Nevertheless the difference in mind between man and the higher animals, great as it is, is certainly one of degree and not of kind
~Darwin, 1871
Following Darwin, the study of human cognition has been properly placed within an evolutionary context. To understand the human mind – how we acquire, process, store, and act on information from the environment – we have to know the long history of our species and the selective pressures that shaped our ancestors. One way of doing this is to compare the differences and similarities among extant species in cognitive processes and the neuroanatomical structures that underlie them. This approach requires understanding the phylogenetic relationships among species to infer the evolutionary changes that occurred in the past. Because the great apes (the primate group that includes chimpanzees, bonobos, gorillas, and orangutans) are the closest living relatives of modern humans, they are an essential basis for comparison. Indirect as it is, the comparative method is one of the most powerful tools we have available, as brains and cognition do not fossilize. Indeed, comparative analysis has provided important insights into the evolution of human behavior and cognition. It has been observed that many behaviors previously thought to be uniquely human are actually present in other species as well, sometimes even to a greater degree (i.e., eusociality in Hymenoptera). Tool-making and use, for example, are not restricted to our species alone. When Jane Goodall reported tool-making in chimpanzees, Louis Leakey famously replied: “Now we must redefine ‘tool’, redefine ‘man’, or accept chimpanzees as humans” [1].
Yet, examples of apparent cognitive and behavioral discontinuity between humans and other species abound. Our syntactically rich language, ability to understand mental states of others, and propensity to generate and manipulate symbols are exceptional [2]. What are the possible evolutionary changes in brain structure that allowed these faculties? Here, we offer a brief overview of the evolution of the human brain and the likely neuroanatomical changes associated with our species’ distinctive cognitive abilities. These changes occurred at several levels of brain organization, providing the neuroanatomical hardware for the complexity of human behavior and cognition.
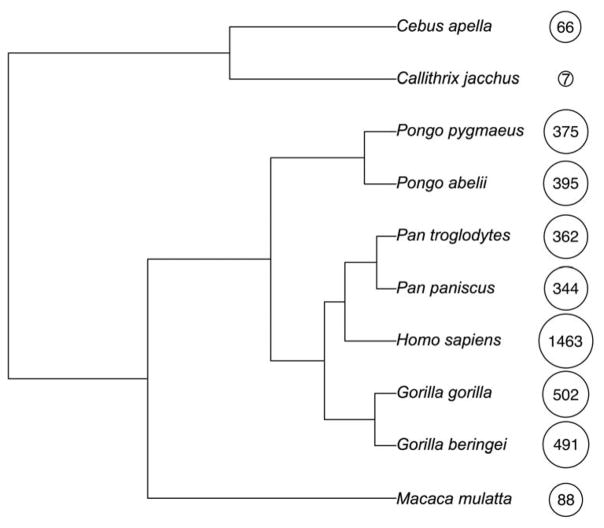
Primates are a very diverse group of mammals of more than 500 living species that includes lemurs, lorises, tarsiers, New World monkeys, Old Word monkeys, and apes. Most comparative neuroanatomical research has focused on a tiny fraction of this variety, concentrating on a few primate model species, including common marmosets (Callithrix jacchus), capuchin monkeys (Cebus apella), rhesus macaques (Macaca mulatta), and chimpanzees (Pan troglodytes) [3]. Our closest living relatives are the chimpanzees and the bonobos with whom we share a common ancestor dated to about 4–8 million years ago [4] (Figure 1).
Figure 1. Phylogenetic tree of primates.
Phylogenetic relationships among the great apes and other primate species mentioned. The numbers indicate the endocranial volumes (in cm3, rounded to the nearest whole number) and the size of the circles shows the natural log transformation of the endocranial volumes to demonstrate the diversity in brain size of the primate species. Data from [5,6].
