Abstract
Purpose
5-chloro-3-[phenylsulfonyl] indole-2-carboxamide (CSIC) is a highly potent non-nucleoside reverse transcriptase inhibitor (NNRTI) of HIV-1 which has been shown to have a more desirable resistance profile than other NNRTIs in development as HIV prevention strategies. This work involves generation of preformulation data for CSIC and systematic development of a cosolvent system to effectively solubilize this hydrophobic drug candidate. This system was then applied to produce a polymeric thin film solid dosage form for vaginal administration of CSIC for use in prevention of sexual acquisition of HIV.
Methods
Extensive preformulation, formulation development, and film characterization studies were conducted. An HPLC method was developed for CSIC quantification. Preformulation tests included solubility, crystal properties, stability, and drug-excipient compatibility. Cytotoxicity was evaluated using both human epithelial and mouse macrophage cell lines. Ternary phase diagram methodology was used to identify a cosolvent system for CSIC solubility enhancement. Following preformulation evaluation, a CSIC film formulation was developed and manufactured using solvent casting technique. The developed film product was assessed for physicochemical properties, anti-HIV bioactivity, and Lactobacillus biocompatibility during 12-month stability testing period.
Results
Preformulation studies showed CSIC to be very stable. Due to its hydrophobicity, a cosolvent system consisting of polyethylene glycol 400, propylene glycol, and glycerin (5:2:1, w/w/w) was developed, which provided a uniform dispersion of CSIC in the film formulation. The final film product met target specifications established for vaginal microbicide application.
Conclusions
The hydrophobic drug candidate CSIC was successfully formulated with high loading capacity in a vaginal film by means of a cosolvent system. The developed cosolvent strategy is applicable for incorporation of other hydrophobic drug candidates in the film platform.
Keywords: Microbicide, ternary phase diagram, drug-excipient compatibility, solvent cast, vaginal drug delivery
Introduction
Over 2 million people are newly infected with HIV each year. Most of these infections arise from unsafe sexual behavior [1]. Women are more vulnerable to HIV infection than men due to physiology and gender inequality issues including, gender abuse [2–4]. Vaginally-applied products represent a potential strategy for administration of antiretroviral drugs either coitally or pericoitally. The use of such products can be controlled by the women who need them. Combining an acceptable vaginal drug delivery platform with potent anti-HIV drug candidates presents a strategy that can be applied globally to minimize the risk of HIV transmission through sexual intercourse.
Topical microbicides are vaginally- (or rectally-) applied products that have potential to prevent or reduce sexual transmission of HIV [2]. In this context, tight-binding non-nucleoside reverse transcriptase inhibitors (NNRTIs) represent good candidates for HIV prevention due to their high potency, rapid penetration into the HIV membrane and capsid core and selective inhibition of HIV-1 reverse transcriptase (RT) [3–5]. A major drawback of this class of inhibitors is their low genetic barrier to resistance. Patients treated with NNRTI monotherapy or who received sub-optimal dosing of combination treatments containing an NNRTI rapidly developed HIV-1 variants resistant to NNRTIs, and in many cases this resistant HIV showed cross-resistance to other NNRTIs [6, 7]. Therefore, it is vital to utilize an NNRTI that is highly potent and exhibits an improved resistance profile. In this study, 5-chloro-3-[phenylsulfonyl] indole-2-carboxamide (CSIC) was utilized because it exhibits a better resistance profile compared to most clinically used NNRTIs, retaining good activity against the clinically important NNRTI resistance mutation K103N compared to other NNRTIs [5]. Due to their hydrophobic nature many NNRTIs have limited systemic uptake following topical application [3, 8]. Finally, CSIC is a highly potent NNRTI, with an EC50 value of approximately 1 nM [3]. CSIC is a tight-binding inhibitor of HIV-1 RT, meaning that it binds rapidly but then dissociates very slowly [3]. This critical property enables the drug to provide prolonged enzyme inhibition even after exogenous drug is removed. We previously showed that CSIC pretreatment of uninfected T-cells protected the cells from subsequent HIV challenge in the absence of exogenous drug [3]. Furthermore, treatment of infected cells with CSIC attenuated infectivity of nascent virus from these cells and prevented cell-to-cell HIV transmission [3].
Polymeric thin films are advantageous for use as vaginally administered products due to their efficient drug release, enhanced bioadhesive properties, negligible vaginal leakage, potential for discreet use, low cost, and ease of insertion without an applicator [9–11]. In comparison to widely utilized gels, films are easy to insert and correct dose can be delivered to target cervicovaginal tissue without the dose leakage. Due to their smaller footprint, films are also easy to carry and they can be concealed easily, which is useful in situations where women have little control over the sexual activity. Vaginal films provide an additional woman-controlled dosage form option that may improve user adherence [12, 13]. This dosage form has been evaluated for vaginal administration of the contraceptive/antimicrobial agent sodium polystyrene sulfonate [14], the antifungal drug itraconazole [15], prostaglandin E2 for labor induction [16], several anti-HIV or microbicide drug candidates including cellulose acetate phthalate [17], the pyrimidinedione IQP-0528 [10], the nucleotide reverse transcriptase inhibitor tenofovir [18] and the NNRTI dapivirine [19]. Vaginal films are also being explored for the delivery of biomolecules such as the protein PSC-RANTES [20], monoclonal antibodies (MB66) [21], and siRNA [22]. Our group has recently reported safety, pharmacokinetics, and pharmacodynamics of vaginal films in humans. In this Phase 1 study, dapivirine films and gels showed comparable safety, female genital tract distribution, and efficacy in an ex vivo HIV-1 challenge model [23, 24]. Both contraceptive and cleansing vaginal films are commercially available, showing the market acceptability of this dosage form for vaginal applications.
The goal of the present work was to formulate CSIC into a rapidly dissolving vaginal film. We conducted preformulation studies to investigate the physicochemical properties of CSIC such as solubility, crystal form, melting point, stability, and drug-excipient compatibility, which are crucial for formulation development. In vitro cytotoxicity of CSIC was also investigated using human epithelial cells and mouse macrophages. Due to the extreme hydrophobicity of CSIC (log P ~ 3), which was identified during preformulation studies, a cosolvent system was developed to facilitate drug solubilization and dispersion in the polymeric film formulation. Evaluations of the produced film products included analysis of drug content, water content, tensile strength, disintegration, dissolution, anti-HIV bioactivity, and Lactobacillus biocompatibility, during a 12-month stability testing of film samples stored under different conditions, as per the FDA Guidance for Industry Q1A(R2) [25]. This study provides a systematic approach to film formulation development based on thorough investigation of the drug substance from preformulation studies and utilization of strategies such as drug solubilization to obtain a stable and acceptable vaginal film product. Most importantly, the cosolvent strategy utilized in this study is applied for the first time in polymeric vaginal film formulation development, which is significantly useful for utilization of this platform to deliver highly hydrophobic compounds.
Materials and methods
Materials
CSIC was synthesized by Dalton Laboratories (Toronto, Canada) and had a validated purity of >98%. The human epithelial cell line HEC-1A and the mouse macrophage cell line J774A.1 were purchased from American Type Culture Collection (ATCC, Manassas, VA). Methylthiazolyldiphenyl-tetrazolium bromide (MTT) was obtained from Sigma-Aldrich (St. Louis, MO). Excipients including polyvinyl alcohol (PVA), polyethylene glycol 400 (PEG 400), propylene glycol, and glycerin were obtained from Spectrum Chemicals and Laboratory Products (Gardena, CA). Hydroxypropyl methylcellulose 4000 (HPMC K4M) was purchased from Colorcon (West Point, PA). Polyethylene glycol 4000 (PEG 4000) was obtained from Dow Chemical (Midland, MI). Cremophor RH40 was obtained from Spectrum Chemicals. HPLC grade acetonitrile and trifluoroacetic acid (TFA) were purchased from Spectrum and Thermo Scientific, respectively. All the other chemicals were analytical grade.
