Abstract
Purpose
While nitric oxide (NO) donors are emerging as treatments for glaucoma, the mechanism by which NO lowers intraocular pressure (IOP) is unclear. NO activates the enzyme guanylyl cyclase (GC) to produce cyclic guanosine monophosphate. We studied the ocular effects of inhaled and topically applied NO gas in mice and lambs, respectively.
Methods
IOP and aqueous humor (AqH) outflow were measured in WT and GC-1α subunit null (GC-1−/−) mice. Mice breathed 40 parts per million (ppm) NO in O2 or control gas (N2/O2). We also studied the effect of ocular NO gas exposure (80, 250, 500, and 1000 ppm) on IOP in anesthetized lambs. NO metabolites were measured in AqH and plasma.
Results
In awake WT mice, breathing NO for 40 minutes lowered IOP from 14.4 ± 1.9 mm Hg to 10.9 ± 1.0 mm Hg (n = 11, P < 0.001). Comparable results were obtained in anesthetized WT mice (n = 10, P < 0.001). In awake or anesthetized GC-1−/− mice, IOP did not change under similar experimental conditions (P ≥ 0.08, n = 20). Breathing NO increased in vivo outflow facility in WT but not GC-1−/− mice (+13.7 ± 14.6% vs. −12.1 ± 9.4%, n = 4 each, P < 0.05). In lambs, ocular exposure to NO lowered IOP in a dose-dependent manner (−0.43 mm Hg/ppm NO; n = 5 with 40 total measurements; P = 0.04) without producing corneal pathology or altering pulmonary and systemic hemodynamics. After ocular NO exposure, NO metabolites were increased in AqH (n = 8, P < 0.001) but not in plasma.
Conclusions
Breathing NO reduced IOP and increased outflow facility in a GC-dependent manner in mice. Exposure of ovine eyes to NO lowers IOP.
Keywords: intraocular pressure, nitric oxide, soluble guanylyl cyclase
Glaucoma is a leading cause of blindness worldwide with an estimated 80 million cases diagnosed by 2020.1 Primary open angle glaucoma (POAG) is the most prevalent type of glaucoma.2 Risk factors for the development of POAG include increased intraocular pressure (IOP), age, race, and genetic factors.3–5 The only risk factor currently amenable to treatment is increased IOP. Several classes of drugs are available to treat glaucoma,6 but they provide only incomplete protection from optic nerve deterioration. These considerations emphasize the need for novel therapeutic approaches to lower IOP.
Nitric oxide (NO) activates the heterodimeric enzyme guanylyl cyclase α1β1 (GC-1), resulting in increased levels of cyclic guanosine monophosphate (cGMP).7 Previous genetic and epidemiologic studies implicate decreased activity of the NO-cGMP signal transduction pathway in POAG pathogenesis.8–13 NO-cGMP signaling regulates aqueous humor (AqH) outflow from the anterior chamber through the trabecular meshwork and the Schlemm's canal,14–18 possibly by decreasing trabecular meshwork (TM) cell volume,19 decreasing Schlemm's canal cell volume,20 and by relaxing cells in the canalicular outflow system.21 In addition, inhibition of endogenous NO production reduced the AqH outflow rate through the trabecular meshwork cells in human eyes,22 and overexpression of NO-synthase 3 (NOS3) was associated with increasing pressure-dependent drainage and decreased IOP in murine eyes.23 In contrast, pressure-dependent drainage was reduced and IOP increased in NOS3-deficient mice.24 Based on these findings, the NO-cGMP signaling system is emerging as an attractive pathway to target increased IOP.25–28 For example, adding an NO-releasing moiety to dexamethasone prevented the increase in IOP observed in rabbits treated topically with dexamethasone itself.10,24 Evidence that GC-1 is important in POAG pathogenesis comes from a candidate gene association study in which an intergenic variant (rs11722059) in the GUCY1A3/GUCY1B3 region (containing the genes encoding the α1 and β1 GC-1 subunits) was associated with a subset of POAG patients (women with early paracentral visual field loss).29 Furthermore, mice that are deficient in GC-1α develop open-angle glaucoma.29 In recent clinical trials, latanoprostene bunod (LBN, Vyzulta; Bausch + Lomb, Rochester, NY, USA), an IOP-lowering agent that combines a prostaglandin analogue with a NO-donating moiety, lowers IOP in POAG patients.30–32
Inhaled NO is a Food and Drug Administration (FDA)-approved therapy for pulmonary hypertension of hypoxic newborns33 and is under development as a treatment for other cardiovascular diseases. Breathing up to 80 ppm NO lowers pulmonary but not systemic blood pressure.34 The therapeutic effect of breathing NO in the cardiovascular system is GC-1–dependent.35
The objective of this study was to determine whether gaseous NO delivered via inhalation in mice or by topical application to the ovine eye without a pharmaceutical carrier lowers IOP. In addition, we sought to determine whether the effects of NO on IOP were mediated by GC-1, identifying GC-1 as a therapeutic target to lower IOP in glaucoma.
Methods
All experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee. Equal numbers of both sexes were used throughout the experiments (mice and lambs respectively).
Animal Preparation and Inhaled NO Gas Delivery in Mice
We studied 12- to 18-week-old male and female 129S6 wild-type (WT) mice (Taconic, Inc. Hudson, NY, USA) and 129S6 mice deficient in the α1-subunit of GC-1 (GC-1−/−).36 Anesthesia was induced with isoflurane in 90% oxygen (O2)/10% nitrogen (N2) using a standardized anesthesia protocol that maintains IOP at a constant level (isoflurane 5% for 15 seconds, 3% for 1 minute, 2% for 1 minute, and subsequently 1.5%). At the final dose of isoflurane, animals breathed spontaneously via a mask. Animals were immobilized in a prone position during the experimental procedures. Mice breathed either 40 parts per million (ppm) NO in 90% O2/10% N2 or 90% O2/10% N2 (control gas) at a gas flow rate of 1000 mL/minute. NO levels (40 ppm) were continuously monitored, using a chemiluminescence analyzer (Sievers 280i; GE Analytical Instruments, Boulder, CO, USA).37
IOP Measurements in Anesthetized and Awake Mice
Rebound tonometry (iCare, Vantaa, Finland) was used to measure IOP in anesthetized mice at baseline and 10 minutes after breathing either NO or control gas (Supplementary Fig. S1).