Compared to other primates, including great apes, humans have very large brains. Weighing approximately 1,400 grams, our brains are roughly three times larger than those of other great apes and also significantly larger than expected for a primate of our body size. Fossil evidence of the cranial capacity of our direct ancestors indicates gradual increase in brain size early in hominin evolution with a period of more accelerated growth within the last 2 million years [7]. However, important as it certainly must be for increasing numbers of neurons, synaptic connections, and related processing capacity, brain size expansion alone cannot explain the cognitive complexity of our species for several reasons. Many species, including non-primates such as elephants and whales, have brains larger than ours (although perhaps fewer neocortical neurons) and yet do not approach the cognitive sophistication of humans. Moreover, species may be comparable in brain size yet differ dramatically in behavior and social skills. Chimpanzees and bonobos, for example, have similar brain size but differ in temperament and social behavior. Lastly, there is about 1,000 g normal range of variation among modern humans in brain mass, however all are capable of commanding language and other human-specific capacities. Therefore, much of the answer probably lies in changes to brain development, neuroanatomical reorganization, and modifications at the molecular level. It is likely that the reorganization at these levels, and not solely absolute brain size or total number of neurons, produces species differences in cognition.
The increase in human brain size is due mostly to expansion of the neocortex [8], particularly heteromodal association regions of the frontal, temporal, and parietal lobes. To some extent, evolutionary changes to human association cortex have involved differential enlargement of regions that are homologous in other primates and alterations to cortical connectivity [9]. Notably, many regions of the prefrontal cortex have been shown to be homologous between humans and other great ape and monkey species, including Broca’s language areas (areas 44/45) (Friederici, 2016). Some of the changes to human association cortex, however, might comprise differentiation of novel and functionally distinct areas that perform increasingly fine-grained information processing. For example, data suggest that, compared to rhesus macaques, human intraparietal sulcus (IPS) includes four additional motion-sensitive areas dedicated to processing of three-dimensional form in relation to motion [10]. As a result, human parietal lobe includes more regions dedicated to processing of shape compared to that of rhesus macaques. Addition of these and other posterior parietal areas likely enhanced processing of visual and somatosensory information necessary for complex manipulative abilities required for tool manufacture and manipulation [11,12]. Using combined functional and structural MRI data from the Human Connectome Project, a recent study identified some 180 distinct cortical areas in the human brain based on variation in cortical thickness, myelination, and connectivity patterns [13]. This number far exceeds estimates of the number of areas for other primate species [14], although such an approach for parcellation has not yet been conducted with brains of non-human species.
A point of disagreement has been the relative size of the frontal lobes and different prefrontal cortical areas in humans versus other primate species. Regions of the prefrontal cortex play a significant role in language, planning, decision-making, working memory, and other higher-order cognitive functions. Some have argued that change in the absolute and proportional size of the prefrontal cortex is tightly correlated with corresponding changes in other brain areas [15,16], while other analyses show that human prefrontal cortex is enlarged beyond what would be predicted from primate brain scaling trends [17,18]. In this context, direct comparisons of various cytoarchitecturally-defined cortical areas in humans and chimpanzees show that frontopolar cortex (area 10), Broca’s area (areas 44/45), and the anterior insular cortex are about six times larger in humans, whereas the primary motor cortex (area 4) and the primary visual cortex (area 17) are much more similar in size between the species [19].
Reorganization of the human brain is also apparent at the level of microstructure, such as the distribution of neurons and glial cells, innervation patterns of neurotransmitters, and expression of genes and proteins. For example, several studies have examined neuron density across different cortical areas in a number of primate [20] and other mammalian species [21]. Results show that neuronal cell density generally decreases with brain size. This decrease in the packing density of neuronal cell bodies seems to be proportionally related to the increase in the space occupied by dendrites, axons, synapses, and glial processes in the surrounding neuropil space, suggesting greater connectivity patterns [22].
Human brains also show specialization in distribution of glial cells. Glial cells are functionally very diverse and, in addition to providing structural and metabolic support to neurons, play an important role in higher cognitive functions, such as learning and memory [23]. These cells are as numerous as neurons – an adult human brain contains an approximately equal number of neuronal and glial cells (about 86 billion each) – and the ratios of these cells vary across cortical and subcortical structures [24]. Comparative analysis of glia-neuron ratios in 18 species of primates showed that human dorsolateral prefrontal cortex (area 9) has a higher ratio (1.65) than other species [25], suggesting a greater glial metabolic support necessary to maintain larger dendritic arborizations and long-range projecting axons of the human brain. Further possible specialization of the human necortex concerns distribution patterns of different subtypes of astrocytes, some of which are absent in non-primate species. These include interlaminar astrocytes that send long processes across upper cortical layers and polarized astrocytes also with long processes that extend vertically and inhabit deeper layers [26]. Moreover, when compared to other species, human astrocytes are both larger in diameter and more elaborate in number of processes [27]. It has been argued that human patterns of glial cell biology not only contribute to our species’ complex cognitive abilities but also provide unique vulnerability to neuropathologies compared to other primates [26, 27].
