Abstract
There is currently a strong interest among both audiologists and hearing researchers to find a physiological measure that can be used as a marker of how amplified sounds are processed by the brain (i.e., hearing aid fitting) or how the brain changes with exposure to amplified sounds (i.e., hearing aid acclimatization). Currently, auditory evoked potentials are used, or proposed to be used, for both of these purposes to some degree. It is clear from the literature that some of these uses are potentially useful clinically and others are quite problematic. The current state of aided cortical auditory evoked potentials will be discussed relative to their application to hearing aid fitting/verification and in understanding hearing aid acclimatization. Future areas of promise as well as current gaps in the literature will be addressed.
Keywords: Auditory evoked potentials (AEPs), event-related potentials (ERPs), hearing aids, acclimatization
Learning Outcomes: As a result of this activity, the participant will be able to identify the potential uses and challenges associated with aided cortical auditory evoked potentials.
A primary goal of fitting hearing aids on an individual is to increase the audibility of various speech cues and to improve speech understanding. One also hopes that as a result of better speech understanding, the quality of life will improve. There are a host of potential variables that can affect the outcome of a hearing aid fitting. Factors specific to the outcome of speech understanding might include variables specific to the listening environment (e.g., signal type, presence of background noise, etc.), the hearing aid (e.g., algorithms, settings, etc.), and the patient (e.g., integrity of the auditory system, age, motivation, lifestyle, etc.). Souza and Tremblay introduced a schematic to help illustrate some of the variables that may contribute to speech understanding in hearing aid users.1 Fig. 1 shows a modified version of that schematic, which includes four stages: (1) the original acoustic signal, (2) the modifications made by the hearing aid, (3) the processing of the peripheral and central auditory systems, and finally (4) the higher-order processes, including the behavioral response.
Figure 1.
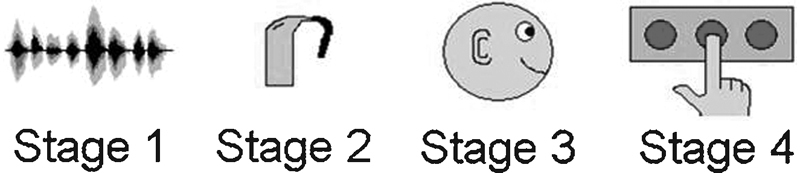
A schematic representing four stages of the listening experience for hearing aid users. Stage 1 includes the incoming signal. Stage 2 addresses the hearing aid and how it modifies the signal. Stage 3 begins when the amplified signal is delivered to the ear and includes transduction and processing by the auditory system. Stage 4 includes higher-order processes up to and including a behavioral response of some kind.
Stage 1 includes the incoming signal and the acoustic properties associated with it. This could include the context in which the signal is presented.
Stage 2 addresses the hearing aid and how it modifies the acoustic signal, including the hearing aid settings such as compression, gain, and frequency response.
Stage 3 begins when the signal is delivered to the ear. Acoustic waveforms are transduced and processed through several parallel processing streams by the peripheral and central auditory systems.
Stage 4 includes the higher-order processes such as those that modulate attention and memory and all processes leading up to, and including, a behavioral response of some kind.
These divisions are somewhat arbitrary and each stage is not independent of the others; in reality, there is considerable interaction and overlap between stages. The purpose of the schematic is to help place the content of this review into the context of the listening experience. The focus will be primarily on stage 2 and stage 3 and the interaction between these two aspects of the hearing aid experience. The purpose of this article is to review the literature that addresses how measures of the brain can contribute to successful rehabilitation of hearing impairment and inform our general understanding of the neural contributions to the process. After providing a brief introduction to evoked potentials, the article reviews the evidence from two main perspectives that are present in the literature: (1) the process of hearing aid fitting and verification and (2) the characterization of hearing aid acclimatization effects.
A Measure of Neural Encoding
In the hearing aid literature, the most common method of measuring neural processing has been electroencephalography (EEG), or the measurement of electrical activity at the level of the scalp. Auditory evoked potentials (AEPs) are a type of EEG that reflect neural activity in response to auditory stimuli, thus allowing the characterization of how the brain encodes sounds. AEPs consist of negative and positive peaks in voltage ranging in time from a few milliseconds to several hundred milliseconds after stimulus onset. These peaks include the auditory brainstem response (ABR) occurring in the first 10 milliseconds after stimulus onset, middle latency responses occurring between 10 and 50 milliseconds, and late latency responses that occur beyond 50 milliseconds. In addition to these transient AEPs, sustained AEPs also can be considered, such as the auditory steady-state response (ASSR) or the related frequency follow response (FFR; for a review see Picton2). AEPs are one tool that can be used to understand how the auditory system is encoding cues important for speech understanding. As a relatively inexpensive tool, they are a clinically relevant way to understand how the brain is encoding amplified sounds and also can help us to determine how the auditory system changes with exposure to hearing-aid-processed sounds. Although some mention is made of ABR and ASSR literature, this article focuses primarily on cortical AEPs (CAEPs) given that most of the current research activity uses these potentials. Fig. 2 illustrates a CAEP waveform and the notable peaks, including N1, P2, and N2. Not only do stimulus onsets evoke these peaks, but acoustic changes within a stimulus and stimulus offsets also can result in similar responses shown as N1OFF, P2OFF, and N2OFF.2 3
Figure 2.
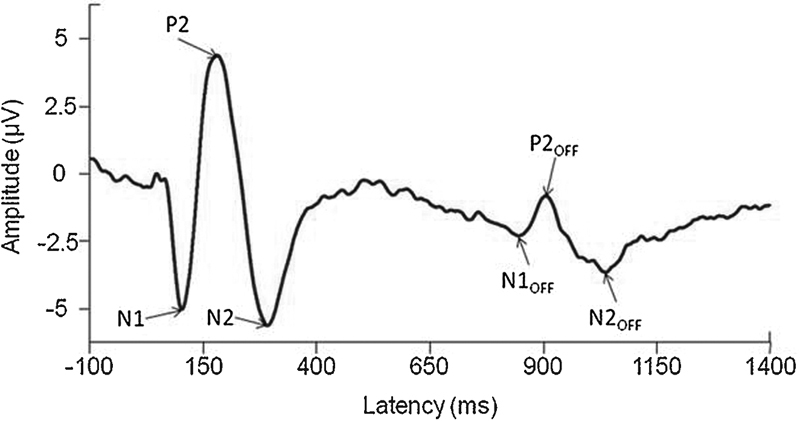
An example of the cortical auditory evoked potential including N1, P2, and N2 waves to a 756-millisecond, 1000-Hz pure tone. Also displayed are subsequent N1OFF, P2OFF, and N2OFF waves that are elicited by an acoustic change in the stimulus; in this case the change was the offset of the signal.