Because isoflurane lowers IOP,38 we also measured ocular tension in awake WT and GC-1−/− mice breathing NO or control gas. Prior to the study, mice were acclimated to manual handling for 3 weeks. During the first week, animals were acclimated to manual handling by holding them by the scruff of their neck for 5 to 10 minutes daily. The following week, animals were familiarized with the probe of the rebound tonometer. Murine eyes were topically anesthetized by proparacaine hydrochloride (0.5%; Bausch + Lomb, Inc.) eye drops (one drop per eye). Ten minutes later, mice were manually restrained and exposed to the rebound tonometer. During the third and last week, the animals were placed in the incubation chamber (World Precision Instruments, Sarasota, FL USA) and exposed to O2 (100%). At predefined time points (0, 40 minutes) animals were removed, restrained and their IOP was measured. After 3 weeks of training, topical anesthesia was no longer used, animal behavior remarkably improved and experiments were conducted. Mice were placed in an anesthesia induction chamber and exposed to either 40 ppm NO in 90% O2/10% N2 or the control gas via a gas inlet at a flow rate of 1000 mL/minute. A gas outlet was open to the environment to maintain the induction chamber at ambient pressure. IOP was measured at baseline and 40 minutes after starting the treatment.
Ex Vivo Mouse Eye Perfusion Protocol to Measure AqH Outflow Rates
A previously established mouse eye perfusion protocol was modified slightly for the present study.39,40 A 5.5 mM D-glucose (DBG) in Dulbecco's PBS was used as mock AqH for perfusions. Enucleated eyes from GC-1−/− or WT mice were fastened to a post in a perfusion chamber using cyanoacrylate gel. Anterior chambers were then cannulated using a 33-gauge needle (World Precision Instruments), guided by a micromanipulator and viewed using a stereo microscope. During the cannulation procedure, eyes were stabilized using a 0.5-mm curret. The needle was connected by pressure tubing to a 50-μL glass syringe (World Precision Instruments), which was mounted in a mini-pump (model 33; Harvard Apparatus, Holliston, MA, USA) and output was controlled by custom software written in LabVIEW (National Instruments, Newbury, UK). The software recorded pressure readings from a Honeywell transducer (model 142PC01G; Honeywell, Fort Washington, PA, USA) that was open to the anterior chamber via a three way stop cock connecting tubing between syringe pump and eye. The computer controlled pump maintained desired pressure steps, adjusting flow rate over time. After a 45-minute equilibration period at 8 mm Hg, using a column of fluid to hold pressure and allow drug/vehicle to access outflow tissues, the software was activated and eyes were perfused for 20 minutes at each of 4 pressure steps (4, 8, 15, 20 mm Hg). To calculate flow rate at each pressure step, a minimum of 10 minutes of stable measurements was used.
Outflow Facility Analysis
To calculate outflow facility (C), the modified Goldmann equation was used: F = C(IOP) + Fu where F represents the flow rate at each pressure step and Fu is an estimate of the pressure-independent outflow rate. This equation is only valid in experimental conditions such as with ex vivo perfusion of enucleated eyes when episcleral venous pressure and AqH inflow is zero. In our experimental paradigm, F reaches equilibrium at each level of IOP, and C and Fu are independent of IOP. The value of C is calculated from the slope of the best-fit linear regression of the flow/pressure relationship.
Measurement of Conventional Outflow Facility in Anesthetized Mice
Outflow facility measurements were performed as previously described.41 Calibration of the flow-through pressure was completed by calculating the pressure of water at two different heights (2.8 mm Hg and 51.4 mm Hg). The transducer was connected to both a reading box to enable digital data transfer to a computer, and a 100-μL syringe (Hamilton), loaded into a microdialysis infusion pump for continuous injection of 0.9 % saline. Mice were anesthetized with isoflurane using the same standardized protocol described above. The anterior chamber was cannulated with a 33-gauge ½-inch stainless steel needle attached to the transducer (ICU Medical, Inc., San Clemente, CA, USA) and pump. Pressure was set at 15, 25, or 35 mm Hg and corresponding outflow capability (μL/minute) was recorded every second for 5 minutes. In the subsequent 10 minutes, 40 ppm NO was added to the inhaled gas and outflow capability was recorded as well as for another 5 minutes after turning off NO. Normalizing outflow rate (μL/minute) to changes in IOP pressure (mm Hg) provided outflow facility measurements (μL/minute/mm Hg).
Animal Preparation and Hemodynamic Monitoring in Anesthetized Lambs
We studied five 3- to 4-month-old Polypay lambs, from a caesarean-derived, specific pathogen-free sheep flock (New England Ovis, Dover, NH, USA). Animals weighed 33 ± 2 kg (mean ± SD). A venous catheter was placed into the jugular vein and both corneas of each lamb were treated with a single dose of NO (80, 250, 500, or 1000 ppm) for 60 minutes on each of four different days (see below for description of gas delivery system). Between experiments, lambs were allowed to recover for at least 2 days. On the last day, lambs were anesthetized and a pulmonary catheter was used to measure the pulmonary arterial pressure, and an arterial catheter was used to measure the systemic blood pressure, during treatment with 1000 ppm NO.37,42 Hemodynamic parameters were measured as previously described.37,42,43
Use of a Custom-Made Device to Deliver NO to the Eyes of Lambs
Commercially available swim goggles were modified to deliver NO directly to the cornea (Supplementary Fig. S3). This device allowed the direct exposure of the eyes to NO, without further dilution by air. At the beginning of the experiment, the device was placed over both eyes. Eyes were treated with NO (80, 250, 500, or 1000 ppm NO in air) or control gas (air) at a flow rate of 500 mL/minute for 1 hour. The fraction of oxygen in air was measured continuously (MiniOX; Ohio Medical, Gurnee, IL, USA) to maintain 21% oxygen in the gas mixture. The gas outlet remained open to maintain normal ambient pressure. IOP was measured every 15 minutes by applanation tonometry after topical saline application to hydrate the ocular surface (Tono-Pen XL; Reichert Technologies, Inc., Depew, NY, USA).
Biochemical Analyses of Ovine Blood and AqH Samples
Ovine blood samples were obtained through a venous catheter at baseline, 45 minutes after topical exposure to the control gas, and after a 1-hour exposure to either NO or the control gas. AqH was sampled in lambs that were treated with 1000 ppm NO using a 25-gauge needle immediately after euthanasia (1 hour after ceasing topical NO delivery). NO oxidation products (nitrate, nitrite) and cGMP levels were measured in both ovine plasma (Supplementary Fig. S2) and AqH samples as previously described.42
Measurement of the Corneal Thickness Before and After Exposure to 1000 ppm NO Using Ultrasound
Ultrasound biomicroscopy images were acquired using a 40 MHz probe (Vevo 770; FUJIFILM-VisualSonics, Toronto, Canada). The probe was positioned perpendicular to the cornea and 2-dimensional images were acquired. The greatest thickness of the cornea was recorded.
