Abstract
Many late adolescents who transition to the college environment perceive changes in psychosocial stress. One such stressor, loneliness, has been associated with numerous health problems among adolescents and adults. The hypothalamic–pituitary–adrenal axis is one mechanism through which loneliness may affect health. Guided by a risk and resilience framework, the present study investigated the association between longitudinal changes in loneliness from high school to college and diurnal cortisol activity (waking levels, cortisol awakening response, diurnal slope) by sampling saliva intensively 5 times a day for 3 weekdays in a US sample of late adolescents in their first semester of college (N = 70; Mage = 18.49, SD = 0.38). The present study also explored how the link between loneliness and cortisol might depend on coping efficacy—one’s belief in successfully coping with future stressors or novel situations. Results from hierarchical linear growth curve models demonstrated that an increase in loneliness across this contextual transition was associated with steeper cortisol slopes in college. Coping efficacy at baseline (in high school) significantly moderated the relation between changes in loneliness and diurnal slopes, such that late adolescents with low levels of coping efficacy who reported increased loneliness across the transition exhibited significantly flatter diurnal slopes in college. Higher levels of coping efficacy at baseline also significantly predicted lower waking cortisol levels during the first semester of college. These results suggest that coping efficacy may serve as a protective factor by contributing to regulation of daily physiological stress activity for late adolescents as they struggle with loneliness across the transition to college.
Keywords: coping efficacy, cortisol, late adolescence, loneliness, transition to college
Loneliness, defined as perceiving a lack of quality or quantity in social relationships (Hawkley & Cacioppo, 2010; Peplau & Perlman, 1982), has been associated with numerous mental and physical health problems, including obesity (Lauder, Mummery, Jones, & Caperchione, 2006), high systolic blood pressure (Hawkley, Masi, Berry, & Cacioppo, 2006), poor sleep efficiency (Hawkley, Preacher, & Cacioppo, 2010), and depression (Alpass & Neville, 2003). Researchers have theorized that the hypothalamic–pituitary–adrenal (HPA) axis is one biological mechanism through which the psychosocial stress of loneliness leads to negative health outcomes (Cacioppo et al., 2000; Hawkley & Cacioppo, 2003). Accumulating evidence has demonstrated relations among cortisol (a biomarker of HPA axis activity), loneliness, and various indicators of health (Doane & Adam, 2010; Okamura, Tsuda, & Matsuishi, 2011; Pressman, Cohen, Miller, Barkin, & Rabin, 2005). These findings from human studies have been complemented by animal models, which have identified loneliness as a causal agent contributing to heightened activation of the HPA axis (Cacioppo, Cacioppo, Capitanio, & Cole, 2015).
The risk and resilience framework (Luthar, Cicchetti, & Becker, 2000; Masten, 2001, 2004) suggests there are important vulnerability and protective factors that can exacerbate or diminish, respectively, the association between a risk factor (e.g., loneliness) and an outcome (e.g., daily regulation of stress physiology). The identification of such factors can reveal how relatively stable characteristics might buffer certain late adolescents from the negative impact of psychosocial stress. In the United States, the transition to college is a time during which many late adolescents experience shifting social contexts and are thus at heightened risk for the psychosocial stress of loneliness (Conley, Kirsch, Dickson, & Bryant, 2014; Shaver, Furman, & Burhmester, 1985). However, as adolescents develop, they also vary in the degree to which they are able to recruit coping resources that may protect them from the potentially deleterious effects of psychosocial stress (e.g., Compas, Connor-Smith, Saltzman, Thomsen, & Wadsworth, 2001).
In the current study, we examined associations between late adolescents’ changes in loneliness across the transition to college and multiple indicators of the diurnal cortisol rhythm using a naturalistic salivary assessment protocol. Although coping efficacy (belief in one’s ability to cope with future stressors; Sandler, Tein, Mehta, Wolchik, & Ayers, 2000) has not yet been formally studied as a protective factor in adolescent stress physiology research, studies have shown positive associations between coping efficacy and successful adaptation to stress (Benight et al., 1997; Keefe et al., 1997; Massey, Garnefski, Gebhardt, & van der Leeden, 2009). Additionally, researchers have hypothesized that a greater sense of perceived self-efficacy (belief in one’s own abilities; Bandura, 1977) might predict improved health outcomes across development (e.g., Bandura, 2004; Strauss, Rodzilsky, Burack, & Colin, 2001), potentially by promoting regulation of physiological stress activity (O’Leary, 1992; O’Leary & Brown, 1995). Based on this literature and classic stress-coping theory (Lazarus & Folkman, 1984), we also explored whether coping efficacy assessed prior to the college transition moderated relations between loneliness and diurnal cortisol activity.
Late adolescence and the transition to college
In a recent review, five key characteristics of the adolescent years (roughly until the age of 18) were identified that place this population at risk for loneliness: changes in companions (e.g., peers instead of parents), increasing need for autonomy and individuation, identity development, improved cognitive abilities and social perspective taking, and physical development (Laursen & Hartl, 2013). Similar to findings in adult samples, loneliness during late adolescence and the college years has been linked to health problems, such as poor cardiovascular health, less efficient sleep, and decreased immune function (Cacioppo, Hawkley, Berntson, et al., 2002; Hawkley, Burleson, Berntson, & Cacioppo, 2003; Pressman et al., 2005). In sum, loneliness is a developmentally salient and unfortunately common risk factor for poor health and well-being in late adolescence.
In the United States, the majority of late adolescents enter the college environment following high school (Bureau of Labor Statistics, 2013). Previous research has indicated that individuals are vulnerable to stress during transitions (Felner, Farber, & Primavera, 1983; Juster et al., 2011), and first-year college students may be particularly vulnerable to the stressors characteristic of this transition: moving away from home for the first time (Compas, Wagner, Slavin, & Vannatta, 1986), handling an increased academic load (Ross, Niebling, & Heckert, 1999), and building new relationships (Hays & Oxley, 1986).
In order to understand the interplay between psychosocial stress and physiological adaptation during this contextual transition, research is needed to investigate how changes in loneliness from high school to college relate to late adolescents’ daily cortisol functioning in college. Although evidence suggests that chronic loneliness remains relatively stable across childhood and adolescence (Qualter, Brown, Munn, & Rotenberg, 2010; Qualter et al., 2013; Vanhalst, Goossens, Luyckx, Scholte, & Engels, 2013), it is unclear if and how perceptions of loneliness change across the relatively brief time period that spans the transition to college. Researchers have focused on the stability of loneliness across development (Bartels, Cacioppo, Hudziak, & Boomsma, 2008; Boomsma, Willemsen, Dolan, Hawkley, & Cacioppo, 2005) but have not yet examined how loneliness relates to biological stress processes across this transition.
HPA axis and cortisol
The HPA axis is one of the body’s major stress response systems and is also involved in maintaining homeostasis and everyday functioning (Kudielka & Kirschbaum, 2005; Lovallo, 2005). Cortisol is the end product of the HPA axis stress response and serves as a marker of HPA axis activation (Kudielka & Kirschbaum, 2005; Stratakis & Chrousos, 1995; Wilcox, Granger, Szanton, & Clark, 2014). Researchers have frequently studied salivary cortisol levels in response to psychosocial stress using controlled lab reactivity paradigms (e.g., Dickerson & Kemeny, 2004). Other researchers have explored alterations in daily cortisol patterns in relation to psychosocial stress in naturalistic settings (e.g., Adam, 2006; Adam & Gunnar, 2001). Cortisol levels follow a normative diurnal rhythm: relatively high levels upon waking, an increase of 50%–65% between waking and 30 minutes after (the cortisol awakening response; CAR), and then an overall decrease across the day with lowest levels at midnight (Adam & Kumari, 2009).