Variations of the term aided AEPs have been used in the literature in different ways. For the purposes of this article, we define the term as AEP responses elicited from an individual using stimuli that have been processed by a hearing aid. There are at least four methods for recording aided AEPs. The most common method found in the literature involves recording evoked potentials while an individual is wearing the hearing aid and stimuli are presented in the sound field. This sound-field approach is most intuitive and representative of how clinical aided AEPs would be measured; however, when testing monaurally in the sound field, it is important to minimize contribution of the nontest ear, often done with an earplug of some kind. Another method is to record the hearing aid output offline either in a coupler or a mannequin such as the Knowles Electronics Manikin for Acoustic Research or the Brüel and Kjær Head and Torso Simulator.4 5 The output recording is then presented through insert earphones to the participant. In the third method, the hearing aid is worn by the individual, and stimuli are presented through the hearing aid using direct audio input.6 Finally, in the fourth method used in the hearing literature, hearing aid processing is simulated with a master hearing aid approach, in which hearing aid processing is applied digitally to a signal using Matlab (Mathworks, Natick, MA) or other signal processing software. The modified stimuli are then presented through insert earphones. Although less real-world, the last three methods have the advantage of minimizing extraneous variables related to sound field testing such as the effects of head movement and standing waves. In addition, variability in how the hearing aid processes sound from presentation to presentation is minimized. When testing monaurally, the contribution of the nontest ear is controlled through earphone presentation. A distinct approach to recording aided AEPs is to make measurements without the hearing aid and apply results to our understanding of the aided condition. This has often been the approach in studying hearing aid acclimatization as will be discussed.
Hearing aid Fitting: Estimating Unaided and Aided Thresholds
Hearing aid fitting and verification has been the primary focus of the aided AEP literature to date. The usefulness of a physiological measure to assist the clinician in the fitting process would be especially beneficial for hard-to-test populations. Unaided use of AEPs continues today as a means of threshold estimation. Historically, CAEPs have been used successfully to estimate behavioral thresholds (approximately within 10 dB of behavioral threshold) of both normal-hearing and hearing-impaired populations7 8 9 10; however, in some cases unaided CAEP thresholds can exceed behavioral thresholds by more than 20 dB.6 11 This wide range of variability in threshold estimation has the potential to cause obvious challenges in the hearing aid fitting process. Other AEPs, such as the ABR and ASSR, also have been used for unaided threshold estimation especially in infants as they can be recorded effectively while the patient is sleeping (for a review see Korczak et al12 and Hood13).
CAEPs have been used to estimate aided thresholds to assist with the hearing aid fitting process with hard-to-test populations such as infants. Similar to the rationale of an aided behavioral threshold, the purpose of aided CAEPs is to verify that the amplified signal is being processed by the brain. Rapin and Graziani were the first to publish aided CAEP data, presenting eight case studies of infants/children.14 Five of the eight individuals demonstrated a 20-dB improvement in aided CAEP response threshold compared with unaided CAEP threshold, two cases showed no improvement in CAEP threshold, and one had insufficient data to determine benefit. This initial report was followed by several case studies that suggested the potential for aided CAEPs to assist in the hearing aid fitting process.15 16 17 18
In parallel with aided CAEP investigations, researchers explored aided ABRs as a possible measure to assist with the hearing aid fitting.19 20 21 22 23 24 However, brief transient stimuli (clicks and tone pips) are used to elicit the ABR, and these stimuli are not ideal for measuring hearing aid function. Researchers concluded that such brief-duration stimuli were problematic because they did not effectively and consistently activate hearing aid circuitry.20 22
The recent development of HearLab by Frye Electronics (Frye Electronics, Inc.; Tigard, OR) and National Acoustic Laboratories (Sydney, Australia) has increased interest in aided CAEPs and encouraged their use for hearing aid fitting in infants.25 26 27 28 Although the potential need for such a physiological measure is apparent, it is only recently that systematic research questioning the use of aided AEPs has emerged. A determination of whether aided AEPs are of value clinically remains unresolved because of varied results across these studies. The main obstacle to clinical implementation is that under certain conditions, no effect of amplification is demonstrated.29 30 31 32 33 That is, there is no statistical difference between CAEP waveforms elicited in an unaided condition and those elicited in an aided condition, despite the additional gain provided by the hearing aid and perceptual measures verifying increased gain. These data are quite problematic from a clinical perspective.
Inconsistent research findings present an important clinical challenge; if aided AEPs can only be used for some subset of subjects or only a specific group of hearing aids, then the clinical utility is greatly diminished. The wide range of variability is a problem that must be addressed before aided AEPs can completely transition into the clinic. It may be that some of the variability can be explained by the stimulus level used relative to the threshold of the individual or group being tested. Reexamination of one of the first larger-scale parametric studies of aided AEPs provides some insight into why an amplification effect may not always be present.34 Korczak and colleagues varied signal level in two different groups of hearing-impaired participants.34 They demonstrated that the effect of amplification measured using aided CAEPs is level dependent. That is, amplification effects (i.e., differences between unaided and aided conditions) were more likely to occur near threshold than at suprathreshold levels. This illustrates an important aspect of the early aided AEP literature: isolating an effect of amplification by comparing a barely audible or inaudible unaided evoked response with a suprathreshold aided evoked response often resulted in significant changes to waveform morphology.
