Abstract
Background
Palliative treatments and stents are necessary for relieving dysphagia in patients with esophageal cancer. The aim of this study was to simultaneously compare available treatments in terms of complications.
Methods
Web of Science, Medline, Scopus, Cochrane Library and Embase were searched. Statistical heterogeneity was assessed using the Chi2 test and was quantified by I2. The results of this study were summarized in terms of Risk Ratio (RR). The random effects model was used to report the results. The rank probability for each treatment was calculated using the p-score.
Results
Out of 17855 references, 24 RCTs reported complications including treatment related death (TRD), bleeding, stent migration, aspiration, severe pain and fistula formation. In the ranking of treatments, thermal ablative therapy (p-score = 0.82), covered Evolution® stent (p-score = 0.70), brachytherapy (p-score = 0.72) and antireflux stent (p-score = 0.74) were better treatments in the network of TRD. Thermal ablative therapy (p-score = 0.86), the conventional stent (p-score = 0.62), covered Evolution® stent (p-score = 0.96) and brachytherapy (p-score = 0.82) were better treatments in the network of bleeding complications. Covered Evolution® (p-score = 0.78), uncovered (p-score = 0.88) and irradiation stents (p-score = 0.65) were better treatments in network of stent migration complications. In the network of severe pain, Conventional self-expandable nitinol alloy covered stent (p-score = 0.73), polyflex (p-score = 0.79), latex prosthesis (p-score = 0.96) and brachytherapy (p-score = 0.65) were better treatments.
Conclusion
According to our results, thermal ablative therapy, covered Evolution® stents, brachytherapy, and antireflux stents are associated with a lower risk of TRD. Moreover, thermal ablative therapy, conventional, covered Evolution® and brachytherapy had lower risks of bleeding. Overall, fewer complications were associated with covered Evolution® stent and brachytherapy.
Introduction
Esophageal cancer includes squamous cell carcinoma and adenocarcinoma. It has an aggressive nature, a poor prognosis and a low five-year survival rate [1]. More than 50% of patients with esophageal cancer are diagnosed with an advanced stage of the disease, for which surgery is not appropriate. Therefore, palliative treatments are necessary for relieving the associated dysphagia [2]. Certain treatment options have been developed to relieve the pain and dysphagia, including the stent, brachytherapy, and external radiotherapy [3]. Different types of stents such as Self-expandable metallic stents (SEMS), Self-expandable plastic stents (SEPS), polyflex, and antireflux, have been developed and compared in randomized control trials (RCT) [4]. However, the optimal treatment intervention has not been determined yet [3]. Up to now, many RCTs have been conducted to assess the effectiveness of stents and palliative treatments among patients with advanced esophageal cancer [5–10]. These RCTs have reported adverse events for stent placement and other palliative treatments such as brachytherapy and thermal ablative treatment in patients, including TRD, bleeding, stent migration, fistula formation, and aspiration. However, there is no consensus as to which stent has lower complications [3].
A network meta-analysis simultaneously comparing all available treatments can prove useful in the selection of better treatments [11]. Therefore, the aim of this network meta-analysis was to simultaneously compare available palliative treatments in terms of complications, including TRD, bleeding, stent migration, aspiration, severe pain and fistula formation, and to rank these treatment interventions in patients with esophageal cancer.
Methods
Search strategy and selection criteria
This network meta-analysis is a part of a comprehensive systematic review which has simultaneously compared all the treatment interventions for esophageal cancer. The protocol of this systematic review has been registered in PROSPERO (ID: CRD42015023950) and has also been published [12].
A search strategy was developed to obtain relevant RCTs on the evaluation of treatment interventions for esophageal cancer. The search strategy for this review included the following Keywords: #1: Esophageal cancer [tw]; #2: Esophageal squamous cell carcinoma [Mesh terms]; #3: Esophageal Neoplasms [Mesh terms]; #4: #1 OR #2 OR #3; #5: Randomized controlled trial [Mesh terms]; #6: Randomized clinical trial [tw]; #7: #5 OR #6; #8: Radiotherapy [Mesh terms]; #9: Stents [Mesh terms]; #10: Brachytherapy [Mesh terms]; #11: Palliative Care [Mesh terms]; #12: #8 OR #9 OR #10 OR #11; #13: #4 AND #7 AND #12.
The international databases searched until July 2017 included Web of Science, Medline, Scopus, Cochrane Library and Embase. During the hand-search, the reference lists of the included RCTs and relevant published systematic reviews and meta-analyses were also scanned. The authors of the included studies were contacted. In addition, the following websites of relevant conferences were searched to obtain unpublished articles:
International Gastric Cancer Association; available from http://www.igca.info/news/dec2012_02.html
The International Society for Diseases of the Esophagus; available from http://www.isde.net/events
Cancer Research UK; available from: http://www.cancercentre.ox.ac.uk/events/sponsored-events/symposium-on-oesophageal-cancer/
World Organization for Specialized Studies on Diseases of the Esophagus; available from: http://www.oeso.org/index.html
Gastroenterology Conference Map; available from: http://www.mdlinx.com/gastroenterology/conference-map.cfm
All RCTs that had evaluated stent placement or palliative treatments of esophageal cancer were retrieved in this review. We put no restriction on the time, location and language of the published RCTs. The inclusion criteria for this review were, RCTs that included patients with either histology of esophageal cancer i.e. squamous cell carcinoma and/or adenocarcinoma. Cohort studies and non-randomized clinical trials were excluded.
Data extraction and quality assessment
Two investigators (ADI & MAM) screened the titles and abstracts of the retrieved RCTs independently. They reviewed the full texts of relevant RCTs to assess the eligibility for inclusion in the network meta-analysis. Any disagreement between the authors was resolved by discussion and -if need be- by the judgment of other authors. The following data were extracted using a predefined data sheet: year of publication, location, duration of study (months), stage of cancer, type of palliative treatment in each arm of the RCT in detail, number of randomized patients in each arm of the RCT, numbers of males and females, mean/median age of participants and the numbers of complications among patients in each arm of the included RCT. The risk of bias was assessed using Cochrane’s tools [13]. The details of used items from Cochrane’ tools in this review were reported in the protocol [12].
Outcome
The outcomes of interest in this study were TRD, bleeding, stent migration, aspiration, severe pain and fistula formation among patients with esophageal cancer who had received palliative treatment interventions.
Statistical analysis
In the first stage, the network of treatment interventions was drawn using the netgraph command in R software. We performed a pairwise meta-analysis for each comparison using the random effect model. A Risk Ratio with 95% CI was calculated for each complication in two by two comparisons. The statistical heterogeneity was assessed using the Chi2 test and the heterogeneity across each comparison was quantified using I2 statistics [14]. The similarity assumption was assessed clinically and epidemiologically in terms of effect modifiers in the arms of the RCTs. The consistency assumption was assessed using loop-specific and design by treatment interaction methods [15]. The publication bias for each complication was assessed visually by the adjusted network funnel plot using Stata 13 (Stata Corp, College Station, TX, USA) [16]. The results of this random effect network meta-analysis were summarized in terms of Risk Ratio with 95% Confidence Interval (CI).
