.8) | 69 (56.6) | 13 (56.5) | 13 (38.2) |
| Warm (May-September) | 140 (47.1) | 86 (42.2) | 53 (43.4) | 10 (43.5) | 21 (61.8) |
Abbreviations: BMI, body mass index
Total is not equal to 680 because some women had more than one CV event.
Table 2.
Distribution of temperature during the week preceding delivery by season.
| Season of Delivery | Days Before Delivery | Temperature (°C)
|
|||
|---|---|---|---|---|---|
| Min | Max | Mean | SD | ||
| Cold (October to April) | 0 (delivery day) | −14.5 | 29.4 | 9.5 | 9.9 |
| 1 | −15.8 | 29.4 | 9.3 | 9.9 | |
| 2 | −17.3 | 28.6 | 9.3 | 10.2 | |
| 3 | −19.6 | 29.0 | 9.3 | 10.5 | |
| 4 | −16.5 | 29.4 | 9.5 | 10.3 | |
| 5 | −16.0 | 29.4 | 9.5 | 10.0 | |
| 6 | −16.8 | 28.6 | 9.2 | 10.1 | |
| 7 | −16.5 | 29.0 | 9.1 | 10.4 | |
| Avg 0–7 | −11.5 | 28.2 | 9.3 | 9.6 | |
|
| |||||
| Warm (May to September) | 0 (delivery day) | 6.9 | 31.3 | 22.0 | 5.5 |
| 1 | 6.4 | 31.3 | 22.1 | 5.5 | |
| 2 | 6.4 | 31.7 | 22.1 | 5.6 | |
| 3 | 5.8 | 32.2 | 21.9 | 5.7 | |
| 4 | 4.4 | 31.4 | 21.9 | 5.7 | |
| 5 | 5.4 | 30.4 | 21.7 | 5.8 | |
| 6 | 5.4 | 30.5 | 21.5 | 5.7 | |
| 7 | 5.2 | 30.4 | 21.5 | 5.9 | |
| Avg 0-7 | 7.6 | 30.3 | 21.8 | 5.3 | |
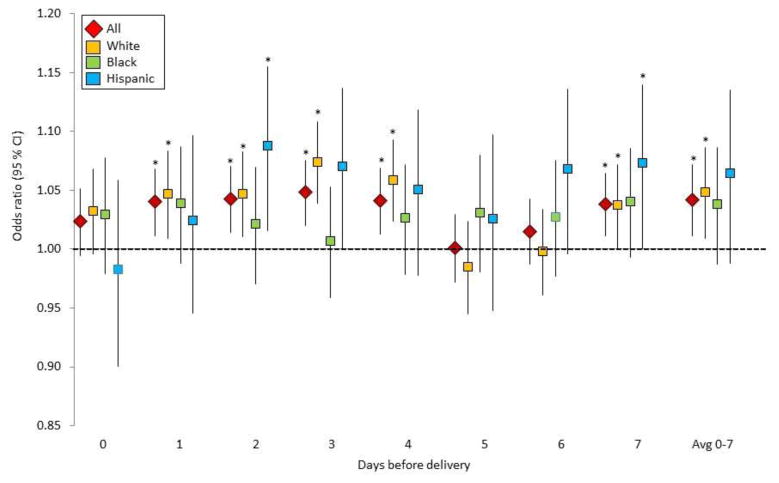
During the cold season, 1°C lower in average temperature during the week prior to delivery was associated with a 4% (OR: 1.04, 95% CI: 1.01–1.07%) increased odds of having any cardiovascular event at labor/delivery (Figure 1, eTable 3). These estimates were generally consistent across race, although we lost some precision due to low sample size among Blacks and Hispanics. When analyzed separately for each of the seven days before delivery, risks were generally more consistent on days 1 to 4 and 7 for all women. In addition, compared to their Non-Hispanic White counterparts, the same change in temperature of 1°C lower, Non-Hispanic Black women had lower risk on day 3 [OR: 1.01(0.96–1.05) vs. OR: 1.07 (1.04–1.11), p for contrast = 0.02], and Hispanic women had higher risk on day 6 [OR: 1.07 (1.04–1.11) vs. OR: 1.00 (0.96–1.03), p for contrast=0.08] (eTable 4).
