Abstract
Background
Rosacea is a chronic inflammatory skin disorder. Inflammation and oxidative stress are involved in the etiopathogenesis of rosacea and chronic kidney disease (CKD). This study aimed to investigate the association between rosacea and CKD.
Methods
This population-based cohort study identified 277 patients with rosacea in the Taiwan National Health Insurance Research Database during 2001–2005. These patients were matched for age, sex, and comorbidities with 2216 patients without rosacea. All subjects were individually followed-up for 8–12 years to identify those who subsequently developed CKD
Results
The incidence rates of CKD per 1000 person-years were 16.02 in patients with rosacea and 10.63 in the non-rosacea reference population. After adjusting for other covariates and considering the competing risk of mortality, patients with rosacea remained at increased risk of CKD (adjusted sub-distribution hazard ratio (aSD-HR) 2.00; 95% confidence interval (CI) 1.05–3.82). The aSD-HRs (95% CI) for CKD were 1.82 (0.83–4.00) and 2.53 (1.11–5.75) for patients with mild and moderate-to-severe rosacea, respectively.
Conclusions
Rosacea is an independent risk factor for CKD. High rosacea severity and old age further increased CKD risk in patients with rosacea. Careful monitoring for CKD development should be included as part of integrated care for patients with rosacea.
Introduction
Rosacea is a chronic inflammatory cutaneous disorder characterized by centrofacial erythema, telangiectasias, papules, and pustules. Aberrations in immune response and dysregulation of the neurovascular system are presumed to be key pathophysiologic components of the disease.[1, 2] Recent studies suggest that rosacea is a systemic disorder and not merely a skin condition. Prior studies reported that it is associated with dyslipidemia, hypertension, metabolic diseases, alcohol consumption, tobacco smoking, cardiovascular diseases, and gastroesophageal reflux disease,[3–5] all of which are also prevalent in patients with chronic kidney disease (CKD).[6–8]
Accumulating evidence suggests that rosacea pathogenesis is linked to overexpression of pro-inflammatory cytokines and higher reactive oxygen species production.[9–11] Similarly, previous studies reported that chronic low-grade inflammation and oxidative stress are important in CKD development.[12, 13] Because rosacea and CKD share some pathogenic mechanisms and associated conditions, it is tempting to posit an association between these diseases. Patients with inflammatory conditions such as psoriasis and rheumatoid arthritis have a high risk of CKD.[14–16] Like that of psoriasis, the underlying mechanism of rosacea is thought to be associated with inflammatory cascades.[17, 18] However, the relationship between rosacea and CKD has not been previously investigated. We therefore assessed the risk of CKD in a large, nationally representative, population-based cohort of Chinese patients with rosacea in Taiwan.
Materials and methods
Study design and data source
The data used in this cohort study were obtained from the Longitudinal National Health Insurance Research Database (LHID) 2000, which is a subset of the National Health Insurance Research Database (NHIRD). The NHIRD is derived from the Taiwanese National Health Insurance (NHI) program, which was launched in 1995 to finance health care for all citizens. For the LHID2000, about 1,000,000 representative individuals were randomly sampled from the NHI Registry of Beneficiaries in 2000. The database includes information on inpatient care, outpatient care, ambulatory care, and prescription drugs for the period from January 1, 1996 through December 31, 2013. And patient diagnoses were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The Taiwanese NHI program provides care for approximately 99% of the Taiwanese population of more than 23 million people and offers unique possibilities for research. To ensure the accuracy and reliability of coding, the Bureau of the NHI of Taiwan performs random cross-checking, requests justifications by invited physicians, imposes heavy fines for false claims and overcharging, and initiates malpractice proceedings for fraudulent claims. Thus, the NHIRD is generally regarded as accurate and reliable. Confidentiality assurances were addressed by abiding by the data regulations of the NHI Bureau, and a formal written waiver for ethical approval was obtained from the local investigational research bureau of the National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan (103-024-E). All patient records and information were anonymized and de-identified before the analysis.
