Abstract
A simple and efficient approach for the synthesis of 2-aminoquinazoline derivatives in moderate to good yields. This reaction employs mild reaction conditions, is metal-free and utilizes readily available starting materials making it a more viable reaction for the scale up synthesis and ligand diversity. Notably, this methodology allows the synthesis of 2-aminoquinazolines using a free amine or cyclic amine enabling structural diversity and good atom economy.
Keywords: Quinazolines, Oxidative Annulation, Metal Free, Heteroaromatic
Graphical abstract
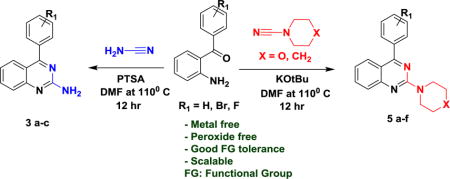
Designing a simple and efficient chemical reaction sequence that provides maximum structural diversity with few synthetic steps to yield small heterocyclic molecules with interesting biological function is a challenge to both medicinal and synthetic chemists.1 As a representative of heterocyclic molecules, 2-aminoquinazolines are drug-like scaffolds and exhibit wide range of biological activities such as potent, selective, and orally efficacious inhibitors of receptor tyrosine kinase c-Kit, represented by the 5,6-dihydro-pyridinone 12 along with a 2¢-deoxynucleotide analogue 23 (figure 1). The 2-aminoquinazoline and quinazoline scaffold is also represented in FDA-approved pharmaceuticals including the antihypertensive agent Prazosin (3) and the anti-cancer agent Gefitinib (4) (figure 1).
Figure 1.
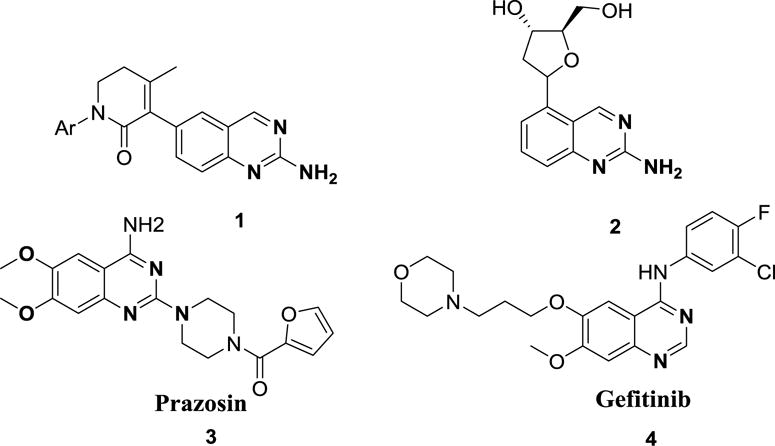
Selected examples of tyrosine kinase receptor inhibitors (1), 2¢-deoxynucleotide analogue (2) and pharmaceutical drugs Prazosin (3) and Gefitinib (4), possessing the quinazoline scaffold.
Diverse ranges of pharmacological activities of 2-aminoquinazoline derivatives have sparked considerable interest in their synthesis using versatile and scalable methods.4–6 There are very few approaches that describe the construction of 2-aminoquinazolines (scheme 1). For example, 2-aminoquinazoline derivatives have been developed using arylboronic acids as a precursor (scheme 1, A).4 In 2005, Mahajan reported a microwave-promoted synthesis of quinazolines from N-arylamidines and aldehydes (scheme 1, B).5 Alternatively, the reaction of 2-halophenyl ketone with guanidine derivatives in the presence of copper catalyst yielded 2-aminoquinazoline derivatives (scheme 1, C).6 These reactions suffer from harsh reaction conditions using microwaves, use of reactive metal catalysts and expensive boronated pre-functionalized starting materials, which limits ligand diversity and scale up processes.
Scheme 1.
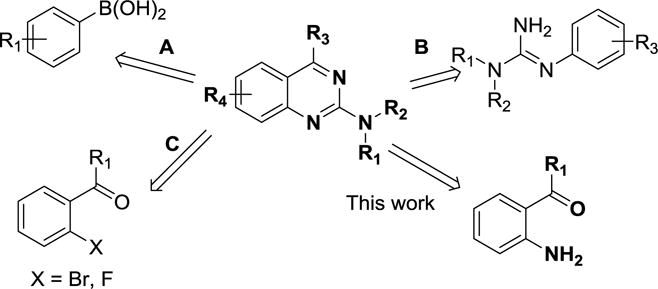
Retrosynthetic analysis of the 2-aminoquinazoline scaffold.
