Abstract
The purpose of this study was to determine the effects of noise type, signal-to-noise ratio (SNR), age, and hearing status on cortical auditory evoked potentials (CAEPs) to speech sounds. This helps to explain the hearing-in-noise difficulties often seen in the aging and hearing impaired population. Continuous, modulated, and babble noise types were presented at varying SNRs to 30 individuals divided into three groups according to age and hearing status. Significant main effects of noise type, SNR, and group were found. Interaction effects revealed that the SNR effect varies as a function of noise type and is most systematic for continuous noise. Effects of age and hearing loss were limited to CAEP latency and were differentially modulated by energetic and informational-like masking. It is clear that the spectrotemporal characteristics of signals and noises play an important role in determining the morphology of neural responses. Participant factors such as age and hearing status, also play an important role in determining the brain’s response to complex auditory stimuli and contribute to the ability to listen in noise.
Keywords: Cortical auditory evoked potentials, electrophysiology, noise type, masking, aging, hearing loss
Introduction
Understanding speech in background noise is a complex process which is dependent upon the integrity of both the auditory system and cognitive functioning. It is generally accepted that acoustically adverse environments affect speech understanding more in older hearing-impaired individuals than in young normal-hearing individuals. However, it is unclear whether perception-in-noise difficulties are predominantly caused by reduced central processing ability (including, but not limited to, cognitive functioning) or by the lack of acoustical information necessary to differentiate the signal from the noise at the level of the peripheral auditory system. In certain cases it may be that cognition compensates for peripheral coding failures or the lack of available acoustic cues. Given the many contributions to accurate speech understanding in background noise, it is not surprising that some types of background noise are more detrimental to speech understanding than others [1]. Understanding how speech in noise is neurally coded in normal and impaired individuals may improve our understanding of the underlying mechanisms that contribute to successful perception in noise, allowing for better management and treatment of individuals with speech-perception-in-noise difficulties.
Cortical auditory speech-in-noise coding is determined by several factors. For example, the level of the signal and its relationship to the noise (i.e, signal-to-noise ratio) affect both the timing and magnitude of cortical neural responses [2]. In addition, the spectrotemporal properties of both signal and noise can interact and affect neural coding. Signals presented in modulated or interrupted noise produce stronger cortical responses than those presented in unmodulated noise [3,4], which is consistent with behavioral data that demonstrate better speech reception thresholds in fluctuating noise [5]. These improvements are thought to be due to the listener’s ability to take advantage of gaps in the noise as a means of identifying the signal [6].
Masking release as a function of age and hearing loss has been studied extensively in the behavioral domain [5,7,8]; however, in the physiological domain, masking release and the effect of different noise types in individuals with hearing loss has not been determined. Age-related changes are reported to be independent of peripheral hearing sensitivity in both animal and human studies [9]. However, physiological studies on the effect of background noise as a function of age are not as conclusive. While some studies have found differences in the evoked responses between younger and older individuals [10–12], others found that the effect persisted when co-varying for age; suggesting that the change in CAEPs to signal in noise were not attributable to normal aging [13].
We aim to clarify the effects of noise type and SNR on cortical neural coding to improve our understanding of the underlying process of signal extraction in a dynamic environment with specific focus on how older individuals differ from younger individuals and how individuals with and without hearing loss differ from each other. A better understanding of the neural coding of signals in noise may help to improve assessment and treatment of perception-in-noise difficulties. We hypothesize that there will be important effects of noise type, SNR, and group, but that these effects will interact such that the effects of noise type differ by both SNR and group.
Methods
Participants
Participants included 30 right-handed individuals recruited into three groups: 10 younger normal-hearing individuals (YNH, mean age = 27.1, SD = 7.0), 10 older normal-hearing individuals (ONH, mean age = 67.2, SD = 5.1), and 10 older hearing-impaired individuals (OHI, mean age = 68.8 years, SD = 5.9). The two older groups did not differ significantly in age (T(18)=−.645; p=.527). All three groups consisted of four male and six female participants. Normal-hearing participants had thresholds below 25 dB HL bilaterally up to 4000 Hz, and hearing-impaired individuals had mild-to-moderate sloping sensorineural hearing loss. Each group’s pure-tone average (average of hearing thresholds at 500, 1000, and 2000 Hz) was calculated (young normal hearing: 6.0 ± 4.4dB); older normal-hearing: 7.9 ± 4.9dB); older hearing impaired: 32.3 ± 7.7dB) and revealed no significant difference between normal-hearing groups (T(18)=−.95; p=.355). The mean thresholds for all participant groups are shown in Table 1. All participants gave their informed consent and the research was completed with the approval of the local institutional review board.