Study population
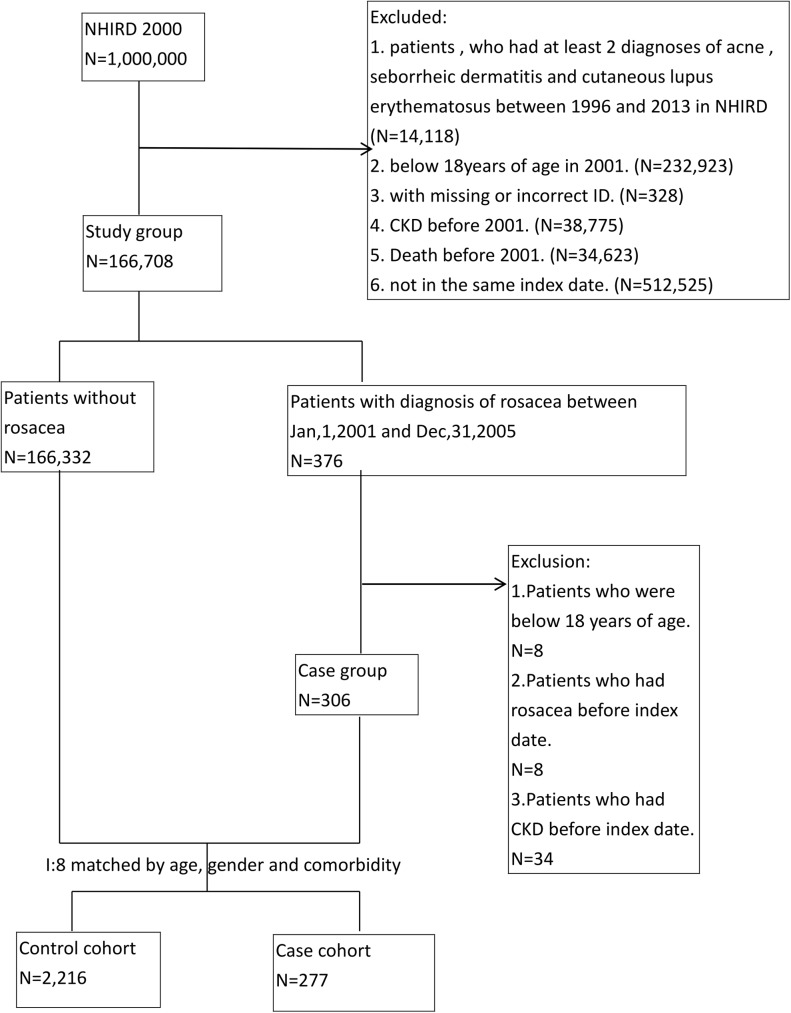
This retrospective cohort study analyzed data from individuals who received a new diagnosis of rosacea (ICD-9-CM code 695.3) during ambulatory visits or inpatient care episodes between January 1, 2001 and December 31, 2005. To ensure diagnostic validity, we required that patients have at least 2 dermatologist diagnoses. Because acne (ICD-9-CM code 706.1), seborrheic dermatitis (ICD-9-CM code 690.1), and cutaneous lupus erythematosus (ICD-9-CM code 695.4) are frequently confused with rosacea, patients with 2 diagnoses of any of these diseases were excluded from the study group. The initial diagnosis date was defined as the index date of entry into the rosacea cohort.
Propensity score matching adjusted for sex, age, and comorbidities was used to assemble a comparison group among subjects without rosacea and CKD in the LHID2000. Each individual with rosacea was paired with 8 individuals without rosacea on the index enrollment date. The matched comorbidities included hypertension (ICD-9-CM codes 401–402), diabetes mellitus (ICD-9-CM 250.xx), dyslipidemia (ICD 9-CM code 272.x), and cardiovascular disease (ICD-9-CM 410–429). Patients in the study cohort and control cohort were excluded if they were younger than 18 years or had CKD or rosacea before the index date (Fig 1). Patients with rosacea were stratified by disease severity as having moderate-to-severe or mild rosacea. Patients who received oral drugs (including doxycycline, minocycline, tetracycline, metronidazole, and isotretinoin) for rosacea at least 3 times during the first year of follow-up were considered to have moderate-to-severe rosacea. The remaining patients were considered to have mild rosacea.
Fig 1. Selection of study population.
CKD, chronic kidney disease; LHID, Longitudinal National Health Insurance Research Database; NHIRD, National Health Insurance Research Database.
Outcome
The primary outcome was defined as the first ambulatory visit, hospitalization, or surgical procedure for CKD (ICD-9-CM codes: 580–589, 753, 403, 404, 250.4, 274.1, 440.1, 442.1, 447.3, 572.4, 642.1, 646.2). To investigate the risk of developing CKD during the follow-up period, all individuals in the study and comparison cohorts were tracked for 8–12 years from their index enrollment date until death or the end of the study period.