Billings and colleagues4 revisited the literature with Korczak et al's34 results in mind and proposed a perspective of thinking about these results that may help understand the current clinical utility of aided AEPs until more can be understood about their limitations. Many of the early studies demonstrated significant changes in waveform morphology in the aided condition relative to the unaided condition, but usually the unaided stimulus was barely audible or not audible.14 15 35 In contrast, several studies failed to show an effect when both aided and unaided stimuli were tested at suprathreshold levels. Fig. 3 and Table 1, taken from Billings et al,4 show the possible effects of testing near threshold (i.e., a physiological detection approach) as opposed to testing at suprathreshold levels (i.e., a physiological discrimination approach). Amplification effects are more likely to be found when the detection approach is used. A related factor to the physiological detection/discrimination perspective in the aided AEP literature that should be considered carefully is the hearing status of the participants. Many of the studies that demonstrate absent amplification effects were completed in individuals with normal hearing as a means to control interactions between an impaired auditory system and AEP results, and many studies that show amplification effects were completed in individuals with hearing impairment. In an attempt to bridge these findings, Billings et al elicited aided AEPs from noise-masked normal-hearing individuals and demonstrated that a detection approach resulted in amplification effects and a discrimination approach did not.4 Additional well-controlled studies with hearing-impaired individuals are needed to verify the validity of a detection approach where the aided AEP threshold is found. It appears that the aided AEP discrimination approach remains problematic clinically.
Figure 3.
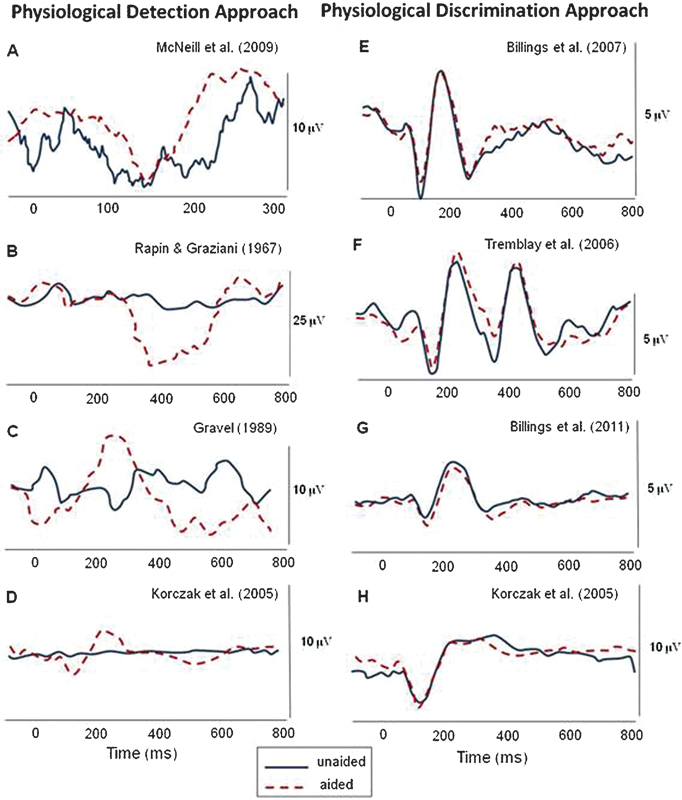
Examples of physiological detection (A to D) and physiological discrimination (E to H) approaches from the aided cortical auditory evoked potential literature. Results across these studies demonstrate significant amplification effects (unaided versus aided) for physiological detection but very limited amplification effects for physiological discrimination. All figures were modified from published figures; the appropriate citation is indicated for each panel (see Table 1 for details). This figure was originally published in Billings et al, 2012.4
Table 1. Experimental conditions used in the studies cited in Fig. 3.
| Subjects | Experimental Design | Source | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Characteristics | Stimulus | Duration (ms) | ISI (ms) | Signal Level | Conditions | ||
| Detection approach examples | ||||||||
| McNeil et al, 200935 | 1 | 68-y-old man; severe to profound SNHL | /ba/ | 115 | 750* | 60-dB nHL | Unaided and aided | Fig. 1, p. 81: unaided and left hearing aid |
| Rapin and Graziani, 196714 | 1 | 21-mo-old girl; rubella, sedated | 500-Hz tone | Not specified | Not specified | 109 dB re: 0.0002 dynes/cm2 | Unaided and aided | Fig. 4, p. 892: 109 dB with and 109 dB without aid |
| Gravel et al, 198915 | 1 | 7-mo-old boy; severe to profound SNHL | /da/ | Not specified | Not specified | Not specified | Unaided and aided (aid set to user settings) | Fig. 3, p. 271 |
| Korczak et al, 200534 | 7 | Adults; severe to profound SNHL | /ba/ and /da/ | 150 | 950 | 80-dB ppeSPL | Unaided and aided (aid set to MCL) | Fig. 3, p. 176: standard responses in lower panel, left |
| Discrimination approach examples | ||||||||
| Billings et al, 200730 | 13 | Young adults; normal hearing | 1000-Hz tone | 757 | 1910 | 50-dB SPL | Unaided and aided (20-dB gain) | Fig. 2, p. 238: 50 dB aided and 50 dB unaided waveforms |
| Tremblay et al, 200629 | 7 | Young adults; normal hearing | /si/ | 655 | 1910 | 64-dB SPL | Unaided and aided (average gain of 19 dB) | Fig. 7, p. 99: top panel |
| Billings et al, 201131 | 9 | Young adults; normal hearing | 1000-Hz tone | 756 | 1910 | 40-dB SPL | Unaided and aided (gain of 20 dB) | Fig. 6, p. 7: panel (a) unaided waveform, panel (b) aided waveform |
| Korczak et al, 200534 | 4 | Adults; moderate SNHL | /ba/ and /da/ | 150 | 950 | 85-dB HL | Unaided and aided (aid set to MCL) | Fig. 3, p. 176: standard responses in lower panel, center |
Table was originally published in Billings et al, 2012.4 Note that the parameters listed are specific to the conditions used to generate the waveform data presented in Fig. 3 and do not necessarily represent all conditions presented in each study. The eight studies included represent aided CAEP data in the literature for which unaided and aided waveforms were able to be reproduced.