Preformulation studies
High performance liquid chromatography (HPLC) analysis
CSIC and degradants were quantified by reverse phase HPLC (Gemini C18 4.6x150 mm; Phenomenex, Torrance, CA), with UV detection at 302 nm. The mobile phase was composed of 0.08% v/v TFA in water (A) and 0.05% v/v TFA in acetonitrile (B). A gradient method was developed starting at 30% B, which increased linearly to 50% B over 15 minutes, a 1 minute hold at 50% B followed by return to 30% B for 4 minutes for re-equilibration prior to the next sample injection. The flow rate was 1.4 mL/min. The limit of detection (LOD) and limit of quantification (LOQ) were determined at a signal-to-noise ratio of 3:1 and 10:1, respectively.
Solubility
Solubility of CSIC in acetonitrile, water, and water/acetonitrile mixture (60/40, v/v) was determined by adding a known quantity of drug (~5 mg) to 1.5 mL of the appropriate solvent and then placing the mixture on a rotating mixer for 5 days at room temperature. Samples were then filtered through a 0.2 μm PTFE (Polytetrafluoroethylene) filter, and the filtrate was assessed for CSIC content using HPLC analysis as described above.
Octanol/water partition coefficient (Log Poct/wat) determination
Octanol (oct) and deionized water (wat) were mixed at different ratios: 1:1, 2:1, and 1:2 (v/v) and then rotated vigorously overnight to reach equilibration. Two phases were then separated by centrifugation. A known quantity of CSIC powder was added, and the samples were rotated at room temperature for 12 hours. The CSIC content of each phase was analyzed using HPLC, and the octanol/water partition coefficient (log Poct/wat) value was calculated using the equation below:
Polarized light microscopy
CSIC powder was placed on a glass slide, fixed with Cytoseal 60, and air-dried overnight. Surface morphology of CSIC powder was then observed using a Zeiss Axioskop 40 inverted phase contrast microscope equipped with a polarized light filter, an AxioCam MRc5 color video camera, and analyzed by AxioVision Rel 4.7 software.
Scanning electron microscope (SEM)
CSIC powder was mounted on an aluminum holder using carbon conductive glue and coated with platinum using a platinum sputter coater. Imaging of the surface morphology of CSIC was carried out using SEM (Philips XL30 FEG) with a 10kV accelerating voltage.
Differential scanning calorimetry (DSC) analysis of CSIC
The thermal properties of CSIC were evaluated using a DSC 1 with STARe controlled by the DB V11.00 software (Mettler Toledo, Columbus, OH). Thermograms were obtained by heating CSIC samples from 25 °C to 350 °C at a rate of 10 °C/min with a constant nitrogen purge at 50.0 mL/min.
CSIC stability studies
pH stability
Standard buffer solutions with pH values of 1.2, 4, 5, 7, 9, and 10 were prepared according to the United States Pharmacopeia (USP) [26]. CSIC was first dissolved in acetonitrile and then mixed with each buffer solution individually to achieve a final nominal concentration of 100 μg/mL. Sample solutions were sonicated in a water bath until the drug particles completely disappeared based on visual observation. Samples were then covered with aluminum foil and stored at room temperature. Aliquots were taken daily for 10 days, and the drug content was analyzed by HPLC as described above. All studies were conducted in triplicate.
Thermal stability
CSIC was dissolved in 40% (v/v) aqueous acetonitrile at a nominal concentration of 100 μg/mL. Samples were incubated at 25°C and 65°C for 10 days. At predetermined time points, aliquots were taken for HPLC analysis as described above.
Oxidation
CSIC was dissolved in 40% (v/v) aqueous acetonitrile containing 3% H2O2 to provide 100 μg/mL final concentration. This solution was stored at room temperature and aliquots were removed at predetermined time intervals, until 10 days, for HPLC analysis as described above.
Photolysis
CSIC solutions at a concentration of 100 μg/mL were prepared in 40% (v/v) aqueous acetonitrile and in 40% (v/v) acetonitrile in phosphate buffer (pH 7.0). Half of the samples in each group were covered by aluminum foil prior to the light exposure, as the control group. All samples were then placed in a 30 °C/65% relative humidity (RH) environmental chamber under a fluorescent light source (2 × 20 watts) for 12 days. Aliquots were taken at predetermined time points for drug quantitation by HPLC analysis as described above.
Cytotoxicity of CSIC
Cytotoxicity was assessed using HEC-1A human endometrial epithelial cells and J774A.1 mouse macrophage cells. Cells were seeded in 96-well plates at a density of 2.5×104 cells per well, for HEC-1A, and 8×104 cells per well, for J774A.1. After a 24-hour incubation period at 37 °C and 5% CO2, cells were treated with serially diluted CSIC in DMEM (containing 0.1% DMSO) at concentrations ranging from 10 μg/mL to 1 ng/mL. After 24 hours of drug exposure, cytotoxicity was evaluated using MTT assay. Briefly, medium in each well was replaced by 180 μL of cell culture medium and 20 μL of MTT solution (5 mg/mL). After incubation at 37 °C for 3 hours, media was removed, and 200μL of DMSO was added in each well. The plates were then covered with aluminum foil and agitated on an orbital shaker for 20 minutes. The absorbance was measured at 595 nm using a microplate reader (DTX 880, Beckman Coulter, Brea, CA).
CSIC-excipient compatibility study
Excipients including PVA, HPMC K4M, PEG 4000, PEG 400, propylene glycol, and glycerin were mixed with CSIC individually, at a ratio of 1:1 (w/w), to facilitate any potential interactions [10]. Physical compatibility of CSIC with each excipient was determined by DSC and compared to that of CSIC alone [27, 28]. Samples were heated from 25 °C to 280 °C (except glycerin, 25 to 350 °C), at a heating rate of 10 °C/min, with a constant nitrogen purge at 50.0 mL/min.
Evaluation of a cosolvent system to increase CSIC solubility
PEG 400, propylene glycol, and glycerin were selected as cosolvents for CSIC. Identification of the optimal ratio of these solvents was carried out by mixing different percentages of solvents according to the ternary phase diagram shown in Figure 1. An excess amount of CSIC was added in each solvent matrix. Samples were then placed on a rotating mixer for 5 days at room temperature to allow for saturation. To determine CSIC solubility, samples were centrifuged to remove the undissolved drug particles, and the supernatant was assayed for CSIC quantification using the HPLC method described above.
Fig. 1.
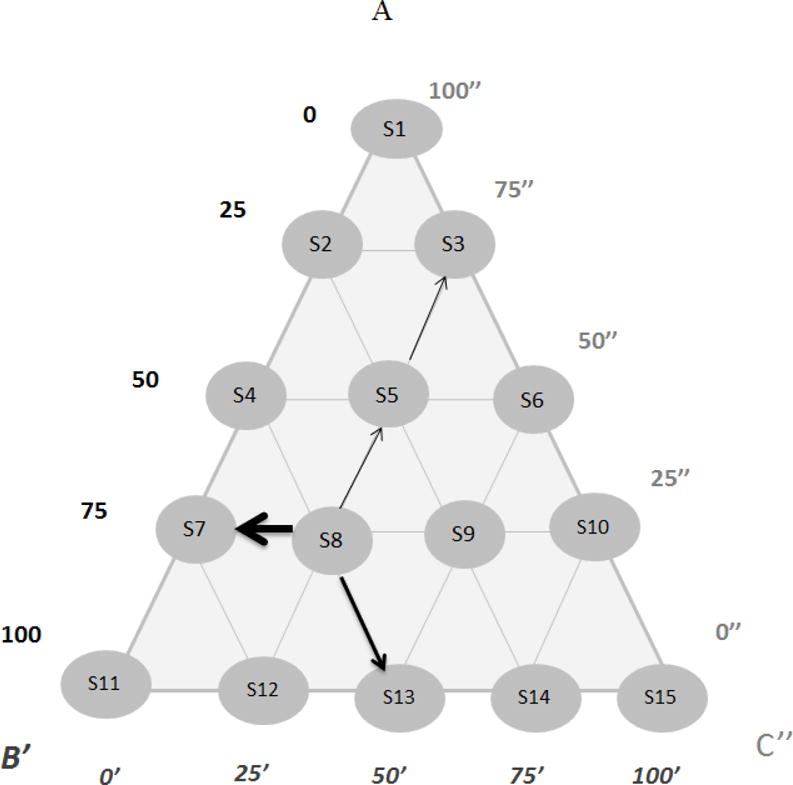
Ternary phase diagram of PEG 400, propylene glycol, and glycerin, aiming to optimize the ratio among the three cosolvents in terms of CSIC solubility. Any point within the triangle represents a given composition of the three cosolvents. Outward arrows from each solvent matrix are drawn parallel to the sides of the triangle. The distances AB′, B′C″ and C″A indicate the parts of PEG 400, propylene glycol, and glycerin, respectively. For example, in the case of solvent matrix (S8), the matrix was composed of 75 parts of A, 50′ parts of B′ and 75″ parts of C″, and the ratio among A, B′ and C″ was 75:50:75, or 3:2:3 (w/w/w)
CSIC film formulation development
CSIC film was manufactured using a solvent casting technique, as described previously, with minor modifications [11]. Briefly, PVA was dissolved in Milli-Q water heated at 90 °C in a water bath. HPMC K4M and PEG 4000 were then added to the PVA solution at room temperature. The polymer solution was stirred overnight using an overhead stirrer (Eurostar Power Control-Visc, IKA Works, Staufen im Breisgau, Germany). CSIC was dissolved in the optimized cosolvent system according to the ternary phase diagram (Fig. 1) and then added to the pre-prepared polymer solution, with stirring over 15 minutes to ensure uniform distribution of CSIC. The mixture was then cast onto a preheated (70 °C) PET liner which was secured on to a film applicator (Elcometer) under vacuum to allow for water evaporation within 15 minutes. Film sheets were removed and cut into 1″×2″ unit doses and then stored in aluminum foil pouches.