Statistical analysis
Frequencies and percentages are used to present descriptive statistics of the study sample. The chi-square test was used to compare distributions of baseline demographic characteristics and selected comorbidities between patients with and without rosacea. Cox proportional hazards regression was used to estimate the effect of rosacea on CKD. Covariates adjusted in multivariable Cox regression analysis included gender, age, diabetes, hypertension, hyperlipidemia, cardiovascular disease, systemic lupus erythematosus, rheumatoid arthritis, polycystic kidney disease, medications putatively associated with CKD risk (non-steroidal anti-inflammatory drugs, angiotensin-converting-enzyme inhibitors/angiotensin II receptor antagonists, loop diuretics, and statin), Charlson comorbidity index score, and annual ambulatory care visits, as detailed in previous studies.[19] The Kaplan–Meier method and log-rank test were used to compare cumulative incidences of CKD between patients with and without rosacea. CKD incidences in the rosacea and control cohorts were calculated by dividing the number of patients with CKD by the total number of person-years. Death before CKD incidence was considered as a competing risk event. In addition, Cox proportional hazard models with competing risk analysis were used to estimate CKD risk among rosacea patients, as described previously.[20] Analyses were performed with SAS v9.3 (SAS Institute, Cary, NC). A P value of <0.05 was considered to indicate statistical significance.
Results
The study comprised 277 patients with rosacea and 2216 age-, sex-, and comorbidity-matched reference subjects. Table 1 shows the sociodemographic characteristics of the patients. After propensity score matching, no significant differences in sex, age, or comorbidities were noted between patients with and without rosacea. Approximately 71% of patients were women and 29% were men in both the study and control groups. Most study participants were aged 30–49 years.
Table 1. Background characteristics and comorbidities of patients with and without rosacea.
| Characteristic | No.(%) of individuals | p-value | |
|---|---|---|---|
| Patients with rosacea N = 277 | Patients without rosacea N = 2216 | ||
| Sex | |||
| Female | 196(70.76) | 1570(70.85) | 0.9751 |
| Male | 81(29.24) | 646(29.15) | |
| Age Group | |||
| 18–29 | 46(16.61) | 370(16.70) | 1.0000 |
| 30–39 | 51(18.41) | 410(18.50) | |
| 40–49 | 107(38.63) | 857(38.67) | |
| 50–59 | 43(15.52) | 342(15.43) | |
| 60+ | 30(10.83) | 237(10.69) | |
| Comorbidity | |||
| Diabetes | 1(0.6) | 8(0.36) | 1.0000 |
| Hypertension | 13(4.69) | 105(4.74) | 0.9734 |
| Hyperlipidemia | 7(2.53) | 56(2.53) | 1.0000 |
| Cardiovascular diseases | 8(2.89) | 52(2.35) | 0.5793 |
| Systemic lupus erythematosus | 2(0.72) | 2(0.09) | 0.0634 |
| Rheumatoid arthritis | 2(0.72) | 7(0.32) | 0.2204 |
| Polycystic kidney disease | 0(0.0) | 0(0.0) | _ |
| Charlson comorbidity index | |||
| 0 | 243(87.73) | 2018(91.06) | 0.0512 |
| 1 | 28(10.11) | 139(6.27) | |
| ≧2 | 6(2.17) | 59(2.66) | |
| Drug use | 107(38.63) | 906(40.88) | 0.4710 |
| Loop diuretics | 1(0.36) | 26(1.17) | 0.3537 |
| Statin | 2(0.72) | 23(1.04) | 1.0000 |
| NSAID | 103(37.18) | 877(39.58) | 0.4423 |
| ACEI | 0(0.00) | 10(0.45) | 0.6144 |
| ARB | 6(2.17) | 28(1.26) | 0.2630 |
| Annual ambulatory care visits† | 19(10–29) | 12(6–21) | <0.0001 |
| <15 | 117(42.24) | 1366(61.64) | <0.0001* |
| ≧15 | 160(57.76) | 850(38.36) | |
* p<0.05 for comparison between patients with versus without rosacea.
†The overall OPD times: Median (Q1-Q3) = 15(6–23) months.
The median interval to CKD was 9.92 years, and there was no significant different between rosacea patients (median 9.80 years; interquartile range (IQR): 8.68–11.34) and controls (median 9.95; IQR: 8.91–11.42). The overall CKD incidence rate was higher in patients with rosacea than in the controls (16.02 vs. 10.63 per 1000 person-years, respectively). The Kaplan–Meier curves for the cumulative CKD incidence rate in patients with and without rosacea are shown in Fig 2. CKD development was 50% more likely in patients with rosacea (hazard ratio (HR) 1.51; 95% confidence interval (CI) 1.08–2.09) than in those without rosacea, and this association remained significant after adjusting for other covariates (adjusted HR 1.40; 95% CI 1.01–1.96). The Cox proportional hazard models with competing risk analysis yielded similar results (adjusted subdistribution (SD)-HR 2.00; 95% CI 1.05–3.82). When patients were stratified by age group, the increase in CKD risk was highest for patients aged 30–39 years. However, in the competing risk model the association between rosacea and CKD was not significant for patients aged 30–39 years. Patients older than 50 years had a significant risk of CKD (adjusted SD-HR for age 50–59 years, 3.68; 95% CI 1.00–13.56; adjusted SD-HR for 60+ years, 4.24; 95% CI 1.63–11.04) (Table 2).