Consistent with prior work in naturalistic settings (e.g., Adam, 2006), our study concentrates on waking levels of cortisol, the CAR, and the diurnal cortisol slope (rate of decline across the day) in relation to changes in loneliness. Among various physiological and psychological factors, researchers have hypothesized that an increased CAR may reflect greater anticipation of demands for the upcoming day (Fries, Dettenborn, & Kirschbaum, 2009). An increased or blunted CAR has also been associated with negative physiological and psychological correlates, such as depression and chronic pain (Adam et al., 2010; Edwards, Hucklebridge, Clow, & Evans, 2003; Geiss, Varadi, Steinbach, Bauer, & Anton, 1997; Stetler & Miller, 2005). A steep, negative cortisol slope across the day typically represents effective physiological regulation, whereas flattened or positive slopes have been associated with chronic stress (Miller, Chen, & Zhou, 2007), breast cancer mortality (Sephton, Sapolsky, Kraemer, & Spiegel, 2000), and chronic fatigue syndrome (Nater et al., 2008). However, evidence also indicates that this pattern of association depends upon the type of stressor (e.g., social, physical) and person-specific characteristics (e.g., perceptions of stress, coping resources; Miller et al., 2007). Across several studies of adolescents at varying ages, researchers have demonstrated that diurnal cortisol indices include both trait-like and state-like variation (Ross, Murphy, Adam, Chen, & Miller, 2014; Shirtcliff et al., 2012).
Loneliness and cortisol
Studies of adults have typically found positive associations between loneliness and cortisol activity, such that loneliness has been associated with higher average cortisol levels and a greater CAR (Adam, Hawkley, Kudielka, & Cacioppo, 2006; Okamura et al., 2011; Steptoe, Kunz-Ebrecht, Brydon, & Wardle, 2004). Some researchers have focused on associations between loneliness and cortisol among late adolescents and college students, but findings have been inconsistent (Cacioppo et al., 2000; Cacioppo, Hawkley, Crawford, et al., 2002; Doane & Adam, 2010; Pressman et al., 2005). In a study of undergraduate students, higher levels of trait loneliness were associated with higher average cortisol levels across a single day (Cacioppo et al., 2000). Doane and Adam (2010) measured salivary cortisol 6 times a day for 3 days in 17–20-year-olds and found that higher levels of trait loneliness were associated with a flatter diurnal slope, while higher levels of feeling lonely/sad the prior day were associated with a greater CAR the next morning. In a study of first-year college students, Pressman et al. (2005) found that momentary experiences of loneliness were associated with higher average cortisol levels in the morning and evening. Despite these findings, some researchers have been unable to find significant associations between loneliness and cortisol. Cacioppo, Hawkley, Crawford, et al. (2002) did not find a main effect of loneliness on average cortisol levels in undergraduates. Analyses only used average levels of cortisol, rather than focusing on indicators that capture the time-varying nature of cortisol across the day (e.g., CAR, diurnal slope).
To our knowledge, only four studies to date have investigated the relation between loneliness and cortisol during late adolescence, and findings have been inconsistent. This is most likely due to the varying methods researchers have used to assess both loneliness and cortisol. Although all of these studies sampled salivary cortisol at multiple instances throughout the day, only two studies sampled for 3 or more days and only one study modeled changes in cortisol across the day to estimate diurnal parameters (e.g., CAR, diurnal slope) rather than averaging daily or morning levels. This methodological difference is critical to note because modeling daily cortisol profiles across the day has the potential to capture meaningful differences in physiological function and adaptation as they relate to changing experiences of loneliness (Adam, 2012). In addition, all four previous studies were cross-sectional and were thus not able to investigate changing developmental and social influences characteristic of life transitions. By measuring at more than one time point, we are able to extend available literature by exploring longitudinal changes in loneliness as late adolescents adapt to the college environment. A longitudinal design afforded us the ability to estimate changes in loneliness rather than assuming that one measurement of loneliness assessed in the social context of high school represented static individual differences. In order to address the inconsistencies of past research and contribute an extension to available literature, the first aim of the present study was to sample salivary cortisol 5 times a day for 3 days, analyse multiple indicators of the diurnal cortisol pattern (e.g., waking levels, CAR, diurnal slope), measure loneliness longitudinally over an important social transition, and explore whether the link between loneliness and cortisol might depend upon available coping resources in late adolescents’ lives.
Coping with loneliness: Coping efficacy
Within the risk and resilience framework (Luthar et al., 2000; Masten, 2001, 2004), developmental researchers are also keenly invested in identifying protective factors that buffer at-risk individuals from the negative impacts of stress on adaptation processes. A wealth of literature suggests that coping responses can serve as effective tools for reducing the maladaptive effects of stress on health and well-being (Compas et al., 2001; Galatzer-Levy, Burton, & Bonanno, 2012; Lazarus & Folkman, 1984). Indeed, prominent coping theory urges researchers to consider transactions between stress and coping processes (Lazarus & Folkman, 1984). As late adolescents enter the college context and experience dramatic shifts in their social support systems, their ability to cope with changing perceptions of support may be critical for their adaptation to this transition (e.g., Abouserie, 1994; Pierceall & Keim, 2007). In a longitudinal study of the transition to adulthood, Masten et al. (2004) identified coping skills as an adaptive resource that protected individuals from potential risks and promoted competence among already successful individuals. Lonely individuals are more likely than non-lonely individuals to adopt passive coping strategies and behaviorally disengage rather than actively cope or seek support from others (Cacioppo et al., 2000).
In addition to how late adolescents cope with problems, the belief that they can successfully cope with a future stressor or novel situation (coping efficacy; Sandler et al., 2000) may be a key predictor of adjustment. Coping efficacy has been associated with successfully adapting to various stressors, including trauma and daily pain (Benight et al., 1997; Keefe et al., 1997; Massey et al., 2009). To our knowledge, there is no available empirical literature exploring coping efficacy as a moderator of the relation between loneliness and diurnal cortisol activity, but prior research has demonstrated that various coping responses moderate the relation between stress and cortisol reactivity (Gunlicks-Stoessel & Powers, 2009; Stowell, Tumminaro, & Attarwala, 2008). In a sample of undergraduate students taking regularly scheduled examinations, test anxiety (worry) was strongly associated with higher cortisol levels only for those with low levels of problem-focused coping (Stowell et al., 2008). Gunlicks-Stoessel and Powers (2009) found that men who more frequently reported seeking social support to cope exhibited higher cortisol levels in response to a conflict task with their romantic partner. Together, these results from laboratory studies reflect how coping responses and stress serve as transactional processes to predict cortisol reactivity.
Given the lack of prior physiological stress research exploring the protective role of late adolescents’ confidence in facing future problems, our second aim was to determine whether the relation between changes in loneliness from high school to college and diurnal cortisol activity in college differed based on individual differences in coping efficacy. Coping behaviors have already been identified as protective factors for adolescents of varying ages and college students perceiving stress in their environment (e.g., Compas et al., 2001; South & Miller, 2014). Thus, we predicted that coping efficacy would also emerge as a protective factor for late adolescents at risk for the negative impact of loneliness as they transitioned to college. More specifically, we expected that those who experienced risk (increased loneliness) but also had initially high levels of coping efficacy would be buffered from potentially maladaptive alterations in daily cortisol functioning.
The present study
We examined associations among changes in loneliness, diurnal cortisol activity, and coping efficacy in a sample of late adolescents as they transitioned to college. Our first aim was to examine whether the change in loneliness from senior year of high school to the first semester of college was associated with cortisol levels at waking, the CAR, and the diurnal cortisol slope in the first semester of college. By measuring changes in loneliness across a relatively short transition period, we expected that increasing loneliness would be associated with diurnal cortisol patterns reflecting physiological adaptation to short-term stress (that is, higher waking levels or increased CAR; Doane & Adam, 2010; Pressman et al., 2005). Based on research suggesting state-like properties of the diurnal cortisol slope (Ross et al., 2014) and cross-sectional studies of loneliness and cortisol (e.g., Doane & Adam, 2010), we predicted that increases in loneliness would be associated with relatively flatter slopes in college. More importantly, our second aim was to determine whether individual differences in coping efficacy (a proposed protective factor) assessed at baseline before the transition to college would moderate associations between the change in loneliness across the transition and diurnal cortisol activity in the first semester of college. We included coping efficacy assessed during high school in order to specifically investigate whether prior individual differences would modify stress responsivity in college and ultimately identify a target for future prevention and intervention programs prior to the transition. Although there is limited available research involving coping efficacy and diurnal cortisol activity, we expected that late adolescents with high levels of coping efficacy would feel relatively more confident when facing increased loneliness (e.g., Massey et al., 2009; Masten et al., 2004). Thus, we expected that coping efficacy would emerge as a protective factor, such that those high on coping efficacy would be buffered from the impact of increased loneliness on alterations in typical diurnal cortisol activity (e.g., increased waking levels or greater CAR; flatter diurnal slope).