Table 1.
Audiometric thresholds (mean and standard deviation in dB) for the right ear across the three participant groups.
| Frequencies (Hz) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| 250 | 500 | 1000 | 2000 | 4000 | 8000 | |
| Younger Normal Hearing Group | 7.5 (4.2) | 7.5 (5.9) | 5.0 (4.7) | 5.5 (4.4) | 4.0 (5.7) | 1.0 (8.4) |
| Older Normal Hearing Group | 9.5 (6.0) | 8.0 (4.8) | 10.0 (6.7) | 6.0 (5.7) | 17.5 (8.9) | 29.4 (20.2) |
| Older Hearing Impaired Group | 19.0 (5.7) | 24.0 (8.1) | 28.0 (9.2) | 45.0 (11.1) | 56.5 (10.3) | 64.0 (10.8) |
Signals and maskers
Naturally produced syllables /ba/ and /da/, shortened to 150 ms by windowing the syllable offset, were used in an oddball test paradigm (see electrophysiological measurement section below). These syllables have been used previously [14]. The signals were monaurally presented to the right ear in quiet and in three types of background noise at three different signal-to-noise ratios (SNRs): −3, 3, and 9 dB SNR. These SNRs were chosen because previous work suggested that such a range would show a main effect of SNR in each group of participants [12,15]. Overall, there were 10 conditions: nine were signal-in-noise conditions and one was a signal-in-quiet condition. For every condition, the level of the signal was kept constant at 65 dB SPL.
The three noise types were (1) a continuous speech-spectrum noise or SSC, (2) a one-talker modulated noise or 1TM, and (3) a four-talker babble or 4TB. All noises were low-pass filtered at 4000 Hz. The continuous noise and four-talker babble were used in our previous work [14,16]. The continuous noise was then modulated with the envelope of 10 concatenated Institute of Electrical and Electronic Engineers (IEEE) sentences to create the one-talker modulated noise, which would make for a better representation of modulated noise in the real world instead of a simple interrupted speech noise as used in the study by Billings and colleagues [14]. The one-talker modulated noise also had greater envelope fluctuations than the four-talker babble, which in theory should result in better CAEP responses, allowing a point for comparison.
Electrophysiological measurements
Evoked potentials were recorded using Neuroscan Synamps RT/Scan 4.5 and a 64-channel electrode cap (Electro-Cap International, Inc). A passive oddball paradigm was used for stimulus presentation, with the probability of presentation of the standard /ba/ at 0.8 and the deviant /da/ at 0.2. Two blocks of trials for each condition were completed, totaling 375 trials (75 deviants and 300 standards). Only the responses to standards following identical standards are presented in this article (i.e., only when /ba/ followed another /ba/, or 225 trials per condition). To ensure that responses obtained were free from the interacting effect of age and interstimulus interval [17], a relatively long interval of 1600 ms (offset to onset) was used. The ordering of test conditions was randomized across participants.
Recordings were completed while participants reclined comfortably in an electro-acoustically shielded booth, watching a silent close-captioned movie of their choice. Each block took eight minutes to complete, during which the participants were instructed to ignore the stimuli and minimize head and body movement. Overall, the CAEP visit lasted 3.5 hours including breaks given throughout testing.
The online reference electrode was located at vertex and the ground electrode was placed on the forehead. Waveforms were digitized at 1000 Hz and recorded from 0 to 100 Hz. Recorded responses were further analyzed offline. The waveforms were epoched using a range of 100-ms pre-stimulus period to 1000-ms post-stimulus period. Trials with blink artifacts were corrected using a procedure that calculates the amount of covariation between each evoked potential channel and a vertical eye channel using a spatial filter, in which singular value decomposition is used to remove the blink activity from each electrode on a point-by-point basis to the degree that the evoked potential and blink activity covaried [18]. Sweeps containing voltages exceeding 70 µV were then rejected, and the remaining sweeps were averaged, filtered from 1 Hz to 30 Hz, and re-referenced using an average reference.