Fig 2. Kaplan–Meier curves.
Cumulative incidence rate of CKD in patients with and without rosacea.
Table 2. Incidences and hazard ratios for chronic kidney disease (CKD) in patients with and without rosacea.
| Patients with rosacea | Patients without rosacea | Crude HR (95%CI) | Adjustedb HR (95%CI) | Crude SD-HRc (95%CI) | Adjustedb SD-HRc (95%CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CKD | PRa | Rate | CKD | PRa | Rate | |||||
| All patients | 42 | 2622.09 | 16.02 | 229 | 21549.18 | 10.63 | 1.51(1.08–2.09)* | 1.40(1.01–1.96)* | 2.18(1.18–4.00)* | 2.00(1.05–3.82)* |
| Gender | ||||||||||
| Female | 26 | 1889.54 | 13.76 | 151 | 15383.86 | 9.82 | 1.40(0.92–2.13) | 1.41(0.93–2.15) | 1.05(0.32–3.47) | 1.20(0.35–4.04) |
| Male | 16 | 732.55 | 21.84 | 78 | 6165.32 | 12.65 | 1.71(1.00–2.93) | 1.41(0.81–2.47) | 3.23(1.56–6.68)* | 2.83(1.33–6.06)* |
| Age | ||||||||||
| 18–29 | 4 | 470.35 | 8.50 | 20 | 3734.02 | 5.36 | 1.63(0.56–4.77) | 1.42(0.46–4.37) | — | — |
| 30–39 | 5 | 482.11 | 10.37 | 12 | 4196.11 | 2.86 | 3.63(1.28–10.31)* | 3.18(1.08–9.39)* | 1.62(0.19–13.81) | 1.22(0.21–7.18) |
| 40–49 | 13 | 1081.54 | 12.02 | 70 | 8513.67 | 8.22 | 1.47(0.82–2.66) | 1.47(0.80–2.67) | 0.46(0.06–3.49) | 0.72(0.08-.6.33) |
| 50–59 | 10 | 360.72 | 27.72 | 59 | 3154.54 | 18.70 | 1.49(0.76–2.91) | 1.41(0.71–2.81) | 5.34(1.79–15.92)* | 3.68(1.00–13.56)* |
| 60+ | 10 | 227.38 | 43.98 | 68 | 1950.84 | 34.86 | 1.27(0.65–2.47) | 1.18(0.60–2.32) | 3.31(1.32–8.32)* | 4.24(1.63–11.04)* |
*P < 0.05.
a. per 1000 person years
b. Adjusted by the variables included in Table 1.
c. SD-HR was estimated from the competing risk model, and there is no subject death in aged at 18~29.
HR, hazard ratio; CI, confidence interval; CKD, chronic kidney disease; PY, person-years; SD-HR, sub-distribution hazard ratio.
When patients with rosacea were further categorized as those with moderate-to-severe rosacea (n = 203 (73.3%)) and those with mild rosacea (n = 74 (26.7%)). Patients with moderate-to-severe rosacea had a higher HR for CKD than those with mild rosacea. After adjustment for all other covariates and considering the competing risk of mortality, only those with moderate-to-severe rosacea had a significantly higher HR for CKD as compared with the non-rosacea controls. (SD-HR 2.53; 95% CI 1.11–5.75; P = 0.026) (Table 3).
Table 3. Hazard ratios for chronic kidney disease (CKD) by rosacea severity.
| N | CKD(%) | Crude HR (95%CI) | Adjusteda HR (95%CI) | P-value | Crude SD-HRb (95%CI) | Adjusteda SD-HRb (95%CI) | P-value | |
|---|---|---|---|---|---|---|---|---|
| Patients without rosacea | 2216 | 229(10.33) | Ref. | Ref. | Ref. | Ref. | ||
| Patients with mild severity of rosacea¶ | 203 | 29(14.29) | 1.41(0.96–2.07) | 1.33(0.90–1.97) | 0.1480 | 2.03(1.00–4.11)* | 1.82(0.83–4.00) | 0.1350 |
| Patients with moderate-to-severe severity of rosacea† | 74 | 13(17.57) | 1.79(1.02–3.13)* | 1.59(0.91–2.81) | 0.1066 | 2.61(0.94–7.21) | 2.53(1.11–5.75) | 0.0266* |
HR, hazard ratio; CI, confidence interval; CKD, chronic kidney disease; Ref., reference group; SD-HR, sub-distribution hazard ratio.
a. Adjusted by the variables included in Table 1.
b. SD-HR was estimated from the competing risk model.