*Reference does not state whether this value refers to onset to onset or offset to onset. All other ISIs refer to offset to onset. HL, hearing level; ISI, interstimulus interval; MCL, most comfortable loudness; ppeSPL, peak-to-peak equivalent sound pressure level; SNHL, sensorineural hearing loss; SPL, sound pressure level.
There is also an emerging interest in other electrophysiological measures. The ASSR and the FFR may be promising as clinical tools to measure the physiological aided threshold (see Clinard and Tremblay in this special issue).36 Several researchers have demonstrated good potential for their use in measuring aided thresholds and demonstrating the effect of amplification.37 38 39 40 41 42 An advantage of these responses may be that they are elicited by longer-duration stimuli than the ABR and are analyzed in the frequency domain, effectively reducing dependence on the size of a particular peak. However, similar difficulties to those experienced with the ABR may be present given that the ASSR is evoked primarily by the initial portions of a stimulus (also see Clinard and Tremblay in this issue).12 36 It remains unknown whether the spectral analyses used to interpret these steady-state potentials will allow this measure to overcome challenges that have been demonstrated with aided CAEPs and aided ABRs. Additional research is needed to determine the extent of the interactions between hearing-aid-processed stimuli and the neural responses.
In summary, a portion of the amplification effect variability that exists in the literature may be explained by differences in approach across studies. Where a detection approach is taken, there is more likely to be significant amplification effects; where a discrimination approach is taken, an absent amplification effect is more likely to result.
What are The Limitations of Aided CAEPs?
Why have various studies employing aided CAEPs found no effect of amplification? As indicated above, in some cases the absence of effects may be due to testing at suprathreshold levels. At threshold, the specifics of the hearing aid processing lose their relevance to some degree because an absent response in the unaided condition is often compared with a present response in the aided condition; in that situation, the overall level is the robust cue being encoded (i.e., a detection task). However, when two suprathreshold conditions are compared, as in a discrimination task, the more subtle hearing aid processing characteristics complicate the picture. At least two major cues have emerged in the literature as contributing to the absent amplification effects at suprathreshold stimulus levels: signal-to-noise ratio (SNR) and stimulus onset characteristics (e.g., rise time). Billings and colleagues demonstrated that SNR plays an important role in aided CAEP testing.30 31 Specifically, they found that when amplification effects were absent, the SNR ratio between the unaided and aided conditions was very similar. Furthermore, they demonstrated that CAEPs have a strong sensitivity to SNR rather than absolute signal level when background noise is present.43 44 This could be problematic for testing amplification effects at suprathreshold levels as shown in Fig. 3. It may be that when signal and noise are present at suprathreshold levels, amplification effects would not be expected. However, although SNR is an important factor, it is also clear that SNR does not account for all aided CAEP results.31 33
Onset characteristics of the stimulus are also critical to CAEP morphology. Hearings aids can dramatically modify the signal onset in ways that will affect AEPs.33 It is worth noting that the onset modifications were not consistent across hearing aids, signal levels, or signal types. The two digital hearing aids that were tested varied dramatically in the resulting modifications to onsets. Jenstad pointed out that SNR and onset modifications together do not account for the lack of amplification effects that they found. In addition, even when rise characteristics such as overshoot and rise time are modified by the hearing aid, there is not always an effect on CAEPs.5 Additional research is needed to determine what other acoustic properties of the hearing-aid-processed stimuli are affecting the AEP.
Aided CAEPs can be reliably recorded for some speech cues and not in other cases. For example, aided CAEPs showed reliable differences between the syllables “see” and “shee,”29 45 but did not distinguish between the syllables “ma,” “ga,” and “ta.”46 Additional research will need to determine how sensitivities to various cues differ in unaided and aided conditions. That information may help to determine what cues affecting AEPs, in addition to SNR and onset cues, are being modified by the hearing aid.
It is important to note that an absent AEP is usually nondiagnostic on its own47; that is, an absent response does not conclusively indicate that the person cannot hear the stimulus. AEPs represent the neural synchrony of some subset of auditory neurons at a given point in time and provide only a glimpse into the complex central auditory nervous system. Furthermore, the absence of a response is determined either by visual inspection or by some automated detection algorithm and does not necessarily indicate that there is not neural response present. It may be that biological noise, subject state, or a host of other factors have prevented our ability to detect a response. For this reason, both the unaided and aided AEP literature has examples of absent evoked potentials to stimuli that are clearly detectable behaviorally. As mentioned previously, although visual CAEP detection thresholds are within 10 dB of behavioral threshold in most cases, they have been found to exceed 20 dB in some studies and individuals.
In summary, at suprathreshold levels, hearing aid processing and AEPs interact in unexpected ways. We are beginning to understand some of these confounds (i.e., SNR and signal onset modifications); however, additional research is needed to determine what other factors are contributing. Furthermore, it appears that the causes of an absent amplification effect vary from individual to individual and hearing aid to hearing aid, a challenge that also will need to be addressed further in the laboratory.