Method
Participants
A total of 82 late adolescents were recruited during the spring semester of their senior year of high school (T1) through e-mail correspondence and orientation sessions at a large southwestern university in the United States. Participants were required to live within 35 miles of the university and plan to attend in the fall. From the original sample, 76 late adolescents participated again during their first semester of college (T2; 93% retention): three participants did not matriculate in the fall, and three declined to participate. These six participants had parents who completed more education (M = 5.00, SD = 1.52) than parents of participants who remained in the study (M = 3.36, SD = 1.47), t(80) = −2.63, p = .01. However, these participants did not differ significantly on other demographic variables, including gender, race/ethnicity and whether or not they lived at home during the first semester of college.
Additionally, six participants deemed noncompliant with saliva sampling procedures were excluded from analyses. These six excluded participants had parents who completed less education (M = 2.42, SD = 0.66) than those remaining in the analyses (M = 3.44, SD = 1.49), t(10.10) = 3.16, p = .01, and reported lower coping efficacy (M = 22.83, SD = 5.49) than those remaining in the analyses (M = 25.80, SD = 3.21), t(74) = 2.04, p = .05, but did not differ significantly on other demographic or focal variables (e.g., cortisol, loneliness). Our final analytic sample comprised 70 late adolescents (23% male; Mage = 18.49, SD = 0.38) from relatively diverse racial/ethnic and socioeconomic backgrounds: 54% European American, 26% Latino/Hispanic, 3% African American, 4% Asian, and 13% multiracial descent; 4.3% of parents completed some high school, 25.7% received a high school diploma, 22.8% completed some college, 12.8% received an associate’s degree, 20% received a bachelor’s degree, and 14.3% received a graduate degree. At T2, 21.4% of the sample lived with their parents and 78.6% lived away from home.
Procedures
The university Institutional Review Board approved all procedures. Participants provided saliva samples 5 times a day for 3 typical consecutive weekdays at both time points. Present analyses focus on diurnal cortisol from T2. Analyses focusing on cortisol at T1 have been reported elsewhere (Doane & Zeiders, 2014). Study personnel delivered materials to participants’ residences, explained study procedures, and collected consent forms. Parental consent forms were completed for participants under the age of 18. Personnel answered questions regarding the protocol via phone and text message throughout the 3 sampling days and then collected study materials from participants’ residences upon completion of the protocol. Participants were compensated $40 at T1 and $50 at T2. Assessments for T1 took place over a 4-month period (March–June) and T2 assessments were completed over a 3-month period (September–November). The average time between T1 and T2 assessments was 5.2 months (SD = .96).
Study materials included daily diary booklets, an actigraph (wrist-based accelerometer), 16 vials for saliva sampling, a MEMS 6™ (Aardax) track cap compliance device with 16 small straws, and several questionnaires. Participants provided saliva samples via passive drool and completed paper-and-pencil diary entries 5 times a day: at waking, 30 minutes after waking, 2 random times throughout the day prompted by pre-programmed alarms from the actigraph to avoid mealtimes (approximately 3 hours and 8 hours after waking), and at bedtime. The average number of saliva samples provided by each participant was 14.55 (SD = 1.09) out of 15 possible samples. In the diary entries, participants recorded their mood and stressful events; caffeine, alcohol, medication and nicotine use; food intake; exercise; and sleeping behavior within the last hour. Participants also completed self-report questionnaires at each time point including demographic information and trait measures of loneliness and coping efficacy.
Noncompliance to sampling procedures can distort cortisol estimates (Kudielka, Broderick, & Kirschbaum, 2003). As such, six participants were entirely excluded from analyses for overall noncompliance with the track cap procedure for saliva sampling (that is, provided no compliant waking samples, necessary to estimate the model intercept). Otherwise, non-compliance was permitted if at least 1 waking sample was available in order to estimate the intercept (that is, waking cortisol level, given the centering of the growth term at time since waking). For the remaining participants, we considered each sample compliant if: 1) the track cap-detected waking sample was within 15 minutes of their actigraph-detected wake time, and 2) the track cap-detected second sample was between 23 and 37 minutes after their actigraph wake time (DeSantis, Adam, Mendelsohn, & Doane, 2010). Based on these criteria, 52 waking samples and 13 samples provided 30 minutes after waking were considered noncompliant and were excluded from analyses. After taking these strict criteria into account, there were 958 momentary data points available for analyses.
Measures
Salivary cortisol
Upon collection of saliva samples from participants’ residences, the samples were stored at −20° C in our laboratory until sent via courier on dry ice over 3 days to the Biochemisches Labor at the University of Trier in Germany. Samples were assayed in duplicate using a competitive solid phase time-resolved fluorescence immunoassay with fluorometric endpoint detection (DELFIA; Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). The intra-assay coefficients of variation (the degree to which two measurements of the same saliva sample differ using the same assay) ranged between 4.0% and 6.7%. The inter-assay coefficients (the degree to which two measurements differ using separate assays) ranged between 7.1% and 9.0%. Both of these reflect acceptable ranges: average intra-assay coefficients of variation should not typically exceed 10% and inter-assay coefficients of variation should not typically exceed 15% (Schultheiss & Stanton, 2009). Cortisol outlier values were windsorized at 1.81 μg/dl (equivalent to 50 nmol/L; Nicolson, 2008) and transformed using the natural log function to correct for a strong positive skew in the distribution of values across the day.
Loneliness
The UCLA Loneliness Scale Version 3 (Russell, 1996) was used to measure trait or global loneliness at T1 and T2. Items assess the extent to which participants generally feel a lack of quality or quantity in their social relationships (1 = never to 4 = always). Example items include: “How often do you feel that you lack companionship?” and “How often do you feel that there are people you can turn to?” Estimates of reliability have been strong across several groups with a test-retest reliability coefficient of .73 and internal consistencies ranging from .89 to .94 (Russell, 1996). Positively worded items were reverse scored and then items were summed to determine a total loneliness score, with higher scores reflecting higher levels of loneliness. Cronbach’s alphas for this sample were .91 at T1 and .90 at T2.
Coping efficacy
The Coping Efficacy Scale (Sandler et al., 2000) was used to measure adolescents’ belief in their ability to use coping strategies to handle future stressors and novel situations at T1 and T2. Participants responded to items using a 4-point fully anchored scale with unique anchors designed for each item. Scores on this measure have demonstrated adequate reliability and validity in samples of children and adolescents (Sandler et al., 2000, 2003). Example items include: “Overall, how good do you think you will be at handling your feelings when problems come up in the future?” and “Overall, compared to other people, how good do you think that you have been in handling your problems?” Scores were summed with higher scores reflecting higher coping efficacy. Cronbach’s alphas for this sample were .85 at T1 and .91 at T2.
Covariates
Participants completed a questionnaire to provide age, gender (1 = male, 0 = female), race/ethnicity, oral contraceptive use (females using a form of oral contraception coded as 1, other females and all males coded as 0), and regular caffeine consumption (1 = regularly consumed caffeine, at least once a day, 0 = not regular consumer). Participants also reported on momentary affect using the Positive and Negative Affective Schedule (PANAS; Watson, Clark, & Tellegen, 1988) and momentary perceived stress level in the daily diary reports. These covariates were included in analyses given past research demonstrating associations with cortisol activity (see Adam & Kumari, 2009), including trends of elevated cortisol responses to stress among males (Kirschbaum, Wüst, & Hellhammer, 1992), flatter diurnal cortisol slopes (less rapid rates of hourly decline) among African American and Hispanic late adolescents (DeSantis et al., 2007), elevated morning cortisol levels among females using oral contraceptives (Meulenberg, Ross, Swinkels, & Benraad, 1987), elevated cortisol levels in relation to momentary experiences of negative affect among late adolescents (Doane & Zeiders, 2014), and increased daily cortisol levels among regular caffeine users (Lovallo et al., 2005). To provide more nuanced information about the temporal sequencing of our proposed research questions, in all analyses we covaried for an overall indicator of the cortisol diurnal rhythm from T1 (area under the curve with respect to ground, AUCg; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) and T2 coping efficacy. Living at home during the first semester of college and days since beginning of the T2 semester were not significantly correlated with T2 loneliness or T2 average diurnal cortisol parameters (see Table 1) and were thus not included as covariates in analyses.
