Abstract
Objective
To use cortical auditory evoked potentials (CAEPs) to understand neural encoding in background noise and the conditions under which noise enhances CAEP responses.
Methods
CAEPs from 16 normal-hearing listeners were recorded using the speech syllable/ba/presented in quiet and speech-shaped noise at signal-to-noise ratios of 10 and 30 dB. The syllable was presented binaurally and monaurally at two presentation rates.
Results
The amplitudes of N1 and N2 peaks were often significantly enhanced in the presence of low-level background noise relative to quiet conditions, while P1 and P2 amplitudes were consistently reduced in noise. P1 and P2 amplitudes were significantly larger during binaural compared to monaural presentations, while N1 and N2 peaks were similar between binaural and monaural conditions.
Conclusions
Methodological choices impact CAEP peaks in very different ways. Negative peaks can be enhanced by background noise in certain conditions, while positive peaks are generally enhanced by binaural presentations.
Significance
Methodological choices significantly impact CAEPs acquired in quiet and in noise. If CAEPs are to be used as a tool to explore signal encoding in noise, scientists must be cognizant of how differences in acquisition and processing protocols selectively shape CAEP responses.
Keywords: electroencephalography, event related potential, hearing, human, presentation rate, speech
1. Introduction
With the ubiquitous nature of sound in our world, the central auditory system is under constant challenge to filter out irrelevant background noise while simultaneously maximizing the encoding of meaningful auditory information. Early obligatory cortical auditory evoked potentials (CAEPs) provide a non-invasive means by which to study the brain’s capacity to encode signals in noise backgrounds, one which may provide important clues regarding how the auditory system adjusts to different listening environments. Most studies addressing the effects of noise on CAEP responses indicate that background noise degrades responses, eliciting smaller amplitudes and longer latencies compared with responses to the same signal presented in quiet (Whiting et al., 1998; Kaplan-Neeman et al., 2006; Billings et al., 2009; Billings et al., 2013). However, two studies report that N1 wave amplitudes are actually increased in low levels of background noise relative to quiet (Alain et al., 2009; Parbery-Clark et al., 2011). Discrepancies in results are likely due to methodological differences such as monaural versus binaural presentations, use of different noise levels, and differences in stimulus presentation rate. The present study is one of the first addressing the impact of multiple stimulus presentation variables on CAEP responses in noise compared to quiet. A better understanding of how stimulus factors influence CAEP measures in quiet and noise is likely to reveal important aspects of how the auditory system adjusts in order to achieve signal encoding in challenging listening environments.
CAEP response amplitudes, and N1 peaks in particular, are well correlated with behavioral measures of speech perception in noise (Anderson et al., 2010; Parbery-Clark et al., 2011; Bennett et al., 2012; Billings et al., 2013). For example, Parbery-Clark and colleagues demonstrated a significant positive correlation between N1 amplitudes and performance on the Hearing in Noise Test (HINT) such that participants with better speech-in-noise perception also had larger N1 amplitudes in response to speech syllables (Parbery-Clark et al., 2009). Further, a recent study by Billings and colleagues demonstrated that of the many possible methods of characterizing CAEP waveforms (e.g. various peak latencies, amplitudes, and area measures), N1 amplitudes measured at Cz were among the strongest predictors of individual speech-in-noise perception thresholds (Billings et al., 2013). Thus, reports of enhanced N1 amplitudes in noise relative to quiet may have important functional consequences. Review of current literature revealed two consistent differences between studies reporting N1 enhancement in noise and those reporting N1 amplitude decrements. Those studies reporting enhanced N1 responses in noise employed binaural presentations of stimuli at relatively fast presentation rates (Alain et al., 2009; Parbery-Clark et al., 2011), whereas studies showing amplitude decrements in noise used monaural presentations at slow presentation rates (Billings et al., 2009; Kaplan-Neeman et al., 2006). Therefore, we chose to investigate the effects of stimulus presentation rate and ear of stimulation on CAEP responses in quiet and in noise. We selected two presentation rates: one fast rate similar to that used in studies showing N1 amplitude enhancement in noise, and a second slower rate similar to that used in studies showing an N1 amplitude decrement. The signal consisted of a naturally spoken/ba/syllable which was presented both monaurally and binaurally in quiet and in two levels of background noise. This paradigm allowed us to replicate the presentation rate, ear of presentation, and background noise levels used in studies showing N1 amplitude decrements and those showing amplitude increments. If N1 enhancement in noise depends upon binaural listening at a fast presentation rate as the literature suggests, we would expect to find similar effects in the present study when stimuli are presented binaurally at a fast presentation rate, regardless of other methodological differences. In addition, use of multiple stimulus rates, noise levels, and binaural and monaural presentations allows for comparison of the relative importance of each of these factors in achieving enhanced N1 amplitudes in noise. Based upon the results of these previous studies, we hypothesized that eliciting enhanced N1 amplitudes in low noise backgrounds requires both binaural stimulation and a relatively fast stimulus presentation rate.
In contrast to N1, available literature suggests that P2 responses are consistently reduced in noise, regardless of ear of presentation or stimulus presentation rate (Whiting et al., 1998; Kaplan-Neeman et al., 2006; Alain et al., 2009; Billings et al., 2009; Parbery-Clark et al., 2011; Billings et al., 2013). Because both N1 and P2 are largely exogenous potentials (Picton and Stuss, 1980), this difference in responses to signals in noise implies that each is driven by distinct stimulus characteristics. Thus, variation in stimulus and recording methodology are likely to affect N1 and P2 differently. Based upon previous accounts of P2 responses in noise, we hypothesized that P2 would be diminished with the addition of noise irrespective of the factors studied herein (i.e., rate, noise level, and monaural/binaural presentation). P1, also known as the P50 potential, is also considered to be an exogenous potential of thalamocortical origin. The few available accounts of the effects of noise on P1 peak responses generally indicate minimal change. Gott and Hughes (1989) reported that increasing background noise from 0 to 40 dB SL resulted in a small but significant increase in the latency of P1 responses to click stimuli, with no significant changes in amplitude. Similarly, Billings et al. (2009) reported that, compared to quiet conditions, P1 amplitudes in response to speech syllables did not change significantly in noise, even at levels of −10-dB SNR. However, Bertoli et al (2005) reported that P1 amplitudes were significantly smaller in the presence of 0 dB SNR contralateral cafeteria noise compared to quiet conditions with no significant change in P1 latency. Variability in the effects of noise on P1 amplitudes in background noise may be due to the inherent variability of this peak that is often reported to be absent (Hayes et al., 2003) or less robust compared to other CAEP peaks (Billings et al., 2009). This variability likely stems from both individual differences as well as variation in acquisition and processing of CAEP data across studies.
With regard to N2 responses in noise, the majority of available reports indicate reduced amplitudes and increased latencies with increases in background noise levels, though significant changes only occurred at relatively high noise levels of 0 to −10-dB SNR (Whiting et al., 1998; Bertoli et al., 2005; Billings et al., 2009). Kaplan-Neeman et al (2006) reported no significant effect of noise on the amplitude of either P2 or N2, and inconsistent latency effects on P2 and N2. These measures were made in response to two speech syllables presented in the oddball paradigm presented in quiet and in background noise from +15 to −6-dB SNR. Hence, use of the oddball paradigm may account for differences between this study and others. Overall, we predict that N2 amplitudes will be reduced by the presence of background noise compared to quiet conditions.
2. Materials and Methods
2.1 Participants
Sixteen young normally hearing listeners participated in this study (mean age = 23.9 yrs; age range between 18 and 33 yrs; nine males, seven females; all right-handed). Participants had normal hearing bilaterally from 250 to 8000 Hz (≤20 dB HL) and normal tympanometry measures (single admittance peak between ±50 daPa to a 226-Hz tone). All participants were in good general health with no report of significant history of otologic or neurologic disorders. All participants provided written informed consent prior to participation, and research was conducted with approval from the Institutional Review Boards of the Portland Veterans Affairs Medical Center and Oregon Health and Science University.