Microscopy
After euthanasia, the sheep globes were enucleated and immersion-fixed for 24 hours in PBS containing paraformaldehyde (4%). The fixed globes were sectioned in the dorsoventral sagittal plane, serially sectioned at a thickness of 5 μm, and stained with hematoxylin and eosin (H&E). A pathologist masked to the type of intervention analyzed lamb corneas using a brightfield microscope (BX43; Olympus Corp., Melville, NY, USA). Images were captured using a mounted digital camera (DP72; Olympus Corp.) with image analysis software (CellSence Dimension 1.4; Olympus Corp.).
Statistical Analyses
All data are expressed as mean ± SD. Statistical analyses were performed using commercial software (GraphPad Prism 6; GraphPad Software, La Jolla, CA, USA). Variables were tested for normality using the Shapiro-Wilk test. Two-group comparisons were performed using the Student's t-test (paired or unpaired, as appropriate) for normally distributed variables. For multigroup comparisons, ANOVA was used. For variables that were measured over time (or at different pressures), a repeated measures ANOVA was used. The exact analyses for each comparison are described in detail below. All analyses were adjusted for multiple comparisons using the Bonferroni correction, where the P value threshold for significance was 0.05/(number of comparisons).
A paired t-test was used to compare differences in murine IOP levels at baseline and after NO treatment in the WT and GC-1−/− mice. We performed 2-way repeated measures ANOVA to compare AqH outflow rates between WT and GC-1−/− mice at various IOP levels. Because the interaction P value between genotype and pressure was significant in the 2-way repeated measures ANOVA, unpaired t-tests were performed for each pressure to compare the two genotypes. An unpaired t-test was used to compare differences in the outflow facility and the percent change in outflow rate between NO-treated WT and GC-1−/− mice. A paired t-test was performed to compare differences in ovine IOP levels before and after exposure to various NO concentrations. Linear regression analysis with slope comparisons of the change in IOP before and after NO exposure of ovine corneas was used to evaluate the dose-response effect between various NO concentrations. Ovine time-response experiments were analyzed by a pairwise comparison of the 45-minute time point with 60-, 75-, 90-, or 105-minute time points, respectively, within a group using a paired t-test. An unpaired t-test was used to compare differences in AqH nitrate and nitrite levels between control-treated and NO-treated eyes. Plasma nitrate, nitrite, and cGMP levels were analyzed by a 1-way repeated measures ANOVA including baseline, 45, 90, and 140 minute levels within each treatment group. For hemodynamic experiments, a 1-way ANOVA was used to compare differences between the control-treated, NO-treated, and post-NO treated groups (P < 0.017 was considered significant).
Results
Inhaled NO Reduces IOP in WT, but not GC-1−/− Mice
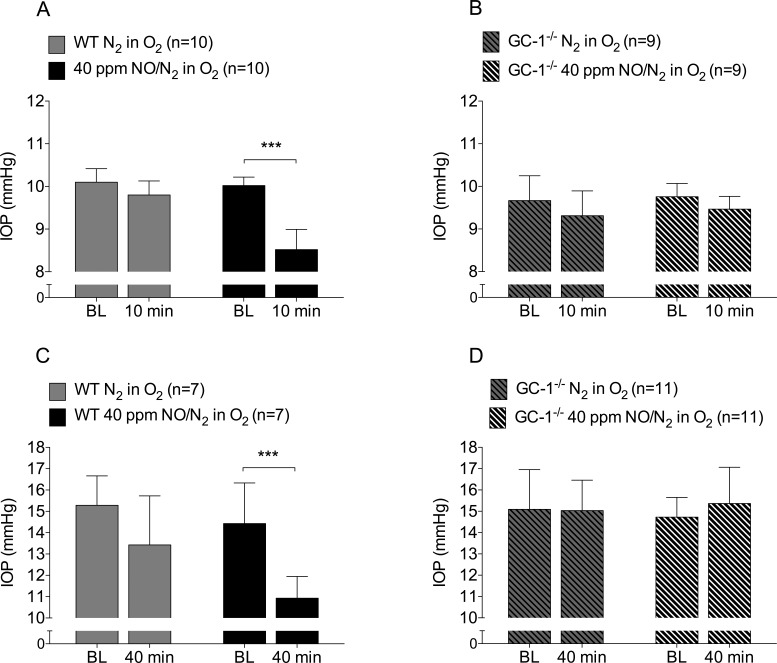
Breathing 40 ppm NO for 10 minutes lowered the IOP in anesthetized WT mice (IOP before and after breathing NO, 10.0 ± 0.2 mm Hg vs. 8.5 ± 0.5 mm Hg, respectively, n = 10 each, P < 0.001, Fig. 1A). Inhalation of NO did not affect IOP in GC-1−/− mice (IOP before and after breathing NO, 9.8 ± 0.3 mm Hg vs. 9.5 ± 0.3 mm Hg, respectively, n = 9 each, P = 0.08, Fig. 1B). Breathing control gas did not change IOP in anesthetized WT mice (IOP before and after breathing control gas, 10.0 ± 0.2 mm Hg vs. 9.8 ± 0.3 mm Hg, respectively, n = 10 each, P = 0.11). Similarly, control gas did not significantly affect IOP in GC-1−/− mice (IOP before and after breathing control gas, 9.7 ± 0.6 mm Hg vs. 9.3 ± 0.6 mm Hg, respectively, n = 9 each, P = 0.052).
Figure 1.
Breathing NO gas lowers IOP in WT but not GC-1−/− mice. (A) Breathing 40 ppm NO (n = 10, ***P < 0.001), but not control gas (n = 10, P = 0.11), lowered IOP in anesthetized WT mice. (B) In contrast, breathing 40 ppm NO did not lower IOP in anesthetized GC-1−/− mice (n = 9, P = 0.08). (C–D) Breathing control gas did not affect IOP in awake WT (n = 7, P = 0.06) or GC-1−/− mice (n = 11, P = 0.93). Breathing 40 ppm NO for 40 minutes lowered IOP in awake WT mice (n = 7, ***P < 0.001) but not in awake GC-1−/− mice (n = 11, P = 0.20). BL, baseline.
Similar to results obtained in anesthetized mice, awake WT mice breathing 40 ppm NO for 40 minutes had lower IOP values (IOP before and after breathing NO, 14.4 ± 1.9 mm Hg vs. 10.9 ± 1.0 mm Hg, respectively, n = 7 each, P < 0.001, Fig. 1C). Breathing NO (IOP before and after, 14.7 ± 0.9 mm Hg vs. 15.4 ± 1.7 mm Hg, respectively, n = 11 each, P = 0.20) for 40 minutes did not change IOP in awake GC-1−/− mice (Fig. 1D). Breathing control gas did not significantly change IOP in WT (n = 7, P = 0.06, Fig. 1C) or GC-1−/− mice (n = 11, P = 0.93, Fig. 1D).