Distribution patterns of neurotransmitters in cortical and subcortical regions are also known to vary between humans and other primates [28,29,30,31]. For example, a recent examination of basal ganglia showed human-specific increase in dopaminergic innervation of the medial caudate nucleus [32], a highly interconnected region of the striatum that is involved in language production, among other functions. Notably, this increase is evident even in the context of a relatively smaller striatum than predicted for a primate of human brain size, suggesting an evolutionary reorganization in microstructure in the absence of concomitant increase in volume. This finding is interesting in that normal activity patterns within the medial caudate nucleus in humans depend on the presence of the functional copy of FOXP2 gene [33], suggesting a link between FOXP2 evolution and distribution of dopamine.
Of particular interest has been the genetic basis of human specialization in brain organization and function. Early analyses revealed a global pattern of increased gene expression in the human brain compared to nonhuman primates, which was not evident in non-brain tissue, such as the heart and the liver [34,35,36]. Examination of these genes revealed an enrichment of those involved in synaptic transmission and plasticity as well as energy metabolism in showing upregulation, supporting the idea that human brain evolution is characterized by molecular modifications to increase levels of neuronal and synaptic activity [37]. Following studies found more examples of genes showing increased levels of expression, such as thrombospondin genes involved in the control of synaptogenesis compared to nonhuman primates [38]. It is important to note, however, that the relationship between mRNA expression and protein abundance is not always straightforward and may depend on biological function. For example, a recent analysis revealed that the relationship between mRNA expression and protein expression in humans and chimpanzees was stronger for some biological functions, such as oxidative metabolism and protein synthesis and modification, and weaker for others [39].
Taken together, these findings show how the human brain is the product of evolutionary changes that occurred over time that underpin the complex cognitive abilities of our species. Human specialization in brain organization and function is apparent at several levels of organization. Changes in overall anatomy, such as increase in absolute and relative size and addition of more cortical areas likely provided the neuroanatomical basis for processing of evermore fine-grained information. Changes in microstructure, such as the distribution patterns of neurons and glial cells and changes in expression of both mRNA and proteins, allowed for plasticity and increased learning capacity that, when coupled with cultural and social forces, shaped human cognition.
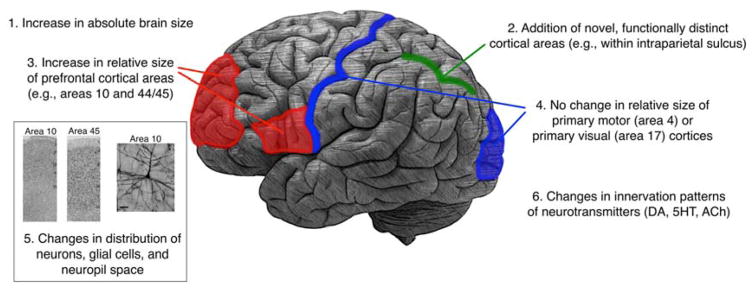
Figure 2. Neuroanatomical changes in human brain evolution.
Summary of the neuroanatomical changes in human brain evolution, including macro- and micro-structural changes (see text for more detail).
HIGHLIGHT.