Evidence of Hearing Aid Acclimatization
Hearing aid acclimatization has been studied from both behavioral and physiological perspectives. Behaviorally, the evidence for perceptual changes over time is quite mixed, with some studies reporting significant increases in benefit and others reporting no significant change (for a review see Turner et al48). This discrepancy in the literature was part of the motivation behind the 1995 Eriksholm Workshop that focused on the deprivation and acclimatization effects associated with hearing impairment and hearing aid use. It was clear that some of the discrepancy in the literature could be due to how acclimatization was being defined. As part of this workshop, researchers defined acclimatization as a change in performance over time that is linked to the available acoustic information and that cannot be attributed to task, procedural, or training effects.49 Probably one of the largest available data sets, and most robust in terms of outcome measures, is a longitudinal study completed by Humes and colleagues in which a battery of speech perception measures and self-report measures were used to investigate changes over time in more than 134 individuals for 1 year, 49 individuals for 2 years, and 9 individuals for 3 years.50 51 Results demonstrated little evidence for acclimatization. A review of the behavioral acclimatization literature is beyond the scope of this article and has been addressed by others48 52 53 54; however, even 18 years after the Eriksholm Workshop, conflicting results remain unexplained and one could conclude as they did in 1995, that although there is evidence for acclimatization, it is difficult to specify under what conditions, the size of the effect, or its time course.49
The mixed behavioral evidence surrounding hearing aid acclimatization has led some to explore physiological measures as a marker of neural changes throughout the auditory system. Gatehouse and Robinson18 applied a clever methodology that had been initially used behaviorally55 to study acclimatization using AEPs; unilaterally aided individuals were tested for acclimatization effects by comparing results for the hearing-aid-fitted ear to the nonfitted ear, effectively using the nonfitted ear as a control. In this early case study, they reported improved intensity discrimination and increases in CAEP amplitudes at the highest intensity tested. This result has been followed by a series of studies from Munro and colleagues as well as others, demonstrating some physiological acclimatization effects using acoustic reflex thresholds, the ABR, and CAEPs.56 57 58 In contrast, a recent doctoral dissertation began to explore acclimatization in the aided condition, but found little evidence for any acclimatization effect as measured using CAEPs.59 Given these mixed results, it remains unclear how the physiological effects relate to perception. It is noteworthy that these physiological acclimatization measures were made without the hearing aid on the individual. Many of the same difficulties and variability that are associated with measuring aided thresholds are present when measuring changes over time as well; however, one might hypothesize that measures would be most sensitive to acclimatization if testing were completed in the aided condition. Several studies by Sharma and colleagues have demonstrated neural change over time with the device being worn during testing60 61; unfortunately details about the hearing aid settings from test to retest and the acoustic modifications resulting from amplification were not reported, making it difficult to judge how these results fit into the literature.
In summary, although acclimatization does occur, the extent of that effect remains unclear. Furthermore, physiological measures have not helped to clarify these issues. It appears that before acclimatization can be demonstrated in the aided condition, the interaction between hearing aid processing and the resulting evoked potentials needs to be better understood.
Future Research Needs and Recommendations
An area not addressed in this review but important to the future of understanding the effects of hearing aids on brain and behavior is the effect of auditory training in hearing aid users (for a review of physiologic effects of training, see Tremblay62). This area will be important for future research as training programs become more user-friendly and self-administered. Data have been mixed on the efficacy of these programs, and more studies are needed to clarify what neural changes, if any, are occurring as a result of these programs.
In the aided evoked potential literature, there is emerging interest in the use of aided change responses.63 64 A change response consists of a repeated N1 and P2 peaks that result from changes within a stimulus. Also called the acoustic change complex in the literature,65 66 the change response has been used with aided AEPs with the syllables “see” and “shee.”29 45 The neural response change associated with stimulus offset also demonstrates promise. Fig. 4 shows data from a previous article.30 We found previously that there was no significant effect of amplification on the P1-N1-P2 onset. We reanalyzed the onset and offset response (Fig. 4) using a rectified area measure rather than peak values (offset peaks were very difficult to identify given the size of the physiological noise relative to the response). The rectified area was calculated over the range of the onset and offset response (60 to 450 and 790 to 1140 milliseconds, respectively) after baseline correction was applied over the same range (baseline correction was used as a means to control for any drift that occurred later in the waveform). Although neural onset amplification effects remained absent as previously shown,30 offset amplification effects were significant (F = 19.8(6,72), p < 0.001) and an interaction between level and amplification (F = 5.6(6,72), p = 0.003) was found using a repeated measures analysis of variance. Subsequent post hoc testing using paired samples t tests revealed significant effects and trends of amplification at 40, 60, 70, and 80 dB (p = 0.023, 0.074, 0.003, 0.008, respectively). It may be that some of the hearing aid modifications to stimulus rise characteristics are not as problematic for offset aided AEPs.33 In addition, perhaps area measures can help improve sensitivity to some of the subtle effects of amplification in a discrimination approach paradigm.
Figure 4.
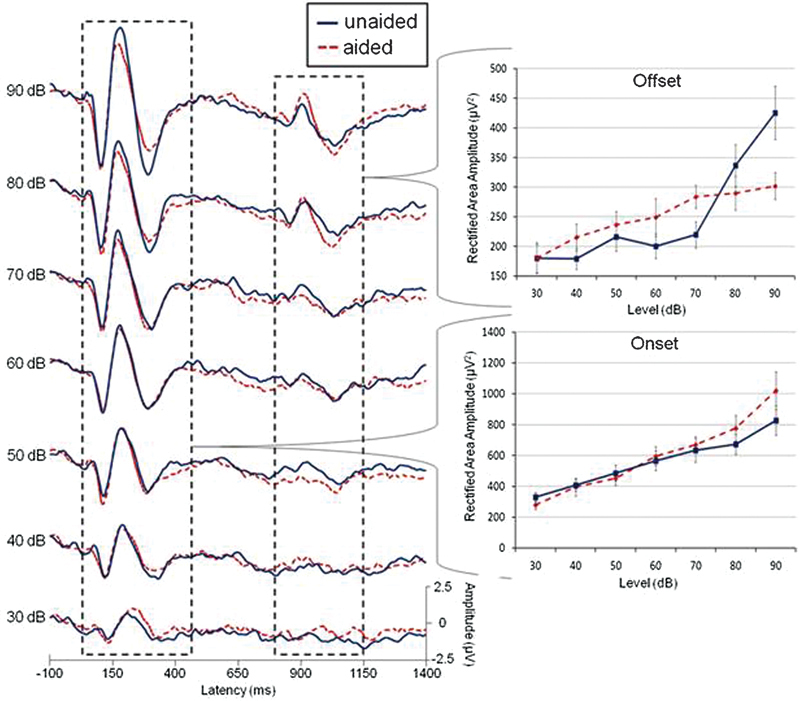
Grand averages (n = 13) and area measures for unaided (dotted) and aided (solid) conditions as a function of signal level. A minimal effect of amplification is seen for the onset at most signal levels, similar to peak results published previously (Billings et al, 200730). However, offset area measures demonstrate a significant difference between unaided and aided conditions. It is possible that an area measure or an acoustic change peak such as the offset is sensitive to amplification effects unlike the onset response.