2.2 Stimuli
Stimulus factors in the current study were carefully selected in order to approximate the parameters used in previous studies, some of which resulted in enhanced N1 amplitudes in noise (Alain et al., 2009; Parbery-Clark et al., 2011) and some of which did not result in enhancement (Billings et al. 2009; Kaplan-Neeman et al., 2006). The signal consisted of a/ba/syllable spoken by a female talker taken from the UCLA Nonsense Syllable Test (Dubno & Schaefer, 1992), shortened to 450 ms and presented at a level of 80 dB C (C-weighting). Two presentation rates were used, including 900 and 1900 ms interstimulus interval (ISI) measured from offset to onset. The signal was presented at three signal-to-noise ratios (SNR):30-dB SNR, 10-dB SNR, and a quiet condition. The background noise spectrum matched the long term spectra of speech (see Billings et al., 2011 for details of noise creation) and was presented continuously during the 30-dB SNR and 10-dB SNR conditions. Subjective reports from study participants as well as the authors’ perceptions of the stimuli in each condition indicate that the stimulus was perceived as a/ba/regardless of either ear of presentation, presentation rate, or noise background. Lastly, each of the two ISIs and the three SNRs were presented to the right ear only, left ear only, and binaurally through ER3A insert earphones (Etymotic, Inc.; Elk Grove Village, IL). Signal levels were calibrated using 10 seconds of a concatenated version of the signal played through an insert earphone coupled to a Brüel & Kjær 2260 Investigator sound level meter set to a slow time weighting. A minimum of 200 stimuli were presented in each condition with a maximum of 226 presentations per condition. Stimulus conditions were run in blocks corresponding to the ear of presentation (e.g. binaural presentations, then monaural presentation, etc.), and the order of ear of presentation was randomized across subjects to control for order effects. Within each block, the order of the six conditions was randomized. Blocks of trials containing the slower presentation rate (1900 ms ISI) required approximately eight minutes, while trial blocks containing the faster presentation rate (900 ms ISI) required approximately four minutes to complete. Overall, the entire test battery required between three and three and a half hours to complete (depending upon the need for breaks) after set-up was complete.
2.3 Electrophysiology
Participants were seated comfortably in a sound attenuating booth during recording sessions. They were instructed to ignore the test stimuli and watch a silent closed-captioned movie of their choice. Cortical responses elicited by stimuli were obtained using a 64-channel cap (Electro-Cap International, Inc.; Eaton, OH) and the Compumedics Neuroscan system (Charlotte, NC). The ground electrode was located on the forehead during CAEP collection, and Cz was the reference electrode. Data were then re-referenced off-line to an average reference of all electrodes. Horizontal and vertical eye movement was monitored with electrodes located inferiorly and at the outer canthi of both eyes. The recording time window consisted of a 200-ms pre-stimulus baseline following by 1100-ms post stimulus onset. Evoked responses were analog low-pass filtered online at 100 Hz (12 dB/octave roll off). All channels were amplified with a gain X 10 and converted using an analog-to-digital sampling rate of 1000 Hz. Trials with eyeblink artifacts were corrected off-line using Neuroscan software. This blink reduction procedure calculates the amount of covariation between each evoked potential channel and a vertical eye channel using a spatial, singular value decomposition and removes the vertical blink activity from each electrode on a point-by-point basis to the degree that the evoked potential and blink activity covaried (Neuroscan 2007). After blink correction, trials containing artifacts exceeding ±70 μV were rejected from averaging. For all individuals and conditions, 70% or more of the collected trials were available for averaging after artifact rejection. After artifact rejection, the remaining sweeps were averaged (an average of 178 sweeps per condition after artifact rejection, and a minimum of 116 sweeps) and filtered off-line from 0.1 Hz (high-pass filter, 24 dB/octave) to 30 Hz (low-pass filter, 12 dB/octave). For further analysis of the contributions of low frequencies to the recordings, average waveforms were also filtered using a higher high-pass filter setting of 3 Hz (24 dB/octave).
2.4 Analysis
The peak latency and amplitude values of waves P1, N1, P2, and N2 were obtained from the central electrode Cz. Responses at Cz were selected for analysis primarily to remain consistent with previous research on the relationship between CAEPs and background noise, and because of the robust nature of responses at this location (Billings et al., 2013, Parbery-Clark et al., 2009). Peak values were determined by two independent judges using temporal electrode inversion, comparison of the average responses from odd and even numbered presentations, global field power traces, and grand average responses. When disagreements arose between the judges, a third expert judge made the final decision based upon extensive experience with CAEP analysis. Amplitude values were measured relative to baseline and latency measures were determined relative to signal onset. Acceptable latency ranges were between 40 – 80 ms, 85 – 150 ms, 180 – 240 ms, and 250 – 450 ms post stimulus onset for P1, N1, P2, and N2, respectively. For all peaks, shorter latencies generally corresponded to quiet and low-noise conditions with longer peak latencies measured for higher noise conditions. Three paired-samples t-tests were used to evaluate the significance of the effects of noise for conditions replicated from previous studies. The first compared N1 response amplitudes measured in quiet and in 30-dB SNR background noise for stimuli presented at a fast rate binaurally (replication of Alain et al., 2009). The second compared N1 amplitudes measured in quiet and 10-dB SNR background noise for stimuli presented at a fast rate binaurally (replication of Parbery-Clark et al., 2011). The final t-test compared N1 response amplitudes obtained in quiet and 30-dB SNR background noise when presented at a slow rate to the right ear only (replication of Billings et al., 2009).
A repeated-measures analysis of variance (ANOVA) was used to examine the main effects and interactions between ear of presentation and presentation rate. Repeated measures ANOVA comparing right and left ear presentations revealed no significant main effects or interactions on either the latency or amplitude of responses measured at vertex. Therefore, peak amplitude and latency data obtained from the right and left ear presentations of each subject were averaged together for all subsequent analysis. The sole exception to the averaging of right and left ear data is the paired t-test analysis which was specifically meant to replicate the conditions of the previous study of Billings et al. 2009. In this case, only responses to right ear stimulation were assessed in order to remain consistent with the previous study. However, all subsequent analyses of monaural conditions were conducted on the average of right and left ear stimulation. In order to simplify the analysis and interpretation, separate repeated-measures ANOVAs were run comparing each noise condition to the quiet condition. Therefore, the effects of rate (900 and 1900 ms), ear (binaural and the average of both monaural conditions), and noise level (quiet and 30-dB SNR, or quiet and 10-dB SNR) on the amplitude and latency of P1, N1, P2, and N2 responses measured at vertex (Cz) was conducted, yielding a 2×2×2 factor analysis. In summary, analysis was completed on responses to a total of 12 different listening conditions including two ISIs (900 and 1900 ms), three SNRs (quiet, 10, and 30 dB), and two ears of presentation (binaural and the average of left and right).
3. Results
3.1. Replicating previously reported effects of noise on N1 amplitudes
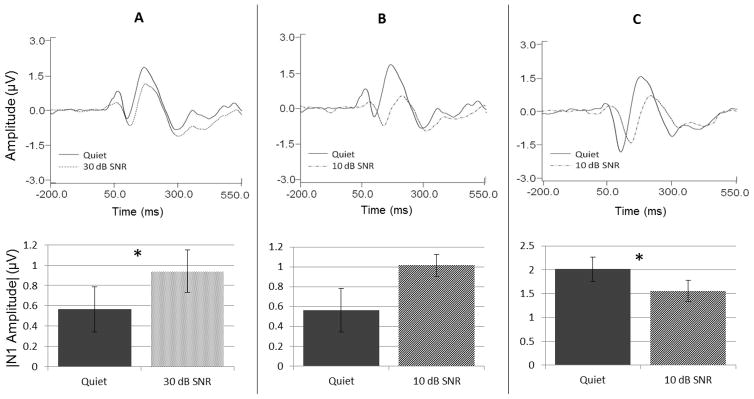
A primary motivation for the present study was the exploration of differences in the effects of noise on N1 amplitudes previously reported in the literature. We hypothesized that amplitude enhancements in noise relative to quiet were the result of binaural stimuli presented at fast presentation rates. In order to test whether these are, in fact, the important factors promoting N1 enhancement in noise, we first replicated the ear of presentation, stimulus rate, and noise levels used in three studies, two of which demonstrated an increase in N1 amplitude in background noise relative to quiet (Alain et al., 2009; Parbery-Clark et al., 2011) and one of which demonstrated a decrease in N1 amplitude relative to quiet (Billings et al., 2009). The results of these replications are shown in Figure 1. Stimulus parameters from Alain et al. 2009 (panel A) and from Parbery-Clark et al. 2011 (panel B) included binaural presentations with an ISI of 900 ms, and noise levels of 30-dB and 10-dB SNR, respectively. Grand average waveform data measured at Cz (top panels) and mean N1 amplitudes plotted as the absolute value (bottom panels) indicate that these parameters elicited greater N1 amplitudes in noise conditions at both noise levels relative to amplitudes recorded in quiet. Paired samples t-tests confirmed that N1 amplitudes were significantly larger in the 30-dB SNR condition relative to the quiet condition (t = 2.377; p = .033), and were trending towards significance in the 10-dB SNR condition (t = 1.817; p = .091; See Table 1 for descriptive statistics for N1 responses in all conditions). This is similar to the trend reported by Parbery-Clark et al. (p = .058) for these stimulus settings. Further, the N1 amplitude enhancement in noise found for binaural presentations at a fast rate recorded at Cz was well conserved across the scalp. Figure 2 depicts the grand averaged waveforms obtained in fast rate binaural presentations measured at each electrode site. This figure clearly shows that enhanced N1 amplitudes were apparent at all measurable electrode locations. In contrast, panel C of Figure 1, obtained using right ear presentations at a 1900-ms ISI (similar to those settings reported in Billings et al. 2009), demonstrates the opposite pattern of results in which N1 amplitudes decrease as a result of noise backgrounds at a level of 10-dB SNR. Paired t-tests confirmed that the reduction in N1 amplitude in noise relative to quiet was significant (t = −2.943; p = .010; See Table 1 for descriptive statistics for N1 responses in all conditions). In summary, use of stimulus parameters similar to those previously reported in the literature successfully replicated reported results such that fast binaural presentations resulted in increased N1 amplitudes in noise relative to quiet and slow monaural presentations resulted in decreased N1 amplitudes in noise.