The rank probabilities of the treatments were calculated using the p-score. The p-score for treatment i is one minus the one-sided p-value p[j] of accepting the alternative hypothesis. Therefore, the p-score for each treatment is the mean of all 1-p[j]. The P-score is a value between zero and one, where a larger value indicates a better treatment [17]. The statistical analysis was performed using R, version 3.3.1 (2016-06-21), with the netmeta package for network meta-analysis.
Results
Overall, 17855 references were retrieved after removing duplicate references. After screening the titles and abstracts, 832 RCTs’ full texts remained for review. Upon checking the eligibility criteria, 24 RCTs [5, 7–10, 18–36] had reported the complications of stents and palliative treatments, so they were included in our network meta-analysis (Fig 1 and Table 1).
Fig 1. A flow chart depicting the stages of retrieving articles and checking the eligibility criteria for network meta-analysis.

Table 1. Characteristics of included randomized control trials.
| Author | Quality | Country | Treatments | n1 | n2 | proportion of male in arm 1 | Proportion of male in arm 2 | Mean age 1 | Mean age 2 | TRD 1 | TRD 2 | Bleeding 1 | Bleeding 2 | Stent migration 1 | Stent migration 2 | Aspiration 1 | Aspiration 2 | Sever pain 1 | Sever pain 2 | Fistula 1 | Fistula 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amdal (2013) | I | Norway | T1: SEMS+BT T2: BT |
21 | 20 | 0.62 | 0.65 | 74 | 73 | 1 | 0 | 1 | 0 | 3 | 0 | ||||||
| Conio (2007) | I | International | T1: Polyflex T2: Ultraflex stent |
47 | 54 | 0.83 | 0.83 | 74.9 | 69.1 | 2 | 4 | 2 | 0 | 7 | 1 | 1 | 2 | ||||
| Dallal (2001) | I | UK | T1: Thermal ablative therapy T2: Metallic stent |
34 | 31 | 0.53 | 0.52 | 75 | 77 | 1 | 2 | 0 | 3 | 0 | 1 | 3 | 0 | ||||
| De Palma (1996) | L | Italy | T1: Plastic stent T2: Metallic stent |
20 | 19 | 0.75 | 0.84 | 69.4 | 67.8 | 3 | 0 | 1 | 0 | ||||||||
| Guo (2008) | H | China | T1: Irradiation stent T2: Conventional stent |
27 | 26 | 0.70 | 0.77 | 72.2 | 69.5 | 6 | 5 | 9 | 7 | 2 | 3 | 1 | 2 | 8 | 7 | 1 | 0 |
| Homs (2004) | I | Netherlands | T1: BT T2: SEMS 18 |
101 | 108 | 0.75 | 0.80 | 69 | 69 | 1 | 6 | 5 | 14 | 3* | 18 | 1 | 1 | 1 | 3 | 3 | 3 |
| Homs (2004) | I | Netherlands | T1: FerX-Ella+ antireflux T2: FerX-Ella |
15 | 15 | 0.80 | 0.80 | 68 | 69 | 0 | 0 | 2 | 1 | 5 | 2 | 0 | 1 | 1 | 1 | ||
| Javed (2012) | H | India | T1: Ultraflex stent T2: Ultraflex stent+RT |
37 | 42 | 0.73 | 0.69 | 58.1 | 58.6 | 0 | 0 | 9 | 6 | ||||||||
| Knyrim (1993) | L | Germany | T1: Plastic stent T2: Metallic stent |
21 | 21 | 0.00 | 0.00 | 68.8 | 64.8 | 6 | 3 | 5 | 0 | 1 | 0 | 1 | 2 | ||||
| O’Donnell (2002) | L | UK | T1: Plastic stent T2: Metallic stent |
25 | 25 | 0.00 | 0.00 | 72.3 | 72.9 | 5 | 6 | 3 | 2 | ||||||||
| Power (2007) | H | Ireland | T1: Conventional stent T2: Antireflux stent |
25 | 24 | 0.68 | 0.58 | 73.9 | 64.8 | 0 | 0 | 0 | 2 | 1 | 2 | ||||||
| Sabharwal (2008) | I | UK | T1: Antireflux stent T2: Ultraflex stent + omeprazole |
22 | 26 | 0.68 | 0.81 | 71.4 | 66.3 | 0 | 1 | 1 | 2 | 7 | 6 | 2 | 9 | 0 | 1 | ||
| Sabharwal (2003) | I | UK | T1: Ultraflex stent T2: Flamingo stent |
31 | 22 | 0.81 | 0.68 | 71.6 | 61.2 | 5 | 4 | 1 | 1 | 2 | 1 | ||||||
| Shenfine (2009) | H | UK | T1: Rigid plastic stent T2: Non-Stent |
57 | 50 | 0.70 | 0.78 | 78.2 | 76.9 | 12 | 6 | 17 | 8 | 2 | 0 | 8 | 7 | ||||
| Vakil (2001) | I | Italy | T1: Metallic stent T2: Uncovered stent |
32 | 30 | 0.00 | 0.00 | 74 | 71 | 8 | 2 | 4 | 2 | 12 | 15 | ||||||
| van Heel (2012) | L | Netherlands | T1: Ultraflex stent T2: Covered Evolution stent |
40 | 40 | 0.80 | 0.65 | 67 | 67 | 10 | 7 | 7 | 0 | 3 | 1 | 3 | 2 | 5 | 6 | ||
| Wenger (2006) | I | Sweden | T1: Antireflux T2: Conventional |
19 | 22 | 0.68 | 0.59 | 75 | 73 | 0 | 0 | 1 | 1 | 2 | 3 | ||||||
| Wenger (2005) | H | Sweden | T1: SEMS T2: BT |
30 | 30 | 0.60 | 0.70 | 74 | 72 | 2 | 2 | 1 | 0 | 2 | - | 2 | 1 | ||||
| White (2015) | H | Kenia | T1: SEMS 18 T2: SEMS 23 |
50 | 50 | 0.62 | 0.58 | 61.8 | 57.1 | 3 | 0 | 1 | 0 | ||||||||
| Zhu (2014) | H | China | T1: Irradiation Stent T2: CSENACS |
80 | 80 | 0.76 | 0.66 | 71 | 71 | 5 | 5 | 0 | 0 | 11 | 14 | 17 | 15 | 6 | 5 | ||
| Laasch (2002) | L | UK | T1: Open Stent T2: Antireflex stent |
25 | 25 | 0.76 | 0.60 | 69 | 69 | 1 | 0 | 3 | 4 | 1 | 0 | ||||||
| Siersema (1998) | I | Netherlands | T1: Latex prosthesis T2: Metallic stent stent |
38 | 37 | 0.76 | 0.70 | 65.2 | 67.6 | 4 | 1 | 5 | 3 | 3 | 0 | 6 | 12 | 1 | 0 | ||
| Siersema (2001) | L | Netherlands | T1: Ultraflex stent T2: Flamingo stent |
34 | 33 | 0.82 | 0.67 | 67.7 | 71.4 | 1 | 0 | 5 | 3 | 6 | 3 | 3 | 6 | ||||
| Verschuur (2008) | I | Italy | T1: Ultraflex stent T2: Polyflex stent |
42 | 41 | 0.67 | 0.68 | 69 | 70 | 1 | 2 | 5 | 5 | 7 | 12 | 1 | 0 | 1 | 0 | 2 | 0 |
H: high quality; I: intermediate quality; L: low quality; T1: treatment 1, T2: treatment 2; BT: Brachytherapy; n1: sample size in arm 1, n2: sample size in arm 2; CSENACS: Conventional self-expandable nitinol alloy covered stent; SEMS: Self-expandable metallic stents.