One inherent difficulty with aided AEPs is that results using one hearing aid may not necessarily translate to other hearing aids, or even to the same hearing aid with different settings. If aided CAEPs are to be used successfully in the clinic on a larger scale, this difficulty of variability needs to be understood. A key to improving understanding will be to characterize the interaction between the hearing aid signal processing, and resulting modification to the stimulus, and the AEP. To move our understanding of aided AEPs forward, it is important that researchers record and characterize the output of the hearing aid. This can be done with in-the-canal recordings using a probe tube microphone (such as the Etymotic ER7c (Etymotic Research, Inc., Elk Grove Village, IL)) to verify what acoustic cues are reaching the ear, or at the very least in a mannequin or coupler. The interaction between hearing aid and EPs is the main contributor to problems currently.
The aided AEP literature has progressed dramatically in the last decade, mostly as a result of moving from case study reports to group data with more statistical power. Additional large sample studies will be needed to determine what subtle variables are important to understanding aided AEPs. Once the contributing factors are identified, translation back to the clinical application in individuals will need to be completed.
In conclusion, the aided CAEP literature demonstrates considerable difficulties in application to the clinic especially when trying to differentiate two present responses; the variability within and across hearing aids, individuals, and studies is quite problematic clinically. However, when used in a physiological detection approach (i.e., comparing an absent response to a response that is present) in conjunction with the battery of other audiological tests, aided AEPs may be useful to determine whether aided input is reaching the auditory cortex. It should be kept in mind, however, that physiological detection of an aided stimulus does not mean that the hearing aid is fit appropriately; in fact, a present response compared with an absent one indicates only that the signal, or some portion of the signal, is being encoded physiologically. In addition, as with unaided AEPs, visual detection thresholds can be quite elevated when compared with behavioral thresholds; this limitation in the aided domain will have to be more completely determined for clinical use of aided AEPs to be of great benefit.
Acknowledgments
Special thanks to Tina Penman, Lucas Baltzell, Angela Eilbes, and Melissa Papesh for assistance with and comments about this manuscript. This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (R03DC10914) and the Veterans Affairs Rehabilitation Research and Development Service (C8006W).
References
- 1.Souza P E, Tremblay K L. New perspectives on assessing amplification effects. Trends Amplif. 2006;10(3):119–143. doi: 10.1177/1084713806292648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picton T W. San Diego, CA: Plural Publishing, Inc.; 2011. Human Auditory Evoked Potentials. [Google Scholar]
- 3.Martin B A, Tremblay K L, Korczak P. Speech evoked potentials: from the laboratory to the clinic. Ear Hear. 2008;29(3):285–313. doi: 10.1097/AUD.0b013e3181662c0e. [DOI] [PubMed] [Google Scholar]
- 4.Billings C J Papesh M A Penman T M Baltzell L S Gallun F J Clinical use of aided cortical auditory evoked potentials as a measure of physiological detection or physiological discrimination Int J Otolaryngol 20122012365752 10.1155/2012/365752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Easwar V, Glista D, Purcell D W, Scollie S D. Hearing aid processing changes tone burst onset: effect on cortical auditory evoked potentials in individuals with normal audiometric thresholds. Am J Audiol. 2012;21(1):82–90. doi: 10.1044/1059-0889(2012/11-0039). [DOI] [PubMed] [Google Scholar]
- 6.Glista D Easwar V Purcell D W Scollie S A pilot study on cortical auditory evoked potentials in children: aided CAEPs reflect improved high-frequency audibility with frequency compression hearing aid technology Int J Otolaryngol 20122012982894. 10.1155/2012/982894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rapin I, Ruben R J, Lyttle M. Diagnosis of hearing loss in infants using auditory evoked responses. Laryngoscope. 1970;80(5):712–722. doi: 10.1288/00005537-197005000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Prasher D, Mula M, Luxon L. Cortical evoked potential criteria in the objective assessment of auditory threshold: a comparison of noise induced hearing loss with Ménière's disease. J Laryngol Otol. 1993;107(9):780–786. doi: 10.1017/s0022215100124429. [DOI] [PubMed] [Google Scholar]
- 9.Yeung K N, Wong L L. Prediction of hearing thresholds: comparison of cortical evoked response audiometry and auditory steady state response audiometry techniques. Int J Audiol. 2007;46(1):17–25. doi: 10.1080/14992020601102238. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda K, Hayashi A, Matsuda O, Sekiguchi T. An ignoring task improves validity of cortical evoked response audiometry. Neuroreport. 2010;21(10):709–715. doi: 10.1097/WNR.0b013e32833b502a. [DOI] [PubMed] [Google Scholar]
- 11.Van Maanen A, Stapells D R. Comparison of multiple auditory steady-state responses (80 versus 40 Hz) and slow cortical potentials for threshold estimation in hearing-impaired adults. Int J Audiol. 2005;44(11):613–624. doi: 10.1080/14992020500258628. [DOI] [PubMed] [Google Scholar]
- 12.Korczak P, Smart J, Delgado R, Strobel T M, Bradford C. Auditory steady-state responses. J Am Acad Audiol. 2012;23(3):146–170. doi: 10.3766/jaaa.23.3.3. [DOI] [PubMed] [Google Scholar]