Physicochemical characterization of CSIC films
Standard measurements of the film dosage form included weight, thickness, film appearance, water content, tensile strength, disintegration time, drug content, content uniformity, and dissolution. Film appearance was evaluated subjectively by texture and color. Water content was determined using the Karl Fisher method (Model 890 Titrando and 832 KF Thermoprep, Herisau, Switzerland). Film tensile strength was measured using a texture analyzer (TA.XTPlus, Texture Technologies, Hamilton, MA) in combination with a TA 96B probe and calculated using the equation below:
Film disintegration analysis was performed by submerging films in 3 mL Milli-Q water, followed by rotation on an orbital shaker. The disintegration time was determined by visual observation when the first piece of film was separated. In order to determine the osmolality of gel mass obtained after film disintegration, CSIC film was dissolved in 3 mL vaginal fluid simulant. Osmolality of this mixture was assessed using a freezing point depression method (Osmometer 3320, Advanced Instruments Inc.). To determine the drug content, the film matrix was first dissolved in Milli-Q water, and CSIC was extracted by mixing with a 70% acetonitrile solution before HPLC analysis. Film drug content uniformity was evaluated by cutting a film into six sections and then quantifying the CSIC content in each sample. The dissolution profile of CSIC films was obtained using a USP class IV dissolution method coupled with a CE7 smart apparatus (Sotax, Westborough, MA). 80 mL of 1% Cremophor RH40 solution was used as the dissolution media at a flow rate of 16 mL/min. At predetermined time points, 1 mL samples were collected for analysis of CSIC content by HPLC as described above.
Lactobacillus compatibility of CSIC films
Compatibility of CSIC film with seven different Lactobacillus strains was conducted using standard microbicide safety techniques [29]. Briefly, the Lactobacillus-containing suspension and film solution were incubated together at a ratio of 1:1 (v/v) for 30 minutes at 37 °C. Colony-forming units determined after film exposure were counted and compared with the units before film treatment.
In vitro anti-HIV bioactivity test
Two types of bioactivity assays were conducted in this study [30], both using single replication cycle analysis in P4R5 cells. Direct antiviral activity was assessed by simultaneously exposing cells with HIV and various concentrations of CSIC film solutions for 48 hours. The protective effect (memory effect) of film-formulated CSIC was also assessed by pre-exposing cells to various concentrations of CSIC film solutions for 2 hours, followed by removal of the drug prior to exposure to HIV-1. In both studies, a single replication cycle assay was used to evaluate HIV replication. As a control group, similar studies were performed for CSIC drug substance.
CSIC film stability
Stability studies were carried out according to FDA Guidance for Industry (Q1A (R2) Stability Testing of New Drug Substances and Products) [25]. Films were individually sealed in aluminum foil packages and stored at 30 °C/65% RH for 12 months as a long-term study and 40 °C/75% RH for 6 months as an accelerated study. At specific time point, film samples were pulled for physicochemical characterization.
Statistical analysis
All results are provided as mean ± standard deviation. Pairwise differences were determined by Student’s t-test. The differences among groups in the photolysis study were tested by a repeated measures mixed model with pairwise comparisons. One-way ANOVA with Bartlett’s test was applied for the CSIC film stability study to determine whether the tested film attributes changed over time (GraphPad Prism 5). A p value <0.05 was considered statistically significant.
Results
In order to obtain a thorough understanding of CSIC’s physiochemical properties, which are crucial for successful formulation development, preformulation studies were conducted. As part of the preformulation studies, a stability indicating HPLC analytical method was developed. The HPLC retention time of CSIC was found to be 10.9 minutes. The LOD was 0.01 μg/mL, and the LOQ was 0.033 μg/mL. The method was linear (R2 > 0.999) between 0.5–200 μg/mL.
CSIC solubility in water, 40% (v/v) acetonitrile, and 100% acetonitrile were estimated to be 0.0014 mg/mL (4.3 μM), 0.36 mg/mL (1.1 mM), and 1.7 mg/mL (5.1mM), respectively. The hydrophobic nature of CSIC was also confirmed by the octanol/water partition coefficient [31]. LogP was determined in octanol water mixtures at three phase ratios. The Log P values of CSIC in the 1:1, 2:1, and 1:2 (v/v) octanol/water mixtures were found to be 3.72, 3.69, and 3.72, respectively.
The physicochemical properties of CSIC, such as surface morphology and thermal behavior, were also investigated. Prism-shaped crystals were observed under light microscopy (Fig. 2a) and the size of CSIC crystals was not uniform. SEM imaging showed that CSIC exists in a prismatic form and aggregated in a plate-like shape (Fig 2b). DSC thermograms showed an endothermic peak at 257 °C (melting point), indicating the transition of CSIC powder from a crystal to a liquid form (Fig. 2c). The presence of one sharp peak suggests that CSIC exists as a single crystal arrangement with no polymorphism.
Fig. 2.
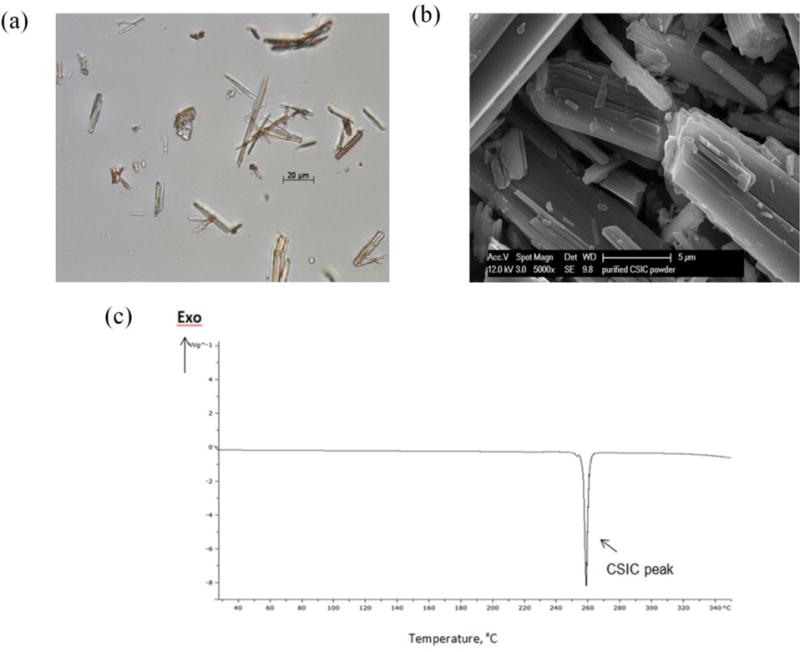
Physicochemical characterization of CSIC compound: (a) Polarized light microscopic image of CSIC (scale bar=20 μm) (b) Observation of CSIC powder using field emission scanning electron microscopy (FESEM) (scale bar=5μm) (c) DSC thermograph of CSIC powder. The melting point of CSIC was determined to be 257°C
For the CSIC solution-stability studies, different buffer solutions were prepared and mixed with acetonitrile, which allowed for complete CSIC solubilization. Any drug loss detected over time is thus assumed to be a result of drug degradation. The susceptibility of the drug across a wide range of pH was investigated for potential hydrolytic degradation (Fig. 3a). CSIC was found to be stable under all pH conditions for at least 10 days (<5% of drug loss). At each time point, more than 95% of the drug was detected, compared to the drug concentration at time zero. In the thermal stability study, the concentration versus time profiles for CSIC exposed to 25 °C/60% RH and 65 °C were plotted. Results showed that CSIC was stable under both conditions and that drug concentrations remained unchanged for at least 10 days (Fig. 3b). Upon H2O2 exposure, a 20% drug loss was observed over time (Fig. 3b). For the photolysis stability study, CSIC stored in the dark was found to be stable for at least 12 days. In comparison, drug concentration declined by 4% over time in samples that were exposed to a fluorescent light source (2 × 20 watts). After 6 days, a significant difference in drug content was confirmed between samples stored under light source and samples stored in dark (Fig 3c). The indole moiety of CSIC is sensitive to light and as a result CSIC could undergo photolytic degradation. However, this low degradation of CSIC is not likely an issue when it is formulated in a solid film dosage form.