*In this RCT some of randomized patients in BT group received SEMS 18.
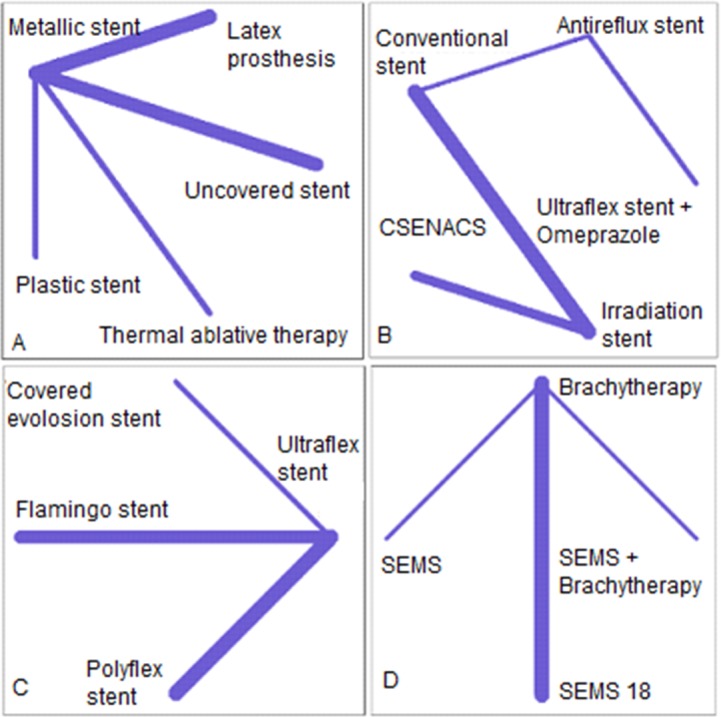
As shown in Figs 2 and 3, and S1, S3, S5 and S7 Figs, there were no closed loops in the networks, so we could not assess the consistency assumption of the complications. However, we assessed the heterogeneity in the two by two comparisons and the entire networks. The results showed no significant heterogeneity in the networks in either of the complications (S1–S6 Tables). Based on the adjusted funnel plot there was no evidence of publication bias for the set of studies related to each complication (S9–S14 Figs).
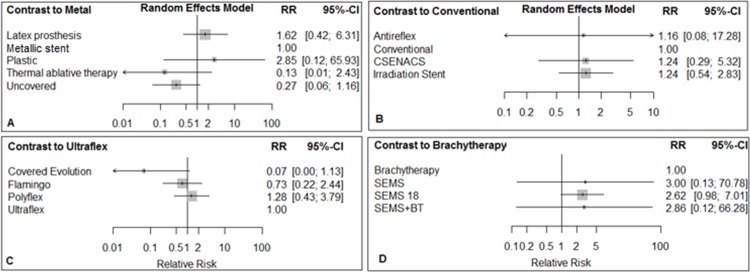
Fig 2.
Network of stent interventions for palliative treatments that had reported TRD in esophageal cancer; A: the metallic stent is a reference treatment, B: Ultraflex is a reference, C: Brachytherapy is a reference, and D: Antireflux is a reference.
Fig 3.
Forest plots for TRD in networks A, B, C, & D. Metallic stent, Ultraflex, Brachytherapy, and Antireflux are reference treatments in networks A, B, C & D, respectively.
Treatment-related death complication
TRD was reported in 16 RCTs, which included 1075 patients with esophageal cancer. The networks of eligible comparisons for TRD and bleeding are shown in Figs 2 and 3. The comparisons of treatments for TRD involved four independent sub-networks. In the networks, A and B, C and D, the metallic stent, the Ultraflex stent, brachytherapy and antireflux stents were reference treatments, respectively (Fig 2). According to the results of the test for heterogeneity, the I2 statistic for network A was 15.2%, and for network B, C, and D was zero (S1 Table).
The comparison of palliative treatments with reference treatments in each network for TRD is shown in Fig 3. In network A, compared with the metallic stent, the latex prosthesis increased the risk of TRD. The relative risk (RR) was 3.89 (95% CI: 0.42, 36.33). The RR for thermal ablative therapy compared with the metallic stent was 0.46 (95% CI: 0.04, 5.19). In network B, covered Evolution® compared with Ultraflex stent decreased the risk of TRD, RR = 0.70 (95% CI: 0.30, 1.66). In network C, SEMS 18 compared to brachytherapy increased the risk of TRD, RR = 5.61, (95% CI: 0.69, 45.80). In network D, both the open stent and ‘Ultraflex plus omeprazole’ compared to antireflux stent increased the risk of TRD (Fig 3).
Results of simultaneous direct and indirect comparisons of treatments for TRD are shown in S1 Table.
In terms of ranking of treatments, thermal ablative therapy (p-score = 0.82), covered Evolution® (p-score = 0.70), brachytherapy (p-score = 0.72) and antireflux stent (p-score = 0.74) were the best treatments in networks A, B, C and D, respectively (Table 2).