Figure 1.
Replication of previous study results depicting differences in the effects of noise on N1 amplitudes. Panels A and B represent data based on parameters from Alain et al. (2009) and Parbery-Clark et al. (2011), respectively, obtained using binaural stimulation at a fast presentation rate. Panel C represents data based on parameters from Billings et al. (2009) obtained using right ear stimulation at a slow presentation rate. Grand average waveform data from Cz are shown in the top panels, and accompanying bar graphs below showing the absolute amplitude of N1 in quiet and noise conditions. Asterisks indicate significance (* indicates significance at < 0.05), and error bars represent the standard error of the mean (SEM).
Table 1.
Mean (SD) for P1, N1, P2, and N2 amplitudes and latencies for all conditions.
| Fast | Slow | Fast | Slow | ||
|---|---|---|---|---|---|
| Mean (SD): P1 amplitude (μV) | Mean (SD): P1 latency (ms) | ||||
|
|
|
||||
| Binaural | |||||
| Quiet | .959 (.2) | .857 (.14) | Quiet | 61.47 (2.45) | 49.19 (3.4) |
| 30 dB SNR | .605 (.11) | .477 (.12) | 30 dB SNR | 61 (4.56) | 62.56 (3.35) |
| 10 dB SNR | .453 (.12) | .226 (.1) | 10 dB SNR | 82 (3.11) | 72.07 (4.72) |
| Monaural | |||||
| Quiet | .582 (.08) | .596 (.09) | Quiet | 58.97 (3.49) | 48.66 (2.89) |
| 30 dB SNR | .465 (.09) | .239 (.12) | 30 dB SNR | 61.72 (3.12) | 56.13 (3.49) |
| 10 dB SNR | .314 (.12) | .311 (.13) | 10 dB SNR | 81.43 (5.47) | 77.03 (3.9) |
| Mean (SD): N1 amplitude (μV) | Mean (SD): N1 latency (ms) | ||||
|
|
|
||||
| Binaural | |||||
| Quiet | −.601 (.22) | −2.435 (.42) | Quiet | 104.13 (2.72) | 102.53 (2.35) |
| 30 dB SNR | −1.01 (.11) | −2.018 (.28) | 30 dB SNR | 113.93 (2.9) | 114.93 (3.46) |
| 10 dB SNR | −1.043 (.12) | −1.764 (.17) | 10 dB SNR | 145.53 (5.76) | 137.06 (2.8) |
| Monaural | |||||
| Quiet | −.982 (.21) | −2.259 (.26) | Quiet | 108.67 (2.48) | 104.1 (1.91) |
| 30 dB SNR | −1.0739 (.17) | −2.2891 (.26) | 30 dB SNR | 115.86 (1.73) | 114.21 (2.17) |
| 10 dB SNR | −1.046 (.13) | −1.7559 (.18) | 10 dB SNR | 143.03 (2.76) | 141.53 (4.14) |
| Mean (SD): P2 amplitude (μV) | Mean (SD): P2 latency (ms) | ||||
|
|
|
||||
| Binaural | |||||
| Quiet | 2.111 (.14) | 3.685 (.28) | Quiet | 174.88 (4.96) | 183.38 (5.04) |
| 30 dB SNR | 1.394 (.16) | 2.467 (.34) | 30 dB SNR | 183.94 (5.18) | 199.31 (4.96) |
| 10 dB SNR | .821 (.17) | 1.085 (.22) | 10 dB SNR | 215.63 (6.38) | 219.31 (5.76) |
| Monaural | |||||
| Quiet | 1.273 (.18) | 1.914 (.2) | Quiet | 178.72 (3.96) | 185.78 (4.4) |
| 30 dB SNR | 1.126 (.18) | 1.319 (.2) | 30 dB SNR | 184.13 (4.54) | 199.28 (5.15) |
| 10 dB SNR | .765 (.17) | .886 (.18) | 10 dB SNR | 221.07 (7.4) | 227.72 (4.14) |
| Mean (SD): N2 amplitude (μV) | Mean (SD): N2 latency (ms) | ||||
|
|
|
||||
| Binaural | |||||
| Quiet | −1.035 (.22) | −1.111 (.26) | Quiet | 303.94 (6.4) | 304.56 (9.75) |
| 30 dB SNR | −1.491 (.2) | −1.52 (.39) | 30 dB SNR | 313.25 (9.12) | 302.56 (6.19) |
| 10 dB SNR | −1.111 (.17) | −1.015 (.19) | 10 dB SNR | 316.88 (8.45) | 332.06 (10.28) |
| Monaural | |||||
| Quiet | −1.334 (.18) | −1.246 (.18) | Quiet | 312.5 (4.49) | 310.13 (5.17) |
| 30 dB SNR | −1.432 (.15) | −1.503 (.18) | 30 dB SNR | 322.75 (9.33) | 326.47 (9.31) |
| 10 dB SNR | −.799 (.13) | −1.019 (.14) | 10 dB SNR | 353.94 (10.14) | 356.78 (9.85) |
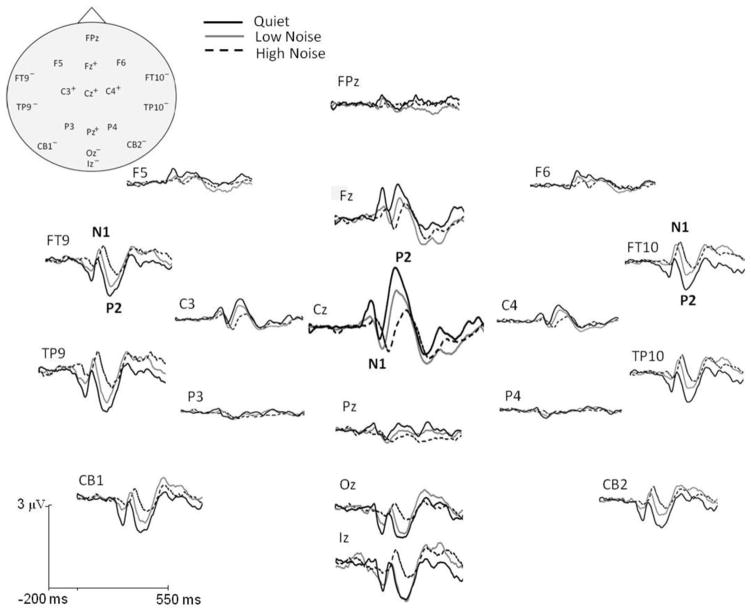
Figure 2.
Grand average cortical responses to speech in quiet and two levels of noise recorded for binaural conditions at fast presentation rates. A subset of electrode locations on opposite sides of the CAEP dipoles illustrate polarity inversions and are denoted by + and – symbols (“+” = typical, negative N1; “−” = an inverted, positive N1) in the schematic representation of the head (top left). The addition of background low-level (solid gray) and high-level (dashed black) noise altered the morphology of the cortical response to speech in quiet (solid black). N1 amplitude trended towards a greater magnitude in both noise levels, whereas P2 amplitude was significantly decreased. Significant latency delays with noise for both N1 and P2 were also evident.
3.2 Presentation Rate and Noise Level Effects
A significant main effect of presentation rate was found for both N1 and P2 amplitudes (Table 2), with slower presentation rates eliciting significantly larger responses than fast presentation rates (Table 1). This is in keeping with previous literature indicating that slower presentation rates produce larger cortical responses (Chapman et al. 1981). However, the amplitude of P1 and N2 peaks was unaffected by variation in presentation rate as demonstrated by lack of significant main effects of or interactions with presentation rate.
Table 2.