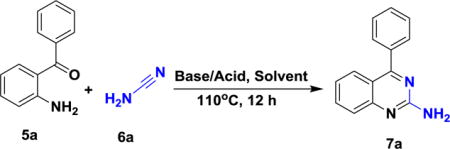
Pandya et al have explored the metal catalyzed transformation of small heterocycles including the C-H functionalization.7,8 Herein, we report a metal-free and simple reaction scheme for the synthesis of 2-aminated (primary and tertiary amines) quinazoline derivatives using readily available starting materials producing moderate to high yields. Our initial efforts commenced by designing unsubstituted 2-amino derivatives of the quinazoline scaffold using unsubstituted cynamide (1 eq.) and 2-aminobenzophenone (1eq.). To identify suitable reaction conditions, we started our investigation with PTSA (p-toluene sulfonic acid) (1 eq.) as a catalyst for the reaction in THF (table 1, entry 1). These conditions produced the desired product (7a) in moderate yields (61%). To our delight, the yield was increased to 75% when changing the solvent to DMF, also in the presence of PTSA (table 1, entry 2). Decreasing the reaction temperature to 70°C produced the desired product in a low yield (table 1, entry 5) and at room temperature the reaction did not produce product (table 1, entry 6). Notably, after basifying reaction conditions using KOtBu, the reaction produced moderate yields (table 1, entry 3 & 4).
Table 1.
Optimization of reaction conditions for the unsubstituted 2-aminoquinazoline derivatives.
| ||||
|---|---|---|---|---|
| entry | acid/base | equivalent | solvent | yields (%)c |
| 1 | PTSA | 1 | THF | 61 |
| 2 | PTSA | 1 | DMF | 75 |
| 3 | KOtBu | 0.5 | DMF | 55 |
| 4 | KOtBu | 1 | DMF | 65 |
| 5a | PTSA | 1 | DMF | 8 |
| 6b | PTSA | 1 | DMF | 0 |
Reaction Carried out at 70° C.
Reaction carried out at room temperature.
Isolated yields (flash chromatography). PTSA: para toluene sulfonic acid
The reaction of 4-morpholinecarbonitrile (1 eq.) with 2-aminobenzophenone (1eq.) produced a cyclic amine at the 2-position of the quinazoline core. To our surprise, the reaction gave good yield when KOtBu used over the PTSA. (table 2, entry 1 & 2).
Table 2.
Optimization of reaction conditions for substituted cyclic 2-aminoquinazoline derivatives.