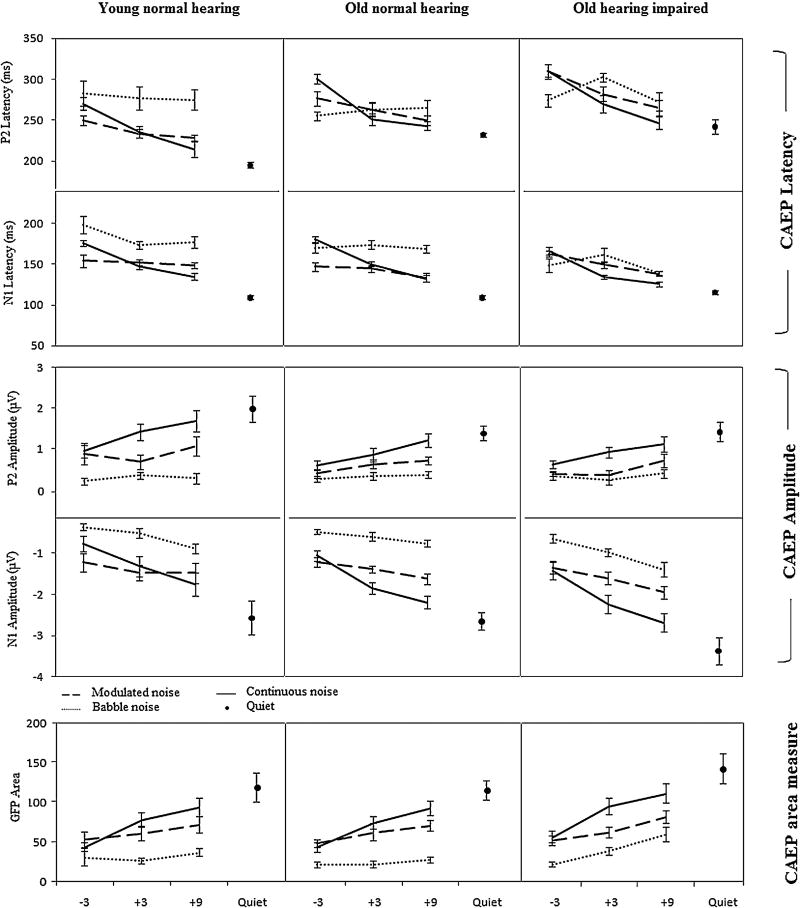
For the purpose of this study, only responses recorded at electrode Cz were analyzed. Based on the CAEP grand average of the 30 participants, we defined P1 and P2 to be the positive peaks that occur prominently within the latency ranges of 40 to 110 ms and 180 to 280 ms respectively, while N1 was defined as the negative peak occurring prominently between 90 ms and180 ms. At −3 dB SNR and for all waveforms recorded in babble noise, 30 ms were added to the allowances used to determine the latency of all of the evoked potentials given the established effect of SNR on CAEPs [2]. The initial peaks were picked automatically by the Neuroscan software which selected the maximum deflection points within the given ranges. Two judges then verified the peaks according to the range defined, temporal electrode inversions, and repetition consistency using odd and even bins. The final latency and amplitude values were decided based on the judges’ agreement. Amplitude was defined as the voltage difference between the peak and the average voltage of the 100-ms pre-stimulus baseline. We also analyzed the rectified area of the waveforms between 30 and 350 ms, providing valuable information on the overall CAEP magnitude for the three subject groups.
Statistical Analysis
A 3 × 3 × 3 analysis of variance for repeated measures was performed, including the variables of Noise Type (SSC, 1TM, and 4TB), SNR (+9, +3 and −3), and the between-subject factor of Group (YNH, ONH, and OHI). The measurements obtained in quiet were not included in the statistical analysis but were included as a visual reference with which to compare latency, amplitude, and area outcomes. The level of significance was set to p = 0.05. Greenhouse-Geisser corrections were used where an assumption of sphericity was not appropriate. Follow-up pairwise comparisons with Bonferroni adjustment were administered to explore the Noise Type × SNR and Noise Type × Group interactions.