Table 2. Ranking of palliative treatments in terms of lower risk of TRD and bleeding, stent migration, aspiration, severe pain and fistula in patients with esophageal cancer.
| TRD | |||||||
| Network A | Network B | Network C | Network D | ||||
| Treatment | P-score | Treatment | P-score | Treatment | P-score | Treatment | P-score |
| Thermal ablative therapy | 0.82 | Covered evolution | 0.70 | Brachytherapy | 0.72 | Antireflux | 0.74 |
| Metallic stent | 0.65 | Polyflex | 0.49 | SEMS | 0.69 | Ultraflex +omeprazole | 0.40 |
| Plastic stent | 0.38 | Flamingo | 0.44 | SEMS18 | 0.09 | Open stent | 0.36 |
| Latex prosthesis | 0.15 | Ultraflex | 0.37 | ||||
| Bleeding | |||||||
| Network A | Network B | Network C | Network D | ||||
| Treatment | P-score | Treatment | P-score | Treatment | P-score | Treatment | P-score |
| Thermal ablative therapy | 0.86 | Conventional | 0.62 | Covered evolution | 0.96 | Brachytherapy | 0.82 |
| Uncovered stent | 0.79 | Antireflux | 0.54 | Flamingo | 0.50 | SEMSBT | 0.41 |
| Metallic stent | 0.41 | CSENACS | 0.49 | Ultraflex | 0.34 | SEMS | 0.40 |
| Latex | 0.24 | Irradiation stent | 0.47 | Polyflex | 0.20 | SEMS18 | 0.36 |
| Plastic stent | 0.20 | Ultraflex + Omeprazole | 0.37 | - | - | - | - |
| Stent migration | |||||||
| Network A | Network B | Network C | |||||
| Treatment | P-score | Treatment | P-score | Treatment | P-score | ||
| Covered Evolution stent | 0.78 | Uncovered stent | 0.88 | Irradiation stent | 0.65 | ||
| Flamingo stent | 0.67 | Metallic stent | 0.69 | Ultraflex stent+ omeprazole | 0.52 | ||
| Ultraflex stent + RT | 0.66 | Plastic stent | 0.27 | Open stent | 0.50 | ||
| Ultraflex stent | 0.36 | Latex prosthesis stent | 0.16 | Conventional stent | 0.49 | ||
| Polyflex stent | 0.03 | Antireflux stent | 0.34 | ||||
| Aspiration | |||||||
| Network A | Network B | Network C | |||||
| Treatment | P-score | Treatment | P-score | Treatment | P-score | ||
| Polyflex stent | 0.69 | Irradiation stent | 0.74 | BT | 0.69 | ||
| Covered evolution | 0.52 | CSENACS | 0.46 | SEMS18 | 0.68 | ||
| Ultraflex stent | 0.29 | Conventional stent | 0.31 | SEMSBT | 0.13 | ||
| Severe pain | |||||||
| Network A | Network B | Network C | Network D | ||||
| Treatment | P-score | Treatment | P-score | Treatment | P-score | Treatment | P-score |
| CSENACS | 0.73 | Polyflex stent | 0.79 | Latex prosthesis | 0.96 | BT | 0.65 |
| Conventional stent | 0.71 | Ultraflex | 0.58 | Metallic stent | 0.44 | SEMS23 | 0.63 |
| Irradiation stent | 0.63 | Covered evolution | 0.45 | Uncovered stent | 0.10 | SEMS18 | 0.22 |
| Antireflux | 0.52 | Flamingo | 0.19 | - | - | - | - |
| Open stent | 0.27 | - | - | - | - | - | - |
| Ultraflex + Omeprazole | 0.14 | - | - | - | - | - | - |
| Fistula | |||||||
| Network A | Network B | Network C | |||||
| Treatment | p-score | Treatment | p-score | Treatment | p-score | ||
| Plastic stent | 0.81 | Conventional stent | 0.72 | SEMS18 | 0.62 | ||
| Metallic stent | 0.64 | CSENACS | 0.46 | BT | 0.59 | ||
| Latex | 0.36 | Irradiation | 0.32 | SEMS | 0.29 | ||
| Thermal ablative therapy | 0.19 | - | - | - | - | ||
Bleeding
Bleeding was reported in 18 RCTs, which included 1374 patients with esophageal cancer. Other characteristics of the included RCTs are shown in Table 1.
In the network of bleeding complications, the metallic stent, conventional stent, Ultraflex stent, and brachytherapy were reference treatments in networks A, B, C, and D, respectively (Fig 4). Based on the results of the test for heterogeneity, the I2 statistic for network A, B, C, and D was zero (S2 Table).
Fig 4.
The network of stent interventions for palliative treatments that reported the bleeding complication in esophageal cancer; A: metallic stent is a reference treatment, B: Conventional is a reference, C: Ultraflex is a reference, and D: Brachytherapy is a reference treatment. CSENACS: Conventional self-expandable nitinol alloy covered stent.
In network A, the latex prosthesis and plastic stent increased the risk of bleeding when compared to the metallic stent. The RR for latex prosthesis was 1.62 (95% CI: 0.42, 6.31) and was 2.85 (95% CI: 0.12, 65.93) for the plastic stent. On the other hand, thermal ablative therapy (RR = 0.13, 95% CI: 0.01, 2.43) and uncovered stent (RR = 0.27, 95% CI: 0.06, 1.16) decreased the risk of bleeding when compared to the metallic stent. In network B, the irradiation stent and CSENACS increased the risk of bleeding when compared to the conventional stent. In network C, the covered Evolution® stent decreased the risk of bleeding (RR = 0.07, 95% CI: 0.00, 1.13) when compared to Ultraflex. In network D, SEMS and SEMS+BT increased the risk of bleeding when compared to brachytherapy (Fig 5).
Fig 5.
Forest plots for bleeding complication in networks A, B, C & D. Metallic stent, Conventional stent, Ultraflex, and Brachytherapy are reference treatments in networks A, B, C & D, respectively.
The results of simultaneous direct and indirect comparisons of treatments for bleeding are shown in S2 Table.
In terms of ranking treatments for the lower risk of bleeding, thermal ablative therapy (p-score = 0.86), the conventional stent (p-score = 0.62), covered Evolution® (p-score = 0.96) and brachytherapy (p-score = 0.82) were the best treatments in networks A, B, C, and D, respectively (Table 2).
Stent migration
Stent migration was reported in 23 RCTs. However, in 4 of the RCTs patients had received BT or thermal ablative therapy [5, 8, 19, 34] in one arm. Since stent migration cannot be a complication of BT and thermal ablative therapy, these RCTs were excluded from the network meta-analysis. Therefore, 19 RCTs involving 1207 patients were included. The available treatments for stent migration involved three dependent networks (S1 Fig). In the network A, and B the I2 statistic was zero, and in the network C the I2 was 16.1% (S3 Table).
In network A, when compared to the Ultraflex stent, the polyflex stent increased the risk of stent migration 2.07 times (95% CI: 1.01, 4.67). The risk of stent migration for covered Evolution® stent, Flamingo stent, and Ultraflex stent plus radiotherapy was lower than the ultraflex stent; however, the 95% CIs involved the null values. In network B, the risk ratio for the latex prosthesis and plastic stents compared to the metallic stent was 6.82 (95% CI: 0.36, 127.54) and 2.87 (95% CI: 0.87, 10.64), respectively. In network C, there were no considerable differences between the conventional, Irradiation, open and ultraflex stents plus omeprazole and the Antireflux stent (S2 Fig). The results of simultaneous direct and indirect comparisons of treatments for stent migration are shown in S3 Table.