Table 1.
Descriptive statistics and zero-order correlations for cortisol, loneliness, coping efficacy, and covariates.
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | M | (SD)a | Range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. T2 Waking levels of cortisol | – | −1.66 | (.70) | − 5.11 – −.61 | ||||||||||||||
| 2. T2 Cortisol awakening response | −.32** | – | .58 | (.45) | −.35 – 2.06 | |||||||||||||
| 3. T2 Cortisol diurnal slope | −.64** | .21† | – | −.11 | (.06) | −.23 – .09 | ||||||||||||
| 4. T1 Cortisol area under the curve (AUC) | .17 | −.03 | .13 | – | 72.62 | (11.01) | 43.61– 110.85 | |||||||||||
| 5. T1 Loneliness | .07 | −.07 | .10 | .10 | – | 36.14 | (8.69) | 18.00 – 57.00 | ||||||||||
| 6. T2 Loneliness | .13 | −.09 | −.16 | −.04 | .61** | – | 37.56 | (8.88) | 19.00 – 57.00 | |||||||||
| 7. T1 Coping efficacy | −.21† | .19 | .10 | .09 | −.38** | −.44* | – | 25.80 | (3.21) | 18.00 – 32.00 | ||||||||
| 8. T2 Coping efficacy | −.07 | .04 | .09 | .07 | −.37** | −.55* | .68** | – | 25.11 | (4.43) | 11.00 – 32.00 | |||||||
| 9. Age | .03 | −.14 | −.09 | −.11 | −.05 | −.01 | −.12 | −.08 | – | 18.49 | (.38) | 17.18 – 19.12 | ||||||
| 10. Gender (male = 1) | .13 | −.07 | −.02 | .19 | −.08 | −.13 | .03 | −.05 | −.01 | – | 23% | – | ||||||
| 11. race/ethnicity | −.09 | .05 | −.06 | .01 | .30* | .09 | −.07 | −.11 | .19 | −.05 | – | 54% | – | |||||
| 12. Caffeine consumption | .11 | −.16 | .13 | −.01 | .02 | .07 | −.22† | −.18 | .06 | .05 | −.02 | – | 45.7% | – | ||||
| 13. Oral contraceptive use | .02 | −.25* | .12 | −.02 | .00 | −.02 | −.11 | .10 | −.02 | −.36** | .10 | .09 | – | 30% | – | |||
| 14. T2 Living at home | .04 | .14 | −.05 | .08 | −.18 | −.03 | −.02 | −.07 | .09 | −.12 | .06 | .15 | .11 | – | 21.4% | – | ||
| 15. T2 Days since beginning of semester | .13 | .09 | −.01 | −.08 | −.04 | .14 | .28* | −.34** | .04 | −.05 | −.07 | .11 | −.01 | .31** | – | 55.40 | (20.32) | 25.00 – 88.20 |
Note. N = 70. Cortisol values reflect the natural log function (μg/dl). Cortisol diurnal slope reflects the unadjusted mean level of decline.
Means and standard deviations presented for continuous variables. Percentages presented for dichotomous variables.
p < .10.
p < .05.
p < .01.
Analytic plan
As the current study’s data were collected across moments, days, and individuals, hierarchical linear growth curve models were employed to test the current study’s hypotheses. These models represent the nesting of moments within days and days nested within individuals (Raudenbush & Bryk, 2002; Singer & Willett, 2003). Simulation multilevel modeling studies have indicated that only sample sizes of 50 or less at the highest level (in our study, the person-level) typically lead to biased estimates of standard errors; sample sizes greater than 50 (as in the present study) typically result in unbiased and accurate estimates of the regression coefficients (fixed effects), variance components (random effects), and standard errors (Maas & Hox, 2005; see Scherbaum & Ferreter, 2009, for an extended treatment of power analysis in multilevel modeling). We used a three-level growth model to effectively illustrate the three levels of changes in cortisol (that is, momentary, daily, and person). At Level 1 (L1), the diurnal cortisol rhythm was modeled using two time variables1 based on individuals’ wake time (linear and quadratic) and a dummy variable corresponding to the CAR sample (Adam, 2006). No variables were included at Level 2, the day level. At Level 3 (L3), person-specific variables (that is, loneliness, coping efficacy) were included. All L1 variables (except time and the CAR dummy-code) were group-mean centered and L3 variables were grand-mean centered.
First, we modeled individuals’ cortisol diurnal rhythm with the inclusion of covariates (adjusting for T1 cortisol patterns; AUCg). Analyses focused on waking cortisol levels, the CAR, and the cortisol diurnal slope from T2. Next, we investigated the between-person effect of T2 loneliness on cortisol parameters (wake levels, CAR, and slope), while accounting for T1 loneliness. Finally, we investigated whether coping efficacy at T1 moderated the association between loneliness at T2 and cortisol (adjusting for coping efficacy at T2). Significant interactions between cortisol, loneliness, and coping efficacy were probed using the simple slopes technique (Aiken & West, 1991) with an online calculator (Preacher, Curran, & Bauer, 2006).
Results
Descriptive statistics and correlations are presented in Table 1 (diurnal cortisol parameters were aggregated at the person level only for descriptive purposes). T1 and T2 loneliness were highly positively correlated, r(68) = .68, p < .001. However, there was a high degree of variability in changes in loneliness across the transition to college. Based on raw change scores, approximately 57% of the sample reported feeling more lonely, 37% reported feeling less lonely, and 6% remained stable. Person-level averages of waking cortisol levels, the size of the CAR, and the diurnal cortisol slope were not significantly correlated with loneliness or coping efficacy at either time point.
Results from the 3-level growth model accounting for covariates (Model 1) demonstrated that individuals exhibited a typical diurnal cortisol rhythm: high morning levels (γ000 = 1.595, p < .001; equivalent to .203 μg/dl), a 73%2 increase in cortisol 30 minutes after waking (CAR; γ100 = .549, p < .001), and a decrease in cortisol across the day at a rate of 8% per hour at waking (γ200 = −.078, p < .001). Next, we investigated the effect of T2 loneliness, while accounting for T1 loneliness, on the diurnal cortisol parameters by including it at L3 (Table 2, Model 2). While accounting for T1 loneliness, T2 loneliness was associated with steeper slopes across the day (linear time; γ206 = −.002, p < .05). There were no associations between T2 loneliness and waking levels or the CAR.
Table 2.
Multilevel regression estimates predicting daily cortisol activity from loneliness and coping efficacy.