Results
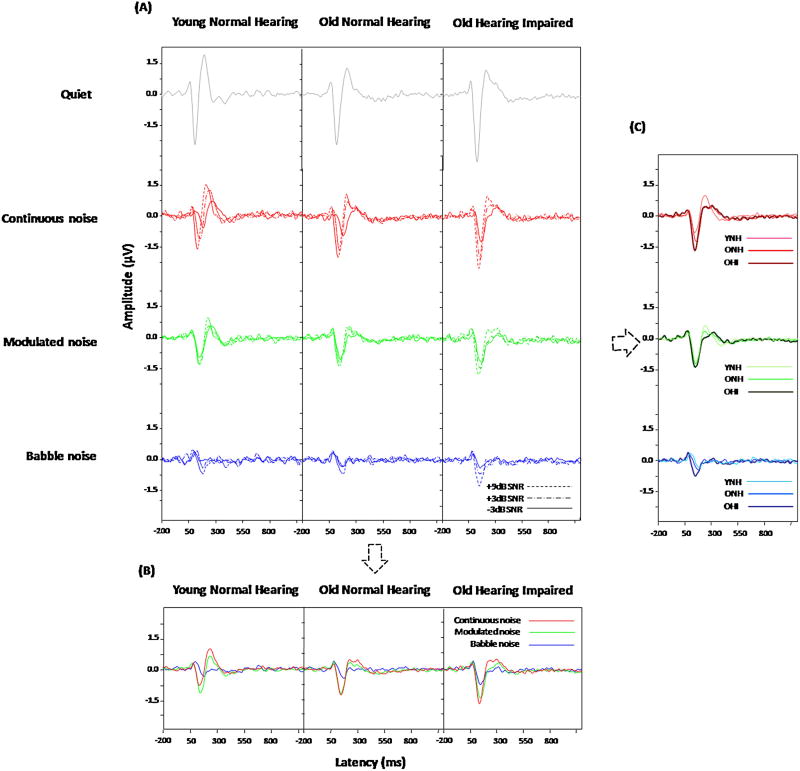
The overall CAEP waveforms recorded at Cz in all listening conditions for all three subject groups are shown in Figure 1A. Generally, when noise was introduced, there was an increase in CAEP latency and a decrease in its amplitude. However, the effect of SNR is not consistent across noise types. The largest SNR effect, showing a systematic decrease in amplitude and increase in latency, can be seen for the continuous noise condition. When collapsed across SNRs, noise type effects (Figure 1B) and group effects (Figure 1C) can be seen. For reference, Appendix A provides the mean and standard deviation of the CAEP latency and amplitude for the three participant groups.
Figure 1.
(A) Grand averaged CAEP waveforms (n=10/condition) for all conditions tested. Group and noise type effects (top/red = continuous; middle/green = modulated; bottom/blue = babble) are displayed as a function of SNR. (B) Noise type effects are shown for each group collapsed across SNR. (C) Group effects are shown for each noise type collapsed across SNR.
A repeated measures ANOVA on CAEP latencies (P1, N1, and P2) revealed main effects of noise type (P1: F(2,48)=20.4, p<.001; N1: F(1.5,32.8)=29.1, p<.001; P2: F(2,42)=9.8, p<.001), SNR (P1: F(2,48)=32.6, p<.001; N1: F(2,44)=43.1, p<.001; P2: F(2,42)=23.5, p<.001), and group (P1: F(2,24)=8.7, p<.001; N1: F(2,22)=14.3, p<.001; P2: F(2,21)=4.5, p=.023) on all CAEP peaks. Significant P1, N1, and P2 latency interactions were found between noise type and SNR (P1: F(4,96)=7.2, p<.001; N1: F(4,88)=9.3, p<.001; P2: F(4,84)=15.9, p<.001) and between noise type and group (P1: F(4,48)=6.0, p=.001; N1: F(2.99,32.8)=7.2, p=.001; P2: F(4,42)=8.4, p<.001), but not between SNR and group. There was a 3-way noise type × SNR × group interaction for the N1 peak only (F(8,88)= 3.0, p = 0.005).