GC-1 Modulates Conventional AqH Outflow in WT Mice
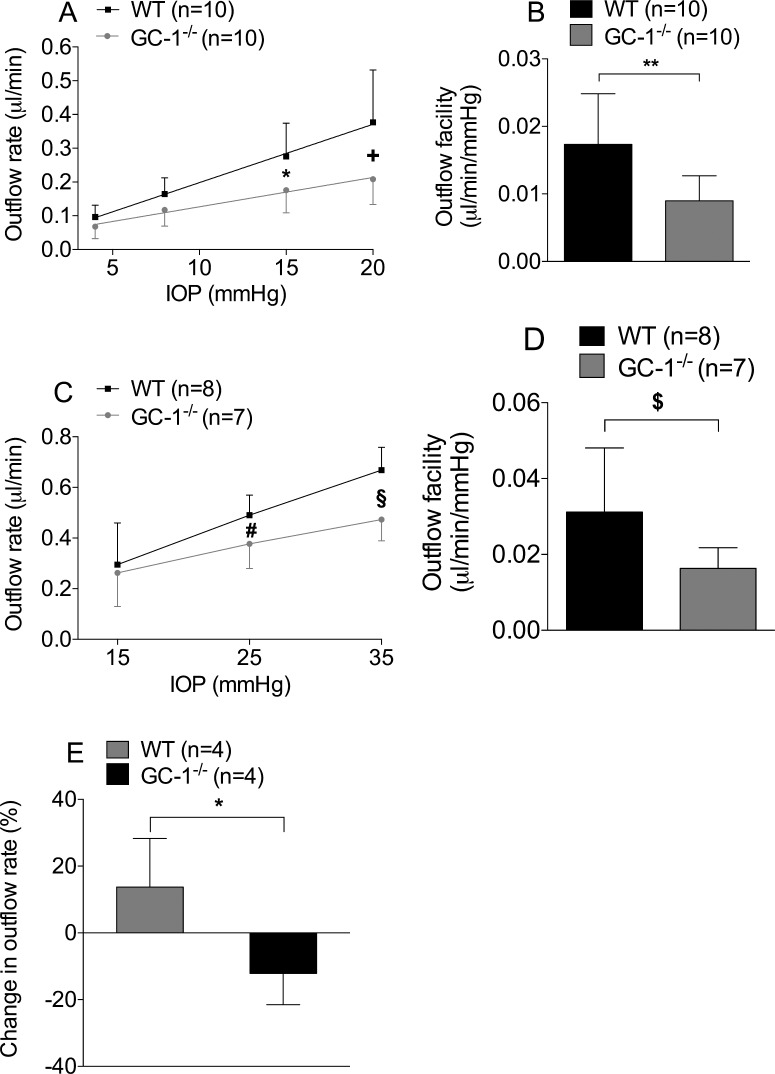
To investigate the mechanism by which NO decreases IOP, we measured AqH outflow rates ex vivo at various IOP levels (4, 8, 15 and 20 mm Hg) in enucleated eyes from GC-1−/− and WT mice. Ex vivo AqH outflow was higher in enucleated WT mouse eyes at IOP levels of 15 and 20 mm Hg, respectively, than in GC-1−/− mouse eyes (outflow rates at 15 and 20 mm Hg, 0.28 ± 0.1 μL/minute vs. 0.18 ± 0.07 μL/minute, P = 0.007 and 0.38 ± 0.16 μL/minute vs. 0.21 ± 0.08 μL/minute, P < 0.0001, n = 10 each, Fig. 2A). To further investigate the ability of GC-1 to modulate ex vivo AqH outflow, the relationship between outflow rates (uL/min) at each pressure (mm Hg) provides a conventional outflow facility estimate (uL/minute/mm Hg). Ex vivo conventional outflow facility was higher in WT mouse eyes than in age-matched GC-1−/− mouse eyes (0.017 ± 0.007 μL/minute/mm Hg vs. 0.008 ± 0.003 μL/minute/mm Hg, respectively, P = 0.005, n = 10 each, Fig. 2B).
Figure 2.
Conventional AqH outflow is lower in GC-1−/− than WT mice. (A, B) Ex vivo AqH outflow measurements in WT and GC-1−/− mice. AqH outflow rates at 15 mm Hg (n = 10, *P = 0.007) and (A) 20 mm Hg (n = 10, +P < 0.0001) and (B) conventional outflow facility (n = 10, **P = 0.005) were lower in GC-1−/− mice than in age-matched WT mice. (C, D) In vivo outflow measurements in WT (n = 8) and GC-1−/− mice (n = 7). Confirming measurements ex vivo, outflow rate at 25 mm Hg (#P = 0.0411) and (C) 35 mm Hg (§P = 0.0018) and (D) outflow facility ($P = 0.0449) were lower in GC-1−/− mice than in age-matched WT mice. (E) NO gas, delivered via inhalation to anesthetized mice increased outflow rate in WT, but not GC-1−/− animals (n = 4 each, P < 0.02).
Next, in vivo AqH outflow was measured at different IOP levels (15, 25, 35 mm Hg) in both GC-1−/− and WT mice. Similar to the ex vivo observations, the AqH outflow rate was higher in WT mice (n = 8) than in age-matched GC-1−/− mice (n = 7; AqH outflow rates at IOP levels of 25 and 35 mm Hg: 0.49 ± 0.17 μL/minute vs. 0.38 ± 0.1 μL/minute, P = 0.04; and 0.67 ± 0.09 μL/minute vs. 0.47 ± 0.09 μL/minute, P = 0.018; respectively, Fig. 2C). Outflow facility was higher in WT mice (n = 8) than in age-matched GC-1−/− mice (n = 7; 0.032 ± 0.017 μL/minute/mm Hg vs. 0.012 ± 0.005 μL/minute/mm Hg, P = 0.0449, Fig. 2D). In addition, breathing NO increased outflow facility in WT but not in GC-1−/− mice (13.7% ± 14.6% vs. −12.1% ± 9.4%, n = 4 each, P < 0.02, Fig. 2E).
Exposure of the Cornea to NO Lowers the IOP in Anesthetized Lambs
To further test that NO by itself can alter IOP, anesthetized lambs were treated with inhaled NO (80 ppm). In contrast to the results observed in mice, breathing NO for 60 minutes did not lower the IOP in healthy lambs (data not shown).