In terms of ranking, the covered Evolution® (p-score = 0.78), Uncovered (p-score = 0.88), and Irradiation stents (p-score = 0.65) were the best treatments in networks A, B and C, respectively (Table 2).
Aspiration
Nine RCTs reported aspiration as a complication of stent placement. These RCTs involved 805 esophageal cancer patients. Three RCTs involved independent comparisons, so they were excluded from the network meta-analysis [20, 21, 27]. These studies have been described in Table 1. The remaining RCTs involved three independent networks (S3 Fig). The I2 statistic for all networks of this complication was zero (S4 Table). Forest plots drawn to compare the treatments with reference treatments in each network are shown in S4 Fig. Results of simultaneous direct and indirect comparisons of treatments for aspiration are shown in S2 Table. In terms of ranking, the Polyflex stent (p-score = 0.69), Irradiation stent (p-score = 0.74) and BT (p-score = 0.69) were the better treatments in networks A, B and C (Table 2).
Severe pain
Severe pain was reported in 14 RCTs. The CSENACS (p-score = 0.73), Polyflex stent (p-score = 0.79), Latex prosthesis (p-score = 0.96) and BT (p-score = 0.65) were better treatments in terms of lower risk of severe pain among patients in networks A, B, C and D, respectively. The network and forest plots are shown in S5 and S6 Figs. According to the results of test for heterogeneity, The I2 statistic for network A, B, C, and D was zero (S5 Table). In addition, the results of direct and indirect comparisons are presented in S2 Table.
Fistula formation
Fistula formation was reported in 10 RCTs. The networks of interventions and forest plots drawn to compare the treatments with reference treatments in each network are shown in S7 and S8 Figs, respectively. The I2 statistic for all networks of this complication was zero (S6 Table). The Plastic stent (p-score = 0.81), Conventional stent (p-score = 0.72), and SEMS 18 (p-score = 0.62) were better treatments in terms of lower risk of fistula formation in networks A, B, and C, respectively (Table 2).
Discussion
In this network meta-analysis, we compared the complications of palliative treatments including stents in patients with esophageal cancer. The treatments were ranked based on their lower risk of complications including TRD, bleeding, stent migration, aspiration, severe pain and fistula formation.
Based on our results, the TRD complication involved four networks. In network A, the latex prosthesis stent increased the risk of TRD 3.89 times when compared to the metallic stent. Thermal ablative therapy decreased the risk of TRD. In network B, there was no considerable difference between the covered Evolution®, Flamingo, and Ultraflex stents compared with the Ultraflex stent. In network C, when compared to brachytherapy, SEMS 18 increased the risk of TRD. In network D, the open stent and Ultraflex plus omeprazole versus antireflux stent increased the risk of TRD among patients with esophageal cancer.
Upon ranking treatments in terms of lower risk of TRD, thermal ablative therapy, covered Evolution®, brachytherapy and antireflux were better treatment interventions in the TRD networks, respectively. However, in network C, there was no considerable difference in the p-score of brachytherapy (p-score = 0.72) and SEMS (p-score = 0.69).
In terms of lower risk of bleeding, Thermal ablative therapy, Conventional stent, Covered Evolution® stent, and Brachytherapy were ranked as best treatments in networks A, B, C, and D, respectively. The results of bleeding complication were similar to those of TRD. Based on the lower risk of stent migration, the covered Evolution® stent, uncovered stent and irradiation stent were ranked as best treatments in the networks of this complication.
The risk of aspiration was lower for the polyflex stent, Irradiation stent, and brachytherapy in the networks of this complication. However, there was no considerable difference between the first (brachytherapy) and second (SEMS 18) ranked treatments in network C. The risk of severe pain in the CSENACS, polyflex stent, latex prosthesis and brachytherapy was lower than in the other treatments in the networks.
In terms of fistula formation, the plastic stent, conventional stent and SEMS 18 were better treatments in the networks of this complication. However, it should be noted the plastic stents compared with other stents, increased the risk of TRD, bleeding, stent migration and aspiration. Therefore, we could not recommend plastic stents as a better treatment, just because the risk of fistula was lower for this stent. In addition, a recently published clinical guideline recommends against for placement of plastic stents for palliation of esophageal strictures [37].
Based on the results of a traditional meta-analysis, the risk of TRD in palliative locoregional modalities was lower than in the metallic stent (RR = 0.58, 95% CI: 0.17, 1.99). Moreover, the odds ratio (OR) for bleeding was 0.54 (95% CI: 0.29, 1.00) compared to the metallic stents [38]. These results are in line with ours.
In the aforementioned meta-analysis [38], the odds ratio of stent migration in patients who had received ultraflex stents versus other types of stents was 1.17 (95% CI: 0.71, 1.93). In our study, the risk of stent migration was higher for ultraflex stent versus covered Evolution® stent, Flamingo stent, and Ultraflex stent plus radiotherapy. However, the risk of stent migration was higher in the polyflex stent than in the ultraflex stent (RR = 2.17(1.01, 4.67). Moreover, in our study, the risk of stent migration for the flamingo stent was lower than in the polyflex and ultraflex stents, a finding that is consistent with the aforementioned traditional meta-analysis.
According to this meta-analysis [38], the odds ratio of severe pain for the ultraflex stent versus the other stents was 0.52; 95% CI (0.19, 1.45). This result is in line with our study regarding the comparison of ultraflex versus covered Evolution® stent and Flamingo stent. However, the risk of severe pain was higher than in the polyflex stent.
The SEMS has been introduced as a selective palliative treatment in patients with esophageal cancer. The TRD in patients in which this stent had been used was reported between 0 to 1.4% [4]. In our study, the SEMS and SEMS 18 were compared with brachytherapy in network C (for TRD) and in network D (for the bleeding complication). When compared with brachytherapy, SEMS 18 increased the risk of TRD and bleeding 5.61 and 2.62 times, respectively. Likewise, compared with brachytherapy, SEMS increased the risk of bleeding by 3 times. These findings confirm the available evidence regarding the role of brachytherapy in the palliative treatment of patients with advanced stages of esophageal cancer [39]. In addition, according to the results of a recently published meta-analysis brachytherapy was recommended as a relatively safe and highly effective treatment for palliation of dysphagia [40] that is in the line of our results in terms of lower risk of TRD and bleeding for this treatment. However, despite the available evidence regarding the safety of this treatment and strong recommendation of the international guidelines [37] for use of this treatment, the lack of experience of radiation oncologist lead to the underuse of brachytherapy [41].