| Model 2
|
Model 3
|
|||||
|---|---|---|---|---|---|---|
| Loneliness
|
Loneliness × Coping
|
|||||
| Coefficient | SEI | CI | Coefficient | SEI | CI | |
| Intercept: waking level, γ000 | −1.593*** | .073 | −1.739 – −1.447 | −1.595*** | .071 | 1.737 – 1.453 |
| White, γ001 | .132 | .153 | −.173 – .437 | .200 | .138 | −.075 – .475 |
| Oral contraceptive use, γ002 | −.072 | .138 | −.347 – .203 | −.167 | .122 | − .410 – .076 |
| Caffeine, γ003 | .049 | .130 | −.210 – .308 | .013 | .121 | −.228 – .254 |
| Cortisol area under the curve T1, γ004 | .012† | .007 | .002 – .026 | .013† | .007 | −.001 – .027 |
| Loneliness T1, γ005 | −.003 | .010 | −.023 – .017 | −.011 | .009 | −.029 – .007 |
| Loneliness T2, γ006 | .005 | .009 | −.013 – .025 | .003 | .008 | −.013 – .019 |
| Coping efficacy T1, γ007 | −.064** | .022 | −.108 – .020 | |||
| Coping efficacy T2, γ008 | .015 | .017 | −.019 – .049 | |||
| Loneliness T2 Coping efficacy T1, γ009 | .003* | .001 | .001 – .005 | |||
| Cortisol awakening response, γ100 | .550*** | .056 | .438 – .662 | .552*** | .056 | .440 – .664 |
| White, γ101 | −.143 | .123 | −.388 – .102 | −.174 | .128 | −.429 – .081 |
| Oral contraceptive use, γ102 | −.184† | .108 | −.400 – .032 | −.119 | .109 | −.337 – .099 |
| Caffeine, γ103 | −.075 | .115 | −.304 – .154 | −.037 | .112 | −.260 – .186 |
| Loneliness T1, γ104 | −.005 | .007 | −.019 – .009 | −.002 | .007 | −.016 – .012 |
| Loneliness T2, γ105 | .002 | .007 | −.012 – .017 | .001 | .009 | −.017 – .019 |
| Coping efficacy T1, γ106 | .035† | .019 | −.003 – .073 | |||
| Coping efficacy T2, γ107 | −.016 | .018 | −.052 – .020 | |||
| Loneliness T2 Coping efficacy T1, γ108 | −.002 | .002 | −.006 – .002 | |||
| Time since waking: linear slope, γ200 | −.079*** | .016 | −.11 – −.047 | −.079*** | .016 | −.111 –.047 |
| White, γ201 | −.021 | .014 | −.049 – .007 | −.029* | .012 | −.053 –.005 |
| Oral contraceptive use, γ202 | .018 | .015 | −.012 – .048 | .029 | .015 | −.001 – .059 |
| Caffeine, γ203 | .014 | .013 | −.012– .040 | .019 | .013 | −.007 – .045 |
| Loneliness T1, γ204 | .002* | .001 | .001 – .004 | .003** | .001 | .001 – .005 |
| Loneliness T2, γ205 | −.002* | .001 | −.004 – −.001 | −.002* | .001 | −.004 – .001 |
| Coping efficacy T1, γ206 | .005† | .003 | −.001 – .011 | |||
| Coping efficacy T2, γ207 | −.002 | .002 | −.006 – .002 | |||
| Loneliness T2 Coping efficacy T1, γ208 | −.004** | .0001 | −.0042 – .0038 | |||
| Time since waking2: quadratic slope, γ300 | −.002* | .001 | −.004 – −.001 | −.002* | .001 | −.004–.001 |
| Momentary negative affect (NA), γ400 | .066** | .024 | .018 – .114 | .045* | .023 | −.001 –.068 |
| Momentary stress level, γ500 | −.008 | .021 | −.050 – .034 | −.010 | .023 | −.056 – .036 |
Note. 958 moments nested within 70 individuals. Intercept (waking cortisol level) reflects log μg/dL. SE = robust standard errors. CI = 95% confidence interval for individual regression estimates.
p < .10,
p .05,
p < .01,
p < .001.
Finally, in Model 3 (Table 2), we included T1 coping efficacy and the interaction between T1 coping efficacy and T2 loneliness to investigate its potential to moderate the relation between T2 loneliness and cortisol. Coping efficacy from T2 was also included in this model to account for any changes in coping efficacy that may have occurred over the transition to college. Coping efficacy from T1 exhibited main effects on cortisol, such that higher coping efficacy was significantly related to lower waking levels of cortisol (γ007 = −.064, p < .01). T1 coping efficacy also significantly moderated the relation between T2 loneliness and the cortisol slope (γ210 = −.0005, p < .05) and waking levels (γ008 = .003, p < .05).
Probing of the significant interaction between T2 loneliness and coping efficacy on waking levels of cortisol at low (1 SD below the mean) and high (1 SD above the mean) values of coping efficacy revealed a marginally significant association between T2 loneliness and waking levels of cortisol at high levels of coping efficacy (β = .0137, p = .10) but not low or average levels. The association between T2 loneliness and waking levels of cortisol was statistically significant at values of coping efficacy greater than 31 (N = 8).
Probing the significant interaction between T1 coping efficacy and T2 loneliness on the linear cortisol slope at low (.5 SD below the mean) and high (.5 SD above the mean)3 values of coping efficacy and T2 loneliness revealed several significant interactions (Figure 1). Individuals who were low on T1 coping efficacy and low on T2 loneliness exhibited waking levels of approximately .227 μg/dl (β = −1.483, p < .001), and a significant decline in cortisol across the day (β = −.108, p < .001), decreasing approximately 11.4% per hour at waking. In contrast, individuals who were low on coping efficacy and high on T2 loneliness exhibited waking levels of .223 μg/dl (β = −1.500, p < .001), and a flatter slope across the day (β = −.063, p < .05), decreasing approximately 6.5% per hour at waking. Interestingly, individuals who were high on coping efficacy and low on T2 loneliness did not display significant cortisol slopes across the day (see Figure 1). Finally, individuals who were high on coping efficacy and high on T2 loneliness exhibited lower waking values, .190 μg/dl (β = −1.661, p < .001), and the steepest cortisol slopes across the day (β = −.113, p < .001), decreasing approximately 12.0% per hour at waking. For individuals with low coping efficacy (N = 24), the association between T2 loneliness and the linear slope of cortisol was statistically significant at values of T2 loneliness less than 47 (N = 19). In contrast, for individuals with high coping efficacy (N = 21), the association between T2 loneliness and the linear slope of cortisol was statistically significant at values of T2 loneliness greater than 35 (N = 8).
Figure 1.
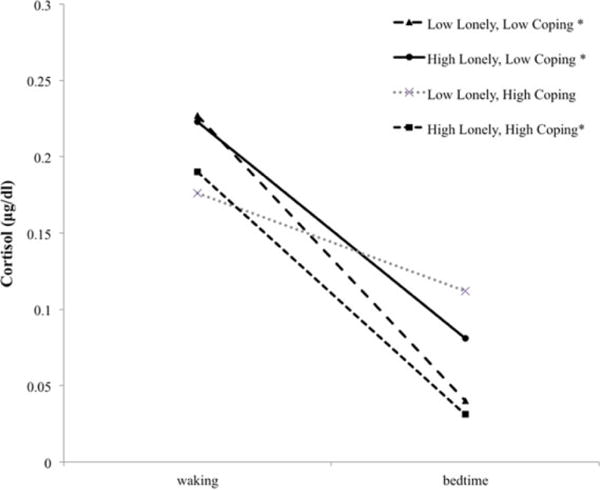
Simple slope plots for diurnal cortisol slopes by high and low levels of loneliness and coping efficacy.
Note. Plots characterized by participants scoring low on loneliness and low on coping (n = 19), high on loneliness and low on coping (n = 13), low on loneliness and high on coping (n = 12), and high on loneliness and high on coping (n = 8). Significant (p ≤ .05) slopes across the day are indicated by*.
Discussion
The transition from late adolescence into adulthood is a developmental period characterized by meaningful changes in interpersonal relationships and increasing demands across social, financial, and academic domains (e.g., Arnett, 2000; Ross et al., 1999). One developmentally salient stressor during this time—loneliness—has been correlated with health problems during late adolescence and adulthood (Hawkley et al., 2010; Pressman et al., 2005). In the present study, we investigated one potential biological mechanism underlying the association between loneliness and health by exploring diurnal cortisol activity in a sample of late adolescents transitioning to college. An increase in loneliness from late adolescents’ senior year of high school to their first semester of college was significantly associated with steeper cortisol slopes in their first semester of college. Coping efficacy significantly moderated this association, such that late adolescents low on coping efficacy in high school who also reported higher levels of loneliness across the transition exhibited a lower rate of decline in daily cortisol output (that is, flatter slopes) in their first year of college, while late adolescents high on coping efficacy who also reported higher levels of loneliness exhibited a higher rate of decline in daily cortisol output (that is, steeper slopes). This significant interaction suggests that late adolescents’ confidence in their ability to handle future stressors prior to the transition serves as a protective factor for those struggling with increasing loneliness by contributing to adaptive regulation of stress physiology. This is the first study to explore the association between changes in loneliness across the transition to college and diurnal cortisol activity, as well as the first to explore how this association differs depending on individual differences in coping efficacy.
Building on prior cross-sectional work with late adolescents (e.g., Doane & Adam, 2010), our first aim was to explore the link between longitudinal changes in loneliness and diurnal cortisol activity during the dynamic transition period late adolescents navigate as they enter the college context. We hypothesized that an increase in loneliness would predict increased morning cortisol levels (that is, higher waking levels or CAR) and less of a decline in cortisol across the day (flatter diurnal slopes). Although there was not a statistically significant association for morning levels or the CAR, increases in loneliness were significantly associated with steeper slopes.