| ||||
|---|---|---|---|---|
| entry | acid/base | equivalent | solvent | yields (%)a |
| 1 | PTSA | 1 | DMF | 66 |
| 2 | KOtBu | 1 | DMF | 78 |
Isolated Yields (Flash Chromatography).
With the optimized reaction conditions in hand, further exploration for the scope of substrate and functional group tolerance was explored. The reaction with strong electron withdrawing groups (7b, 9b, 9e) present on the aromatic ring of the acyl phenyl ring resulted in the good yield compared to weak electron withdrawing (7c, 9c, 9f) and unsubstituted aromatic ring (7a, 9a, 9d) shown in scheme 2.
Scheme 2.
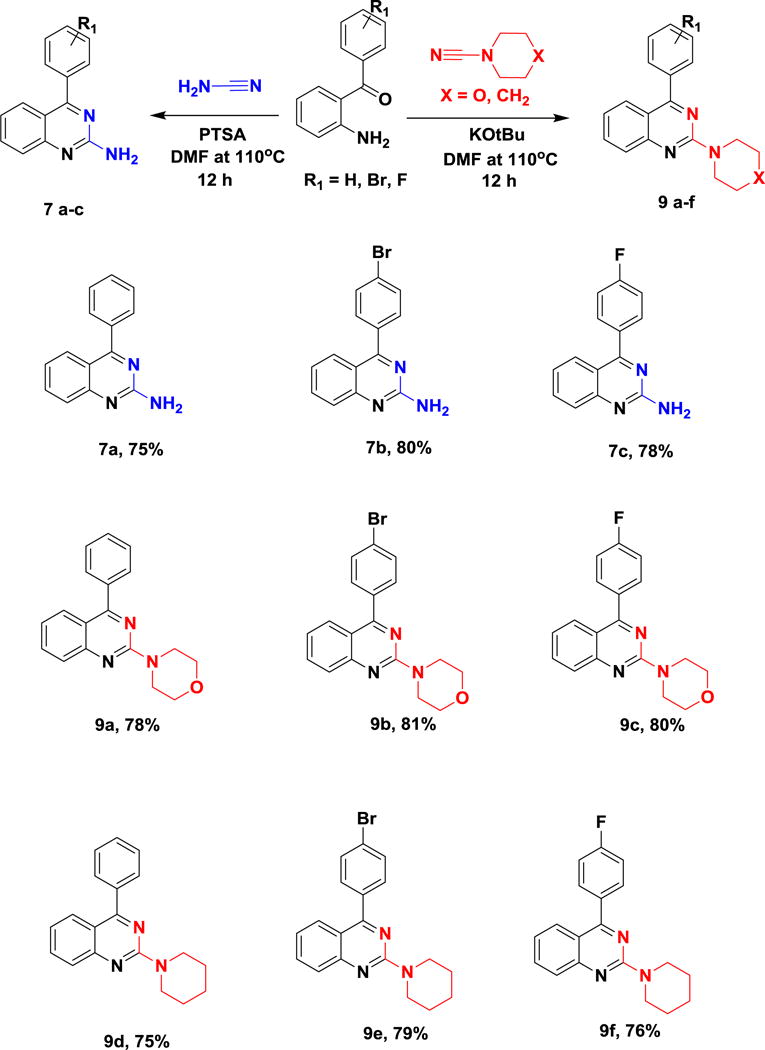
Substrate scope used in the synthesis of 2-aminoquinazoline derivatives.
Structure conformation of the synthesized compounds was confirmed by 1H & 13C NMR and mass spectrometry (supplemental information). Lastly, to dually confirm the synthesis of 2-aminoquinazolines, overnight evaporation of the compound 9e dissolved in ethyl acetate and hexane (3:1) gave a single crystal for the x-ray analysis illustrated in figure 2.
Figure 2.
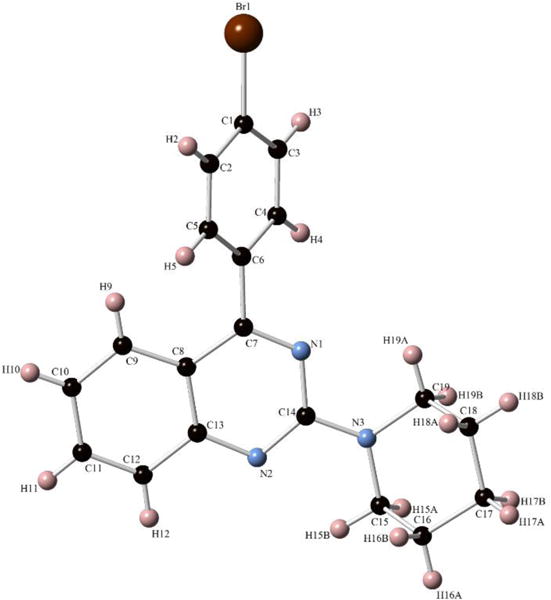
X-ray crystal structure of 2-aminoquinazoline 9e.
Plausible reaction mechanism for 2-aminoquinazoline formation mediated by acid or base is shown in scheme 3. Weak acid PTSA carried out the protonation of the cyano group’s nitrogen, which increases the electrophilic nature of the cyanamide carbon.9 This allows the amine to attack the electrophilic carbon forming the amidine intermediate. This intermediate undergoes cyclization followed by elimination of a water molecule to produce the 2-aminoquinazoline. Conversely, base-mediated proton removal of the amine yields a strong nucleophile capable of reacting with the cyanamide carbon, followed by ring closure afforded product. In summary, acid increases cyanamide carbon electrophilicity, while base increases nucleophilicity, which is one of the key steps in the reaction kinetics.
Scheme 3.
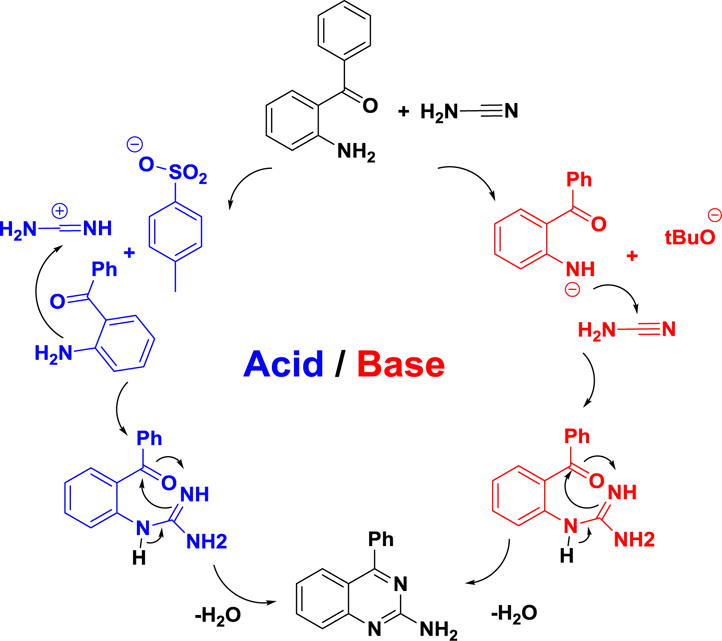
Plausible reaction mechanism for a metal free cascade cyclization.