According to the results of one RCT, the antireflux stent had no additional advantages over the open stent [42]. But in our study, the risk of TRD in patients who had received the open stent was more than in those who had received the antireflux stent. Moreover, the p-score for the antireflux and open stents were 0.74 and 0.36, respectively.
In our network meta-analysis, the confidence intervals for RRs involved the null value in some of the networks. The reason may be due to the low number of RCTs in each network. However, the point estimates of RR in the networks of complications were considerable.
The statistical heterogeneity among the networks of complications was low. However, the statistical power of the Cochrane test is low when the number of studies included is low [3]. So these results may have been affected by the low number of studies in the networks.
According to current literature, there is controversy over the selection of the ideal stent [4]. To our knowledge, our study is the first network meta-analysis to compare the available stents in terms of six major complications (treatment-related death, bleeding, stent migration, aspiration, severe pain and fistula formation) among patients with esophageal cancer. We have provided useful evidence on the ranking of stents with lower risks of the aforementioned complications. In addition, the advantage of our study -as a network meta-analysis- over the traditional meta-analysis is the simultaneous comparison of all available treatments in each network, while traditional meta-analysis compares the stents two by two or one stent versus another [38].
There are certain limitations in this study. Firstly, palliative treatments and stent interventions involved four networks of TRD, bleeding, and severe pain, and three networks for stent migration, aspiration, and fistula formation. Therefore, we could not compare all treatment interventions simultaneously for each complication in one network. Secondly, the number of included RCTs in each network was low, so the power of networks for estimating the indirect comparison was low and the confidence intervals for indirect estimates were wide [43], and this issue can be lead to spars-data bias [44]. Therefore, more RCTs are required for future network meta-analysis.
Secondly, because of the small number of RCTs in each network and consequently low power and lack of validity of statistical tests for publication bias [45, 46], we could not be assessed the publication bias in this network meta-analysis.
We recommend that future RCTs report ‘all’ the complications of stent placement and other palliative treatments in patients with esophageal cancer. Thus, future systematic reviews and network meta-analyses will be able to compare stents in terms of all the major complications simultaneously.
Conclusion
Overall, the results of this network meta-analysis showed that thermal ablative therapy, covered Evolution® stents, brachytherapy and antireflux stents are associated with a lower risk of TRD. In terms of lower risk of bleeding, thermal ablative therapy, conventional stent, covered Evolution® stent and brachytherapy were better palliative treatments for patients with esophageal cancer. Based on the lower risk of stent migration, the covered Evolution®, uncovered, and Irradiation stents were better treatments. In terms of lower risk of severe pain as another major complication the CSENACS, polyflex stent, latex prosthesis and brachytherapy were better treatments.
Supporting information
(DOCX)
(DOCX)
Network of stent interventions for palliative treatments that reported the stent migration in esophageal cancer; A: Ultraflex stent is reference treatment, B: Metallic stent is reference, and C: Antireflux stent is reference.
(TIF)
Forest plots for stent migration in network A, B, C; Ultraflex stent, Metallic stent, Antireflux stent are reference treatments in network A, B, and C respectively.
(TIF)
Network of stent interventions for palliative treatments that reported the aspiration in patients; A: Ultraflex stent is reference treatment, B: Irradiation stent is reference, and C: Brachytherapy is reference.
(TIF)
Forest plots for aspiration in network A, B, C; covered evolution stent, Irradiation stent, brachytherapy are reference treatments in network A, B, and C respectively.
(TIF)
Network of stent interventions for palliative treatments that reported the severe pain in patients; Irradiation stent, Polyflex stent, Metallic stent and SEMS 18 are reference treatments in networks A, B, C and D respectively.
(TIF)
Forest plots for severe pain in network A, B, C and D; Antireflux stent, Ultraflex stent, Metallic stent, and brachytherapy are reference treatments in network A, B, C and D respectively.
(TIF)
Network of stent interventions for palliative treatments that reported the fistula in patients; Irradiation stent, Polyflex stent, Metallic stent and SEMS 18 are reference treatments in networks A, B, C and D respectively.
(TIF)
Forest plots for fistula in network A, B, and C. Metallic stent, Irradiation stent, and brachytherapy are reference treatments in network A, B, and C respectively.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This study supported by Tehran University of Medical Sciences (TUMS). We would like to thank Vic-Chancellor of Research and Technology of TUMS for financial support of this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study supported by Tehran University of Medical Sciences (TUMS). We would like to thank Vic-Chancellor of Research and Technology of TUMS for financial support of this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International journal of cancer. 2010;127(12):2893–917. doi: 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 2.Tytgat G, Bartelink H, Bernards R, Giaccone G, Van Lanschot J, Offerhaus G, et al. Cancer of the esophagus and gastric cardia: recent advances. Diseases of the Esophagus. 2004;17(1):10–26. doi: 10.1111/j.1442-2050.2004.00371.x [DOI] [PubMed] [Google Scholar]
- 3.Sreedharan A, Harris K, Crellin A, Forman D, Everett SM. Interventions for dysphagia in oesophageal cancer. The Cochrane database of systematic reviews. 2009;(4):Cd005048 Epub 2009/10/13. doi: 10.1002/14651858.CD005048.pub2 . [DOI] [PubMed] [Google Scholar]
- 4.Irani S, Kozarek R. Esophageal stents: past, present, and future. Techniques in Gastrointestinal Endoscopy. 2010;12(4):178–90. [Google Scholar]
- 5.Amdal CD, Jacobsen AB, Sandstad B, Warloe T, Bjordal K. Palliative brachytherapy with or without primary stent placement in patients with oesophageal cancer, a randomised phase III trial. Radiotherapy and Oncology. 2013;107(3):428–33. doi: 10.1016/j.radonc.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 6.Bergquist H, Wenger U, Johnsson E, Nyman J, Ejnell H, Hammerlid E, et al. Stent insertion or endoluminal brachytherapy as palliation of patients with advanced cancer of the esophagus and gastroesophageal junction. Results of a randomized, controlled clinical trial. Diseases of the Esophagus. 2005;18(3):131–9. doi: 10.1111/j.1442-2050.2005.00467.x [DOI] [PubMed] [Google Scholar]
- 7.Conio MMD, Repici AMD, Battaglia GMD, De Pretis GMD, Ghezzo LMD, Bittinger MMD, et al. A Randomized Prospective Comparison of Self-Expandable Plastic Stents and Partially Covered Self-Expandable Metal Stents in the Palliation of Malignant Esophageal Dysphagia. American Journal of Gastroenterology. 2007;102(12):2667–77. doi: 10.1111/j.1572-0241.2007.01565.x [DOI] [PubMed] [Google Scholar]
- 8.Dallal HJ, Smith GD, Grieve DC, Ghosh S, Penman ID, Palmer KR. A randomized trial of thermal ablative therapy versus expandable metal stents in the palliative treatment of patients with esophageal carcinoma. Gastrointestinal Endoscopy. 2001;54(5):549–57. . [DOI] [PubMed] [Google Scholar]
- 9.Guo JH, Teng GJ, Zhu GY, He SC, Fang W, Deng G, et al. Self-expandable esophageal stent loaded with 125I seeds: initial experience in patients with advanced esophageal cancer. Radiology. 2008;247(2):574–81. Epub 2008/03/20. doi: 10.1148/radiol.2472070999 . [DOI] [PubMed] [Google Scholar]
- 10.Javed A, Pal S, Dash NR, Ahuja V, Mohanti BK, Vishnubhatla S, et al. Palliative stenting with or without radiotherapy for inoperable esophageal carcinoma: A randomized trial. Journal of Gastrointestinal Cancer. 2012;43(1):63–9. doi: 10.1007/s12029-010-9206-4 [DOI] [PubMed] [Google Scholar]
- 11.Caldwell DM, Ades A, Higgins J. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. British Medical Journal. 2005;7521:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doosti-Irani A, Mansournia MA, Rahimi-Foroushani A, Cheraghi Z, Holakouie-Naieni K. Simultaneous Comparison of Efficacy and Adverse Events of Interventions for Patients with Esophageal Cancer: Protocol for a Systematic Review and Bayesian Network Meta-analysis. Asian Pacific journal of cancer prevention: APJCP. 2016;17(2):867–72. Epub 2016/03/02. . [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]: The Cochrane Collaboration; 2011. Available from: http://handbook.cochrane.org/.