Fig. 3.
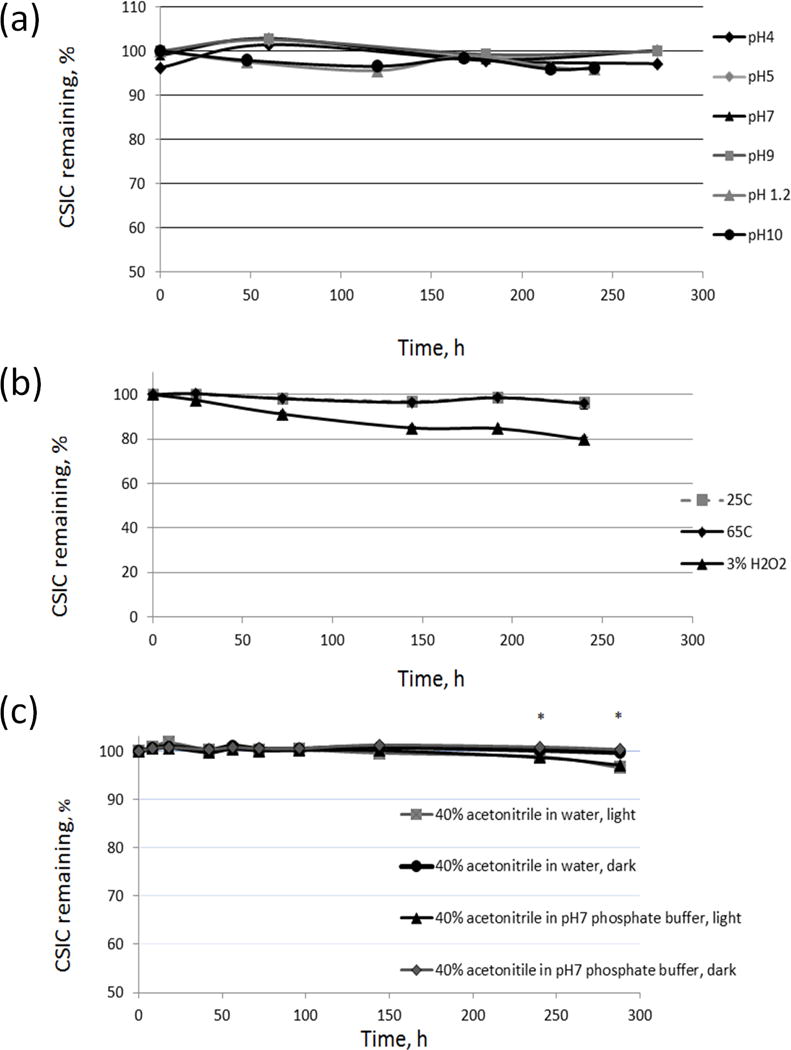
CSIC stability profiles: (a) at various pHs, (b) in 25 °C/60% RH and 65 °C environmental chambers and 3% H2O2 solutions, and (c) with or without light exposure (2 × 20 watts, fluorescent luminaire) in water (40% ACNH2O, v/v) or pH7 phosphate buffer solution (40% ACNpH7, v/v), respectively. Drug substance was found to be susceptible to oxidation and photolysis. (*p<0.05, n=3)
CSIC showed dose-dependent cytotoxicity in both HEC-1A and J774A.1 cells. As shown in Figure 4, the 50% cytotoxic concentration (CC50) of CSIC was greater than 5 μg/mL (15 μM) for both cell lines. This measured CC50 value is several orders of magnitude higher than the antiviral EC50 of CSIC (1 nM), indicating an in vitro selectivity index of 15,000.
Fig. 4.
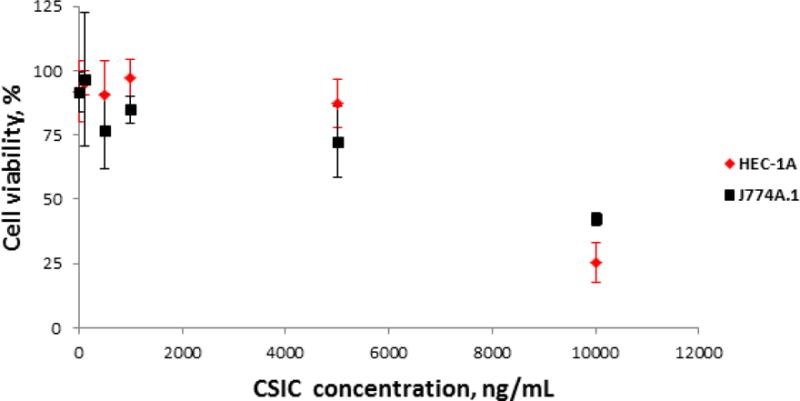
In vitro cytotoxicity of CSIC at various concentrations against HEC-1A and J774.A1 cell lines. The cytotoxicity of CSIC was measured at concentrations ranging from 10 μg/mL to 1 ng/mL. Following 24 h drug treatment, cell viability was determined by MTT assay. The absorbance was measured at 595 nm using a microplate reader (Beckman Coulter DTX 880) (n=6). The 50% cytotoxic concentrations (CC50) of CSIC, for both cell lines, were found to be greater than 5 μg/mL
Prior to film formulation development and optimization, the compatibility of CSIC with each individual excipient was investigated by monitoring the impact of the excipient on the melting point of CSIC. The melting point of CSIC remained same when it was mixed with PVA, HPMC K4M, and propylene glycol (Fig. 5 a–c). CSIC melting peak disappeared in the CSIC/PEG 400 mixture, indicating the solubilization of CSIC in this excipient (Fig. 5d). A similar finding was observed for the CSIC/PEG 4000 mixture (Fig. 5e). In contrast, the melting peak of CSIC shifted to a lower temperature of 217°C (Fig. 5f) in the presence of glycerin.
Fig. 5.