Results of repeated-measures analysis of P1, N1, P2, and N2 amplitudes and latencies for all conditions
| Statistical Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| P1 Amplitude | P1 Latency | |||||||
|
|
|
|||||||
| Quiet versus 30 dB SNR | Quiet versus 10 dB SNR | Quiet versus 30 dB SNR | Quiet versus 10 dB SNR | |||||
| F(1,12) | p | F(1,13) | p | F(1, 13) | p | F(1,11) | p | |
|
|
|
|
|
|||||
| Ear | 7.902 | 0.016 | 11.1983931 | 0.005 | 2.37270247 | 0.147 | 0.83120221 | 0.381 |
| Noise Level | 24.872 | < 0.0001 | 37.9689452 | < 0.0001 | 5.77223154 | 0.032 | 115.74468 | < 0.0001 |
| Rate | 1.764 | 0.2088 | 0.49517496 | 0.494 | 43.1132024 | < 0.0001 | 24.4809697 | < 0.0001 |
| Ear*Noise | 1.716 | 0.215 | 0.85213729 | 0.373 | 0.02111283 | 0.887 | 5.24054859 | 0.043 |
| Ear*Rate | 0.028 | 0.869 | 2.77404323 | 0.120 | 0.1839705 | 0.675 | 1.23976265 | 0.289 |
| Noise*Rate | 2.857 | 0.117 | 0.23869125 | 0.633 | 4.72125921 | 0.049 | 0.83028538 | 0.382 |
| Ear*Rate*Noise | 4.729 | 0.057 | 0.2947377 | 0.596 | 1.61179489 | 0.226 | 0.00301521 | 0.957 |
| N1 Amplitude | N1 Latency | |||||||
|
|
|
|||||||
| Quiet versus 30 dB SNR | Quiet versus 10 dB SNR | Quiet versus 30 dB SNR | Quiet versus 10 dB SNR | |||||
| F(1,13) | p | F(1,12) | p | F(1, 13) | p | F(1,14) | p | |
|
|
|
|
|
|||||
| Ear | 0.097 | 0.76 | 0.173 | 0.685 | 1.782 | 0.205 | 0.795 | 0.388 |
| Noise Level | 0.099 | 0.758 | 0.622 | 0.446 | 31.02 | <0.0001 | 197.605 | <0.0001 |
| Rate | 81.644 | < 0.0001 | 67.696 | <0.0001 | 0.645 | 0.436 | 2.858 | 0.113 |
| Ear*Noise | 0.087 | 0.773 | 0.25 | 0.626 | 2.417 | 0.144 | 0.363 | 0.556 |
| Ear*Rate | 4.051 | 0.065 | 1.7 | 0.217 | 5.779 | 0.032 | 0.592 | 0.455 |
| Noise*Rate | 7.688 | 0.016 | 17.624 | 0.001 | 0.803 | 0.386 | 0.261 | 0.618 |
| Ear*Rate*Noise | 3.428 | 0.087 | 1.88 | 0.195 | 0.074 | 0.790 | 2.715 | 0.122 |
| P2 Amplitude | P2Latency | |||||||
|
|
|
|||||||
| Quiet versus 30 dB SNR | Quiet versus 10 dB SNR | Quiet versus 30 dB SNR | Quiet versus 10 dB SNR | |||||
| F(1,13) | p | F(1,12) | p | F(1,13) | p | F(1,14) | p | |
|
|
|
|
|
|||||
| Ear | 40.864 | <0.0001 | 31.04 | <0.0001 | 0.037 | 0.850 | 5.079 | 0.041 |
| Noise Level | 19.868 | 0.001 | 62.115 | <0.0001 | 11.658 | 0.005 | 135.509 | <0.0001 |
| Rate | 45.935 | <0.0001 | 38.166 | <0.0001 | 22.202 | <0.0001 | 3.727 | 0.074 |
| Ear*Noise | 12.451 | 0.004 | 47.727 | <0.0001 | 1.064 | 0.321 | 0.673 | 0.426 |
| Ear*Rate | 19.763 | 0.001 | 6.994 | 0.021 | 1.855 | 0.196 | 0.128 | 0.726 |
| Noise*Rate | 4.811 | 0.047 | 24.721 | <0.0001 | 1.623 | 0.225 | 0.023 | 0.882 |
| Ear*Rate*Noise | 0.078 | 0.784 | 4.61 | 0.053 | 0.11 | 0.746 | 0.006 | 0.941 |
| N2 Amplitude | N2 Latency | |||||||
|
|
|
|||||||
| Quiet versus 30 dB SNR | Quiet versus 10 dB SNR | Quiet versus 30 dB SNR | Quiet versus 10 dB SNR | |||||
| F(1,15) | p | F(1,15) | p | F(1, 15) | p | F(1,15) | p | |
|
|
|
|
|
|||||
| Ear | 0.427 | 0.523 | 0.11288572 | 0.742 | 10.1430202 | 0.006 | 13.0622372 | 0.003 |
| Noise Level | 6.409 | 0.023 | 3.19735975 | 0.094 | 5.80524058 | 0.029 | 22.1127009 | <0.0001 |
| Rate | 0.023 | 0.8806 | 0.13562666 | 0.718 | 0.26315469 | 0.615 | 1.16727831 | 0.297 |
| Ear*Noise | 2.363 | 0.145 | 5.6738652 | 0.031 | 1.20553428 | 0.290 | 5.8789435 | 0.028 |
| Ear*Rate | 0.083 | 0.777 | 0.12684605 | 0.727 | 1.15815666 | 0.299 | 0.72371211 | 0.408 |
| Noise*Rate | 0.088 | 0.771 | 0.16332793 | 0.692 | 0.12560866 | 0.728 | 1.69596487 | 0.212 |
| Ear*Rate*Noise | 0.598 | 0.451 | 2.15263918 | 0.255 | 2.15843309 | 0.162 | 0.18083919 | 0.677 |
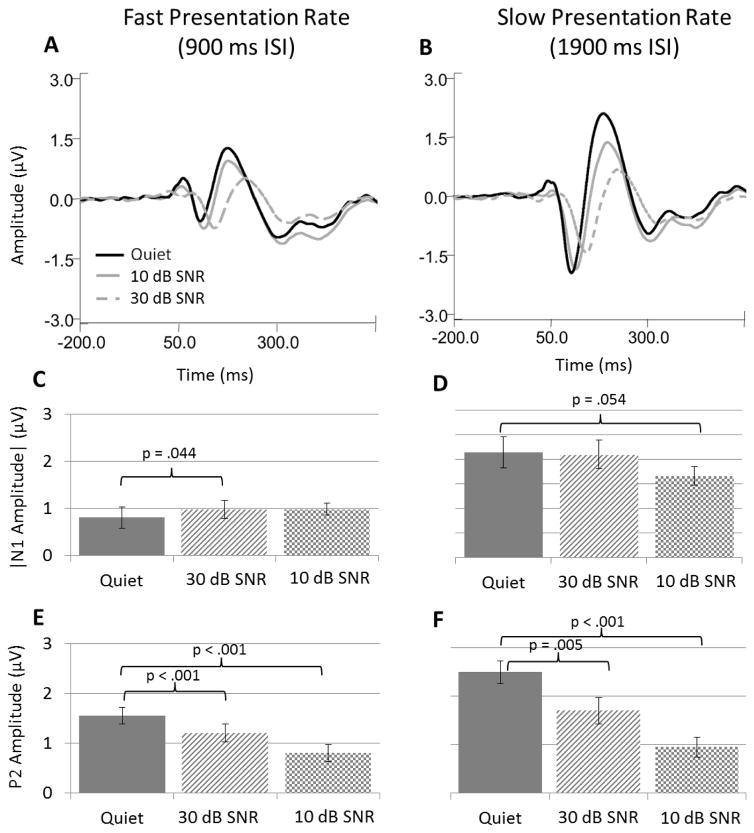
Though no main effect of noise was found for N1 amplitudes, there was a significant interaction between presentation rate and noise level on N1 amplitudes (Table 2). Panels A and B of Figure 3 show grand averaged waveforms collapsed across ear of presentation for fast and slow presentation rate conditions, respectively. Notice that in the fast presentation rate conditions, the average N1 amplitude is larger in 10- and 30-dB SNR conditions (solid and broken gray lines, respectively) compared to the quiet condition (solid black line). In contrast, slow presentation rates elicited progressively smaller N1 amplitudes with increasing background noise level. The effect of presentation rate on N1 amplitudes in quiet compared to noise is further illustrated in panels C and D, which show the absolute value of the average of individual peak N1 amplitudes measured in each background noise condition for fast and slow rates, respectively. To further explore the interaction between presentation rate and noise level, response amplitudes were collapsed across ear of presentation and submitted to post-hoc paired t-tests comparing response amplitudes in quiet with those measured in each background noise condition at both fast and slow rates (p values shown in respective panels of Figure 3). This analysis revealed that N1 responses obtained in 30-dB SNR background noise produced significantly larger amplitudes than those measured in quiet conditions (t(15) = 2.202, p = 0.044) (Panel C). At the slow presentation rate, however, 10-dB SNR conditions yielded generally smaller N1 amplitudes compared to quiet conditions (t(15) = −2.091, p = 0.054) while background noise levels of 30-dB SNR produced amplitudes similar to those measured in quiet (Panel D). Therefore, the significant interaction between rate and noise level is driven by a significant increase in N1 responses in 30-dB SNR noise backgrounds when presented at a fast rate compared to a significant decrease in N1 responses in 10-dB SNR noise backgrounds at a slow rate. Hence, presentation rate is an important factor effecting the direction of N1 amplitude change in noise relative to quiet conditions.
Figure 3.