Figure 1.
Risk of cardiovascular events in the cold season associated with 1°C lower in average temperature during the week prior to delivery. Asterisk indicates statistical significance at p<0.05
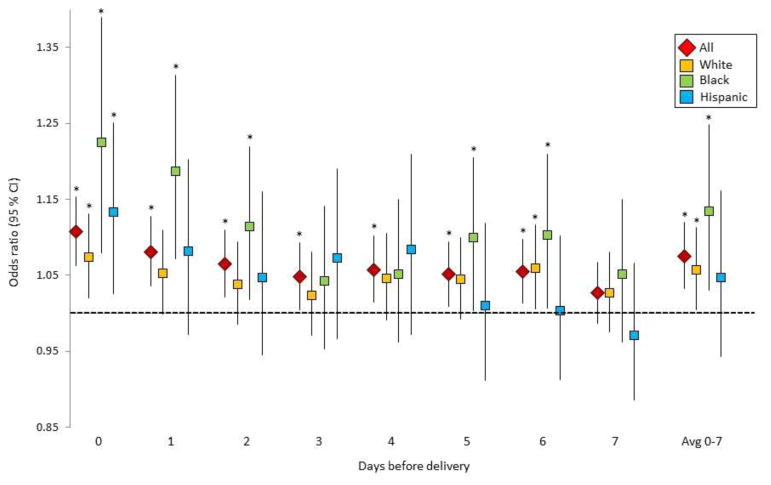
During the warm season, 1°C higher in average temperature during the week prior to delivery was associated with a 7% (OR: 1.07, 95% CI: 1.03–1.12%) increased odds of cardiovascular events (Figure 2, eTable 4). These risks also varied by race with evidence of stronger associations among Black women and on days more proximal to delivery date. For example, on the day of delivery, 1°C higher was associated with an 11% increased risk among all women combined (OR: 1.11, 95% CI: 1.06–1.15%). These estimates were stronger among Non-Hispanic Black women compared to Non-Hispanic White women (OR: 1.22, 95% CI: 1.08–1.39) vs. OR: 1.07, 95% CI: 1.02–1.13, p for contrast = 0.06].
Figure 2.
Risk of cardiovascular events in the warm season associated with 1°C higher in average temperature during the week prior to delivery. Asterisk indicates statistical significance at p<0.05
Sensitivity analyses restricting to the warmest/coldest months yielded consistent findings but we observed stronger effects for days closer to delivery for both seasons (eTable 5). Additional adjustment for other pollutants such as nitric oxides, sulfur dioxides, and carbon monoxide did not change the results (not shown).
Attributable risk
Attributable risk calculations were based on our findings for study-specific background risk given the lack of national data on the incidence of labor/delivery affected by cardiovascular events. During the cold season, with a season-specific overall background rate of approximately 320 per 100 000 singleton pregnancies, the 4% increase in risk associated with 1°C lower in temperature during the week prior to delivery translated to approximately 13.4 (95% CI: 3.6–22.9) excess cases of cardiovascular events per 100 000 singleton pregnancies (Table 3). This is equivalent to about 289 excess cases each year in the US for each 1°C lower in temperature (assuming 53.9% of the 4 annual million deliveries occurred in the cold season). In the warm season, 1°C higher in temperature during the week prior to delivery was associated with an excess (per 100 000) of 23.9 (95% CI: 10.2–38.2) cases among all races, 19.6 among Whites (95% CI: 2.8–36.4), and 52.0 among Blacks (95% CI: 8–104). The number of excess cases was highest for increased temperature during the day of delivery with 34.2 excess cases overall (95% CI: 19.9–49.2), 20.7 for Whites (95% CI: 5.5–36.7), 45.2 for Hispanics (95% CI: 8.8–85.3), and 89.9 for Blacks (95% CI: 31.8–155.8). These estimates translate to an excess of about 442 excess cases per year in the US for each 1°C higher in temperature.