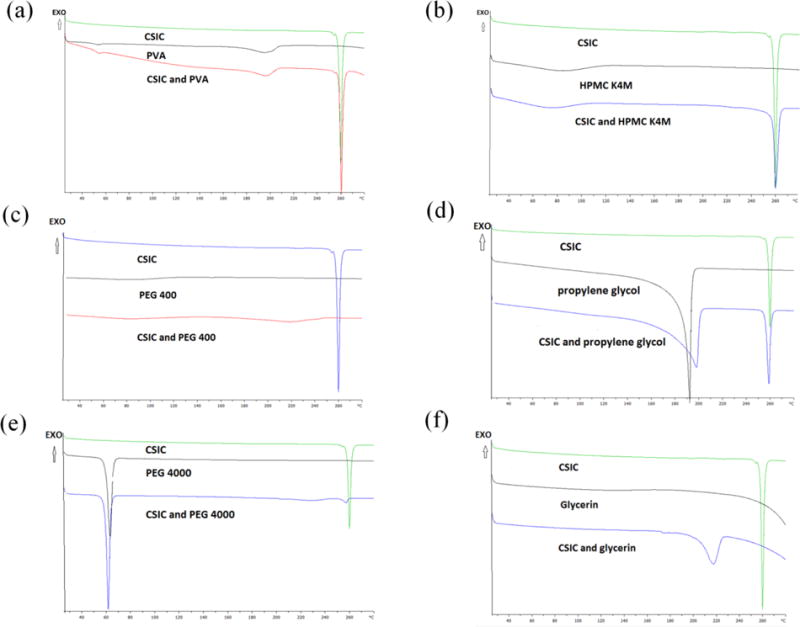
The compatibility studies between CSIC and various excipients conducted by DSC. The melting peak of CSIC did not change when drug was mixed with PVA (a), HPMC K4M (b), and propylene glycol (c) separately. Drug dissolved in PEG 400 (d) and PEG 4000 (e). The melting peak of CSIC has shifted to a lower temperature at 217°C in the presence of glycerin (f), which might indicate some incompatibility issues
To improve the solubility of CSIC in the film formulation, a cosolvent system consisting of PEG 400, propylene glycol, and glycerin was designed. The three cosolvents were chosen due to their favorable safety properties in vaginal application and their ability to improve the drug solubility. A ternary phase diagram was utilized to identify the optimal ratio of use for these three solvents (Fig. 1). Data showed that the utilization of PEG 400 significantly increased the solubility of CSIC, followed by propylene glycol (Table 1a). Among all the solvent matrices illustrated in Figure 1, solvent matrix No.15 (S15), composed of 50% PEG 400 and 50% propylene glycol (w/w), presented the maximum solubility of CSIC at 28.64 mg/mL. To further optimize the cosolvent system, solvent matrices containing higher percentages of PEG 400, such as 67.5% and 75%, were prepared (Table 1b) to achieve greater drug solubilization. Maximum CSIC solubility was obtained at 30.2 mg/mL for the solvent matrix No.18 (S18) when the ratio among PEG 400, propylene glycol, and glycerin was set at 5:2:1 (w/w/w).
Table 1. CSIC solubility in different co-solvent matrixes.
CSIC solubility in (a) solvent matrixes prepared according to the ternary phase diagram and (b) CSIC solubility in solvent matrixes with higher proportion of PEG 400. The percentage of PEG 400 used in each sample is noted in the parenthesis. Solvent matrix 18 represented the highest drug solubility of CSIC.
| (a) | ||||
|---|---|---|---|---|
| Sample No. | PEG 400 % | Propylene glycol % | Glycerin % | Solubility mg/mL |
| S1 | 0 | 50 | 50 | 5.23 |
| S2 | 12.5 | 37.5 | 50 | 10.60 |
| S3 | 12.5 | 50 | 37.5 | 12.85 |
| S4 | 25 | 25 | 50 | 12.85 |
| S5 | 25 | 37.5 | 37.5 | 17.08 |
| S6 | 25 | 50 | 25 | 19.99 |
| S7 | 37.5 | 12.5 | 50 | 20.46 |
| S8 | 37.5 | 25 | 37.5 | 21.81 |
| S9 | 37.5 | 37.5 | 25 | 24.63 |
| S10 | 37.5 | 50 | 12.5 | 22.59 |
| S11 | 50 | 0 | 50 | 22.86 |
| S12 | 50 | 12.5 | 37.5 | 23.67 |
| S13 | 50 | 25 | 25 | 24.31 |
| S14 | 50 | 37.5 | 12.5 | 28.21 |
| S15 | 50 | 50 | 0 | 28.64 |
| (b) | ||||||
|---|---|---|---|---|---|---|
| Component | Sample No. | S16 | S17 | S18 | S19 | S20 |
| PEG 400 | 10 (62.5%) | 10 (62.5%) | 10 (62.5%) | 6 (75%) | 3 (75%) | |
| Propylene glycol | 2 | 3 | 4 | 1 | 1 | |
| Glycerin | 4 | 3 | 2 | 1 | 0 | |
| Solubility, mg/mL | 22.7 | 20.7 | 30.2 | 29.1 | 28.8 | |
Polymeric vaginal films comprising PVA, HPMC K4M, PEG 4000, PEG 400, propylene glycol, glycerin, and CSIC at a mass ratio of 50:20:20:25:10:5:1 were manufactured by solvent casting. The CSIC films were found to be soft, flexible, smooth and translucent. No precipitation of either drug particles or dehydrated polymers was observed on the film surface. These results suggest that the composition of the cosolvents and polymers utilized in the current formulation were adequate for drug solubilization and dispersion.
Numerous physicochemical properties of the CSIC films were evaluated in this study. The average weight and thickness of the films were 137 ± 11.5 mg and 133 ± 0.013 μm, respectively. Drug content in the CSIC films was quantified and found to be 1.19 ± 0.1 mg/film, and the drug substance was distributed uniformly within each individual film (RSD < 5%). In addition, the residual water in the films was less than 10% (6.8 ± 0.5 %).
To determine the mechanical properties of CSIC films, tensile strength was measured and it was found to be in the range of 1000–1400 kg/m2, which was only slightly lower than that obtained for the marketed vaginal contraceptive film (VCF®, 1400–2200 kg/m2). Moreover, in the disintegration study, once the films were exposed to water, initial film disintegration was observed within 3 minutes, suggesting a quick dissolving and fast-disintegrating film was achieved. Drug release from the film formulation was evaluated in a USP class IV dissolution apparatus. Within 3 minutes, the released drug concentration was found to be 3 μg/mL. 50% CSIC was released from the film formulation in 10 minutes, and over 85% of the drug was released in 60 minutes.
Bioactivity of the film-formulated CSIC against HIV replication was tested in P4R5 indicator cells. The antiviral activity (EC50-antiviral) and the protective effect (EC50-memory) of CSIC in the film dosage form were found to be <1 nM and <20 nM, respectively, similar to those of free CSIC (Fig. 6) [8], suggesting that incorporation of CSIC in a polymeric vaginal film had no substantial effect on its in vitro anti-HIV bioactivity.
Fig. 6.
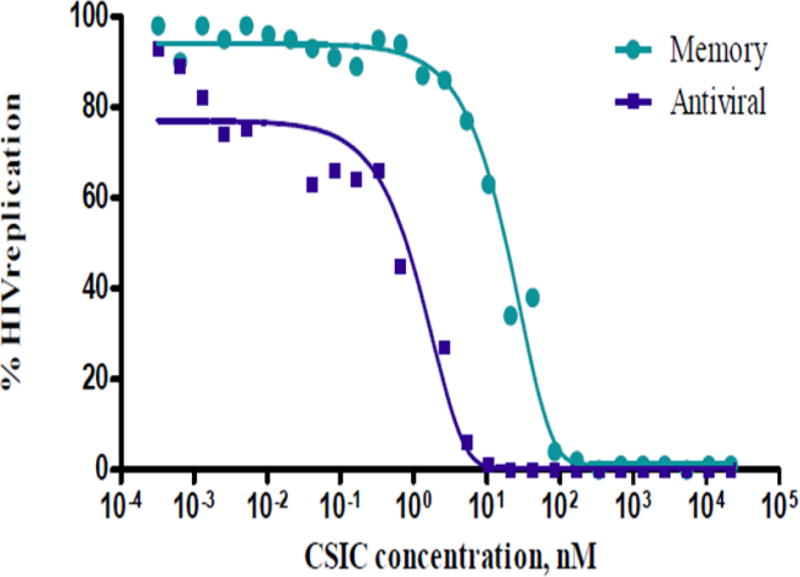
In vitro bioactivity of film-formulated CSIC in P4R5 HIV infection indicator cells. The antiviral activity (EC50-antiviral) and the protective effect (EC50-memory) of CSIC in the film dosage form were found to be <1 nM and <20 nM, respectively
Since Lactobacillus is the major organism found in healthy vaginal microflora and it plays an important role against viral infection by maintaining vaginal acidic pH and producing hydrogen peroxide, the compatibility between CSIC film formulations and Lactobacillus was assessed. As shown in Table 2, the seven major strains of Lactobacillus were unaffected by exposure to the CSIC film formulation.
Table 2. Evaluation of CSIC film compatibility with Lactobacillus.