Effects of presentation rate on N1 and P2 responses in noise and quiet measured at Cz. Panels on the left (A, C, and E) show data obtained in fast rate conditions, with panels on the right (B, D, and F) showing data obtained in slow rate conditions. All data shown are collapsed across ear of presentation in order to highlight presentation rate effects. Panels A and B show grand average waveform data for recordings made in quiet (black line), low-noise (gray line), and high-noise (dashed gray line) conditions at both fast (A) and slow (B) presentation rates. Panels C and D contain average N1 peak amplitudes in fast and slow rate conditions, respectively. Absolute values of N1 amplitude are displayed for ease of interpretation. Panels E and F display average peak P2 amplitudes in fast and slow conditions, respectively. Error bars indicate SEM. p values displayed in panels C – F show significant differences between amplitudes recorded in quiet versus amplitudes recorded in noise based on paired t-test analysis. Please note that statistical analysis was based upon responses in quiet compared to noise conditions, hence no comparison between noise conditions was made.
The effects of noise and presentation rate were quite different between N1 and P2, overall. Inclusion of background noise significantly reduced P2 amplitudes regardless of noise level or presentation rate as evidenced by a main effect of noise level and a main effect of rate for both 10-dB SNR and 30-dB SNR conditions. The amplitude of P2 responses was also significantly affected by the interaction between presentation rate and background noise (Table 2), though the pattern of effects were quite different from the results of N1 amplitudes. Visual inspection of P2 amplitude in the grand averaged waveforms shows that noise reduced P2 response magnitude during both fast and slow presentation rates (Figure 3, panels A and B). This effect is further demonstrated in Panels E and F of Figure 3 which show the average of individual peak P2 amplitudes measured in each background noise condition for fast and slow rates, respectively. Both noise levels clearly reduced P2 response amplitudes, with higher noise levels eliciting greater amplitude reductions. Thus, the significant interaction between noise level and presentation rate is due to the significantly larger effects of noise on amplitudes obtained at the slow presentation rate compared with those obtained at the fast presentation rate.
Though stimulus presentation rate appeared to have minimal impact on P1 and N2 response amplitudes, a main effect of noise level was found for P1 amplitudes at both noise levels, for N2 amplitudes when comparing quiet to 30-dB SNR conditions, and was trending toward significance in the comparison of quiet and 10-dB SNR conditions on N2 amplitudes (Table 2). Though both P1 and N2 demonstrated significant amplitude changes in noise, the effect of noise on each of these waves was quite different. For P1, the inclusion of noise significantly reduced response amplitudes (Table 2), with greater response decrements found for higher noise levels (Table 1). This pattern is quite similar to the pattern reported for P2 response amplitudes in quiet and in noise. N2 amplitudes, however, demonstrated a pattern much more similar to N1 responses such that response amplitudes were significantly increased in 30-dB SNR background noise compared to quiet. In contrast to N1 responses, enhancement of N2 amplitudes in 30-dB SNR conditions occurred regardless of presentation rate. In response to 10-dB SNR background noise, N2 amplitudes were generally reduced in comparison to quiet (Table 1). However, the reduction in amplitude was greater for monaural presentations compared to binaural presentations, leading to a significant interaction between noise level and ear of presentation on N2 amplitudes (Table 2).
With regard to latency effects, all peak values were affected similarly by both noise level and presentation rate. Overall, latencies were more affected by changes in noise level than changes in presentation rate, as evidenced by a significant main effect of noise level for P1, N1 P2, and N2 latencies (Table 2). Increasing levels of noise elicited progressively longer latencies. The effect of presentation rate on N1 and N2 latencies was not significant for either noise condition (Table 2), however P1 latencies were significantly shorter in slow presentation rate conditions compared to fast conditions for both noise conditions (Tables 1 and 2). The effect of presentation rate on P2 latency was significant only for analysis of quiet and 30- dB SNR conditions (Table 2). No significant interactions were found between rate and either noise level or ear of presentation for P2 latencies, though a significant interaction was found between ear and rate of presentation for N1 latency in the comparison of quiet and 30-dB SNR background noise. To explore this interaction, N1 latency data for each subject was averaged across quiet and 30-dB SNR listening conditions, and a repeated-measures ANOVA was conducted on two levels of “Presentation Rate” and two levels of “Ear of Presentation”. This analysis revealed that during monaural presentations, slower presentation rates generally elicited shorter N1 latencies compared to fast presentation rate conditions (average latencies of 109 ms and 112.2 ms, respectively, when averaged across quiet and 30 dB SNR conditions). However, during binaural presentations, N1 latencies were similar regardless of fast or slow presentation rates (average latencies of 109 ms and 109.4 ms, respectively).
Comparison of P1 response latencies obtained in quiet and 30-dB SNR background noise revealed a significant interaction between noise level and rate of presentation. When P1 latencies were collapsed across ear of presentation conditions for each subject, it was apparent that this interaction was driven by the fact that latencies in response to quiet conditions were shorter during slow presentation rates, while no difference was found between the P1 latencies across presentation rates in the presence of 30-dB SNR background noise. Though the inclusion of 10-dB SNR background noise increased P1 response latencies for both monaural and binaural presentations compared to quiet, the increase was larger during monaural presentations compared to binaural presentations, thus leading to a significant ear by noise level interaction.
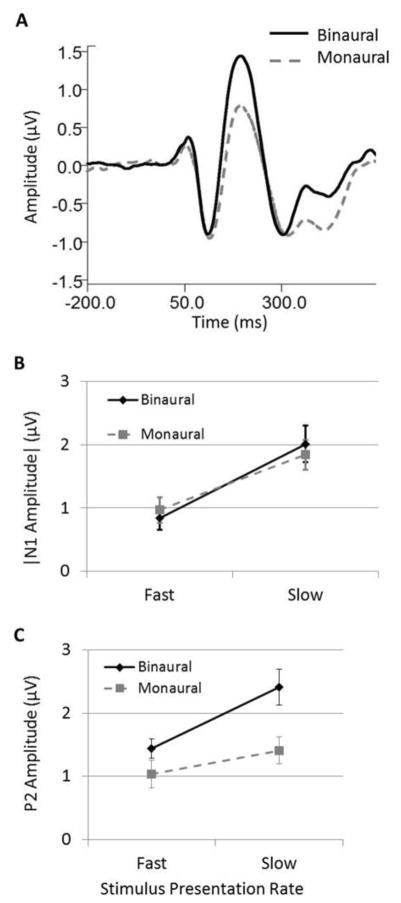
3.3 Ear of Presentation Effects
Statistical analysis indicated no main effect of ear of presentation on N1 response amplitudes or latencies (Table 2). This effect is apparent in Figure 4 panel A, which depicts the grand averaged data for binaural and monaural listening conditions collapsed across presentation rate and noise level. In this figure, it is clear that monaural compared to binaural presentations yielded minimal change in N1 peak responses. A similar effect was found for N2 responses, such that binaural versus monaural presentations did not significantly affect response amplitudes (Table 2). In contrast, P1 and P2 responses, were highly dependent upon ear of stimulation (Table 1 and Table 2), with amplitudes being significantly greater in binaural listening conditions compared to monaural conditions. A significant interaction was also found between ear of presentation and presentation rate on P2 amplitudes, with N1 amplitudes for 30 dB noise showing a trend toward significance. Panels B and C of Figure 4 display the average of individual N1 and P2 response amplitudes, respectively, for binaural and monaural presentations as a function of presentation rate collapsed across background noise level. Although slow presentation rates elicited larger amplitudes during both binaural and monaural conditions compared to fast presentation rates, the difference in amplitude as a function of rate was greater for binaural conditions than monaural conditions, especially for P2 responses. Significant interactions were also found between ear and noise level for P2 amplitudes both for quiet vs 10 dB SNR noise and for quiet vs 30 dB comparisons (Table 2). In both cases, amplitude reductions in background noise conditions compared to quiet were greater during binaural presentations compared to monaural presentations (Table 1). This interaction likely stems from the significantly larger P2 amplitudes obtained in binaural conditions compared to monaural conditions which is evident in quiet and in both noise conditions. The larger amplitudes obtained with binaural presentations allow for greater amplitude reductions with the inclusion of background noise, leading to significantly larger amplitude reductions in the binaural noise conditions compared to the monaural noise conditions.
Figure 4.
Ear of presentation effects on CAEPs. Panel A shows grand average Cz waveforms for binaural (solid black) and monaural (dashed gray) presentations collapsed across presentation rate and background noise conditions. Panels B and C depict the average of N1 and P2 peak amplitudes, respectively, in fast and slow rate presentations collapsed across background noise conditions. For the purposes of illustration, N1 amplitudes are displayed as absolute values. Error bars indicate SEM.
A significant interaction was also found between ear of presentation and noise level on N2 amplitudes and latencies when comparing responses obtained in quiet and in 10-dB SNR conditions (Table 2). In 10-dB SNR background noise, N2 responses were smaller in amplitude and longer in latency compared to quiet conditions for both monaural and binaural presentations. However, latency and amplitude changes in 10-dB SNR background noise were somewhat larger in the monaural condition compared to the binaural condition (Table 1), leading to a significant interaction between ear of presentation and noise level for N2 responses.