- 13.Hood L J. Clifton Park, NY: Delmar Cengage Learning; 1998. Clinical Applications of the Auditory Brainstem Response. [Google Scholar]
- 14.Rapin I, Graziani L J. Auditory-evoked responses in normal, brain-damaged, and deaf infants. Neurology. 1967;17(9):881–894. doi: 10.1212/wnl.17.9.881. [DOI] [PubMed] [Google Scholar]
- 15.Gravel J S, Kurtzberg D, Stapells D R, Vaughan H G, Wallace I F. Case studies. Semin Hear. 1989;10(3):272–287. [Google Scholar]
- 16.Kurtzberg D. Cortical event-related potential assessment of auditory system function. Semin Hear. 1989;10(3):252–261. [Google Scholar]
- 17.Kraus N, McGee T J. Mismatch negativity in the assessment of central auditory function. Am J Audiol. 1994;3:39–51. doi: 10.1044/1059-0889.0302.39. [DOI] [PubMed] [Google Scholar]
- 18.Gatehouse S, Robinson K. Singapore: World Scientific; 1996. Acclimatization to monaural hearing aid fitting-effects on loudness functions and preliminary evidence for parallel electrophysiological and behavioural effects; pp. 319–330. [Google Scholar]
- 19.Beauchaine K A, Gorga M P, Reiland J K, Larson L L. Application of ABRs to the hearing-aid selection process: preliminary data. J Speech Hear Res. 1986;29(1):120–128. doi: 10.1044/jshr.2901.120. [DOI] [PubMed] [Google Scholar]
- 20.Brown E, Klein A J, Snydee K A. Hearing-aid-processed tone pips: electroacoustic and ABR characteristics. J Am Acad Audiol. 1999;10(4):190–197. [PubMed] [Google Scholar]
- 21.Gerling I J. In search of a stringent methodology for using ABR audiometric results. Hear J. 1991;44(26):28–30. [Google Scholar]
- 22.Gorga M P, Beauchaine K A, Reiland J K. Comparison of onset and steady-state responses of hearing aids: implications for use of the auditory brainstem response in the selection of hearing aids. J Speech Hear Res. 1987;30(1):130–136. doi: 10.1044/jshr.3001.130. [DOI] [PubMed] [Google Scholar]
- 23.Hecox K E. Role of auditory brain stem response in the selection of hearing aids. Ear Hear. 1983;4(1):51–55. doi: 10.1097/00003446-198301000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Kileny P. Auditory brainstem responses as indicators of hearing aid performance. Ann Otol Rhinol Laryngol. 1982;91(1 Pt 1):61–64. doi: 10.1177/000348948209100114. [DOI] [PubMed] [Google Scholar]
- 25.Purdy S C, Katsch R, Dillon H, Storey L, Sharma M, Agung K. Basel, Switzerland: Phonak AG; 2005. Aided cortical auditory evoked potentials for hearing instrument evaluation in infants; pp. 115–127. [Google Scholar]
- 26.Frye Electronics Inc. HEARLab System. Operator's Manual Available at: http://www.frye.com/wp/hearlab. Accessed on September 11, 2013
- 27.Golding M, Pearce W, Seymour J, Cooper A, Ching T, Dillon H. The relationship between obligatory cortical auditory evoked potentials (CAEPs) and functional measures in young infants. J Am Acad Audiol. 2007;18(2):117–125. doi: 10.3766/jaaa.18.2.4. [DOI] [PubMed] [Google Scholar]
- 28.Dillon H. So baby, how does it sound? Cortical assessment of infants with hearing aids. Hear J. 2005;58(10):10–17. [Google Scholar]
- 29.Tremblay K L, Billings C J, Friesen L M, Souza P E. Neural representation of amplified speech sounds. Ear Hear. 2006;27(2):93–103. doi: 10.1097/01.aud.0000202288.21315.bd. [DOI] [PubMed] [Google Scholar]
- 30.Billings C J, Tremblay K L, Souza P E, Binns M A. Effects of hearing aid amplification and stimulus intensity on cortical auditory evoked potentials. Audiol Neurootol. 2007;12(4):234–246. doi: 10.1159/000101331. [DOI] [PubMed] [Google Scholar]
- 31.Billings C J, Bennett K O, Molis M R, Leek M R. Cortical encoding of signals in noise: effects of stimulus type and recording paradigm. Ear Hear. 2011;32(1):53–60. doi: 10.1097/AUD.0b013e3181ec5c46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marynewich S Jenstad L M Stapells D R Slow cortical potentials and amplification—part I: N1-P2 measures Int J Otolaryngol 20122012921513 10.1155/2012/921513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenstad L M, Marynewich S, Stapells D R. Slow cortical potentials and amplification—part II: acoustic measures. Int J Otolaryngol. 2012:1–14. doi: 10.1155/2012/386542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korczak P A, Kurtzberg D, Stapells D R. Effects of sensorineural hearing loss and personal hearing aids on cortical event-related potential and behavioral measures of speech-sound processing. Ear Hear. 2005;26(2):165–185. doi: 10.1097/00003446-200504000-00005. [DOI] [PubMed] [Google Scholar]
- 35.McNeill C Sharma M Purdy S C Are cortical auditory evoked potentials useful in the clinical assessment of adults with cochlear implants? Cochlear Implants Int 2009100178–84. 10.1002/cii.391 [DOI] [PubMed] [Google Scholar]
- 36.Clinard C, Tremblay K. What brainstem recordings may or may not be able to tell us about hearing aid-amplified signals. Semin Hear. 2013;34(4):270–277. [Google Scholar]
- 37.Picton T W, Durieux-Smith A, Champagne S C. et al. Objective evaluation of aided thresholds using auditory steady-state responses. J Am Acad Audiol. 1998;9(5):315–331. [PubMed] [Google Scholar]
- 38.Dimitrijevic A, John M S, Picton T W. Auditory steady-state responses and word recognition scores in normal-hearing and hearing-impaired adults. Ear Hear. 2004;25(1):68–84. doi: 10.1097/01.AUD.0000111545.71693.48. [DOI] [PubMed] [Google Scholar]
- 39.Stroebel D, Swanepoel W, Groenewald E. Aided auditory steady-state responses in infants. Int J Audiol. 2007;46(6):287–292. doi: 10.1080/14992020701212630. [DOI] [PubMed] [Google Scholar]
- 40.Shemesh R, Attias J, Magdoub H, Nageris B I. Prediction of aided and unaided audiograms using sound-field auditory steady-state evoked responses. Int J Audiol. 2012;51(10):746–753. doi: 10.3109/14992027.2012.700771. [DOI] [PubMed] [Google Scholar]