¶Rosacea patients who did not receive oral medications for the disease
†Rosacea patients who received oral medications for the disease, including doxycycline, minocycline, tetracycline, metronidazole, and isotretinoin
*P-value<0.05.
Discussion
Rosacea has long been considered a disease limited to the skin, but an increasing number of studies have observed an association between rosacea and extracutaneous diseases, which suggests it has far-reaching systemic effects. A recent study compared cardiovascular disease (CVD) risk factors among 60 rosacea patients and 50 age- and sex-matched controls and found that patients with rosacea were more likely than controls to have higher levels of total cholesterol (199 vs. 163 mg/dL, P < 0.001), low-density lipoprotein cholesterol (121 vs. 101 mg/dL, P = 0.002), and C-reactive protein (0.43 vs. 0.24 mg/L, P = 0.007) and a family history of premature CVD (P = 0.002).[3] Similarly, subsequent studies reported higher prevalences of insulin resistance and CVD risk factors (elevated fasting blood glucose, total cholesterol, and systolic and diastolic blood pressures, P < 0.05) in patients with rosacea.[4, 5, 21] Moreover, Rainer et al. suggested that rosacea is associated with increased risk of CVD,[5] and a very recent population-based study reported a significant association between rosacea and coronary artery disease.[4] These associations between rosacea and certain comorbidities do not appear to be attributable solely to shared CVD risk factors. Prior studies showed that the associations of rosacea with some comorbidities did not substantially change after adjustment for traditional CVD risk factors [4, 5, 22] and that the strength of the associations increased in relation to rosacea severity.[5, 22] These findings suggest that other factors intrinsically linked to rosacea contribute to the development of these comorbidities. Our study provided some support for the hypothesis. Most of the abovementioned CVD risk factors are shared by rosacea and CKD.[23] Nevertheless, using a propensity score matched-pair procedure and multivariable Cox proportional hazards models, we found that rosacea was significantly associated with CKD, even after adjusting for the effects of comorbidities.
Because of its increasing incidence and prevalence and progression to end-stage renal disease, CKD is a global public health burden. CKD limits longevity and increases costs to health-care systems worldwide.[24] Moreover, as compared with the general population, patients with inflammatory diseases such as rheumatoid arthritis, systemic lupus erythematosus, Sjögren syndrome, systemic scleroderma, and psoriatic disease are more likely to have CKD.[14–16, 25] Accumulating evidence indicates that chronic low-grade inflammation resulting in endothelial injury, impaired vasodilation, and glomerulosclerosis plays a major role in CKD development and that various inflammatory cytokines are related to CKD pathogenesis and progression.[12, 26–28] Rosacea is a common chronic inflammatory skin condition characterized by dysfunction in the innate and/or adaptive immune response. Pro-inflammatory cytokines such as interleukin (IL)-1 β, IL-6, IL-8, and tumor necrosis factor-α are involved in rosacea pathogenesis, and inflammasome-related genes (CASP-1 and NALP-3) are overexpressed in skin samples from rosacea patients. [9, 10, 29] These inflammatory mediators may have an important role in CKD development.[27, 30–32] Consistent with these past studies, our data indicate that patients with moderate-to-severe rosacea, who might have a greater inflammation burden, had a higher risk of developing CKD than did patients with mild rosacea. The result lends support to the hypothesis that inflammatory mediators are involved in CKD pathogenesis and progression in patients with rosacea.
Previous studies of rosacea patients reported that the activity of paraoxone-1, an antioxidant enzyme, was decreased and that oxidative stress was increased.[33] In addition, there was a significant positive correlation between the magnitude of the reduction in cutaneous antioxidant capacity and rosacea severity, indicating that oxidative stress may have a role in the pathophysiology of rosacea.[33, 34] Similarly, evidence from numerous clinical studies confirms the importance of oxidative stress in CKD, as it can trigger the inflammatory process and accelerate progression of renal injury.[13, 35] Although the evidence is not conclusive, mechanisms involving inflammatory pathways and oxidative stress appear to contribute to the association between rosacea and CKD risk.