Table 3.
Excess cardiovascular events at labor/delivery associated with a 1°C lower temperature in the cold season and 1°C higher temperature in the warm season (per 100,000 singleton deliveries).
| Season | Days before delivery | Overall | White | Black | Hispanics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iua | N excess | (95% CI) | Iua | N excess | (95% CI) | Iua | N excess | (95% CI) | Iua | N excess | (95% CI) | ||
| Cold | 0 (delivery day) | 320 | 7.5 | (−1.7–16.5) | 280 | 9.1 | (−1.2–19.1) | 450 | 13.3 | (−9.5–34.9) | 360 | −6.3 | (−35.8–21.0) |
| 1 | 12.9 | (3.6–21.9) | 13.1 | (2.5–23.3) | 17.5 | (−5.4–39.2) | 8.6 | (−19.6–34.8) | |||||
| 2 | 13.7 | (4.6–22.6) | 13.2 | (2.9–23.1) | 9.6 | (−13.2–31.3) | 31.6 | (5.6–55.7) | |||||
| 3 | 15.5 | (6.5–24.2) | 20.8 | (10.9–30.4) | 3.1 | (−18.7–23.9) | 25.4 | (−0.2–49.2) | |||||
| 4 | 13.2 | (4.1–22.0) | 16.5 | (6.6–26.1) | 11.8 | (−9.6–32.2) | 18.1 | (−8.1–42.5) | |||||
| 5 | 0.3 | (−9.0–9.4) | −4.2 | (−15.4–6.6) | 14.1 | (−8.8–35.8) | 9.2 | (−18.7–35.1) | |||||
| 6 | 4.9 | (−4.1–13.6) | −0.6 | (−11.0–9.5) | 12.3 | (−10.4–33.9) | 24.6 | (−1.5–48.8) | |||||
| 7 | 12.3 | (3.7–20.6) | 10.4 | (0.4–20.1) | 18.3 | (−3.0–38.5) | 26.2 | (0.2–50.4) | |||||
| Avg 0–7 | 13.4 | (3.6–22.9) | 13.6 | (2.6–24.2) | 17.1 | (−5.9–38.9) | 23.3 | (−4.3–48.7) | |||||
| Warm | 0 (delivery day) | 320 | 34.2 | (19.9–49.2) | 280 | 20.7 | (5.5–36.7) | 400 | 89.9 | (31.8–155.8) | 340 | 45.2 | (8.8–85.3) |
| 1 | 25.8 | (11.5–40.8) | 14.8 | (−0.3–30.6) | 74.7 | (28.7–125.5) | 27.6 | (−9.6–69.0) | |||||
| 2 | 20.7 | (6.8–35.2) | 10.7 | (−4.2–26.3) | 45.7 | (7.1–87.9) | 16.1 | (−18.5–54.4) | |||||
| 3 | 15.3 | (1.6–29.6) | 6.7 | (−8.3–22.5) | 17.1 | (−18.7–56.2) | 24.7 | (−11.3–64.8) | |||||
| 4 | 18.3 | (4.6–32.6) | 12.9 | (−2.6–29.3) | 20.6 | (−15.2–59.8) | 28.6 | (−9.7–71.2) | |||||
| 5 | 16.3 | (3.0–30.2) | 12.5 | (−2.2–28.0) | 39.8 | (1.4–81.9) | 3.2 | (−30.3–40.4) | |||||
| 6 | 17.4 | (4.2–31.1) | 16.7 | (1.5–32.6) | 41.3 | (2.5–83.9) | 1.0 | (−29.6–34.6) | |||||
| 7 | 8.4 | (−4.3–21.6) | 7.4 | (−7.0–22.6) | 20.7 | (−15.3–60.0) | −9.8 | (−39.0–22.3) | |||||
| Avg 0–7 | 23.9 | (10.2–38.2) | 16.0 | (1.2–31.6) | 53.7 | (12.1–99.6) | 15.8 | (−19.5–55) | |||||
Abbreviations: CI, confidence interval; I, incidence
Iu: season-specific background risk, or risk among the unexposed, expressed as per 100 000 singleton pregnancies. This number is calculated using singleton pregnancies from the whole Consortium on Safe Labor cohort (n=223 385).