The positive value indicates increased cell viability. In contrast, negative values illustrate decreased cell viability. The loss of viability has to be < 1 log10 to meet the safety requirement.
| Lactobacillus strain tested | Log Difference (T30 minute Plate Count – T0 minute Plate Count) |
|---|---|
| L.crisp ATCC 33197 | 0.113 |
| L.jen LBP 28Ab | −0.154 |
| L.jen ATCC 25258 | −0.068 |
| L.crisp ATCC 20225 | −0.082 |
| L.crisp LBP 90Aa | −0.143 |
| L.crisp 6099 | −0.100 |
| L.crisp 2116V | −0.023 |
CSIC film stability studies were carried out at 30 °C/65 %RH and 40 °C/75 %RH. Based on the physicochemical evaluations performed, CSIC films were found to be stable at 30 °C/65 %RH for a period of 12 months and at least 6 months under accelerated condition (40 °C/75 %RH). Figure 7 shows that drug content remained unchanged during this period relative to the drug content detected at time zero. Additionally, film disintegration was found to be within 3 minutes, and the residual water content in the film products was lower than 10% at all the time points. Moreover, the bioactivity of CSIC was maintained in the film formulation throughout the stability assessment period. No Lactobacillus compatibility issue was found throughout the stability testing period, confirming the compatibility between the CSIC film and vaginal Lactobacillus.
Fig. 7.
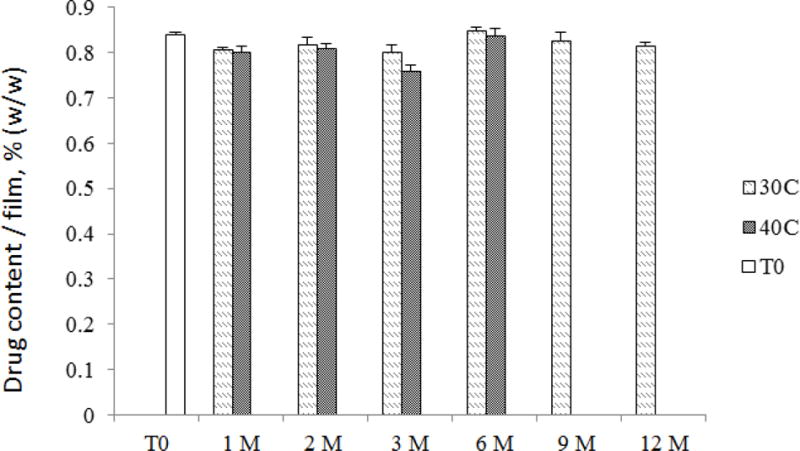
CSIC drug content results from the stability study. CSIC films were stored for 12 months in 30°C/65% RH and 6 months in 40°C/75% RH as per International Council for Harmonisation (ICH) guidelines. Film units were pulled at various time points for evaluation of drug content. The change in drug content was always less than 10% (n=5)
Discussion
The goal of preformulation work in this study is to evaluate the physicochemical and biological properties of the active pharmaceutical ingredient (API) and its stability profile under various conditions in order to identify any potential challenges for formulation or delivery to the target site for action. In the current work, CSIC was found to be very stable under a variety of stressed conditions.
The cytotoxicity profiles of CSIC were evaluated in the epithelial cell line HEC-1A and the mouse macrophage cell line J774A.1. It is well known that epithelial cells in the female reproductive tract are the primary site of sexually transmitted HIV infection and act as a physical barrier against initial HIV acquisition [32, 33]. Any damage to the epithelium will result in an increased risk of infection. It is critical to maintain the integrity/viability of epithelia when developing vaginal delivery products, especially for HIV prevention [34, 35]. In addition, HIV targets macrophages in the female reproductive tract. These cells are actively involved in the phagocytosis of exogenous components and transport of the virus to the draining lymph nodes, which facilitates the establishment of systemic infection [32]. Therefore, the cytotoxic information of the drug with respect to both epithelium and macrophages is essential. A dose-dependent reduction in cell viability was observed in HEC-1A and macrophage cell lines. A non-toxic concentration (CC50), 5 μg/mL (15 μM), was chosen for further biological evaluation. This concentration was expected to provide sufficient anti-HIV activity since it is several orders of magnitude higher than the EC50 of CSIC (~1 nM) [36, 3].
Despite the promising bioactivity and low toxicity of CSIC, the high hydrophobicity of the compound leads to poor aqueous solubility, which will impact the dissolution rate of CSIC in the vaginal lumen [37] as well as drug uniformity in the film dosage form. This low availability of dissolved drug in the vaginal lumen can lead to reduced drug uptake by the cervicovaginal tissue, which is essential for protection from HIV transmission. Therefore, pharmaceutical solubilization strategies were explored in order to improve the CSIC solubility during formulation process.
To improve the solubility of hydrophobic drugs, many pharmaceutical strategies have been developed, such as salt formation, crystal form modification, solid dispersion, micronization, complexation, and self-emulsification and so forth [38, 39]. Co-solvent strategy for drug solubilization has been widely utilized for dosage forms such as soft gels, emulsions, solutions, etc. Since polymeric film is a solid dosage form prepared from a semisolid intermediate, cosolvent strategy is highly applicable. In the current study, a cosolvent system consisting of PEG 400, propylene glycol, and glycerin (5:2:1, w/w/w) was employed to enhance the water miscibility of CSIC (Table 1). This increased miscibility could be due to the hydrogen bonding between their hydrophilic regions and simultaneous disruption of water self-association by the hydrophobic groups [40]. In addition to their ability to enhance the aqueous solubility of CSIC, these three solubilizing agents also act as plasticizers, which improved the flexibility and manufacturability of the film dosage form. In the absence of propylene glycol and glycerin, film products were found to be rigid and curly in the early stages of our investigation. Glycerin also acts as a humectant, absorbing moisture and enhancing film softness. Importantly, the three co-solvents are generally considered as safe for vaginal exposure.
It is well known that a number of polymers have been used for the manufacture of pharmaceutical film dosage form, including hydroxyethyl cellulose (HEC), PVA, hydroxypropyl methylcellulose (HPMC), poly(vinyl pyrrolidone), and Carbopol [41]. In this study, PVA and HPMC were selected as the film-forming polymer base. PVA was chosen because it is safe for vaginal preparations and it is the base polymer used in the marketed vaginal contraceptive film, VCF®. PVA can also be used as a lubricant and a viscosity-increasing agent with moderate mucoadhesive property [41]. The increased viscosity and mucoadhesiveness will enhance the residence time of the film in the vagina, which in turn, may increase drug concentration in the cervicovaginal tissue and improved efficacy. In addition to PVA, a second film-forming polymer, HPMC, was included in the CSIC film formulation. Previous in vitro release studies have shown that the utilization of HPMC provided an initial burst release of API from the film formulation [42, 11]. Dissolution results showed that more than 50 % of the loaded CSIC was released from the film matrix within 30 minutes, which is an essential attribute for coitally dependent drug delivery system for HIV prevention.
In addition, drug-excipient compatibility study was conducted in the solid state by monitoring the thermal events of CSIC using DSC technique. A reduction in melting point of CSIC in the CSIC-glycerin mixture was found (Fig. 5). Hydrogen bonding between CSIC and glycerin may contribute to the observed decrease in the melting peak of CSIC, indicating intimate mixing of CSIC in glycerin. Furthermore, the bioactivity of CSIC and the drug content remained unchanged during the 12-month stability testing period, indicating that the interaction between CSIC and the film components has no effect on the bioactivity. Although 1 mg CSIC per film was chosen as the initial target dose, it is important to note that with the selected cosolvent system, the current film formulation could accommodate a much higher dose of CSIC if needed [43].