The present competing risk model showed a significant association between rosacea and CKD for patients older than 50 years, and CKD risk was highest among adults older than 60 years. A possible explanation for these findings is that age-associated decline in kidney function and loss of renal functional reserve in elderly adults increases the effects of rosacea-induced inflammation and oxidative stress on CKD development. Nevertheless, more research is needed in order to confirm this hypothesis.
Although this study was based on a large, high-quality, nationwide, population-based database, several limitations must be considered. First, diagnosis of rosacea was based on secondary claims data, and misclassification is thus possible. To minimize this bias, the presence of disease was defined as at least 2 ICD-9-CM diagnoses made by a relevant specialist. Moreover, the reliability and validity of using claims data to identify patients with rosacea and CKD have been demonstrated in previous epidemiologic studies.[4, 14–16, 36–38] This study relied on ICD diagnoses for identification of rosacea and CKD. It is likely that some asymptomatic patients did not seek medical treatment and therefore were not captured by this study design. Some misclassification of patients with acute kidney injury or without CKD may have occurred in the rosacea and control groups. However, this misclassification would have occurred in both the rosacea and control groups, would thus be nondifferential, and would bias effect estimates toward the null. Second, the fact that hospital visits are more frequent for patients with rosacea than for the general population may result in potential surveillance bias. However, laboratory testing of serum creatinine concentration and urine analysis, which are required for CKD diagnosis, were not routinely performed during therapeutic monitoring of rosacea. Moreover, the present results remained robust after adjustment for number of doctor visits in both groups. Third, the NHIRD did not include information on rosacea subtypes, lifestyle factors, or laboratory findings. Thus, we were unable to estimate glomerular filtration rate or detect proteinuria at baseline and could not adjust for these unmeasured potential confounders. Fourth, we used treatment with systemic therapies as a surrogate marker for severe disease. Doxycycline, minocycline, and tetracycline are also used to treat acne vulgaris and some infectious diseases. However, the probability of misclassification within this specific cohort with an ICD diagnosis of rosacea is likely to be negligible. Lastly, the present Taiwanese study population mainly consisted of Taiwanese with Han ethnicity and mostly type III/IV Fitzpatrick skin types.[39] Therefore, caution is advised when attempting to extrapolate our results to patients of other ethnicities.
In conclusion, the present results indicate that patients with rosacea, particularly those with moderate-to-severe disease, have an increased risk of CKD that is not completely explained by traditional risk factors. Future research should attempt to identify the mechanisms underlying this increase in CKD risk. Patients with rosacea and their physicians should be aware of this potential link with CKD. Careful monitoring of renal function and avoidance of long-term use of nephrotoxic drugs should be considered as part of integrated care for patients with rosacea, particularly those older than 50 years.
Acknowledgments
This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the Taiwan National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes.
Data Availability
The data on the study population that were obtained from the NHIRD (http://w3.nhri.org.tw/) are maintained in the NHRI (http://nhird.nhri.org.tw/). The NHIRD is limited for research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration. All applications are reviewed for approval of data release. Interested researchers may submit queries related to data access to nhird@nhri.org.tw.
Funding Statement
This work was supported in part by a grant from the National Taiwan University Hospital Hsin-Chu Branch (106-HCH002). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.Steinhoff M, Buddenkotte J, Aubert J, Sulk M, Novak P, Schwab VD, et al. (2011) Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc 15:2–11. Epub 2011/11/15. doi: 10.1038/jidsymp.2011.7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spoendlin J, Voegel JJ, Jick SS, Meier CR.(2013) Migraine, triptans, and the risk of developing rosacea: a population-based study within the United Kingdom. J Am Acad Dermatol 69:399–406. doi: 10.1016/j.jaad.2013.03.027 . [DOI] [PubMed] [Google Scholar]