Discussion
In this nationwide US study, we found consistent evidence suggesting that even small acute changes in temperature during the week prior to delivery were associated with the risk of having a labor/delivery affected by cardiovascular event(s). We also observed that these risks varied by maternal race/ethnicity where Black women appeared more susceptible to adverse effects of higher temperature during the warm season.
The literature generally suggests that exposures to both high and low temperatures are associated with cardiovascular events in non-pregnant populations around the world (Basu, 2009; Bhaskaran et al., 2012; Ha et al., 2014; Honda et al., 2016; Phung et al., 2016; Sartini et al., 2016; Wang et al., 2016; Yang et al., 2015) with potentially higher sensitivity among Blacks women, those with lower socioeconomic status, the elderly, and young children (Basu, 2009); but data among pregnant women are currently lacking. One large international multicenter case-control study among reproductive age women ages 15 to 49 showed that each 5°C decrease in monthly average temperature was associated with a 7% and 12% increase in hospital admission rates for stroke and acute myocardial infarction, respectively; and these effects were more prominent within one month of exposure (Chang et al., 2004). Meanwhile, elevated temperature has also been associated with increased risk for mortality related to cardiovascular diseases, with potentially higher susceptibility among Black racial/ethnic groups and women (Basu, 2009). Similar findings were also observed in a case-crossover analysis consisting of 231,676 non-accidental deaths (both men and women), where daily temperature increase of 10°F (4.7°C) was associated with a 2.6% increased risk for all cardiovascular deaths; and ethnic minorities, especially Blacks, had an elevated risk (Basu and Ostro, 2008).
The reason for this marked racial/ethnic disparity is unclear. As noted by other authors (Basu and Ostro, 2008; Basu et al., 2016), we suspect that Black women may have a higher burden of comorbidity that increases their susceptibility. In fact, Black women in our study had higher pre-pregnancy BMI and had higher prevalence of hypertensive disorders of pregnancy than other groups. It is also possible that socioeconomic factors may also play a role. For example, Black households are typically less likely to have air conditioners compared to other groups (O'Neill et al., 2005). Since each woman is her own control, factors that do not change over the short time window of several weeks, such as chronic comorbidity, demographics, genetic susceptibility and other social and behavioral factors, do not explain the observed risk.
This study is the first to report on excess risk of cardiovascular events at labor/delivery associated with temperature changes. These findings may have important public health implications and build on the prior literature in two ways, first by extending the population at risk to include pregnant women and second, by examining risks associated with smaller temperature changes in a temperate zone. Although the magnitude of association (for 1°C change in temperature) is small, the risks are serious and the prevalence of exposure is high, particularly given the expected increase in the intensity and frequency of extreme temperature events (IPCC, 2013). During the last few decades, ambient temperature in the US has increased by more than 2 °F (>1°C) and is expected to further increase (NOAA, 2011). We are also concerned that the recent increase in the prevalence of maternal risk factors (e.g., obesity, maternal age, gestational complications) will likely exacerbate population-level cardiovascular risk during pregnancy (Ferrara, 2007; Lu et al., 2001; Wallis et al., 2008). We also note that temperature in the US is relatively moderate and our findings may be even more concerning for other parts of the world where the frequency of temperature extremes is more common and cardiovascular risk during pregnancy may be higher.