3.4 Determinants of N1 and N2 amplitude enhancement in noise
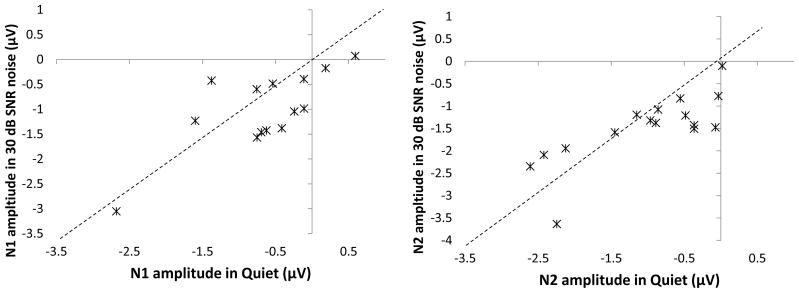
Both N1 and N2 responses demonstrated significant increases in response amplitude in 30-dB SNR background noise compared to quiet conditions, though each displayed unique properties. For example, N2 responses were larger in 30-dB SNR background noise regardless of ear or rate of presentation (Table 1) as evidenced by the significant main effect of noise level (Table 2). In contrast, analysis of N1 responses clearly indicates that stimulus presentation rate is an important factor in eliciting larger N1 responses in noise backgrounds compared to quiet conditions (Figure 3). Although ear of presentation had minimal effect on N1 response amplitudes overall, there was a strong trend toward a significant interaction between ear of presentation and presentation rate, as well as between ear of presentation, presentation rate, and noise level when comparing responses in quiet and 30-dB SNR background noise. In order to more closely address the influence of ear of presentation and stimulus presentation rate on N1 response amplitudes in quiet and 30-dB SNR noise, paired-samples t-tests were conducted. The results of this analysis are shown in Table 3. Notice that of the four total comparisons made, only binaural presentations at a fast rate yielded significantly larger N1 response amplitudes in the 30-dB SNR condition compared to quiet. Therefore, both presentation rate and ear of presentation are important factors for eliciting enhanced N1 responses in background noise, a phenomenon which requires binaural presentations at a fast rate. The enhancement of N1 and N2 response amplitudes in noise compared to quiet during fast binaural stimulus presentations was fairly consistent across most, but not all, subjects. Figure 5 displays individual N1 and N2 response amplitudes measured in quiet using fast binaural presentations as a function of response amplitudes measured under the same conditions but with 30-dB SNR background noise. The dashed lines represent unity at which response amplitudes did not change between noise conditions. N1 responses, shown in the left panel, indicate that 10 of the 14 subjects display larger (e.g. more negative) N1 responses in the 30-dB SNR condition compared to quiet and four subjects show the opposite pattern. With regard to N2 responses shown in the right panel, 11 of 16 individuals demonstrated increased amplitudes in 30-dB SNR background noise as evidenced by data points lying below the unity line, and five demonstrated reduced N2 response amplitudes in the noise condition compared to quiet as evidenced by data points lying above the unity line. The four individuals that demonstrated smaller N1 amplitudes in noise were not the same individuals who showed this effect for N2 amplitude. Thus, while the enhancement of N1 and N2 responses in background noise was significant and found in the majority of participants, some did demonstrate reduced responses in background noise even when stimuli were presented binaurally at a fast rate.
Table 3.
Results of paired t-test analyses of N1 peak amplitudes for each listening condition
| Condition | Fast | Slow | ||
|---|---|---|---|---|
|
|
|
|||
| (Quiet x 30 dB SNR) | t(df) | p | t(df) | p |
| Binaural | 2.377(13) | 0.033 | −1.231(15) | 0.237 |
| Monaural | 0.744(14) | 0.469 | 0.647(15) | 0.527 |
Figure 5.
Individual variability in N1 (left panel) and N2 (right panel) amplitude enhancement in 30-dB SNR compared to quiet conditions. Plotted data represent responses to stimuli presented binaurally at a fast presentation rate as these conditions were necessary to elicit significant enhancement in noise. Amplitudes measured in quiet conditions are plotted on the horizontal axis, and amplitudes measured in 30-dB SNR background noise are plotted on the vertical axis. The dashed black line represents the point at which response amplitudes between quiet and noise were identical. Points falling below this line indicate individuals who displayed larger (e.g. more negative) response amplitudes in noise compared to quiet conditions.
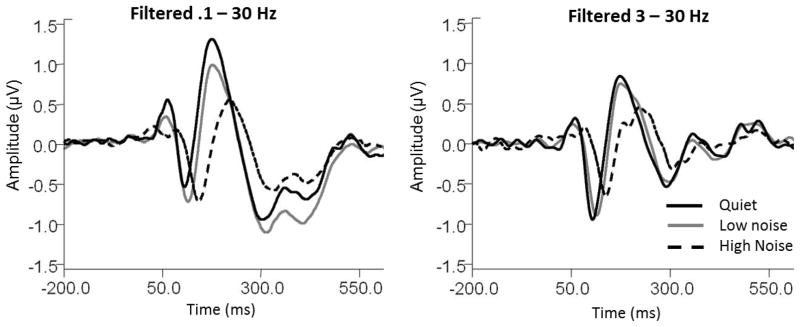
Lastly, it is important to note that response filtering can have significant impacts on N1 and N2 response enhancement in noise. The present study employed off-line bandpass filtering between 0.1–30 Hz. However, higher high pass cut-offs are also frequently cited in the literature (Debreyune et al. 1984, Wang et al., 2008, Tremblay et al., 2010). Figure 6 demonstrates the effect of altering the high pass filter setting on responses in quiet and background noise. The left panel displays grand averaged responses for fast binaural presentations filtered using the original band pass filter of 0.1 – 30 Hz. At these settings, both N1 and N2 amplitudes appear larger in the 30-dB SNR noise conditions (solid gray lines) compared to quiet (solid black line), and N1 is also larger in the 10-dB SNR condition (dashed black lines) compared to quiet. However, this effect disappears when the high pass filter cut-off is raised to 3 Hz (right panel). Hence, eliminating low frequency portions of the response effectively abolishes N1 and N2 amplitude enhancement in noise.
Figure 6.
Effects of response filtering on grand average Cz waveforms to speech presented binaurally at fast presentation rates. For responses filtered with the original filter settings or .1 to 30 Hz (left panel), both low (gray) and high (black dashed) noise conditions elicited significantly larger N1 amplitudes than when elicited in quiet (solid black). However, when responses were filtered to remove response energy below 3 Hz (right panel), N1 amplitudes in both noise conditions are significantly smaller than responses measured in quiet. This indicates that noise enhancement of the N1 response is dependent upon low frequency response energy.
4. Discussion
The aim of this study was to investigate the effects of stimulus presentation factors on cortical responses to speech in quiet and noise, particularly with regard to the conditions under which N1 amplitudes are enhanced by background noise relative to quiet. Based upon procedural differences between studies reporting N1 enhancement and those reporting decrements in noise, we hypothesized that both binaural presentations and fast stimulus presentation rates were critical to eliciting noise-enhanced N1 responses. Consistent with our expectations, only those stimuli presented binaurally at a fast presentation rate elicited enhanced N1 amplitudes in noise compared to quiet (Figs. 1–3; Tables 1 – 3). Although several aspects of the stimuli used in the current study were different from those reporting N1 amplitude enhancement in noise (e.g. stimulus and noise type, duration, etc.), use of binaural presentations at a rate of 900 ISI was sufficient to elicit enhancement. In addition to contributing to understanding the effects of noise on the N1 peak component, the present work also revealed that the amplitude of the N2 peak component is enhanced in low levels of background noise, regardless of ear or rate of stimulus presentation, and that P2 response amplitudes are highly dependent upon ear of stimulation.
4.1. N1 and N2 Enhancement in Noise: Ear, Rate, and Filtering Effects
A main finding of the present study was that both N1 and N2 peak responses can actually be enhanced in noise backgrounds relative to quiet. However, certain conditions must be met in order to observe noise-induced increases in N1 magnitudes, including binaural presentations, fast presentation rates, and inclusion of low frequency response components. Previous studies have speculated that enhanced responses in low noise backgrounds could reflect stochastic resonance (Stufflebeam et al., 2000; Alain et al., 2009), a process which has been shown to improve behavioral and physiological signal detection and discrimination in noise (Moss et al., 2004; Ries, 2007; Henry, 1999; Zeng et al., 2000; Lewis & Henry, 1995). While the possibility of stochastic resonance should not be discounted, enhanced N1 and N2 amplitudes in noise found in the current study should not be construed as indicating improved signal detection in noise as no behavioral data were collected to substantiate such a claim. Further, the relationship between the processes underlying stochastic resonance and the methodological constraints governing N1 enhancement in noise are unclear. Behaviorally, binaural stimulus presentations usually result in improved signal detection and discrimination, even when stimuli are homophasic as in the current study (Marks, 1978). Such behavioral performance improvement is likely due, in part, to binaural summation of loudness (Reynolds and Stevens, 1960). However, it seems unlikely that binaural summation of loudness alone can account for the N1 and N2 amplitude enhancements in noise found in the current study. First, the lack of significant effect of ear of presentation on N1 and N2 amplitudes implies that responses obtained during binaural conditions were not larger than those obtained during monaural listening conditions (Tables 1 & 2; Fig. 4). Second, significant amplitude enhancements were only found during the lower of the two noise conditions (30-dB SNR) compared to quiet condition (Table 3). If binaural loudness summation accounted for N1 and N2 amplitude enhancement in noise, then the largest amplitudes should have occurred during the highest noise condition (10-dB SNR).