In conclusion, we have developed a simple and efficient approach for the synthesis of 2-aminoquinazoline derivatives in moderate to good yields. The advantage of this reaction is its mild and metal free reaction conditions. This methodology allows the synthesis of a 2-aminoquinazoline core functionalized with a free amine as chemical handle for the further structural diversity generating a potentially biologically important compound library.
Supplementary Material
Highlights.
Metal-free reaction conditions for the synthesis of 2-aminoquinazolines
Mild reaction conditions for the synthesis of 2-aminoquinazolines
Functionalization of the biologically relevant quinazoline scaffold
Scalable synthesis for 2-aminoquinazolines
Acknowledgments
This project was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases research grant AI116525, American Associations of Colleges of Pharmacy (AACP) New Investigator Award and Creighton University (LB692) to EJN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
General experimental procedures, Mass and NMR spectral data for compounds are provided in supporting information.
References
- 1.Schreiber SL. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science. 2000;287(5460):1964–1969. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 2.Hu E, Tasker A, White RD, Kunz RK, Human J, Chen N, Burli R, Hungate R, Novak P, Itano A, Zhang X, Yu V, Nguyen Y, Tudor Y, Plant M, Flynn S, Xu Y, Meagher KL, Whittington DA, Ng GY. Discovery of Aryl Aminoquinazoline Pyridones as Potent, Selective, and Orally Efficacious Inhibitors of Receptor Tyrosine Kinase c-Kit. Journal of Medicinal Chemistry. 2008;51(11):3065–3068. doi: 10.1021/jm800188g. [DOI] [PubMed] [Google Scholar]
- 3.Li JS, Fan YH, Zhang Y, Marky LA, Gold B. Design of triple helix forming C-glycoside molecules. Journal of the American Chemical Society. 2003;125(8):2084–2093. doi: 10.1021/ja028033x. [DOI] [PubMed] [Google Scholar]
- 4.Tran LQ, Li J, Neuville L. Copper-Catalyzed Domino Three-Component Approach for the Assembly of 2-Aminated Benzimidazoles and Quinazolines. The Journal of organic chemistry. 2015;80(12):6102–6108. doi: 10.1021/acs.joc.5b00614. [DOI] [PubMed] [Google Scholar]
- 5.Kumar V, Mohan C, Gupta M, Mahajan MP. A catalyst-and solvent-free selective approach to biologically important quinazolines and benzo [g] quinazoline. Tetrahedron. 2005;61(14):3533–3538. [Google Scholar]
- 6.(a) Huang X, Yang H, Fu H, Qiao R, Zhao Y. Efficient copper-catalyzed synthesis of 2-amino-4 (3H)-quinazolinone and 2-aminoquinazoline derivatives. Synthesis. 2009;2009(16):2679–2688. [Google Scholar]; (b) Hynes JB, Campbell JP, Hynes JD. Synthesis of 2-aminoquinazolines from ortho-fluoroketones. Journal of heterocyclic chemistry. 1995;32(4):1185–1187. [Google Scholar]
- 7.Pandya AN, Agrawal DK. A concise synthesis of highly substituted imidazoles via copper-mediated oxidative C–H functionalization. Tetrahedron letters. 2014;55(10):1835–1838. doi: 10.1016/j.tetlet.2014.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandya AN, Fletcher JT, Villa EM, Agrawal DK. Silver-mediated synthesis of indolizines via oxidative C–H functionalization and 5-endo-dig cyclization. Tetrahedron letters. 2014;55(50):6922–6924. doi: 10.1016/j.tetlet.2014.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalani HB, Pandya AN, Baraiya AB, Kaila JC, Pandya DH, Sharma JA, Sudarsanam V, Vasu KK. An efficient synthesis of 2-aminopyrroles from enaminone–amidine adduct and phenacyl/benzyl/heteroalkyl-halides. Tetrahedron Letters. 2011;52(48):6331–6335. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