Previous research using naturalistic assessment of diurnal cortisol has demonstrated that chronic loneliness at one time point was concurrently associated with flatter cortisol slopes in a sample of 17–20-year-olds (Doane & Adam, 2010). This previous cross-sectional finding was consistent with results from a meta-analysis, which indicated that flatter cortisol slopes are typically indicative of chronic, cumulative stress (Miller et al., 2007). However, the results of the meta-analysis also highlighted that much of the variability in the link between stress and diurnal cortisol patterns can be attributable to features of the stressor (e.g., loneliness in particular), person (e.g., coping resources), and timing (e.g., changes in loneliness over time). Specifically, stressors that threaten physical integrity, involve trauma, and are uncontrollable are those most likely to elicit a high, flat diurnal pattern of cortisol secretion (Miller et al., 2007). Although certainly critical to the social and emotional adjustment of the adolescents in our sample, changes in loneliness across a relatively short time interval would not be characterized as any of these types of stressors (that is, traumatic, entirely uncontrollable). By measuring across a 6-month period in the present study, we believe we captured relatively short-term rather than enduring changes in loneliness that would be considered chronic stress. This could explain why increases in loneliness predicted a diurnal cortisol profile reflecting effective physiological adaptation (that is, steeper slopes).
Alternatively, it could be that late adolescents in our sample who increased in loneliness were those who did not perceive a lack in their social networks in high school; the association with steeper diurnal cortisol slopes in college might be a residual physiological index of adaptation in high school rather than adaptation in college. Given the high degree of variability in our sample, the complexity of changing loneliness across the college transition requires further exploration with more detailed repeated assessments in order to better estimate the specific time at which diurnal rhythms may covary with changes in loneliness. Finally, we caution full interpretation of this main effect without considering how the association might depend upon levels of coping efficacy. A strong theoretical tradition (and more recently, empirical tradition) encourages researchers to consider the dynamic interplay between stress and coping processes in physiology, developmental, and health research (e.g., Lazarus & Folkman, 1984; Nicolson, 1992). In line with this tradition, we encourage future researchers to continue examining stress and coping processes (risk and resilience processes) in tandem in order to move beyond direct risk pathways.
Guided by this tradition and the risk and resilience framework, our second aim was to explore whether individual differences in coping efficacy (an adaptive resource) assessed prior to the transition would interact with the change in loneliness to predict diurnal cortisol activity in the first year of college. Based on prior literature identifying coping skills as a protective factor (e.g., Masten et al., 2004), we hypothesized that late adolescents with high levels of coping efficacy in high school would be buffered from alterations in diurnal cortisol activity in relation to increases in loneliness across the college transition. Coping efficacy assessed during late adolescents’ senior year of high school significantly moderated the relations between loneliness and waking cortisol levels and diurnal slope in the first semester of college. The interaction was not significant for the CAR. The interaction between loneliness and coping efficacy in the prediction of waking cortisol levels is preliminary given that the simple slopes were only significant for a small portion of our sample. However, these findings suggest that late adolescents with increasing loneliness who were also high on coping efficacy had higher levels of cortisol at waking compared to late adolescents with decreasing or stable loneliness and high levels of coping efficacy.
Regarding diurnal cortisol slopes, coping efficacy emerged as a significant protective factor. Although all variables in the current study were continuous (and were treated as such in analyses), for interpretive purposes we outline important differences for three groups exhibiting statistically significant diurnal cortisol slopes: low loneliness and low coping efficacy, high loneliness and low coping efficacy, and high loneliness and high coping efficacy. Late adolescents who reported low levels of loneliness in college (after adjusting for loneliness in high school) who were also low on coping efficacy displayed significantly negative diurnal slopes, with the rate of decline at waking (11.4% decrease per waking hour) comparable to estimates across various normative samples of adolescents of varying ages (e.g., Adam, 2006; Doane & Adam, 2010). Although this group of individuals had low confidence in their ability to handle stress, they did not report experiencing heightened loneliness when transitioning to the college environment. According to dynamic models of stress and coping (e.g., Lazarus & Folkman, 1984), stress occurs only when perceived demands from the environment exceed an individual’s ability to cope with them. Given that this group of late adolescents did not perceive heightened loneliness across the transition to college, it is likely they did not require a high degree of confidence in their ability to handle future stress in order to facilitate effective physiological regulation. As such, they exhibited a fairly normative rate of decline in cortisol at waking.
However, late adolescents who experienced heightened loneliness across the college transition exhibited different diurnal cortisol slopes depending on their levels of coping efficacy. Specifically, those high on loneliness and low on coping efficacy displayed the flattest slopes (6.5% decrease per waking hour), while those high on loneliness and high on coping efficacy displayed the steepest slopes (12.0% decrease per waking hour, comparable to low lonely–low coping). Prior research has demonstrated that flatter diurnal cortisol slopes are linked to chronic stress (Kumari et al., 2009; Nater et al., 2008), whereas steeper diurnal slopes are typically associated with effective adaptation to one’s environment evidenced by better emotional and physical health (Adam & Kumari, 2009). Although late adolescents in both of these groups experienced perceptions of a lack of quantity or quality in their social relationships, prior levels of coping efficacy modulated their daily stress physiology in the new college environment. This finding expands upon prior literature demonstrating the protective role of coping skills in general (e.g., Compas et al., 2001; Masten et al., 2004) and coping efficacy specifically (e.g., Massey et al., 2009).
Based on the results of our interaction reported here, we hypothesize that coping efficacy may act as a protective mechanism that promotes physiological regulation when adolescents perceive psychosocial stress. Late adolescents with the confidence to handle stressful situations (particularly before a developmental transition period) may benefit from coping resources when facing uncertainty and shifting sources of social support (Masten et al., 2004). Our findings support the notion that one’s belief in his/her ability to cope may promote resiliency among late adolescents transitioning to a new developmental and social context by increasing adaptive stress regulation.
Interestingly, although we did not expect to find main effects of coping efficacy on cortisol activity, higher coping efficacy assessed in high school significantly predicted lower waking levels during the first semester of college. Previous studies have shown that coping skills are directly related to lower total cortisol output (O’Donnell, Badrick, Kumari, & Steptoe, 2008) and steeper diurnal slopes (Sjögren, Leanderson, & Kristenson, 2006). Evidence also suggests that morning cortisol levels may reflect anticipation for the upcoming day (see Fries et al., 2009, for a review). We hypothesize that late adolescents low on coping efficacy may have perceived day-to-day demands in the first semester of college as more stressful, and therefore exhibited higher cortisol levels immediately post-awakening. Because coping efficacy reflects one’s belief in his/her ability to cope with stress and not actual coping behaviors (Sandler et al., 2000), intervention and prevention programs prior to college should consider incorporating curriculum designed to increase self-efficacy. Efforts aimed at improving coping efficacy could potentially help late adolescents manage a variety of stressors as they transition to college, including loneliness.
Although this study contributes novel findings, there are several limitations. First, our sample size was modest and disproportionately female. However, participants were racially and socioeconomically diverse, which suggests our results may generalize to diverse groups of adolescents. Second, our findings may only be relevant to late adolescents in the United States attending a 4-year public university close to home. National survey data show, though, that a large majority of high school seniors in the US now plan to attend college, specifically 4-year colleges or universities (Bureau of Labor Statistics, 2013). Future work should consider how the college transition experience differs for students moving farther away from home. Third, loneliness was not measured in daily diary assessments. Thus, we were not able to capture the simultaneous influences of momentary/state and trait levels of loneliness on the cortisol rhythm.
Future research needs to include multiple time courses of loneliness experiences to parse apart the influence of momentary, state, and trait levels of loneliness on cortisol rhythms across the transition to college. Additionally, future studies should explore both coping efficacy and actual coping behaviors in relation to psychosocial stressors and physiological adjustment. Such a comparison would allow researchers to uncover whether coping efficacy predicts adaptive physiological functioning over and above the predictive utility of observed coping behaviors used to deal with psychosocial stress such as loneliness.
This is the first study to examine changes in loneliness across the transition to college, coping efficacy, and diurnal cortisol activity in a late adolescent sample. Coping efficacy emerged as a significant protective factor, such that those high in coping efficacy who increased in loneliness across the transition exhibited more effective physiological regulation compared to those low in coping efficacy who also experienced increased loneliness. Our results highlight how late adolescents’ belief in their ability to cope with future problems may be an important protective resource as they attempt to adjust to college life. Our findings also highlight the adaptability of the HPA axis to changing social contexts and stressors, as well as the moderating role of coping efficacy in stress and adaptation processes.