The importance of fast presentation rates for N1 enhancement and inclusion of low frequency response components for N1 and N2 amplitude enhancement in noise may be linked to entrainment. Entrainment refers to the ability of cortical oscillations to phase lock to repetitive low frequency amplitude fluctuations in periodic and quasi-periodic signals (Lakatos et al., 2005; Giraud & Poeppel, 2012; Miller et al., 2012). Behaviorally, entrainment makes use of stimulus predictability across time to improve detection, discrimination (Jones et al., 2002), and attention to specific auditory streams amid competing streams (Lakatos et al., 2005). Studies of cortical entrainment indicate an optimal range of presentations rates with a high-pass cut-off of approximately 1 Hz (Schroeder and Lakatos, 2009). Therefore, it is possible that the faster presentation rate used in the current study was better able to stimulate entrainment to stimulus onsets compared to the slow presentation rate. The ability to entrain to the higher presentation rate might also explain why removal of low frequency response components via filtering effectively abolishes N1 and N2 amplitude enhancement in noise since these response frequencies correspond to the stimulus repetition rate.
4.2. P1 and P2 responses
All previous reports of the effect of noise on CAEP responses show that the addition of background noise yields decreased P2 response amplitudes compared to those measured in quiet (Whiting et al., 1998; Kaplan-Neeman et al., 2006; Alain et al., 2009; Billings et al., 2009; Parbery-Clark et al., 2011; Billings et al., 2013). Therefore, we hypothesized that P2 amplitudes measured in noise backgrounds would be smaller than those measured in quiet regardless of ear of presentation or presentation rate. This hypothesis was supported by our results, including the initial comparisons replicating the conditions of earlier studies (Figure 1) and in all subsequent analyses of the data set (Figures 2 – 4). Further, though previous studies had reported variable effects of noise on P1 responses, the present results indicate that P1 amplitudes are reduced even in fairly low levels of background noise. Regardless of ear or rate of presentation, the presence of noise significantly reduced both P1 and P2 amplitudes relative to quiet conditions (Table 2, see “Noise Level” effect). Such a result is likely due to the partial masking effects of noise which reduce the synchrony of neural responses.
Another interesting difference between the responses of negative-going and positive going waves was the effect of monaural compared to binaural presentations. While N1 and N2 responses were minimally affected by ear of presentation, both P1 and P2 amplitudes were found to be highly sensitive to binaural compared to monaural stimulation such that binaural stimulus presentations consistently led to significantly larger P1 and P2 amplitudes compared with monaural recordings (Table 2, see “Ear” effect). This binaural amplitude enhancement was quite robust for P2 in that all subjects demonstrated the effect, and the margin of difference in amplitude between monaural and binaural presentations was considerable (Table 2 and Fig. 4). P1 amplitudes were substantially larger in response to binaural compared to monaural conditions in the majority of participants, leading to a main effect of Ear of presentation. However the effect was slightly less robust compared to P2 responses in that three participants demonstrated slightly larger amplitudes in monaural conditions compared to binaural, while a fourth participant produced equivalent P1 amplitudes regardless of monaural or binaural presentations. The effect of ear of presentation highlights a potentially important contrast between negative- and positive-going responses. Neither N1 nor N2 amplitudes showed significant differences between monaural and binaural stimulation, while both P1 and P2 were sensitive to these contrasts. Unfortunately, comparatively little work has been done regarding the significance of P1 or P2 relative to N1. However, the available information supports the idea that each of these waves are anatomically and functionally distinct. Studies employing source analysis indicate that the neural generators of the P1 response include the superior temporal gyrus, hippocampus, dorsolateral prefrontal cortex, and thalamus (Williams et al., 2011). Functionally, the P1 response is known to reflect preattentive filtering of sensory information in order to enhance cortical responses to novel or relevant stimuli while minimizing responses to extraneous or redundant information (Boutros and Belger, 1999). Studies using source analysis of electroencephalography and magnetoencephalography data indicate that P2 is generated by multiple cortical sources located anterior to the site of N1 generation (Hari et al., 1987; Godey et al., 2001), implicating auditory association and possibly sensory integration areas.
The finding in the current study that P1 responses were significantly enhanced by binaural listening is corroborated by previous studies showing similar effects of ear of presentation (Butler et al., 1969; Kelly-Ballweber et al., 1984; Weihing and Musiek, 2008). If P1 reflects sensory filtering, this binaural enhancement may indicate that binaurally presented sounds are filtered less than monaural sounds and are thus more likely to be robustly represented in the later stages of auditory processing. With regard to monaural versus binaural impact on P2 amplitudes, reports are somewhat conflicting. Butler et al. (1969) reported significant increases in P2 amplitude in binaural presentation conditions relative to monaural presentations. However, the majority of studies have reported no significant P2 amplitude differences resulting from monaural versus binaural stimulation (Debruyne, 1984; Pantev et al., 1986; McPherson & Starr, 1993; Gilmore et al., 2009). Part of the discrepancy in results may stem from differences in stimulation and recording paradigms. For example, the present study employed speech stimuli in contrast to the simpler tone and click stimuli typical of most CAEP studies. Stimulus duration may also be a critical difference since studies showing P2 enhancement under binaural stimulation have used longer duration stimuli (450 ms in the present study; 800 ms in (Butler et al., 1969), while those studies showing no effect of ear of stimulation have used click or tone burst stimuli. This suggests that perhaps the difference in P2 results across studies may be related to a longer window of temporal integration for binaural presentations compared to monaural presentations which is not identified by short duration stimuli. This hypothesis could easily be tested by measuring P2 responses to short and long duration signals presented monaurally and binaurally.
4.3 Summary and Implications for Auditory Research
The present findings refute the notion that CAEP peak responses simply reflect serial processing of stimuli in the auditory system. Different CAEP components are sensitive to different methodological constraints, and thus reflect distinct features of neural stimulus encoding. Our results clearly indicate that background noise can elicit enhanced N1 and N2 response amplitudes to a speech syllable provided that certain listening conditions are met. In addition, both P1 and P2 waves are considerably larger in response to binaural stimulation compared to monaural stimulation. These findings have important implications for how the auditory cortex adjusts in different listening conditions in order to maximize signal detection. However, it is important to remember that no behavioral measures were made in the current study. It is, therefore, impossible to state whether the enhanced amplitudes found in the current study actually reflect improved behavioral signal detection, discrimination, or recognition. Such a behavioral result is possible given the correspondence between CAEP amplitudes and behavioral performance (Billings et al., 2012; Chang et al., 2012; Parbery-Clark et al., 2011; Billings et al., 2013). Further research employing both physiological measures and behavioral measures is needed to assess whether the N1 and N2 noise-enhancement effects or the P1 and P2 binaural-enhancement effects found in the current study in fact reflect improved behavioral outcomes.
Highlights.
This is the first study to systematically explore the effects of both presentation rate and ear of presentation on cortical evoked potential responses in noise.
Cortical N1 and N2 response amplitudes can be enhanced in low levels of background noise.
Both P1 and P2 responses are significantly larger during binaural presentations compared to monaural presentations
Acknowledgments
This work was supported by NIH-NIDCD (R03DC010914) and VA-RR&D (CoE C4844C). These funding sources had no role in the design, interpretation, or publishing of this work. Further, there are no current or potential conflicts of interest, either personal or financial, to be disclosed with regard to the current work. The authors would like to thank Drs. Tina Penman, Sara Blankenship, and Jay Vachhani for donating their time, effort, and bountiful brains to the present project. We would also like to thank Dr. David Lilly for helpful comments during the design of the present study.