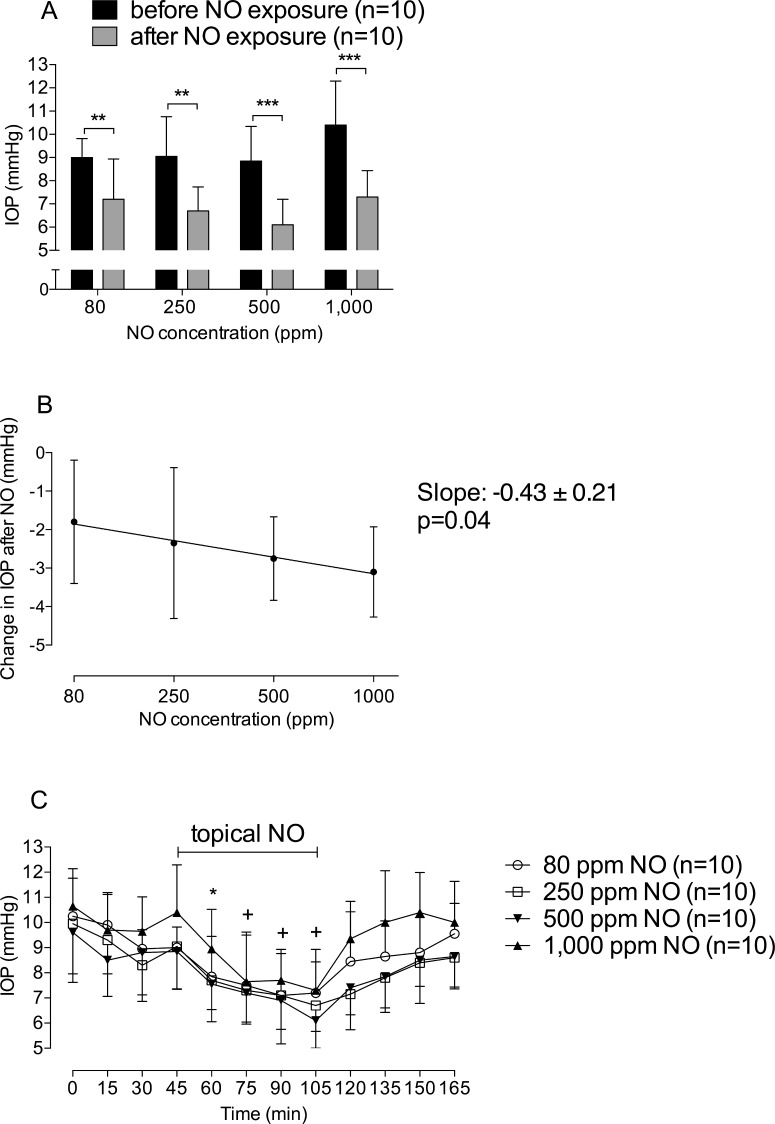
We next examined whether direct NO exposure to lamb eyes would lower IOP. Modified swim goggles were placed over both eyes and NO was delivered in air without admixture by room air. Exposing lamb eyes to 1000 ppm NO for 60 minutes lowered IOP (before versus after application of 1000 ppm NO: 10.2 ± 1.8 mm Hg vs. 7.0 ± 1.2 mm Hg, n = 10 measurements, P < 0.0001, Fig. 3A). Next, lamb eyes were exposed to various NO concentrations of 80, 250, 500, and 1000 ppm. Topical exposure of the lamb cornea to NO lowered IOP in a dose-dependent manner (0.43 mm Hg/ppm NO, Fig. 3B). cGMP levels in the AqH were below the detection limit, before and after topical administration of NO gas.
Figure 3.
Topical exposure of eyes to NO gas lowers IOP in anesthetized lambs in a dose- and time-dependent manner. (A) IOP decreased in lambs treated with either 80, 250, 500, or 1000 ppm NO for 1 hour, when compared to baseline; “before NO exposure” represents the 45-minute time point of (C); “after NO exposure” represents the 105-minute time point of (C). **P < 0.01; ***P < 0.0001. (B) Dose-response analysis of the change in IOP before (45-minute time point of [C]) and after (105-minute time point of [C]) topical NO exposure. (C) IOP values in anesthetized lambs before, during, and up to 60 minutes after treatment with either 80 (circle); 250 (square); 500 (triangle facing up); or 1000 ppm NO (triangle facing down). Animals received topical NO between the 45- and 105-minute time points. IOP decreased until the NO exposure was terminated. *P < 0.05 values in the 80, 500, and 1000 ppm NO groups differ from baseline. +P < 0.05 values differ from baseline in all 80, 250, 500, and 1000 ppm NO groups.
To investigate whether the IOP-lowering effect of topical NO was time-dependent and reversible, IOP measurements were performed during and after NO exposure. When administering 80, 250, 500, or 1000 ppm NO, IOP values decreased as early as 15 minutes after commencing exposure (Fig. 3C). Upon terminating NO treatment, IOP returned to baseline levels within 30 minutes (Fig. 3C).
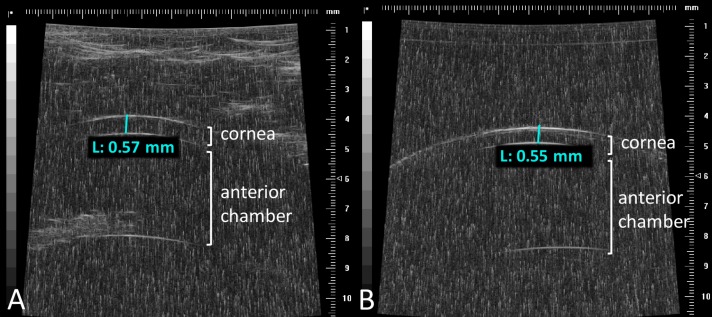
The ovine central corneal thickness was measured before and after a 1-hour exposure to 1000 ppm NO using ultrasound. Lambs aged 3- to 4-months had a central corneal thickness of approximately 570 μm, similar to what is typically observed in humans (Fig. 5A). The corneal thickness was similar before and after exposure to 1000 ppm NO (n = 3, P = 0.52, Supplementary Fig. S4).
Figure 5.
Ultrasound measurement of lamb cornea before and after a 1-hour exposure to 1000 ppm NO. Representative ultrasound images of the ocular anterior segment, obtained in lambs (A) before and (B) after topical eye exposure to 1000 ppm NO for 1 hour. The corneal thickness was similar (A) before and (B) after exposure to 1000 ppm NO. The cornea, anterior chamber, and the iris are evident. Depth of the cornea did not differ in NO-treated versus control-gas treated eyes.