To further optimize the film formulation, the ratio of glycerin and propylene glycol was modified to adjust the film’s physical characteristics and manufacturability. Additionally, in terms of safety, previous studies have reported that the application of high doses of glycerin and propylene glycol in the vaginal or rectal compartment could result in some adverse effects, such as epithelial damage, excessive mucosal fluid secretion, and increased HIV infection risk [44–46]. These side effects are mainly caused by the dehydrating effects of these excipients, which make the product hyperosmotic and tend to absorb water from the surrounding tissues. For example, in our earlier tenofovir gel study, high percentage of glycerin (20%) caused a high product osmolality (3111 ± 10 mOsmol/kg), which induced epithelial stripping in an explant model [46]. In contrast, when the percentage of glycerin was reduced to 5%, gel osmolality was decreased to 836 ± 11 mOsmol/kg and epithelial damage was absent. World health organization (WHO) notes that the osmolality of a vaginal product should be maintained below 1200 mOsm/kg in order to avoid epithelial damage. Although osmolality applies only to liquid or semi-solid dosage forms, in a biological environment, the solid dosage form film will become a gel-like product upon contact with vaginal fluid. If the gel-like product cannot be distributed rapidly in the vaginal lumen, local solute concentration will increase, which may create a hyperosmotic condition and cause safety issue. In order to avoid this potential adverse effect, the percentages of PEG 400, propylene glycol, and glycerin in the optimized film formulation were kept low, and set at 2.5%, 1%, and 0.5% (w/w), respectively. To confirm that, upon hydration in the vaginal vault, the osmolality of the resulting gel-like product would not pose any safety issue; the CSIC film was dissolved in 3 mL vaginal fluid simulant and tested for osmolality. The resulting data showed that the osmolality of the hydrated CSIC film (334 ± 4.04 mOsm/kg) was similar to isosmotic normal vaginal secretions (260–290 mOsm/kg).
After the CSIC film was manufactured, the physicochemical and biological properties of the film product were thoroughly investigated. The limited amount of water (< 10%) retained in the films will help maintain long term stability of the film properties while ensuring that the film is soft and flexible during storage. It was found that there was a fast drug release from the film formulation, with a disintegration time of less than 3 minutes, and nearly 50% of drug released in the first 10 minutes of dissolution testing. This release profile was similar to that of DPV-containing vaginal film reported by Akil et al. [11]. The observed burst release in CSIC film could be attributed to the presence of the disintegrant PEG 4000. This excipient has been reported to be able to accelerate the disintegration and subsequent drug dissolution in immediate-release tablet formulations [47].
The bioactivity of film-formulated CSIC was found to be comparable to CSIC API (Fig. 6) [8]. This result suggests that the presence of the additional pharmaceutical excipients and the manufacturing procedures used for film formulation did not impact the anti-HIV activity of the drug substance. The stability assessments of the developed CSIC film confirmed that the CSIC film product remains stable with little change in the drug content (Fig. 7), dissolution (Table 3), bioactivity, or innate microflora biocompatibility. In summary, CSIC is a highly potent anti-HIV agent with a favorable stability profile supporting its further pharmaceutical development as a vaginally administered product. Although it’s hydrophobic nature poses a challenge to the formulation development, a cosolvent system (consisting of PEG 400, propylene glycol and glycerin) explored in this study showed significant enhancement in the CSIC aqueous solubility and facilitated the drug dispersion into a polymeric film matrix. A thin, soft, flexible, and stable CSIC film was developed using a solvent casting technique. This cosolvent-based polymeric film formulation has potential to be used as a platform for other hydrophobic microbicide candidates. Future studies evaluating the safety of the film in animal models such as the nonhuman primate which has been commonly used for safety, pharmacokinetic evaluation, and efficacy are warranted.
Table 3. Percent cumulative drug release after 10 minutes during the stability study.
CSIC films were stored for 12 months at 30°C/65% RH and 6 months at 40°C/75% RH as per ICH guidelines. Data presented as Mean ± SD (n≥3).
| 30 °C/65% RH | 40 °C/75% RH | |
|---|---|---|
| 1 M | 53.7±5.0 | 55.5±2.0 |
| 2 M | 51.9±0.5 | 44.1±5.4 |
| 3 M | 52.9±4.5 | 57.9±4.4 |
| 6 M | 50.1±5.9 | 53.7±14.4 |
| 9 M | 45.1±2.1 | |
| 12 M | 52.5±8.7 |
Acknowledgments
The current work was funded through the Integrated Pre-clinical/Clinical Program for Microbicide Development (grant number: U19 AI082623) and the Microbicide Innovation Program (grant number: AI079801) of the National Institute of Allergy and Infectious Diseases, Division of AIDS. We would like to acknowledge Eva Nagy (University of Pittsburgh, School of Medicine) for her assistance with the bioactivity study. We also would like to thank Albert Stewart for his kind help with the SEM imaging conducted in this study. We would like to acknowledge Sravan Patel for his help with the editing/proofreading of this manuscript.
This study was funded by Integrated Pre-clinical/Clinical Program for Microbicide Development (grant number: U19 AI082623) and the Microbicide Innovation Program (grant number: AI079801) of the National Institute of Allergy and Infectious Diseases, Division of AIDS. There was no involvement from any pharmaceutical companies. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest
The authors declare that they have no conflicts of interest.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic. 2013 [Google Scholar]
- 2.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New England Journal of Medicine. 2016 doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motakis D, Parniak MA. A Tight-Binding Mode of Inhibition Is Essential for Anti-Human Immunodeficiency Virus Type 1 Virucidal Activity of Nonnucleoside Reverse Transcriptase Inhibitors. Antimicrobial Agents and Chemotherapy. 2002;46(6):1851–6. doi: 10.1128/AAC.46.6.1851-1856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prajapati DG, Ramajayam R, Yadav MR, Giridhar R. The search for potent, small molecule NNRTIs: A review. Bioorganic & medicinal chemistry. 2009;17(16):5744–62. doi: 10.1016/j.bmc.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 5.Williams TM, Ciccarone TM, MacTough SC, Rooney CS, Balani SK, Condra JH, et al. 5-Chloro-3-(phenylsulfonyl)indole-2-carboxamide: a novel, non-nucleoside inhibitor of HIV-1 reverse transcriptase. Journal of Medicinal Chemistry. 1993;36(9):1291–4. doi: 10.1021/jm00061a022. [DOI] [PubMed] [Google Scholar]
- 6.Miller V, de Béthune M-P, Kober A, Stürmer M, Hertogs K, Pauwels R, et al. Patterns of Resistance and Cross-Resistance to Human Immunodeficiency Virus Type 1 Reverse Transcriptase Inhibitors in Patients Treated with the Nonnucleoside Reverse Transcriptase Inhibitor Loviride. Antimicrobial Agents and Chemotherapy. 1998;42(12):3123–9. doi: 10.1128/aac.42.12.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaugerre C, Rohban R, Simon A, Mouroux M, Tricot C, Agher R, et al. Resistance profile and cross-resistance of HIV-1 among patients failing a non-nucleoside reverse transcriptase inhibitor-containing regimen*. Journal of Medical Virology. 2001;65(3):445–8. [PubMed] [Google Scholar]
- 8.Parniak MA. Nonnucleoside reverse transcriptase inhibitors as anti-HIV-1 microbicides. AIDS. 2001;15:S56. [Google Scholar]