Although the biologic mechanisms underlying the relationship between temperature and cardiovascular risk are not well-understood, our findings are biologically plausible. The endothelial, rheological, oxidative, and inflammatory effects of extreme temperature may underlie the pathologic pathways of cardiovascular outcomes (Cai et al., 2016; Halonen et al., 2010; Hong et al., 2012; Martarelli et al., 2011). For example, extreme temperatures can affect blood pressure and blood viscosity, which can ultimately affect cardiovascular risk (Davies and Maconochie, 2009; Zhang et al., 2014b). Some studies have found that these effects occur shortly after exposure (Cai et al., 2016; Halonen et al., 2010; Hong et al., 2012). The stronger associations we observed during days more proximal to delivery and in our sensitivity analyses where we restricted the season to more extreme months further support this biologic plausibility. However, more research is still needed to fully understand the biologic link, especially for the mechanisms underlying the racial disparity observed in our study.
While novel, this study has some limitations. We do not have residential address due to the anonymized clinical data and used the hospital referral region as a proxy for residence and local mobility. While accounting for emission, meteorology and photochemical properties of pollutants, CMAQ estimates cannot account for daily activities patterns data (e.g., times spent indoors/outdoors or AC/heater use), which may be another source of potential misclassification, and can be a contributor to the heterogeneity by race/ethnicity. However, if present, we expect this misclassification to be non-differential and to bias our overall results towards the null. Furthermore, we did not have information on the time of event, which may have affected the definition of our daily lags, particularly for day 0. However, all events occur within the delivery hospital admission and many were associated with delivery complications so the time windows for event occurrence are likely to be relatively close to delivery. The misclassification, if exists, is unlikely to be differential and should bias our findings towards the null. Our analyses based on the weekly average temperatures provide similar estimates which is reassuring with respect to this issue. Another potential limitation is our assumption of a linear temperature effect in the case-crossover model. We assessed the linearity assumption by including a quadratic term, and found no serious departures from linearity, likely because extreme temperatures were sparse in our data. If more extreme temperature exposures are encountered, it is worthwhile to explore potential nonlinear temperature effects. Lastly, we recognize that the different types of cardiovascular events in this study may not be related to a primary cardiovascular cause; however, given low sample size, analyses further stratified by types of cardiovascular event were not informative.
We report acute associations between ambient temperature and cardiovascular events at labor and delivery among a large sample of women across the US, which contributes to the generalizability of our findings. The use of a case-crossover study design allowed us complete control for measured and unmeasured time-invariant confounders since women serve as their own controls. In addition, since cardiovascular events were considered as a joint occurrence with labor/delivery, labor/delivery characteristics were also controlled for by design. Lastly, we were able to adjust for key pollutants and humidity to ensure our findings were not confounded by other ambient environmental factors.
Conclusion
Even small acute changes in temperature may affect the risk of cardiovascular events at labor/delivery. Both colder temperature in the cold season and higher temperature in the warm season were associated with increased risk. These risks varied by race/ethnicity with Black women appearing more susceptible to higher temperature during the warm season. A significant number of cardiovascular events at labor and delivery each year in the US may be attributable to temperature. Given concerns related to global warming and changes in population risk factors, these findings merit attention.
Supplementary Material
Highlights.
Lower temperature increased the risk of cardiovascular event in the cold season
Higher temperature was associated with risk during the warm season
In the warm season, risks were more pronounced on days closer to delivery
Black women were more susceptible to higher temperature in the warm season
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NICHD); including Contract No. HHSN267200603425C (Consortium on Safe Labor), Contract No. HHSN275200800002I, and Task Order No. HHSN27500008 (Air Quality and Reproductive Health).
Role of funding source: Although NICHD cleared this manuscript for publication, it played no other role in the conduct or writing of this study.
We thank all the participants and participating clinical centers involved in the Consortium on Safe Labor: Baystate Medical Center, Springfield, MA; Cedars-Sinai Medical Center Burnes Alllen Research Center, Los Angeles, CA; Christiana Care Health System, Newark, DE; Georgetown University Hospital, MedStar Health, Washington, DC; Indiana University Clarian Health, Indianapolis, IN; Intermountain Healthcare and the University of Utah, Salt Lake City, UT; Maimonides Medical Center, Brooklyn, NY; MetroHealth Medical Center, Cleveland, OH; University of Illinois at Chicago, Chicago, IL; University of Miami, Miami, FL; Summa Healthcare, Akron, OH; and University of Texas Health Science Center at Houston, Houston, TX. We also thank the Emmes Corporation, Rockville, MD, which provided data coordination; and the Texas A&M Supercomputing Facility and the Texas Advanced Computing Center, which provided computing resources essential to completing exposure estimations in this study.