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42(1):332–45. doi: 10.1093/ije/dys222 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PloS one. 2013;8(10):e76654 Epub 2013/10/08. doi: 10.1371/journal.pone.0076654 ; PubMed Central PMCID: PMCPmc3789683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC medical research methodology. 2015;15:58 Epub 2015/08/01. doi: 10.1186/s12874-015-0060-8 ; PubMed Central PMCID: PMCPmc4521472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Palma GD, di Matteo E, Romano G, Fimmano A, Rondinone G, Catanzano C. Plastic prosthesis versus expandable metal stents for palliation of inoperable esophageal thoracic carcinoma: a controlled prospective study. Gastrointest Endosc. 1996;43(5):478–82. Epub 1996/05/01. . [DOI] [PubMed] [Google Scholar]
- 19.Homs MY, Steyerberg EW, Eijkenboom WM, Tilanus HW, Stalpers LJ, Bartelsman JF, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet (London, England). 2004;364(9444):1497–504. Epub 2004/10/27. doi: 10.1016/s0140-6736(04)17272-3 . [DOI] [PubMed] [Google Scholar]
- 20.Homs MYV, Wahab PJ, Kuipers EJ, Steyerberg EW, Grool TA, Haringsma J, et al. Esophageal stents with antireflux valve for tumors of the distal esophagus and gastric cardia: a randomized trial. Gastrointestinal Endoscopy. 2004;60(5):695–702. http://dx.doi.org/10.1016/S0016-5107(04)02047-4. [DOI] [PubMed] [Google Scholar]
- 21.Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. New England Journal of Medicine. 1993;329(18):1302–7. doi: 10.1056/NEJM199310283291803. [DOI] [PubMed] [Google Scholar]
- 22.Laasch H-U, Marriott A, Wilbraham L, Tunnah S, England RE, Martin DF. Effectiveness of Open versus Antireflux Stents for Palliation of Distal Esophageal Carcinoma and Prevention of Symptomatic Gastroesophageal Reflux 1. Radiology. 2002;225(2):359–65. doi: 10.1148/radiol.2252011763 [DOI] [PubMed] [Google Scholar]
- 23.O'Donnell CA, Fullarton GM, Watt E, Lennon K, Murray GD, Moss JG. Randomized clinical trial comparing self-expanding metallic stents with plastic endoprostheses in the palliation of oesophageal cancer. British Journal of Surgery. 2002;89(8):985–92. doi: 10.1046/j.1365-2168.2002.02152.x [DOI] [PubMed] [Google Scholar]
- 24.Power C, Byrne PJ, Lim K, Ravi N, Moore J, Fitzgerald T, et al. Superiority of anti-reflux stent compared with conventional stents in the palliative management of patients with cancer of the lower esophagus and esophago-gastric junction: results of a randomized clinical trial. Diseases of the Esophagus. 2007;20(6):466–70. doi: 10.1111/j.1442-2050.2007.00696.x [DOI] [PubMed] [Google Scholar]
- 25.Sabharwal T, Gulati MS, Fotiadis N, Dourado R, Botha A, Mason R, et al. Randomised comparison of the FerX Ella antireflux stent and the ultraflex stent: Proton pump inhibitor combination for prevention of post-stent reflux in patients with esophageal carcinoma involving the esophago-gastric junction. Journal of Gastroenterology & Hepatology. 2008;23(5):723–8. [DOI] [PubMed] [Google Scholar]
- 26.Sabharwal T, Hamady MS, Chui S, Atkinson S, Mason R, Adam A. A randomised prospective comparison of the Flamingo Wallstent and Ultraflex stent for palliation of dysphagia associated with lower third oesophageal carcinoma. Gut. 2003;52(7):922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenfine J, McNamee P, Steen N, Bond J, Griffin SM. A randomized controlled clinical trial of palliative therapies for patients with inoperable esophageal cancer. American Journal of Gastroenterology. 2009;104(7):1674–85. doi: 10.1038/ajg.2009.155 [DOI] [PubMed] [Google Scholar]
- 28.Siersema PD, Hop WC, Dees J, Tilanus HW, van Blankenstein M. Coated self-expanding metal stents versus latex prostheses for esophagogastric cancer with special reference to prior radiation and chemotherapy: a controlled, prospective study. Gastrointestinal endoscopy. 1998;47(2):113–20. [DOI] [PubMed] [Google Scholar]
- 29.Siersema PD, Hop WC, Van Blankenstein M, van Tilburg AJ, Bac D-J, Homs MY, et al. A comparison of 3 types of covered metal stents for the palliation of patients with dysphagia caused by esophagogastric carcinoma: a prospective, randomized study. Gastrointestinal endoscopy. 2001;54(2):145–53. [DOI] [PubMed] [Google Scholar]
- 30.Vakil N, Morris AI, Marcon N, Segalin A, Peracchia A, Bethge N, et al. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. American Journal of Gastroenterology. 2001;96(6):1791–6. doi: 10.1111/j.1572-0241.2001.03923.x . [DOI] [PubMed] [Google Scholar]
- 31.van Heel NCM, Haringsma J, Boot H, Cats A, Vanhoutvin SALW, Kuipers EJ. Comparison of 2 expandable stents for malignant esophageal disease: a randomized controlled trial. Gastrointestinal Endoscopy. 2012;76(1):52–8. doi: 10.1016/j.gie.2012.02.050 [DOI] [PubMed] [Google Scholar]
- 32.Verschuur EM, Repici A, Kuipers EJ, Steyerberg EW, Siersema PD. New design esophageal stents for the palliation of dysphagia from esophageal or gastric cardia cancer: a randomized trial. The American journal of gastroenterology. 2008;103(2):304–12. doi: 10.1111/j.1572-0241.2007.01542.x [DOI] [PubMed] [Google Scholar]
- 33.Wenger U, Johnsson E, Arnelo U, Lundell L, Lagergren J. An antireflux stent versus conventional stents for palliation of distal esophageal or cardia cancer: a randomized clinical study. Surgical endoscopy. 2006;20(11):1675–80. CN-00572069 UPDATE. doi: 10.1007/s00464-006-0088-2 [DOI] [PubMed] [Google Scholar]