Topical NO Exposure Does Not Induce Systemic Side Effects in Lambs
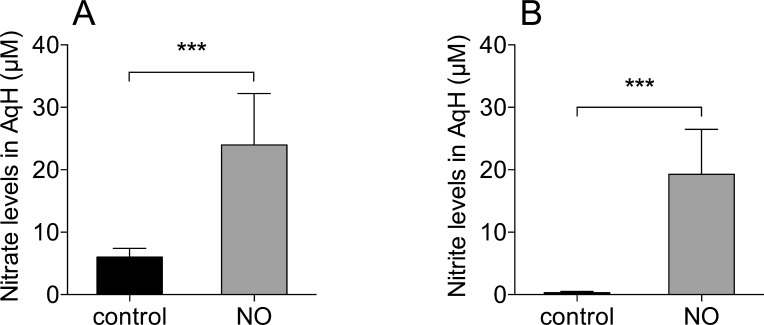
To investigate whether topical NO exposure was associated with systemic side effects, hemodynamic parameters including heart rate, mean arterial pressure, mean pulmonary arterial pressure, pulmonary capillary wedge pressure, central venous pressure, and cardiac output were monitored before, during, and after exposing the corneas to 1000 ppm NO. Systemic and pulmonary hemodynamic parameters did not change (n = 6, P values from 0.58 to 0.98, Supplemental Material, Table S1). Similarly, when administered topically, NO gas did not affect cGMP levels or the NO metabolites nitrate and nitrite in plasma (Supplementary Fig. S2). In contrast, nitrate and nitrite levels of NO-treated eyes were elevated in AqH when compared to control gas-treated eyes (nitrate: control [n = 6] versus NO [n = 8]; P < 0.001, nitrite: control [n = 6] versus NO [n = 8], P < 0.001, Fig. 4).
Figure 4.
Topical exposure of ovine eyes with 1000 ppm NO is associated with increased nitrate and nitrite levels in AqH. (A) Nitrate and (B) nitrite levels increased when lamb eyes were exposed to 1000 ppm NO gas for an hour (nitrate: control [n = 6] versus NO [n = 8], P < 0.001; nitrite: control [n = 6] versus NO [n = 8]). ***P < 0.001.
Exposure to High Levels of NO Did Not Cause Corneal Injury
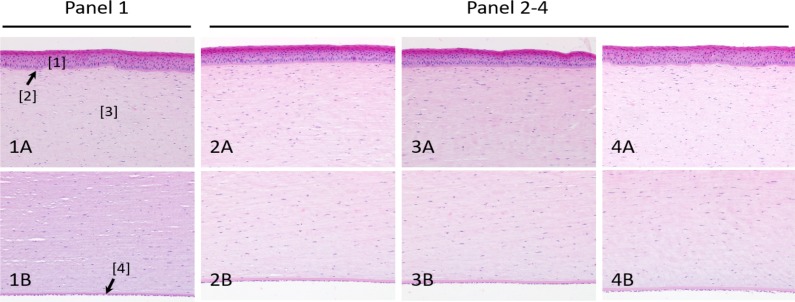
To assess whether exposure of the lamb's eyes to NO results in corneal changes, lamb eyes were exposed to 1000 ppm NO gas (a level 12.5 times higher than the maximum dose commonly used for inhalation therapy33 for 1 hour. Corneas from both control gas (Fig. 6, panel 1) and NO-treated lamb eyes (Fig. 6, panels 2–4) maintained their structural integrity: no microscopic lesions to the various corneal layers were observed. Minor foci of keratinization were observed in both control gas- and NO-treated eyes, suggesting that these small lesions were not treatment-related.
Figure 6.
H&E staining of lamb corneas before and after exposure to 1000 ppm NO. Panel 1: control animal. Panels 2–4: NO-treated animals. (A) Corneal epithelium and anterior stroma. (B) Posterior stroma, Descemet's membrane, and endothelium. No microscopic lesions or differences between groups were detected. H&E, ×20 objective. [1] Epithelium; [2] Bowman's membrane; [3] Stroma; [4] Descemet's membrane.
Discussion
In this study, we provide evidence that NO can directly lower IOP in mice and lambs. Breathing NO decreased IOP and increased conventional AqH outflow in WT mice but not GC-1−/− mice. Our results in GC-1−/− mice demonstrate that the ability of NO to lower IOP and increase AqH outflow is dependent on GC-1, identifying GC-1 as a therapeutic target for lowering IOP. In addition, topical NO exposure of lamb's eyes lowered IOP in a transient and dose-dependent manner within 15 minutes without altering corneal structure or affecting systemic hemodynamics.
The NO-releasing compound LTB combines a prostaglandin analogue with a nitrate moiety. It is metabolized in situ to latanoprost acid and an organic nitrate, which has additional IOP-lowering effects. LTB lowered IOP in clinical phase 3 trials30–32 and is under FDA review for the reduction of IOP in open-angle glaucoma. In a direct comparison to latanoprost 0.005%, LTB 0.024% was superior in lowering diurnal IOP at day 28, but the concentrations of prostaglandin analogue were not identical. The ability of NO to directly lower IOP supports the concept that the greater capacity of LTB than of latanoprost to lower IOP reflects a dual mechanism of action, involving both a latanoprost-dependent effect on uveoscleral outflow and an NO-mediated effect on conventional AqH outflow. Breathing NO leads to increased blood levels of NO-metabolites such as nitrite; nitrate; nitroso (RSNO, RNNO); and nitrosyl (NO-heme), which are possible reservoirs of NO within the bloodstream.44 Nitrite reacts with hemeproteins and is converted to NO, making NO available for organs and tissues.45
In addition to NO, other gasotransmitters (carbon monoxide [CO] and hydrogen sulfate [H2S]) can modulate cGMP levels, lower IOP, increase retinal blood flow, or provide retinal neuroprotection.46–52 Novel classes of oral pharmacological agents directly targeting GC—such as FDA-approved riociguat53,54 (Bayer Healthcare Pharmaceuticals LLC, Berlin, Germany) that stimulates GC synergistically with NO, or GC-activators capable of activating oxidized NO-insensitive GC55—are emerging as promising therapies for cardiovascular diseases. A GC-stimulator (IWP-953) was recently reported to increase conventional AqH outflow facility in murine eyes.40 Combining NO-donors with an GC-stimulator (IWP-953) resulted in a dose-dependent increase in cGMP levels in primary trabecular meshwork cells.40 Since our results identify a GC-dependent IOP lowering mechanism, GC-activators and clinically available GC-activators may represent novel therapeutics for lowering IOP. Whether GC mediates the previously reported effect of NO on AqH formation,56–58 remains to be determined.
A limitation of this study is that we studied the effect of NO gas on IOP in healthy mice and sheep, with IOPs in the normal range. Data from the LBN clinical trials, suggest that NO, when delivered in combination with a prostaglandin analogue, can lower IOP in glaucoma patients.30–32 However, it remains to be determined whether NO alone can increase AqH outflow and lower IOP in a pathologic setting of elevated IOP. We cannot exclude the possibility that the results will not translate to pathologic states with increased IOP or that the IOP-lowering ability of NO (or another gasotransmitter) depends on the starting IOP. Our findings need to be further confirmed in a model of experimental glaucoma. Also, the explanation of why breathing NO reduces IOP in mice but not sheep remains to be determined. Finally, translating administration of an IOP-lowering drug via inhalation to the clinical may prove to be challenging, but our data provide important proof of principle that NO alone may favorably alter AqH dynamics.