References
- Adler G, Adler J. Influence of stimulus intensity on AEP components in the 80- to 200-millisecond latency range. Audiol. 1989;28:316–324. doi: 10.3109/00206098909081638. [DOI] [PubMed] [Google Scholar]
- Alain C, Quan J, McDonald K, Van Roon P. Noise-induced increase in human auditory evoked neuromagnetic fields. Eur J Neurosci. 2009;30:132–142. doi: 10.1111/j.1460-9568.2009.06792.x. [DOI] [PubMed] [Google Scholar]
- Anderson S, Chandrasekaran B, Yi1 H, Kraus N. Cortical-evoked potentials reflect speech-in-noise perception in children. Eur J Neurosci. 2010;32:1407–1413. doi: 10.1111/j.1460-9568.2010.07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett K, Billings C, Molis M, Leek M. Neural Encoding and Perception of Speech Signals in Informational Masking. Ear Hear. 2012;33(2):231–238. doi: 10.1097/AUD.0b013e31823173fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli S, Smurzynski J, Probst R. Effects of Age, Age-Related Hearing Loss, and Contralateral Cafeteria Noise on the Discrimination of Small Frequency Changes: Psychoacoustic and Electrophysiological Measures. J Assoc Res Oto. 2005;6:207–222. doi: 10.1007/s10162-005-5029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, Bennett KO, Molis MR, Leek MR. Cortical encoding of signals in noise: effects of stimulus type and recording paradigm. Ear Hear. 2011;32:53–60. doi: 10.1097/AUD.0b013e3181ec5c46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, McMillan G, Penman T, Ong S. Predicting perception in noise using cortical auditory evoked potentials. J Otolaryngol. 2013;14:891–903. doi: 10.1007/s10162-013-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, Papesh MA, Penman TM, Baltzell LS, Gallun FJ. Clinical use of aided cortical auditory evoked potentials as a measure of physiological detection or physiological discrimination. Int J Otolaryngol. 2012;33:213–238. doi: 10.1155/2012/365752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings CJ, Tremblay KL, Stecker GC, Tolin WM. Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hear Res. 2009;254:15–24. doi: 10.1016/j.heares.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros NN, Belger A. Midlatency evoked potentials attenuation and augmentation reflect different aspects of sensory gating. Biol Psychiat. 1999;45(7):917–922. doi: 10.1016/s0006-3223(98)00253-4. [DOI] [PubMed] [Google Scholar]
- Butler RA, Keidel WD, Spreng M. An investigation of the human cortical evoked potential under conditions of monaural and binaural stimulation. Acta Otolaryngol Suppl. 1969;68:317–326. doi: 10.3109/00016486909121570. [DOI] [PubMed] [Google Scholar]
- Chang H, Dillon H, Carter L, Van Dun B, Young S. The relationship between cortical auditory evoked potential (CAEP) detection and estimated audibility in infants with sensorineural hearing loss. Int J Audiol. 2012;51:663–670. doi: 10.3109/14992027.2012.690076. [DOI] [PubMed] [Google Scholar]
- Chapman CR, Colpitts YH, Mayeno JK, Gagliardi GJ. Rate of stimulus repetition changes evoked potential amplitude: dental and auditory modalities compared. Exp Brain Res. 1981;43:246–52. doi: 10.1007/BF00238365. [DOI] [PubMed] [Google Scholar]
- Debruyne F. Binaural interaction in early, middle and late auditory evoked responses. Scand Audiol. 1984;13:293–296. doi: 10.3109/01050398409042139. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Schaefer AB. Comparison of frequency selectivity and consonant recognition among hearing-impaired and masked normal-hearing listeners. J Acoust Soc Am. 1992;91:2110–2121. doi: 10.1121/1.403697. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Clementz BA, Berg P. Hemispheric differences in auditory oddball responses during monaural versus binaural stimulation. Int J Psychophysiol. 2009;73:326–333. doi: 10.1016/j.ijpsycho.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godey B, Schwartz D, de Graaf JB, Chauvel P, Liégeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: a comparison of data in the same patients. Neurophysiol Clin. 2001;112:1850–1859. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- Gott PS, Hughes EC. Effect of noise masking on the brain-stem and middle-latency auditory evoked potentials: central and peripheral components. Electroen Clin Neuro. 1989;74:131–138. doi: 10.1016/0168-5597(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, Zecker SG, Kraus N. Neural plasticity following auditory training in children with learning problems. Clin Neurophysiol. 2003;114:673–684. doi: 10.1016/s1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Hari R, Pelizzone M, Mäkelä JP, Hällström J, Leinonen L, Lounasmaa OV. Neuromagnetic responses of the human auditory cortex to on- and offsets of noise bursts. Audiol. 1987;26:31–43. doi: 10.3109/00206098709078405. [DOI] [PubMed] [Google Scholar]
- Henry KR. Noise improves transfer of near-threshold, phase-locked activity of the cochlear nerve: evidence for stochastic resonance? J Comp Physiol [A] 1999;184:577–584. doi: 10.1007/s003590050357. [DOI] [PubMed] [Google Scholar]
- Jones MR, Moynihan H, MacKenzie N, Puente J. Temporal Aspects of Stimulus-Driven Attending in Dynamic Arrays. Psychol Sci (Wiley-Blackwell) 2002;13:1313–1319. doi: 10.1111/1467-9280.00458. [DOI] [PubMed] [Google Scholar]
- Kaplan-Neeman R, Kishon-Rabin L, Henkin Y, Muchnik C. Identification of syllables in noise: Electrophysiological and behavioral correlates. J Acoust Soc Am. 2006;120:926–933. doi: 10.1121/1.2217567. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94:1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Henry KR. Nonlinear effects of noise on phase-locked cochlear-nerve responses to sinusoidal stimuli. Hear Res. 1995;92:1–16. doi: 10.1016/0378-5955(95)00189-1. [DOI] [PubMed] [Google Scholar]
- Marks LE. Binaural summation of loudness of pure tones. J Acoust Soc Am. 1978;64:107–114. doi: 10.1121/1.381976. [DOI] [PubMed] [Google Scholar]
- McPherson DL, Starr A. Binaural interaction in auditory evoked potentials: brainstem, middle- and long-latency components. Hear Res. 1993;66:91–98. doi: 10.1016/0378-5955(93)90263-z. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Foster BL, Honey CJ. Does rhythmic entrainment represent a generalized mechanism for organizing computation in the brain? Front Comp Neurosci. 2012;6:85–89. doi: 10.3389/fncom.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss F, Ward LM, Sannita WG. Stochastic resonance and sensory information processing: a tutorial and review of application. Neurophysiol Clin. 2004;115:267–278. doi: 10.1016/j.clinph.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Pantev C, Lütkenhöner B, Hoke M, Lehnertz K. Comparison between simultaneously recorded auditory-evoked magnetic fields and potentials elicited by ipsilateral, contralateral and binaural tone burst stimulation. Audiol. 1986;25:54–61. doi: 10.3109/00206098609078369. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Marmel F, Bair J, Kraus N. What subcortical-cortical relationships tell us about processing speech in noise. Eur J Neurosci. 2011;33:549–557. doi: 10.1111/j.1460-9568.2010.07546.x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT. The component structure of the human event-related potentials. Prog Brain Res. 1980;54:17–49. doi: 10.1016/S0079-6123(08)61604-0. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multi-channel evoked potentials. Neurophysiol Clin. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Ries DT. The influence of noise type and level upon stochastic resonance in human audition. Hear Res. 2007;228:136–143. doi: 10.1016/j.heares.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Roth WT, Ford JM, Lewis SJ, Kopell BS. Effects of Stimulus Probability and Task-Relevance on Event-Related Potentials. Psychophysiol. 1976;13:311–317. doi: 10.1111/j.1469-8986.1976.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Reynolds GS, Stevens SS. Binaural Summation of Loudness. J Acoust Soc Am. 1960;32:1337–1342. [Google Scholar]
- Sheehan KA, McArthur GM, Bishop DVM. Is discrimination training necessary to cause changes in the P2 auditory event-related brain potential to speech sounds? Cog Brain Res. 2005;25:547–553. doi: 10.1016/j.cogbrainres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stufflebeam SM, Poeppel D, Roberts TP. Temporal encoding in auditory evoked neuromagnetic fields: stochastic resonance. Neuroreport. 2000;11:4081–4085. doi: 10.1097/00001756-200012180-00034. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Inoue K, McClannahan K, Ross B. Repeated Stimulus Exposure Alters the Way Sound Is Encoded in the Human Brain. PLoS ONE. 2010;5:1–11. doi: 10.1371/journal.pone.0010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AL, Mouraux A, Liang M, Iannetti GD. The Enhancement of the N1 Wave Elicited by Sensory Stimuli Presented at Very Short Inter-Stimulus Intervals Is a General Feature across Sensory Systems. PLoS ONE. 2008;3:1–8. doi: 10.1371/journal.pone.0003929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihing J, Musiek FE. An Electrophysiological Measure of Binaural Hearing in Noise. J Am Acad Audiol. 2008;19:481–495. doi: 10.3766/jaaa.19.6.4. [DOI] [PubMed] [Google Scholar]
- Whiting KA, Martin BA, Stapells DR. The effects of broadband noise masking on cortical event-related potentials to speech sounds/ba/and/da/ Ear Hear. 1998;19:218–231. doi: 10.1097/00003446-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Williams TJ, Nuechterlein KH, Subotnik KL, Yee CM. Distinct neural generators of sensory gating in schizophrenia. Psychophysiology. 2011:48470–478. doi: 10.1111/j.1469-8986.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Effects of stimulus frequency and complexity on the mismatch negativity and other components of the cortical auditory-evoked potential. J Acoust Soc Am. 2001;109:1526–1537. doi: 10.1121/1.1349184. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Fu QJ, Morse R. Human hearing enhanced by noise. Brain Res. 2000;869:251–255. doi: 10.1016/s0006-8993(00)02475-6. [DOI] [PubMed] [Google Scholar]