Human brain shows examples of specialization at several levels of organization
Increase in absolute brain size
Increase in relative size of some brain areas (e.g., prefrontal areas 10 and 44/45) but not others (e.g., primary motor (area 4) and primary visual (area 17))
Addition of novel, functionally distinct cortical areas that process increasingly fine-grained information
Human-specific changes in distribution of neurons, glial cells, and neuropil space
Human-specific changes in innervation patterns of neurotransmitters (e.g., dopamine)
Human-specific changes in gene and protein expression
Acknowledgments
This work was supported by the National Institutes of Health (NS042867 and NS073134) as well as the James S. McDonnell Foundation (220020293).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodall J. Through a Window: 30 Years Observing the Gombe Chimpanzees. Weidenfeld and Nicolson; 1990. [Google Scholar]
- 2.Sherwood CC, Subiaul F, Zawidzki TW. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J Anat. 2008;212:426–454. doi: 10.1111/j.1469-7580.2008.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, Hart BA, Hopkins WD, Hu S-L, Miller LA, Nader MA, Nathanielsz PW, Rogers J, Shively CA, Voytko ML. Why primate models matter. Am J Primatol. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley BJ. Reconstructing phylogenies and phenotypes: a molecular view of human evolution. J Anat. 2008;212:337–353. doi: 10.1111/j.1469-7580.2007.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isler K, Christopher Kirk E, Miller JMA, Albrecht GA, Gelvin BR, Martin RD. Endocranial volumes of primate species: scaling analyses using a comprehensive and reliable data set. J Hum Evol. 2008;55:967–978. doi: 10.1016/j.jhevol.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 6.de Sousa A, Cunha E. Hominins and the emergence of the modern human brain. Prog Brain Res. 2012;195:293–322. doi: 10.1016/B978-0-444-53860-4.00014-3. [DOI] [PubMed] [Google Scholar]
- 7.Reyes LD, Sherwood CC. Neuroscience and Human Brain Evolution. In: Bruner E, editor. Human Paleoneurology. Springer; 2015. pp. 11–37. [Google Scholar]
- 8.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL, Krienen FM. The evolution of distributed association networks in the human brain. Trends in Cogn Sci. 2013;17:648–665. doi: 10.1016/j.tics.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand J-B, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Stout D, Chaminade T. The evolutionary neuroscience of tool making. Neuropsychologia. 2007;45:1091–1100. doi: 10.1016/j.neuropsychologia.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Verendeev A, Sherwood CC, Hopkins WD. Organization and evolution of the neural control of the hand in primates: Motor systems, sensory feedback, and laterality. In: Kivell TL, Lemelin P, Richmond BG, Schmitt D, editors. The Evolution of the Primate Hand. Springer; 2016. pp. 131–153. [Google Scholar]
- **13.Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–178. doi: 10.1038/nature18933. Using multi-modal approach to parcellation, the authors identified some 180 functionally and cytoarchitecturally distinct cortical areas in the human brain. The method developed in this study will dramatically improve future studies of structural and functional organization of the cerebral cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Essen DC, Dierker DL. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron. 2007;56:209–225. doi: 10.1016/j.neuron.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Barton RA, Venditti C. Human frontal lobes are not relatively large. Proc Natl Acad Sci. 2013;110:9001–9006. doi: 10.1073/pnas.1215723110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabi M, Neves K, Masseron C, Ribeiro PFM, Ventura-Antunes L, Torres L, Mota B, Kaas JH, Herculano-Houzel S. No relative expansion of the number of prefrontal neurons in primate and human evolution. Proc Natl Acad Sci. 2016;113:9617–9622. doi: 10.1073/pnas.1610178113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherwood CC, Smaers JB. What’s the fuss over human frontal lobe evolution? Trends Cogn Sci. 2013;17:432–433. doi: 10.1016/j.tics.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Passingham RE, Smaers JB. Is the prefrontal cortex especially enlarged in the human brain allometric relations and remapping factors. Brain Behav Evol. 2014;84:156–166. doi: 10.1159/000365183. [DOI] [PubMed] [Google Scholar]
- 19.Sherwood CC, Bauernfeind AL, Bianchi S, Raghanti MA, Hof PR. Human brain evolution writ large and small. Prog Brain Res. 2012;195:237–254. doi: 10.1016/B978-0-444-53860-4.00011-8. [DOI] [PubMed] [Google Scholar]