In summary, we studied the direct effect of gaseous NO on IOP and AqH dynamics in two different animal models, mice and lambs. Our results demonstrate that inhaled NO increases conventional outflow facility and reduces IOP in mice. Our data prove, for the first time, that the effect of NO on AqH outflow rate and IOP is mediated by GC-1, identifying GC-1 as a potential therapeutic target for glaucoma. In addition, topical exposure of ovine corneas to NO gas lowers IOP and increases NO metabolites in AqH but not systemically in the ovine plasma.
Supplementary Material
Acknowledgments
Supported by funds of the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and the Harvard Glaucoma Center of Excellence, Massachusetts Eye and Ear Infirmary, Boston, MA, USA; the German Academic Exchange Service (WSL, GF; DAAD); NIH Grant No. R01DK082971 and a grant from the Leducq Foundation (DBB); NIH Grant No. R01EY022746 (ESB); and Grant No. R01EY022359 (WDS). The authors alone are responsible for the content and writing of the paper.
Disclosure: S. Muenster, None; W.S. Lieb, None; G. Fabry, None; K.N. Allen, None; S.S. Kamat, None; A.H. Guy, None; A.C. Dordea, None; L. Teixeira, None; R.E. Tainsh, None; B. Yu, None; W. Zhu, None; N.E. Ashpole, None; R. Malhotra, P. Brouckaert, None; D.B. Bloch, None; M. Scherrer-Crosbie, None; W.D. Stamer, None; M.H. Kuehn, None; L.R. Pasquale, Bausch&Lomb, Inc. (C), Eyenovia (C, S); E.S. Buys, Nicox (C)
References
- 1. Quigley HA,, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedman DS, Wolfs RC, O'Colmain BJ, et al. . Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004; 122: 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Danford ID, Verkuil LD, Choi DJ, et al. . Characterizing the “POAGome”: a bioinformatics-driven approach to primary open-angle glaucoma. Prog Retin Eye Res. 2017; 58: 89–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwon YH,, Fingert JH,, Kuehn MH,, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009; 360: 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abu-Amero K,, Kondkar AA,, Chalam KV. An updated review on the genetics of primary open angle glaucoma. Int J Mol Sci. 2015; 16: 28886–28911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weinreb RN,, Khaw PT. Primary open-angle glaucoma. Lancet. 2004; 363: 1711–1720. [DOI] [PubMed] [Google Scholar]
- 7. Friebe A,, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003; 93: 96–105. [DOI] [PubMed] [Google Scholar]
- 8. Doganay S,, Evereklioglu C,, Turkoz Y,, Er H. Decreased nitric oxide production in primary open-angle glaucoma. Eur J Ophthalmol. 2002; 12: 44–48. [DOI] [PubMed] [Google Scholar]
- 9. Kang JH, Wiggs JL, Rosner BA, et al. . Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010; 51: 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galassi F,, Renieri G,, Sodi A,, Ucci F,, Vannozzi L,, Masini E. Nitric oxide proxies and ocular perfusion pressure in primary open angle glaucoma. Br J Ophthalmol. 2004; 88: 757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thorleifsson G, Walters GB, Hewitt AW, et al. . Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010; 42: 906–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiggs JL, Kang JH, Yaspan BL, et al. . Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011; 20: 4707–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu Y, Vitart V, Burdon KP, et al. . Genome-wide association analyses identify multiple loci associated with central corneal thickness and keratoconus. Nat Genet. 2013; 45: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kotikoski H,, Vapaatalo H,, Oksala O. Nitric oxide and cyclic GMP enhance aqueous humor outflow facility in rabbits. Curr Eye Res. 2003; 26: 119–123. [DOI] [PubMed] [Google Scholar]
- 15. Ellis DZ,, Dismuke WM,, Chokshi BM. Characterization of soluble guanylate cyclase in NO-induced increases in aqueous humor outflow facility and in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009; 50: 1808–1813. [DOI] [PubMed] [Google Scholar]
- 16. Khoobehi B, Chiroli V, Ronchetti D, et al. . Enhanced oxygen saturation in optic nerve head of non-human primate eyes following the intravitreal injection of NCX 434, an innovative nitric oxide-donating glucocorticoid. J Ocul Pharmacol Ther. 2011; 27: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buys ES,, Potter LR,, Pasquale LR,, Ksander BR. Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Front Mol Neurosci. 2014; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krauss AH, Impagnatiello F, Toris CB, et al. . Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2alpha agonist, in preclinical models. Exp Eye Res. 2011; 93: 250–255. [DOI] [PubMed] [Google Scholar]
- 19. Dismuke WM,, Sharif NA,, Ellis DZ. Human trabecular meshwork cell volume decrease by NO-independent soluble guanylate cyclase activators YC-1 and BAY-58-2667 involves the BKCa ion channel. Invest Ophthalmol Vis Sci. 2009; 50: 3353–3359. [DOI] [PubMed] [Google Scholar]
- 20. Ellis DZ,, Sharif NA,, Dismuke WM. Endogenous regulation of human Schlemm's canal cell volume by nitric oxide signaling. Invest Ophthalmol Vis Sci. 2010; 51: 5817–5824. [DOI] [PubMed] [Google Scholar]
- 21. Wiederholt M,, Sturm A,, Lepple-Wienhues A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Invest Ophthalmol Vis Sci. 1994; 35: 2515–2520. [PubMed] [Google Scholar]
- 22. Schneemann A,, Dijkstra BG,, van den Berg TJ,, Kamphuis W,, Hoyng PF. Nitric oxide/guanylate cyclase pathways and flow in anterior segment perfusion. Graefes Arch Clin Exp Ophthalmol. 2002; 240: 936–941. [DOI] [PubMed] [Google Scholar]
- 23. Stamer WD,, Lei Y,, Boussommier-Calleja A,, Overby DR,, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011; 52: 9438–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lei Y,, Zhang X,, Song M,, Wu J,, Sun X. Aqueous humor outflow physiology in NOS3 Knockout Mice. Invest Ophthalmol Vis Sci. 2015; 56: 4891–4898. [DOI] [PubMed] [Google Scholar]
- 25. Huang Q, Rui EY, Cobbs M, et al. . Design, synthesis, and evaluation of NO-donor containing carbonic anhydrase inhibitors to lower intraocular pressure. J Med Chem. 2015; 58: 2821–2833. [DOI] [PubMed] [Google Scholar]
- 26. Impagnatiello F, Toris CB, Batugo M, et al. . Intraocular Pressure-Lowering Activity of NCX 470, a Novel nitric oxide-donating bimatoprost in preclinical models. Invest Ophthalmol Vis Sci. 2015; 56: 6558–6564. [DOI] [PubMed] [Google Scholar]