The authors also wish to thank Dr. Enrique Schisterman for his expert advice on the case- crossover design.
Footnotes
Conflict of interest: The authors report no conflict of interest.
Ethic statement: This project was approved by institutional review boards from all participating centers. Informed consent was not required because data were anonymous.
Potential Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;8:40. doi: 10.1186/1476-069X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Ostro BD. A multicounty analysis identifying the populations vulnerable to mortality associated with high ambient temperature in California. Am J Epidemiol. 2008;168:632–7. doi: 10.1093/aje/kwn170. [DOI] [PubMed] [Google Scholar]
- Basu R, Pearson D, Malig B, Broadwin R, Green R. The effect of high ambient temperature on emergency room visits. Epidemiology. 2012;23:813–20. doi: 10.1097/EDE.0b013e31826b7f97. [DOI] [PubMed] [Google Scholar]
- Basu R, Sarovar V, Malig BJ. Association Between High Ambient Temperature and Risk of Stillbirth in California. Am J Epidemiol. 2016;183:894–901. doi: 10.1093/aje/kwv295. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology. 1999;10:539–44. [PubMed] [Google Scholar]
- Bhaskaran K, et al. Heat and risk of myocardial infarction: hourly level case-crossover analysis of MINAP database. BMJ. 2012;345:e8050. doi: 10.1136/bmj.e8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, et al. The cold effects on circulatory inflammation, thrombosis and vasoconstriction in type 2 diabetic patients. Sci Total Environ. 2016;568:271–277. doi: 10.1016/j.scitotenv.2016.06.030. [DOI] [PubMed] [Google Scholar]
- CDC. Pregnancy Mortality Surveillance System Centers for Disease Control and Prevention; Atlanta, GA. July 27, 2016; 2016. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pmss.html. [Google Scholar]
- Chang CL, Shipley M, Marmot M, Poulter N. Lower ambient temperature was associated with an increased risk of hospitalization for stroke and acute myocardial infarction in young women. J Clin Epidemiol. 2004;57:749–57. doi: 10.1016/j.jclinepi.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Chen G, et al. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environ. 2014;485–486:563–574. doi: 10.1016/j.scitotenv.2014.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, et al. High temperature induces apoptosis and oxidative stress in pufferfish (Takifugu obscurus) blood cells. J Therm Biol. 2015;53:172–9. doi: 10.1016/j.jtherbio.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Creanga AA, et al. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125:5–12. doi: 10.1097/AOG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- Dahlquist M, et al. Short-term departures from an optimum ambient temperature are associated with increased risk of out-of-hospital cardiac arrest. Int J Hyg Environ Health. 2016;219:389–97. doi: 10.1016/j.ijheh.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Davies P, Maconochie I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg Med J. 2009;26:641–3. doi: 10.1136/emj.2008.061598. [DOI] [PubMed] [Google Scholar]
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30(Suppl 2):S141–6. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- Ha S, Talbott EO, Kan H, Prins CA, Xu X. The effects of heat stress and its effect modifiers on stroke hospitalizations in Allegheny County, Pennsylvania. Int Arch Occup Environ Health. 2014;87:557–65. doi: 10.1007/s00420-013-0897-2. [DOI] [PubMed] [Google Scholar]
- Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Associations between outdoor temperature and markers of inflammation: a cohort study. Environ Health. 2010;9:42. doi: 10.1186/1476-069X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Fujimoto K, Miyao Y. Influence of weather conditions on the frequent onset of acute myocardial infarction. J Cardiol. 2016;67:42–50. doi: 10.1016/j.jjcc.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Hong YC, et al. Association of cold ambient temperature and cardiovascular markers. Sci Total Environ. 2012;435–436:74–9. doi: 10.1016/j.scitotenv.2012.02.070. [DOI] [PubMed] [Google Scholar]