- 20.Sherwood CC, Raghanti MA, Stimpson CD, Spocter MA, Uddin M, Boddy AM, Wildman DE, Bonar CJ, Lewandowski AH, Phillips KA, Erwin JM, Hof PR. Inhibitory interneurons of the human prefrontal cortex display conserved evolution of the phenotype and related genes. Proc R Soc Lond [Biol] 2010;277:1011–1020. doi: 10.1098/rspb.2009.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charvet CJ, Cahalane DJ, Finlay BL. Systematic, cross-cortex variation in neuron numbers in rodents and primates. Cereb Cortex. 2015;25:147–160. doi: 10.1093/cercor/bht214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherwood CC, Bauernfeind AL, Verendeev A, Raghanti MA, Hof PR. Evolutionary specializations of human brain microstructure. In: Kaas J, editor. Evolution of Nervous Systems. 2. Academic Press; 2016. pp. 121–139. [Google Scholar]
- **23.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Alcino J, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. Human glial progenitor cells (GPCs) were engrafted into neonatal mice. These matured into astrocytes that displayed characteristics of human astrocytes in their morphology and physiology. Mice engrafted with human GPCs showed enhanced synaptic plasticity and improvement in learning compared to control mice engrafted with mouse GPCs. This study unequivocally demonstrates the importance of glial cells in cognitive function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Filho WJ, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci. 2006;103:13606–13611. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Oberheim NA, Takano T, Han X, He W, Lin JHC, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cholinergic innervation of the frontal cortex: differences among humans, chimpanzees, and macaque monkeys. J Comp Neurol. 2008;506:409–424. doi: 10.1002/cne.21546. [DOI] [PubMed] [Google Scholar]
- 29.Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cereb Cortex. 2008;18:584–597. doi: 10.1093/cercor/bhm089. [DOI] [PubMed] [Google Scholar]
- 30.Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Neurosci. 2008;155:203–220. doi: 10.1016/j.neuroscience.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghanti MA, Spocter MA, Stimpson CD, Erwin JM, Bonar CJ, Allman JM, Hof PR, Sherwood CC. Species-specific distributions of tyrosine hydroxylase-immunoreactive neurons in the prefrontal cortex of anthropoid primates. Neurosci. 2009;158:1551–1559. doi: 10.1016/j.neuroscience.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *32.Raghanti MA, Edler MK, Stephenson AR, Wilson LJ, Hopkins WD, Ely JJ, Erwin JM, Jacobs B, Hof PR, Sherwood CC. Human-specific increase of dopaminergic innervation in a striatal region associated with speech and language: A comparative analysis of the primate basal ganglia. J Comp Neurol. 2016;524:2117–2129. doi: 10.1002/cne.23937. This study examined dopaminergic innervation of the striatum in several primate species. The authors found evidence for human-specific increase in dopaminergic innervation of the medial caudate nucleus, even in the context of a relatively smaller striatum than predicted for a primate of human brain size. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci. 1998;95:12695–12700. doi: 10.1073/pnas.95.21.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enard W, Khaitovich P, Klose J, Zöllner S, Heissig F, Giavalisco P, Nieselt-Struwe K, Muchmore E, Varki A, Ravid R, Doxiadis GM, Bontrop RE, Pääbo S. Intra- and interspecific variation in primate gene expression patterns. Science. 2002;296:340–343. doi: 10.1126/science.1068996. [DOI] [PubMed] [Google Scholar]
- 35.Cáceres M, Lachuer J, Zapala MA, Redmond JC, Kudo L, Geschwind DH, Lockhart DJ, Preuss TM, Barlow C. Elevated gene expression levels distinguish human from non-human primate brains. Proc Natl Acad Sci. 2003;100:13030–13035. doi: 10.1073/pnas.2135499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uddin M, Wildman DE, Liu G, Xu W, Johnson RM, Hof PR, Kapatos G, Grossman LI, Goodman M. Sister grouping of chimpanzees and humans as revealed by genome-wide phylogenetic analysis of brain gene expression profiles. Proc Natl Acad Sci. 2004;101:2957–2962. doi: 10.1073/pnas.0308725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preuss TM, Cáceres M, Oldham MC, Geschwind DH. Human brain evolution: insights from microarrays. Nature Rev Genet. 2004;5:850–860. doi: 10.1038/nrg1469. [DOI] [PubMed] [Google Scholar]
- 38.Cáceres M, Suwyn C, Maddox M, Thomas JW, Preuss TM. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex. 2007;17:2312–2321. doi: 10.1093/cercor/bhl140. [DOI] [PubMed] [Google Scholar]
- **39.Bauernfeind AL, Soderblom EJ, Turner ME, Moseley MA, Ely JJ, Hof PR, Sherwood CC, Wray GA, Babbitt CC. Evolutionary divergence of gene and protein expression in the brains of humans and chimpanzees. Genome Biol Evol. 2015;7:2276–2288. doi: 10.1093/gbe/evv132. Using transcriptomic and proteomic analyses, this study examined the relationship between gene and protein expression in human and chimpanzee brains. The authors found the correlation between the two to be lower than previously reported for other tissue types. This finding is significant in highlighting the importance of assessing both gene and protein expression in studies of evolutionary divergence between species. [DOI] [PMC free article] [PubMed] [Google Scholar]