Acknowledgments
Portions of these data were presented at the 2014 Biennial Meeting of the Society for Research on Adolescence. We would like to thank Dr. Clark Presson, Dr. Kathryn Lemery-Chalfant, and Dr. Douglas Granger for their valued feedback on earlier versions of this manuscript and mentorship.
Funding
This work was partially supported by the Institute for Social Science Research at Arizona State University and by a fellowship from the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1311230 to M. R. Sladek. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of these agencies.
Footnotes
These variables were constructed by subtracting the day specific wake time from each of the individual time points (e.g., 0 = wake-up, .5 = wake-up + 30 min, 2.5 first watch alarm, etc.).
As cortisol variables were log transformed, percentage changes in cortisol per 1 unit change were calculated using the following formula: β% change = e^(β) −1.
Simple slopes were also probed at 1 SD above and below the mean but we did not have enough individuals in our sample represented in each quadrant (e.g., 1 SD high on coping efficacy and 1 SD high on T2 loneliness), and therefore we chose to use .5 SD to capture more variability and representativeness of our sample.
References
- Abouserie R. Sources and levels of stress in relation to locus of control and self-esteem in university students. Educational Psychology. 1994;14:323–330. [Google Scholar]
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK. Emotion-cortisol transactions occur over multiple time scales in development: Implications for research on emotion and the development of emotional disorders. Monographs of the Society for Research in Child Development. 2012;77:17–27. [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffin JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Gunnar MR. Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology. 2001;26:189–208. doi: 10.1016/s0306-4530(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Aiken L, West S. Testing and interpreting interactions in multiple regression. Newbury Park, CA: SAGE; 1991. [Google Scholar]
- Alpass FM, Neville S. Loneliness, health, and depression in older males. Aging and Mental Health. 2003;7:212–216. doi: 10.1080/1360786031000101193. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: A theory of development from late teens through the twenties. American Psychologist. 2000;55:469–480. [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Health promotion by social cognitive means. Health Education & Behavior. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- Bartels M, Cacioppo JT, Hudziak JJ, Boomsma DI. Genetic and environmental contributions to stability in loneliness throughout childhood. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B:385–391. doi: 10.1002/ajmg.b.30608. [DOI] [PubMed] [Google Scholar]
- Benight CC, Antoni MH, Kilbourn K, Ironson G, Kumar MA, Redwine L, Schneidman N. Coping self-efficacy buffers psychological and physiological disturbances in HIV-infected men following a natural disaster. Health Psychology. 1997;16:248–255. doi: 10.1037//0278-6133.16.3.248. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Willemsen G, Dolan CV, Hawkley LC, Cacioppo JT. Genetic and environmental contributions in adults: The Netherlands twin register study. Behavior Genetics. 2005;35:745–752. doi: 10.1007/s10519-005-6040-8. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. College enrollment and work activity of 2012 high school graduates. 2013 Retrieved from http://www.bls.gov/news.release/hsgec.nr0.htm.
- Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW. The neuroendocrinology of social isolation. Annual Review of Psychology. 2015;66:733–767. doi: 10.1146/annurev-psych-010814-015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Ernst JM, Burleson MH, McClintock MK, Malarkey WB, Hawkley LC, Berntson GG. Lonely traits and concomitant physiological processes: The MacArthur social neuroscience studies. International Journal of Psychopathology. 2000;35:143–154. doi: 10.1016/s0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, Hobson JA. Do lonely days invade the nights?: Potential social modulation of sleep efficiency. Psychological Science. 2002;13:384–387. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernest JM, Burleson MH, Kowalkweski RB, Berntson GG. Loneliness and health: Potential mechanisms. Psychosomatic Medicine. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, Wadsworth ME. Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin. 2001;127:87–127. [PubMed] [Google Scholar]
- Compas BE, Wagner BM, Slavin LA, Vannatta K. A prospective study of life events, social support, and psychological symptomatology during the transition from high school to college. American Journal of Community Psychology. 1986;14:241–257. doi: 10.1007/BF00911173. [DOI] [PubMed] [Google Scholar]
- Conley CS, Kirsch AC, Dickson DA, Bryant FB. Negotiating the transition to college: Developmental trajectories and gender differences in psychological functioning, cognitive-affective strategies, and social well-being. Emerging Adulthood. 2014;2:195–210. [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Mendelsohn KA, Doane LD. Concordance between self-reported and objective wakeup times in ambulatory cortisol salivary cortisol research. International Journal of Behavioral Medicine. 2010;17:74–78. doi: 10.1007/s12529-009-9053-5. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Doane LD, Adam EK. Loneliness and cortisol: Momentary, day-to-day, and trait associations. Psychoneuroendocrinology. 2010;35:430–441. doi: 10.1016/j.psyneuen.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane LD, Zeiders KH. Contextual moderators of momentary cortisol and negative affect in adolescents’ daily lives. Journal of Adolescent Health. 2014;54:536–542. doi: 10.1016/j.jadohealth.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immuoassay for salivary cortisol measurement. The Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Edwards S, Hucklebridge F, Clow A, Evans P. Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psychosomatic Medicine. 2003;65:320–327. doi: 10.1097/01.psy.0000033123.70631.8e. [DOI] [PubMed] [Google Scholar]
- Felner RD, Farber SS, Primavera J. Transitions and stressful life events: A model for primary prevention. In: Felner RD, Jason LA, Mortisugu JN, Farber SS, editors. Preventive psychology: Theory, research, and practice. New York, NY: Pergamon; 1983. pp. 199–215. [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Burton CL, Bonanno GA. Coping flexibility, potentially traumatic life events, and resilience: A prospective study of college student adjustment. Journal of Social and Clinical Psychology. 2012;31:542–567. [Google Scholar]
- Geiss A, Varadi E, Steinbach K, Bauer HW, Anton F. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent disectomy. Neuroscience Letters. 1997;237:65–68. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- Gunlicks-Stoessel ML, Powers SI. Romantic partners’ coping strategies and patterns of cortisol reactivity and recovery in response to relationship conflict. Journal of Social and Clinical Psychology. 2009;28:630–649. doi: 10.1521/jscp.2009.28.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Burleson MH, Berntson GG, Cacioppo JT. Loneliness in everyday life: Cardiovascular activity, psychosocial context, and health behaviors. Journal of Personality and Social Psychology. 2003;85:105–120. doi: 10.1037/0022-3514.85.1.105. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness and pathways to disease. Brain, Behavior, and Immunity. 2003;17:S98–S105. doi: 10.1016/s0889-1591(02)00073-9. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Annals of Behavioral Medicine. 2010;40:218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age related differences in systolic blood pressure. Psychology and Aging. 2006;21:152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Preacher KJ, Cacioppo JT. Loneliness impairs daytime functioning but not sleep duration. Healthy Psychology. 2010;29:124–129. doi: 10.1037/a0018646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RB, Oxley D. Social network development and functioning during a life transition. Journal of Personality and Social Psychology. 1986;50:305–313. doi: 10.1037//0022-3514.50.2.305. [DOI] [PubMed] [Google Scholar]
- Juster RP, Bizik G, Picard M, Arsenault-Lapierre G, Sindi S, Trapanier L, Lupien SJ. A transdisciplinary perspective of chronic stress in relation to psychopathology throughout life span development. Development and Psychopathology. 2011;23:725–776. doi: 10.1017/S0954579411000289. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Affleck G, Lefebvre JC, Starr K, Caldwell DS, Tennen H. Pain coping strategies and coping efficacy in rheumatoid arthritis: A daily process analysis. Pain. 1997;69:35–42. doi: 10.1016/s0304-3959(96)03246-0. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Medicine. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid daytime profiles in non-compliant subjects. Psychosomatic Medicine. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Chandola T, Adam EK, Stafford M, Marmot MG, Kivimaki M. Cortisol secretion and fatigue: Associations in a community based cohort. Psychoneuroendocrinology. 2009;34:1476–1485. doi: 10.1016/j.psyneuen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Lauder W, Mummery K, Jones M, Caperchione C. A comparison of health behaviors in lonely and non-lonely populations. Psychology, Health, & Medicine. 2006;11:233–245. doi: 10.1080/13548500500266607. [DOI] [PubMed] [Google Scholar]