- 3.Duman N, Ersoy Evans S, Atakan N.(2014) Rosacea and cardiovascular risk factors: a case control study. J Eur Acad Dermatol Venereol 28:1165–1169. doi: 10.1111/jdv.12234 . [DOI] [PubMed] [Google Scholar]
- 4.Hua TC, Chung PI, Chen YJ, Wu LC, Chen YD, Hwang CY, et al. (2015) Cardiovascular comorbidities in patients with rosacea: A nationwide case-control study from Taiwan. J Am Acad Dermatol 73:249–254. doi: 10.1016/j.jaad.2015.04.028 . [DOI] [PubMed] [Google Scholar]
- 5.Rainer BM, Fischer AH, Luz Felipe da Silva D, Kang S, Chien AL.(2015) Rosacea is associated with chronic systemic diseases in a skin severity-dependent manner: results of a case-control study. J Am Acad Dermatol 73:604–608. doi: 10.1016/j.jaad.2015.07.009 . [DOI] [PubMed] [Google Scholar]
- 6.Shankar A, Klein R, Klein BE.(2006) The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol 164:263–271. doi: 10.1093/aje/kwj173 . [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi Y, Mine T, Kawana I, Yasuzaki H, Kokuho T, Toya Y, et al. (2009) Gastroesophageal reflux disease in chronic renal failure patients: evaluation by endoscopic examination. Tokai J Exp Clin Med 34:80–83. . [PubMed] [Google Scholar]
- 8.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108:2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80 . [DOI] [PubMed] [Google Scholar]
- 9.Casas C, Paul C, Lahfa M, Livideanu B, Lejeune O, Alvarez-Georges S, et al. (2012) Quantification of Demodex folliculorum by PCR in rosacea and its relationship to skin innate immune activation. Exp Dermatol 21:906–910. doi: 10.1111/exd.12030 . [DOI] [PubMed] [Google Scholar]
- 10.Bakar O, Demircay Z, Yuksel M, Haklar G, Sanisoglu Y.(2007) The effect of azithromycin on reactive oxygen species in rosacea. Clin Exp Dermatol 32:197–200. doi: 10.1111/j.1365-2230.2006.02322.x . [DOI] [PubMed] [Google Scholar]
- 11.Zhong S, Sun N, Liu H, Niu Y, Chen C, Wu Y. Topical tranexamic acid improves the permeability barrier in rosacea.(2015) Dermatologica Sinica 33:112–117. doi: 10.1016/j.dsi.2015.04.012 [Google Scholar]
- 12.Kang HT, Kim JK, Shim JY, Lee HR, Linton JA, Lee YJ.(2012) Low-grade inflammation, metabolic syndrome and the risk of chronic kidney disease: the 2005 Korean National Health and Nutrition Examination Survey. J Korean Med Sci 27:630–635. doi: 10.3346/jkms.2012.27.6.630 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaysen GA, Eiserich JP.(2004) The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol 15:538–548. . [DOI] [PubMed] [Google Scholar]
- 14.Chiu HY, Huang HL, Li CH, Yin YJ, Chen HA, Hsu ST, et al. (2015) Increased risk of glomerulonephritis and chronic kidney disease in relation to the severity of psoriasis, concomitant medication, and comorbidity: a nationwide population-based cohort study. Br J Dermatol 173:146–154. doi: 10.1111/bjd.13599 . [DOI] [PubMed] [Google Scholar]
- 15.Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM.(2013) Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ 347:f5961 doi: 10.1136/bmj.f5961 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu HY, Huang HL, Li CH, Chen HA, Yeh CL, Chiu SH, et al. (2015) Increased Risk of Chronic Kidney Disease in Rheumatoid Arthritis Associated with Cardiovascular Complications—A National Population-Based Cohort Study. PLoS One 10:e0136508 doi: 10.1371/journal.pone.0136508PONE-D-15-23571. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki K, Gallo RL.(2009) The molecular pathology of rosacea. J Dermatol Sci. 55:77–81. doi: 10.1016/j.jdermsci.2009.04.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu H-Y, Cheng Y-P, Tsai T-F.(2012) T helper type 17 in psoriasis: From basic immunology to clinical practice. Dermatologica Sinica 30:136–141. doi: 10.1016/j.dsi.2012.08.002 [Google Scholar]
- 19.Hung SC, Chang YK, Liu JS, Kuo KL, Chen YH, Hsu CC, et al. (2015) Metformin use and mortality in patients with advanced chronic kidney disease: national, retrospective, observational, cohort study. Lancet Diabetes Endocrinol. 3:605–614. doi: 10.1016/S2213-8587(15)00123-0 . [DOI] [PubMed] [Google Scholar]
- 20.Tseng CW, Lin CL, Chen YT, Jeng LB. (2017) Ischemic Bowel Syndrome in Patients with Spinal Cord Injury: A Nationwide Study. PLoS One. 12:e0169070 doi: 10.1371/journal.pone.0169070 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akin Belli A, Ozbas Gok S, Akbaba G, Etgu F, Dogan G.(2016) The relationship between rosacea and insulin resistance and metabolic syndrome. Eur J Dermatol 26:260–264. doi: 10.1684/ejd.2016.2748 . [DOI] [PubMed] [Google Scholar]