- IPCC. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC; Cambridge, United Kingdom and New York, NY, USA: 2013. Climate Change 2013: The Physical Science Basis. [Google Scholar]
- Lu GC, et al. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. Am J Obstet Gynecol. 2001;185:845–9. doi: 10.1067/mob.2001.117351. [DOI] [PubMed] [Google Scholar]
- Männistö T, et al. Acute and recent air pollution exposure and cardiovascular events at labour and delivery. Heart. 2015;101:1491–8. doi: 10.1136/heartjnl-2014-307366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martarelli D, Cocchioni M, Scuri S, Spataro A, Pompei P. Cold exposure increases exercise-induced oxidative stress. J Sports Med Phys Fitness. 2011;51:299–304. [PubMed] [Google Scholar]
- Medina-Ramon M, Schwartz J. Temperature, temperature extremes, and mortality: a study of acclimatisation and effect modification in 50 US cities. Occup Environ Med. 2007;64:827–33. doi: 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navidi W. Bidirectional case-crossover designs for exposures with time trends. Biometrics. 1998;54:596–605. [PubMed] [Google Scholar]
- NOAA. State of the Climate, Global Analysis: Annual 2010 National Oceanic and Atmospheric Administration. 2011 http://www.ncdc.noaa.gov/sotc/global/2010/13.
- O'Neill MS, Zanobetti A, Schwartz J. Disparities by race in heat-related mortality in four US cities: the role of air conditioning prevalence. J Urban Health. 2005;82:191–7. doi: 10.1093/jurban/jti043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30:317–29. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Phung D, et al. Ambient temperature and risk of cardiovascular hospitalization: An updated systematic review and meta-analysis. Sci Total Environ. 2016;550:1084–102. doi: 10.1016/j.scitotenv.2016.01.154. [DOI] [PubMed] [Google Scholar]
- Quindry J, et al. Environmental temperature and exercise-induced blood oxidative stress. Int J Sport Nutr Exerc Metab. 2013;23:128–36. doi: 10.1123/ijsnem.23.2.128. [DOI] [PubMed] [Google Scholar]
- Sartini C, et al. Effect of cold spells and their modifiers on cardiovascular disease events: Evidence from two prospective studies. Int J Cardiol. 2016;218:275–83. doi: 10.1016/j.ijcard.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwa K, Bohm M. Incidence and prevalence of pregnancy-related heart disease. Cardiovasc Res. 2014;101:554–60. doi: 10.1093/cvr/cvu012. [DOI] [PubMed] [Google Scholar]
- Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- Wang X, et al. Ambient Temperature and Stroke Occurrence: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2016:13. doi: 10.3390/ijerph13070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann J, Rosengren A, Sjoberg K, Barregard L, Sallsten G. Association between ambient temperature and acute myocardial infarction hospitalisations in Gothenburg, Sweden: 1985–2010. PLoS One. 2013;8:e62059. doi: 10.1371/journal.pone.0062059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, et al. Outdoor temperature, blood pressure, and cardiovascular disease mortality among 23 000 individuals with diagnosed cardiovascular diseases from China. Eur Heart J. 2015;36:1178–85. doi: 10.1093/eurheartj/ehv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. Evaluation of a seven-year air quality simulation using the Weather Research and Forecasting (WRF)/Community Multiscale Air Quality (CMAQ) models in the eastern United States. Sci Total Environ. 2014a;473–474:275–85. doi: 10.1016/j.scitotenv.2013.11.121. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326e1–326e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang S, Wang C, Wang B, Guo P. Effects of moderate strength cold air exposure on blood pressure and biochemical indicators among cardiovascular and cerebrovascular patients. Int J Environ Res Public Health. 2014b;11:2472–87. doi: 10.3390/ijerph110302472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, et al. Low Ambient Temperature and Intracerebral Hemorrhage: The INTERACT2 Study. PLoS One. 2016;11:e0149040. doi: 10.1371/journal.pone.0149040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.