- 34.Wenger U, Johnsson E, Bergquist H, Nyman J, Ejnell H, Lagergren J, et al. Health economic evaluation of stent or endoluminal brachytherapy as a palliative strategy in patients with incurable cancer of the oesophagus or gastro-oesophageal junction: results of a randomized clinical trial. European journal of gastroenterology & hepatology. 2005;17(12):1369–77. CN-00561518 UPDATE. [DOI] [PubMed] [Google Scholar]
- 35.White RE, Chepkwony R, Mwachiro M, Burgert SL, Enders FT, Topazian M. Randomized trial of small-diameter versus large-diameter esophageal stents for palliation of malignant esophageal obstruction. Journal of Clinical Gastroenterology. 2015;49(8):660–5. doi: 10.1097/MCG.0000000000000333 [DOI] [PubMed] [Google Scholar]
- 36.Zhu HD, Guo JH, Mao AW, Lv WF, Ji JS, Wang WH, et al. Conventional stents versus stents loaded with (125)iodine seeds for the treatment of unresectable oesophageal cancer: a multicentre, randomised phase 3 trial. The Lancet. 2014;Oncology. 15(6):612–9. CN-00988108 UPDATE. doi: 10.1016/S1470-2045(14)70131-7 [DOI] [PubMed] [Google Scholar]
- 37.Spaander MC, Baron TH, Siersema PD, Fuccio L, Schumacher B, Escorsell À, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48(10):939–48. doi: 10.1055/s-0042-114210 [DOI] [PubMed] [Google Scholar]
- 38.Sgourakis G, Gockel I, Radtke A, Dedemadi G, Goumas K, Mylona S, et al. The use of self-expanding stents in esophageal and gastroesophageal junction cancer palliation: a meta-analysis and meta-regression analysis of outcomes. Digestive diseases and sciences. 2010;55(11):3018–30. Epub 2010/05/05. doi: 10.1007/s10620-010-1250-1 . [DOI] [PubMed] [Google Scholar]
- 39.Lettmaier S, Strnad V. Intraluminal brachytherapy in oesophageal cancer: defining its role and introducing the technique. Journal of Contemporary Brachytherapy. 2014;6(2):236–41. doi: 10.5114/jcb.2014.43780. PMC4105652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuccio L, Mandolesi D, Farioli A, Hassan C, Frazzoni L, Guido A, et al. Brachytherapy for the palliation of dysphagia owing to esophageal cancer: A systematic review and meta-analysis of prospective studies. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2017;122(3):332–9. Epub 2017/01/21. doi: 10.1016/j.radonc.2016.12.034 . [DOI] [PubMed] [Google Scholar]
- 41.Fuccio L, Guido A, Hassan C, Frazzoni L, Arcelli A, Farioli A, et al. Underuse of brachytherapy for the treatment of dysphagia owing to esophageal cancer. An Italian survey. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2016;48(10):1233–6. Epub 2016/08/02. doi: 10.1016/j.dld.2016.07.003 . [DOI] [PubMed] [Google Scholar]
- 42.Sabharwal T, Gulati MS, Fotiadis N, Dourado R, Botha A, Mason R, et al. Randomised comparison of the FerX Ella antireflux stent and the ultraflex stent: proton pump inhibitor combination for prevention of post-stent reflux in patients with esophageal carcinoma involving the esophago-gastric junction. Journal of gastroenterology and hepatology. 2008;23(5):723–8. Epub 2008/04/16. doi: 10.1111/j.1440-1746.2008.05396.x . [DOI] [PubMed] [Google Scholar]
- 43.Mills EJ, Ghement I, O'Regan C, Thorlund K. Estimating the power of indirect comparisons: a simulation study. PloS one. 2011;6(1):e16237 Epub 2011/02/02. doi: 10.1371/journal.pone.0016237 ; PubMed Central PMCID: PMCPmc3025012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ. 2016;352 doi: 10.1136/bmj.i1981 [DOI] [PubMed] [Google Scholar]
- 45.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. Journal of clinical epidemiology. 2000;53(11):1119–29. [DOI] [PubMed] [Google Scholar]
- 46.Trinquart L, Chatellier G, Ravaud P. Adjustment for reporting bias in network meta-analysis of antidepressant trials. BMC medical research methodology. 2012;12(1):150 doi: 10.1186/1471-2288-12-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Network of stent interventions for palliative treatments that reported the stent migration in esophageal cancer; A: Ultraflex stent is reference treatment, B: Metallic stent is reference, and C: Antireflux stent is reference.
(TIF)
Forest plots for stent migration in network A, B, C; Ultraflex stent, Metallic stent, Antireflux stent are reference treatments in network A, B, and C respectively.
(TIF)
Network of stent interventions for palliative treatments that reported the aspiration in patients; A: Ultraflex stent is reference treatment, B: Irradiation stent is reference, and C: Brachytherapy is reference.
(TIF)
Forest plots for aspiration in network A, B, C; covered evolution stent, Irradiation stent, brachytherapy are reference treatments in network A, B, and C respectively.
(TIF)
Network of stent interventions for palliative treatments that reported the severe pain in patients; Irradiation stent, Polyflex stent, Metallic stent and SEMS 18 are reference treatments in networks A, B, C and D respectively.
(TIF)
Forest plots for severe pain in network A, B, C and D; Antireflux stent, Ultraflex stent, Metallic stent, and brachytherapy are reference treatments in network A, B, C and D respectively.
(TIF)
Network of stent interventions for palliative treatments that reported the fistula in patients; Irradiation stent, Polyflex stent, Metallic stent and SEMS 18 are reference treatments in networks A, B, C and D respectively.
(TIF)
Forest plots for fistula in network A, B, and C. Metallic stent, Irradiation stent, and brachytherapy are reference treatments in network A, B, and C respectively.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.