- 41.Damarla V KS, Manjula P. Application of ASSR in the hearing aid selection process. Aust N Z J Audiol. 2007;29(2):89–97. [Google Scholar]
- 42.Anderson S Kraus N The potential role of the cABR in assessment and management of hearing impairment Int J Otolaryngol 20132013604729 10.1155/2013/604729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Billings C J, Tremblay K L, Stecker G C, Tolin W M. Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hear Res. 2009;254(1-2):15–24. doi: 10.1016/j.heares.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billings C J, McMillan G P, Penman T M, Gille S. Predicting perception in noise using cortical auditory evoked potentials. http://dx.doi.org/10.1077/s10162-013-0415-y. J Assoc Res Otolaryngol. 2013 doi: 10.1007/s10162-013-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tremblay K L, Kalstein L, Billings C J, Souza P E. The neural representation of consonant-vowel transitions in adults who wear hearing aids. Trends Amplif. 2006;10(3):155–162. doi: 10.1177/1084713806292655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munro K J, Purdy S C, Ahmed S, Begum R, Dillon H. Obligatory cortical auditory evoked potential waveform detection and differentiation using a commercially available clinical system: HEARLab™. Ear Hear. 2011;32(6):782–786. doi: 10.1097/AUD.0b013e318220377e. [DOI] [PubMed] [Google Scholar]
- 47.Stapells D R. Baltimore, MD: Lippincott, Williams & Wilkins; 2002. Cortical event-related potentials to auditory stimuli. [Google Scholar]
- 48.Turner C W Humes L E Bentler R A Cox R M A review of past research on changes in hearing aid benefit over time Ear Hear 199617(3, Suppl):14S–25S. [DOI] [PubMed] [Google Scholar]
- 49.Arlinger S Gatehouse S Bentler R A et al. Report of the Eriksholm Workshop on auditory deprivation and acclimatization Ear Hear 199617(3, Suppl):87S–98S. [DOI] [PubMed] [Google Scholar]
- 50.Humes L E, Wilson D L, Barlow N N, Garner C. Changes in hearing-aid benefit following 1 or 2 years of hearing-aid use by older adults. J Speech Lang Hear Res. 2002;45(4):772–782. doi: 10.1044/1092-4388(2002/062). [DOI] [PubMed] [Google Scholar]
- 51.Humes L E, Wilson D L. An examination of changes in hearing-aid performance and benefit in the elderly over a 3-year period of hearing-aid use. J Speech Lang Hear Res. 2003;46(1):137–145. doi: 10.1044/1092-4388(2003/011). [DOI] [PubMed] [Google Scholar]
- 52.Palmer C V, Nelson C T, Lindley G A IV. The functionally and physiologically plastic adult auditory system. J Acoust Soc Am. 1998;103(4):1705–1721. doi: 10.1121/1.421050. [DOI] [PubMed] [Google Scholar]
- 53.Kuk F K, Potts L, Valente M, Lee L, Picirrillo J. Evidence of acclimatization in persons with severe-to-profound hearing loss. J Am Acad Audiol. 2003;14(2):84–99. doi: 10.3766/jaaa.14.2.4. [DOI] [PubMed] [Google Scholar]
- 54.Munro K J. Reorganization of the adult auditory system: perceptual and physiological evidence from monaural fitting of hearing aids. Trends Amplif. 2008;12(3):254–271. doi: 10.1177/1084713808323483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gatehouse S. Apparent auditory deprivation effects of late onset: the role of presentation level. J Acoust Soc Am. 1989;86(6):2103–2106. doi: 10.1121/1.398469. [DOI] [PubMed] [Google Scholar]
- 56.Munro K J, Pisareva N Y, Parker D J, Purdy S C. Asymmetry in the auditory brainstem response following experience of monaural amplification. Neuroreport. 2007;18(17):1871–1874. doi: 10.1097/WNR.0b013e3282f1b003. [DOI] [PubMed] [Google Scholar]
- 57.Munro K J, Walker A J, Purdy S C. Evidence for adaptive plasticity in elderly monaural hearing aid users. Neuroreport. 2007;18(12):1237–1240. doi: 10.1097/WNR.0b013e32822025f4. [DOI] [PubMed] [Google Scholar]
- 58.Bertoli S, Probst R, Bodmer D. Late auditory evoked potentials in elderly long-term hearing-aid users with unilateral or bilateral fittings. Hear Res. 2011;280(1-2):58–69. doi: 10.1016/j.heares.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 59.McCullagh J P. Mansfield, CT: University of Connecticut; 2010. An Investigation of Central Auditory Nervous System Plasticity Following Amplification [Ph.D. thesis] [Google Scholar]
- 60.Sharma A, Tobey E, Dorman M F. et al. Central auditory maturation and babbling development in infants with cochlear implants. Arch Otolaryngol Head Neck Surg. 2004;130(5):511–516. doi: 10.1001/archotol.130.5.511. [DOI] [PubMed] [Google Scholar]
- 61.Sharma A, Dorman M F, Kral A. The influence of a sensitive period on central auditory development in children with unilateral and bilateral cochlear implants. Hear Res. 2005;203(1-2):134–143. doi: 10.1016/j.heares.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Tremblay K L. Training-related changes in the brain: evidence from human auditory-evoked potentials. Semin Hear. 2007;28(2):120–132. [Google Scholar]
- 63.Hinchey T Korczak P Martin B A Pallett S Effects of hearing aid gain on slow cortical auditory evoked potentials Association for Research in Otolaryngology Mid-Winter Meeting; 2009
- 64.Kirby B Abbas P J Brown C J Verification of frequency compression parameters using the auditory change complex Poster presentation at American Auditory Society Scientific and Technology Meeting; Scottsdale, AZ; March 2012
- 65.Martin B A, Boothroyd A. Cortical, auditory, event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear Hear. 1999;20(1):33–44. doi: 10.1097/00003446-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Martin B A, Boothroyd A. Cortical, auditory, evoked potentials in response to changes of spectrum and amplitude. J Acoust Soc Am. 2000;107(4):2155–2161. doi: 10.1121/1.428556. [DOI] [PubMed] [Google Scholar]