- 22.Aldrich N, Gerstenblith M, Fu P, Tuttle MS, Varma P, Gotow E, et al. (2015) Genetic vs Environmental Factors That Correlate With Rosacea: A Cohort-Based Survey of Twins. JAMA Dermatol 151:1213–1219. 2429555 doi: 10.1001/jamadermatol.2015.2230 . [DOI] [PubMed] [Google Scholar]
- 23.Said S, Hernandez GT.(2014) The link between chronic kidney disease and cardiovascular disease. J Nephropathol 3:99–104. doi: 10.12860/jnp.2014.19 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. (2008) All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371:2173–2182. doi: 10.1016/S0140-6736(08)60952-6 . [DOI] [PubMed] [Google Scholar]
- 25.Kronbichler A, Mayer G.(2013) Renal involvement in autoimmune connective tissue diseases. BMC Med 11:95 doi: 10.1186/1741-7015-11-95 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. (2004) The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 140:167–174. . [DOI] [PubMed] [Google Scholar]
- 27.Carrero JJ, Stenvinkel P. (2009) Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol 4 Suppl 1:S49–55. doi: 10.2215/CJN.02720409 . [DOI] [PubMed] [Google Scholar]
- 28.Arici M, Walls J.(2001) End-stage renal disease, atherosclerosis, and cardiovascular mortality: is C-reactive protein the missing link? Kidney Int 59:407–414. doi: 10.1046/j.1523-1755.2001.059002407.x . [DOI] [PubMed] [Google Scholar]
- 29.Buhl T, Sulk M, Nowak P, Buddenkotte J, McDonald I, Aubert J, et al. (2015) Molecular and Morphological Characterization of Inflammatory Infiltrate in Rosacea Reveals Activation of Th1/Th17 Pathways. J Invest Dermatol 135:2198–2208. Epub 2015/04/08. doi: 10.1038/jid.2015.141 . [DOI] [PubMed] [Google Scholar]
- 30.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, et al. (2005) IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int 67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x . [DOI] [PubMed] [Google Scholar]
- 31.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, et al. (2010) The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21:1732–1744. doi: 10.1681/ASN.2010020143 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granata S, Masola V, Zoratti E, Scupoli MT, Baruzzi A, Messa M, et al. (2015) NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS One 10:e0122272 doi: 10.1371/journal.pone.0122272 PONE-D-14-31723. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tisma VS, Basta-Juzbasic A, Jaganjac M, Brcic L, Dobric I, Lipozencic J, et al. (2009) Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol 60:270–276. doi: 10.1016/j.jaad.2008.10.014 . [DOI] [PubMed] [Google Scholar]
- 34.Oztas MO, Balk M, Ogus E, Bozkurt M, Ogus IH, Ozer N.(2003) The role of free oxygen radicals in the aetiopathogenesis of rosacea. Clin Exp Dermatol 28:188–192. . [DOI] [PubMed] [Google Scholar]
- 35.Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC.(2012) Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 17:311–321. doi: 10.1111/j.1440-1797.2012.01572.x . [DOI] [PubMed] [Google Scholar]
- 36.Egeberg A, Hansen PR, Gislason GH, Thyssen JP.(2016) Assessment of the risk of cardiovascular disease in patients with rosacea. J Am Acad Dermatol 75:336–339. doi: 10.1016/j.jaad.2016.02.1158 . [DOI] [PubMed] [Google Scholar]
- 37.Egeberg A, Hansen PR, Gislason GH, Thyssen JP.(2016) Patients with Rosacea Have Increased Risk of Depression and Anxiety Disorders: A Danish Nationwide Cohort Study. Dermatology 232:208–213. doi: 10.1159/000444082 . [DOI] [PubMed] [Google Scholar]
- 38.Egeberg A, Hansen PR, Gislason GH, Thyssen JP.(2016) Exploring the Association Between Rosacea and Parkinson Disease: A Danish Nationwide Cohort Study. JAMA Neurol 73:529–534. doi: 10.1001/jamaneurol.2016.0022 . [DOI] [PubMed] [Google Scholar]
- 39.Li YW, Chu CY.(2007) The minimal erythema dose of broadband ultraviolet B in Taiwanese. J Formos Med Assoc 106:975–978. doi: 10.1016/S0929-6646(08)60071-6 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data on the study population that were obtained from the NHIRD (http://w3.nhri.org.tw/) are maintained in the NHRI (http://nhird.nhri.org.tw/). The NHIRD is limited for research purposes only. Applicants must follow the Computer-Processed Personal Data Protection Law (http://www.winklerpartners.com/?p=987) and related regulations of National Health Insurance Administration. All applications are reviewed for approval of data release. Interested researchers may submit queries related to data access to nhird@nhri.org.tw.