- Laursen B, Hartl AC. Understanding loneliness during adolescence: Developmental changes that increase the risk of perceived isolation. Journal of Adolescence. 2013;36:1261–1268. doi: 10.1016/j.adolescence.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York, NY: Springer; 1984. [Google Scholar]
- Lovallo WR. Stress & health. Thousand Oaks, CA: SAGE; 2005. [Google Scholar]
- Lovallo WR, Whitsett TL, al’Absi M, Sung BH, Vincent AS, Wilson MF. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosomatic Medicine. 2005;67:734–739. doi: 10.1097/01.psy.0000181270.20036.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS, Cicchetti D, Becker B. The construct of resilience: A critical evaluation and guidelines for future work. Child Development. 2000;7:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas CJM, Hox JJ. Sufficient sample sizes for multilevel modeling. Methodology. 2005;1:86–92. [Google Scholar]
- Massey EK, Garnefski N, Gebhardt WA, van der Leeden R. Daily frustration, cognitive coping and coping efficacy in adolescent headache: A daily diary study. Headache. 2009;49:1198–1205. doi: 10.1111/j.1526-4610.2009.01492.x. [DOI] [PubMed] [Google Scholar]
- Masten AS. Ordinary magic: Resilience processes in development. American Psychologist. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- Masten AS. Regulatory processes, risk, and resilience in adolescent development. Annals of the New York Academy of Sciences. 2004;1021:310–319. doi: 10.1196/annals.1308.036. [DOI] [PubMed] [Google Scholar]
- Masten AS, Burt KB, Roisman GI, Obradovic J, Long JD, Tellegen A. Resources and resilience in the transition to adulthood: Continuity and change. Development and Psychopathology. 2004;16:1071–1094. doi: 10.1017/s0954579404040143. [DOI] [PubMed] [Google Scholar]
- Meulenberg PMM, Ross HA, Swinkels LMJW, Benraad TJ. The effect of oral contraceptives on plasma-free and salivary cortisol and cortisone. Clinical Chimica Acta. 1987;165:379–385. doi: 10.1016/0009-8981(87)90183-5. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nater UM, Youngblood LS, Jones JF, Unger ER, Miller AH, Reeves WC, Heim C. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosomatic Medicine. 2008;70:298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- Nicolson NA. Stress, coping, and cortisol dynamics in daily life. In: de Vries MW, editor. The experience of psychopathology: Investigating mental disorders in their natural settings. Cambridge, UK: Cambridge University Press; 1992. pp. 219–232. [Google Scholar]
- Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of Physiological Methods in Health Psychology. Thousand Oaks, CA: SAGE; 2008. pp. 37–73. [Google Scholar]
- Okamura H, Tsuda A, Matsuishi T. The relationship between perceived loneliness and cortisol awakening responses on work days and weekends. The Japanese Psychological Association. 2011;53:113–120. [Google Scholar]
- O’Donnell K, Badrick E, Kumari M, Steptoe A. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology. 2008;33:601–611. doi: 10.1016/j.psyneuen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- O’Leary A. Self-efficacy and health: Behavioral and stress-physiological mediation. Cognitive Therapy and Research. 1992;16:229–245. [Google Scholar]
- O’Leary A, Brown S. Self-efficacy and the physiological stress response. In: Maddux JE, editor. Self-efficacy, adaptation, and adjustment: Theory, research, and application. New York, NY: Plenum Press; 1995. pp. 227–246. [Google Scholar]
- Peplau LA, Perlman D. Loneliness: A sourcebook of current theory, research and therapy. New York, NY: Wiley Interscience; 1982. [Google Scholar]
- Pierceall EA, Keim MC. Stress and coping strategies among community college students. Community College Journal of Research and Practice. 2007;31:703–712. [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Pressman SD, Cohen S, Miller GE, Barkin A, Rabin B. Loneliness, social network size, and immune response to influenza vaccination in college freshman. Health Psychology. 2005;24:297–306. doi: 10.1037/0278-6133.24.3.297. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Qualter P, Brown SL, Munn P, Rotenberg KJ. Childhood loneliness as a predictor of depressive symptoms: An 8-year longitudinal study. European Child & Adolescent Psychiatry. 2010;19:493–501. doi: 10.1007/s00787-009-0059-y. [DOI] [PubMed] [Google Scholar]
- Qualter P, Brown SL, Rotenberg KJ, Vanhalst J, Harris RA, Goossens L, Munn P. Trajectories of loneliness during childhood and adolescence: Predictors and health outcomes. Journal of Adolescence. 2013;36:1283–1293. doi: 10.1016/j.adolescence.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Application and data analysis methods. Thousand Oaks, CA: SAGE; 2002. [Google Scholar]
- Ross KM, Murphy MLM, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Niebling BC, Heckert TM. Sources of stress among college students. College Student Journal. 1999;33:841–846. [Google Scholar]
- Russell DW. UCLA loneliness scale (version 3): Reliability, validity, and factor Structure. Journal of Personality Assessment. 1996;66:20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- Sandler IN, Tein JY, Mehta P, Wolchik S, Ayers T. Coping efficacy and psychological problems of children on divorce. Child Development. 2000;71:1099–1118. doi: 10.1111/1467-8624.00212. [DOI] [PubMed] [Google Scholar]
- Sandler IN, Ayers TS, Wolchik SA, Tein JY, Kwok OM, Haine RA, Griffin WA. The family bereavement program: Efficacy evaluation of a theory-based prevention program for parentally bereaved children and adolescents. Journal of Consulting and Clinical Psychology. 2003;71:587–600. doi: 10.1037/0022-006x.71.3.587. [DOI] [PubMed] [Google Scholar]
- Scherbaum CA, Ferreter JM. Estimating statistical power and required sample sizes for organization research using multilevel modeling. Organization Research Methods. 2009;12:347–367. [Google Scholar]
- Schultheiss OC, Stanton SJ. Assessment of salivary hormones. In: Harmon-Jones E, Beer JS, editors. Methods in social neuroscience. New York, NY: Guilford Press; 2009. pp. 17–44. [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of National Cancer Institute. 2000;12:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shaver P, Furman W, Buhrmester D. Transition to college: Network changes, social skills, and loneliness. In: Duck S, Perlman D, editors. Understanding personal relationships: An interdisciplinary approach. Thousand Oaks, CA: SAGE; 1985. pp. 193–219. [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental Psychobiology. 2012;54:493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren E, Leanderson P, Kristenson M. Diurnal saliva cortisol levels and relations to psychosocial factors in a population sample of middle-age Swedish men and women. International Journal of Behavioral Medicine. 2006;13:193–200. doi: 10.1207/s15327558ijbm1303_2. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- South SC, Miller ML. Measuring momentary stress, affect, and cognition: Relationships with the internalizing and externalizing spectra. Journal of Psychopathology and Behavioral Assessment. 2014;36:93–104. [Google Scholar]
- Steptoe A, Kunz-Ebrecht SR, Brydon L, Wardle J. Central adiposity and cortisol responses to waking in middle-aged men and women. International Journal of Obesity. 2004;28:1168–1173. doi: 10.1038/sj.ijo.0802715. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. Journal of Abnormal Psychology. 2005;114:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Stowell JR, Tumminaro T, Attarwala M. Moderating effects of coping on the relationship between test anxiety and negative mood. Stress and Health. 2008;24:313–321. [Google Scholar]
- Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Annals New York Academy of Sciences. 1995;771:1–18. doi: 10.1111/j.1749-6632.1995.tb44666.x. [DOI] [PubMed] [Google Scholar]
- Strauss RS, Rodzilsky D, Burack G, Colin M. Psychosocial correlates of physical activity in healthy children. Archives of Pediatrics & Adolescent Medicine. 2001;155:897–902. doi: 10.1001/archpedi.155.8.897. [DOI] [PubMed] [Google Scholar]
- Vanhalst J, Goossens L, Luyckx K, Scholte RHJ, Engels RCME. The development of loneliness from mid- to late adolescence: Trajectory classes, personality traits, and psychosocial functioning. Journal of Adolescence. 2013;36:1305–1312. doi: 10.1016/j.adolescence.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilcox RR, Granger DA, Szanton S, Clark F. Cortisol diurnal patterns, associations with depressive symptoms, and the impact of intervention in older adults: Results using modern robust methods aimed at dealing with power due to violations of standard assumptions. Hormones and Behavior. 2014;65:219–225. doi: 10.1016/j.yhbeh.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