- 9.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. Journal of Antimicrobial Chemotherapy. 2005;56(5):954–6. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 10.Ham A, Rohan L, Boczar A, Yang LW, Buckheit K, Buckheit R., Jr Vaginal Film Drug Delivery of the Pyrimidinedione IQP-0528 for the Prevention of HIV Infection. Pharmaceutical Research. 2012;29(7):1897–907. doi: 10.1007/s11095-012-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, et al. Development and Characterization of a Vaginal Film Containing Dapivirine, a Non-nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug delivery and translational research. 2011;1(3):209–22. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christopher Elias CC. Acceptability Research on Female-Controled Barrier Methods to Prevent Heterosexual Transmission of HIV: Where Have We Been? Where Are We Going? J Wemens Health & Gender Based Med. 2001;10(2):11. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- 13.Raymond E, Alvarado G, Ledesma L, Diaz S, Bassol S, Morales E, et al. Acceptability of two spermicides in five countries. Contraception. 1999;60(1):45–50. doi: 10.1016/s0010-7824(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 14.Garg S, Vermani K, Garg A, Anderson RA, Rencher WB, Zaneveld LJD. Development and Characterization of Bioadhesive Vaginal Films of Sodium Polystyrene Sulfonate (PSS), a Novel Contraceptive Antimicrobial Agent. Pharmaceutical Research. 2005;22(4):584–95. doi: 10.1007/s11095-005-2490-1. [DOI] [PubMed] [Google Scholar]
- 15.Dobaria N, Badhan AC, Mashru RC. A Novel Itraconazole Bioadhesive Film for Vaginal Delivery: Design, Optimization, and Physicodynamic Characterization. AAPS PharmSciTech. 2009;10(3):951–9. doi: 10.1208/s12249-009-9288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad RNV, Adaikan PG, Arulkumaran S, Ratnam SS. Preinduction cervical priming with PGE2 vaginal film in primigravidae — A randomised, double blind, placebo controlled study. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1989;36(3):185–8. doi: 10.1016/0952-3278(89)90060-4. [DOI] [PubMed] [Google Scholar]
- 17.Neurath A, Strick N, Li Y-Y. Water dispersible microbicidal cellulose acetate phthalate film. BMC Infectious Diseases. 2003;3(1):27. doi: 10.1186/1471-2334-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Hu M, Shi Y, Gong T, Dezzutti CS, Moncla B, et al. Vaginal microbicide film combinations of two reverse transcriptase inhibitors, EFdA and CSIC, for the prevention of HIV-1 sexual transmission. Pharmaceutical research. 2015;32(9):2960–72. doi: 10.1007/s11095-015-1678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akil A, Devlin B, Cost M, Rohan LC. Increased Dapivirine Tissue Accumulation through Vaginal Film Codelivery of Dapivirine and Tenofovir. Molecular Pharmaceutics. 2014;11(5):1533–41. doi: 10.1021/mp4007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ham A, Cost M, Sassi A, Dezzutti C, Rohan L. Targeted Delivery of PSC-RANTES for HIV-1 Prevention using Biodegradable Nanoparticles. Pharmaceutical Research. 2009;26(3):502–11. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costanzo CJ. Immune defense of the female lower reproductive tract and the use of monoclonal antibody-based topical microbicide films to protect against HIV infection. BOSTON UNIVERSITY; 2015. [Google Scholar]
- 22.Gu J, Yang S, Ho EA. Biodegradable Film for the Targeted Delivery of siRNA-Loaded Nanoparticles to Vaginal Immune Cells. Molecular Pharmaceutics. 2015;12(8):2889–903. doi: 10.1021/acs.molpharmaceut.5b00073. [DOI] [PubMed] [Google Scholar]
- 23.Bunge KE, Dezzutti CS, Rohan LC, Hendrix CW, Marzinke MA, Richardson-Harman N, et al. A Phase 1 Trial to Assess the Safety, Acceptability, Pharmacokinetics, and Pharmacodynamics of a Novel Dapivirine Vaginal Film. Jaids-J Acq Imm Def. 2016;71(5):498–505. doi: 10.1097/Qai.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson JA, Marzinke MA, Bakshi RP, Fuchs EJ, Radebaugh CL, Aung W, et al. Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B) AIDS Res Hum Retroviruses. 2016 doi: 10.1089/AID.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.<Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products.pdf>.
- 26.Reagents, Indicators, and Solutions. United States Pharmacopeia. :1209–10. [Google Scholar]
- 27.Freire FD, Aragão CFS, Lima e Moura TFA, Raffin FN. Compatibility study between chlorpropamide and excipients in their physical mixtures. Journal of Thermal Analysis and Calorimetry. 2009;97(1):355–7. [Google Scholar]
- 28.Roumeli E, Tsiapranta A, Pavlidou E, Vourlias G, Kachrimanis K, Bikiaris D, et al. Compatibility study between trandolapril and natural excipients used in solid dosage forms. Journal of Thermal Analysis and Calorimetry. 2012;111(3):2109–15. [Google Scholar]
- 29.Moncla BJ, Hillier SL. Why nonoxynol-9 may have failed to prevent acquisition of Neisseria gonorrhoeae in clinical trials. Sexually transmitted diseases. 2005;32(8):491–4. doi: 10.1097/01.olq.0000170444.13666.e9. [DOI] [PubMed] [Google Scholar]
- 30.Abram ME, Parniak MA. Virion Instability of Human Immunodeficiency Virus Type 1 Reverse Transcriptase (RT) Mutated in the Protease Cleavage Site between RT p51 and the RT RNase H Domain. Journal of Virology. 2005;79(18):11952–61. doi: 10.1128/JVI.79.18.11952-11961.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooke D, Dobbs A, Williams N. Octanol: Water partition coefficients (P): Measurement, estimation, and interpretation, particularly for chemicals with P> 10 5. Ecotoxicology and environmental safety. 1986;11(3):251–60. doi: 10.1016/0147-6513(86)90099-0. [DOI] [PubMed] [Google Scholar]
- 32.Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes and Infection. 2003;5(1):59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 33.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. Journal of Reproductive Immunology. 2002;57(1–2):61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 34.D’Cruz OJ, Uckun FM. Clinical Development of Microbicides for the Prevention of HIV Infection. Current Pharmaceutical Design. 2004;10(3):315–36. doi: 10.2174/1381612043386374. [DOI] [PubMed] [Google Scholar]
- 35.Stafford MK, Ward H, Flanagan A, Rosenstein IJ, Taylor-Robinson D, Smith JR, et al. Safety Study of Nonoxynol-9 as a Vaginal Microbicide: Evidence of Adverse Effects. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1998;17(4):327–31. doi: 10.1097/00042560-199804010-00006. [DOI] [PubMed] [Google Scholar]
- 36.Williams T, Ciccaro T, MacTough S, Rooney C, Balani S, Condra J, et al. 5-Chloro-3-(phenylsulfonyl)indole-2-carboxamide: a novel, non-nucleoside inhibitor of HIV-1 reverse transcriptase. J Med Chem. 1993;36:1291–4. doi: 10.1021/jm00061a022. [DOI] [PubMed] [Google Scholar]
- 37.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–5. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 38.Savjani KT, Gajjar AK, Savjani JK. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharmaceutics. 2012;2012:10. doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawabata Y, Wada K, Nakatani M, Yamada S, Onoue S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. International Journal of Pharmaceutics. 2011;420(1):1–10. doi: 10.1016/j.ijpharm.2011.08.032. [DOI] [PubMed] [Google Scholar]
- 40.Patil Satish K, W KS, Parik Venkatesh B, Akarte Anup M, Baviskar Dheeraj T. Strategies for solubility enhancement of poorly soluble drugs. International Journal of Pharmaceutical Sciences Review and Research. 2011;8(2):7. [Google Scholar]
- 41.Morales JO, McConville JT. Manufacture and characterization of mucoadhesive buccal films. European Journal of Pharmaceutics and Biopharmaceutics. 2011;77(2):187–99. doi: 10.1016/j.ejpb.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Garg S, Garg S, Kumar G. Development and evaluation of a buccal bioadhesive system for smoking cessation therapy. Die Pharmazie - An International Journal of Pharmaceutical Sciences. 2007;62(4):266–72. [PubMed] [Google Scholar]
- 43.Obitte N, Rohan L, Adeyeye C, Parniak M, Esimone C. The utility of self-emulsifying oil formulation to improve the poor solubility of the anti HIV drug CSIC. AIDS Research and Therapy. 2013;10(1):14. doi: 10.1186/1742-6405-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuchs EJ, Lee LA, Torbenson MS, Parsons TL, Bakshi RP, Guidos AM, et al. Hyperosmolar Sexual Lubricant Causes Epithelial Damage in the Distal Colon: Potential Implication for HIV Transmission. Journal of Infectious Diseases. 2007;195(5):703–10. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 45.Lacey CJ, Woodhall S, Qi Z, Sawant S, Cowen M, McCormack S, et al. Unacceptable side-effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. International Journal of STD & AIDS. 2010;21(10):714–7. doi: 10.1258/ijsa.2010.010215. [DOI] [PubMed] [Google Scholar]
- 46.Dezzutti CS, Rohan LC, Wang L, Uranker K, Shetler C, Cost M, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. Journal of Antimicrobial Chemotherapy. 2012;67(9):2139–42. doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perissutti B, Rubessa F, Moneghini M, Voinovich D. Formulation design of carbamazepine fast-release tablets prepared by melt granulation technique. International journal of pharmaceutics. 2003;256(1):53–63. doi: 10.1016/s0378-5173(03)00062-0. [DOI] [PubMed] [Google Scholar]