- 27. Cavet ME,, Vollmer TR,, Harrington KL,, VanDerMeid K,, Richardson ME. Regulation of endothelin-1-induced trabecular meshwork cell contractility by latanoprostene bunod. Invest Ophthalmol Vis Sci. 2015; 56: 4108–4116. [DOI] [PubMed] [Google Scholar]
- 28. Drago F,, Bucolo C. Therapeutic potential of nitric oxide modulation in ocular diseases. Drug News Perspect. 2010; 23: 430–437. [DOI] [PubMed] [Google Scholar]
- 29. Buys ES, Ko YC, Alt C, et al. . Soluble guanylate cyclase alpha1-deficient mice: a novel murine model for primary open angle glaucoma. PLoS One. 2013; 8: e60156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weinreb RN, Scassellati Sforzolini B, Vittitow J, Liebmann J. Latanoprostene bunod 0.024% versus timolol maleate 0.5% in subjects with open-angle glaucoma or ocular hypertension: the APOLLO study. Ophthalmology. 2016; 123: 965–973. [DOI] [PubMed] [Google Scholar]
- 31. Araie M,, Sforzolini BS,, Vittitow J,, Weinreb RN. Evaluation of the effect of latanoprostene bunod ophthalmic solution, 0.024% in lowering intraocular pressure over 24 h in healthy Japanese subjects. Adv Ther. 2015; 32: 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weinreb RN, Ong T, Scassellati Sforzolini B, et al. . A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol. 2015; 99: 738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bloch KD,, Ichinose F,, Roberts JD, Jr,, Zapol WM. Inhaled NO as a therapeutic agent. Cardiovasc Res. 2007; 75: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Frostell C,, Fratacci MD,, Wain JC,, Jones R,, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991; 83: 2038–2047. [DOI] [PubMed] [Google Scholar]
- 35. Nagasaka Y, Buys ES, Spagnolli E, et al. . Soluble guanylate cyclase-alpha1 is required for the cardioprotective effects of inhaled nitric oxide. Am J Physiol Heart Circ Physiol. 2011; 300: H1477–H1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buys ES, Sips P, Vermeersch P, et al. . Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res. 2008; 79: 179–186. [DOI] [PubMed] [Google Scholar]
- 37. Yu B,, Muenster S,, Blaesi AH,, Bloch DB,, Zapol WM. Producing nitric oxide by pulsed electrical discharge in air for portable inhalation therapy. Sci Transl Med. 2015; 7: 294ra107. [DOI] [PubMed] [Google Scholar]
- 38. Ding C,, Wang P,, Tian N. Effect of general anesthetics on IOP in elevated IOP mouse model. Exp Eye Res. 2011; 92: 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lei Y,, Overby DR,, Boussommier-Calleja A,, Stamer WD,, Ethier CR. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011; 52: 1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ge P, Navarro ID, Kessler MM, et al. . The soluble guanylate cyclase stimulator IWP-953 increases conventional outflow facility in mouse eyes. Invest Ophthalmol Vis Sci. 2016; 57: 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu W, Gramlich OW, Laboissonniere L, et al. . Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc Natl Acad Sci U S A. 2016; 113: E3492–E3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baron DM, Yu B, Lei C, et al. . Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012; 116: 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muenster S, Beloiartsev A, Yu B, et al. . Exposure of stored packed erythrocytes to nitric oxide prevents transfusion-associated pulmonary hypertension. Anesthesiology. 2016; 125: 952–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gladwin MT,, Lancaster JR, Jr,, Freeman BA,, Schechter AN. Nitric oxide's reactions with hemoglobin: a view through the SNO-storm. Nat Med. 2003; 9: 496–500. [DOI] [PubMed] [Google Scholar]
- 45. Vitturi DA,, Patel RP. Current perspectives and challenges in understanding the role of nitrite as an integral player in nitric oxide biology and therapy. Free Radic Biol Med. 2011; 51: 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Coletta C, Papapetropoulos A, Erdelyi K, et al. . Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012; 109: 9161–9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salomone S,, Foresti R,, Villari A,, Giurdanella G,, Drago F,, Bucolo C. Regulation of vascular tone in rabbit ophthalmic artery: cross talk of endogenous and exogenous gas mediators. Biochem Pharmacol. 2014; 92: 661–668. [DOI] [PubMed] [Google Scholar]
- 48. Stagni E,, Privitera MG,, Bucolo C,, Leggio GM,, Motterlini R,, Drago F. A water-soluble carbon monoxide-releasing molecule (CORM-3) lowers intraocular pressure in rabbits. Br J Ophthalmol. 2009; 93: 254–257. [DOI] [PubMed] [Google Scholar]
- 49. Bucolo C,, Drago F. Carbon monoxide and the eye: implications for glaucoma therapy. Pharmacol Ther. 2011; 130: 191–201. [DOI] [PubMed] [Google Scholar]
- 50. Salvi A, Bankhele P, Jamil J, et al. . Effect of hydrogen sulfide donors on intraocular pressure in rabbits. J Ocul Pharmacol Ther. 2016; 32: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Resch H,, Zawinka C,, Weigert G,, Schmetterer L,, Garhofer G. Inhaled carbon monoxide increases retinal and choroidal blood flow in healthy humans. Invest Ophthalmol Vis Sci. 2005; 46: 4275–4280. [DOI] [PubMed] [Google Scholar]
- 52. Huang S, Huang P, Liu X, et al. . Relevant variations and neuroprotecive effect of hydrogen sulfide in a rat glaucoma model. Neuroscience. 2017; 341: 27–41. [DOI] [PubMed] [Google Scholar]
- 53. Ghofrani HA, Simonneau G, Rubin LJ, et al. for the PATENT-1 Study Group. . Riociguat for pulmonary hypertension. N Engl J Med. 2013; 369: 2268. [DOI] [PubMed] [Google Scholar]
- 54. Ghofrani HA, D'Armini AM, Grimminger F, et al. . Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013; 369: 319–329. [DOI] [PubMed] [Google Scholar]
- 55. Stasch JP, Schmidt PM, Nedvetsky PI, et al. . Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006; 116: 2552–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Millar JC,, Shahidullah M,, Wilson WS. Intraocular pressure and vascular effects of sodium azide in bovine perfused eye. J Ocul Pharmacol Ther. 2001; 17: 225–234. [DOI] [PubMed] [Google Scholar]
- 57. Shahidullah M,, Yap M,, To CH., Cyclic GMP, sodium nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br J Pharmacol. 2005; 145: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miller TL,, Willis AM,, Wilkie DA,, Hoshaw-Woodard S,, Stanley JR. Description of ciliary body anatomy and identification of sites for transscleral cyclophotocoagulation in the equine eye. Vet Ophthalmol. 2001; 4: 183–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.