The analysis of CAEP amplitudes also revealed main effects of noise type (N1: F(1.6,34.2)=73.3, p<.001; P2: F(1.4,28.6)=41.6, p<.001), SNR (P1: F(2,48)=5.2, p=.009; N1: F(1.5,32.3)=83.1, p<.001; P2: F(2,42)=18.4, p<.001), and group (N1: F(2,22)=6.7, p=.005) on most peaks, except for P1 noise type effects, and P1 and P2 group effects. Significant noise type × SNR amplitude interactions were found for N1 (F(2.95,69.3)=11.8, p<.001) and P2 (F(4,84)=10.1, p<.001) but not for P1. In addition, a significant group × SNR interaction was found for N1 amplitude only (F(2.93,32.3)= 3.5, p = 0.028). No significant noise type × group or 3-way interactions were found.
With respect to rectified CAEP area for global field power, significant main effects were found for SNR (F(1.5,39.9)=66.6, p<.001) and noise type (F(2,54)=82.5, p<.001) but not for group effects. Interaction effects were only present for the noise type × SNR interaction (F(4,108) = 15.3, p<.001).
A primary focus of this experiment was the possible interaction effects between (1) noise type and SNR and (2) noise type and group. Therefore, additional paired comparisons were completed on the robust N1 and P2 peaks to clarify the interactions that were found. First, to improve our understanding of the noise type × SNR interaction, all data were collapsed across group and an SNR difference score [i.e., the difference between the two extreme SNRs: (9 dB SNR) – (-3 dB SNR)] was then computed for each noise type. Paired comparisons between each combination of the three noise types revealed significant latency and amplitude effects for SSC vs 1TM and SSC vs 4TB using a Bonferroni-corrected alpha level of 0.008 (i.e., six comparisons per peak). For the 1TM vs. 4TB comparison, only P2 latency resulted in a significant effect. Generally, these results indicate that the noise type × SNR interaction is driven primarily by the SSC condition. The second interaction of interest (i.e., noise type × group) was further addressed by collapsing the latency data across SNR and calculating difference scores that approximated a release from masking (SSC – 1TM) and an informational masking effect (SSC – 4TB) for each group. Only latency data were analyzed because interactions were specific to latency. Paired comparisons intended to determine the effects of noise type as a function of age (YNH vs. ONH) and hearing impairment (ONH vs. OHI) were then completed. Using a Bonferroni-corrected alpha level of 0.025 (i.e., two comparisons for each peak), age effects were only seen for P2 latency, and specifically only for the SSC minus 4TB comparison, with the younger group showing a larger difference between noise types. Hearing impairment effects were only seen for N1 latency, and only for the SSC minus 1TM comparison, with those with hearing loss showing a smaller difference between noise types. These results indicate that the noise type × Group interaction was driven by both age and hearing impairment effects. It should be noted that differences between YNH and OHI groups could be important contributors to the interaction; however, they were not compared here given our specific interest in separating out age and hearing impairment effects.
Discussion
The results demonstrate significant effects of noise type, SNR, and group on CAEP amplitudes and latencies. In addition, consistent amplitude and latency noise type × SNR interactions and latency noise type × group interactions were found.
Main effects of Noise Type, SNR, and Group on CAEP
Generally, we found that the largest and earliest CAEP peaks were produced in the continuous noise condition, while the smallest and latest peaks were found in the babble noise condition, with waveform morphology in the modulated noise condition somewhere in between. These results are consistent with the acoustic characteristics of the different noise types. The spectral energy of a continuous speech noise is steady across time with very little fluctuation in temporal envelope or spectral change, resulting in more limited masking effects; whereas, the one-talker modulated noise contains similar spectral energy to the continuous noise with the addition of a very different temporal envelop, resulting in more effective masking due to onsets/offsets similar to the signal. The four-talker babble had variations in both temporal envelope and spectrum, resulting in the greatest similarity to the signals being used, which may explain the poor response waveform morphology in babble noise compared to responses in other types of noises. There may also be cognitive factors at play, resulting in poor waveform morphology in four-talker babble; however, cognitive contributions were likely minimized given that data were collected under passive listening conditions.
Group differences were mainly found for latency measures and were most notable for P2 latencies where morphology of the P2 peak was dramatically weaker for both the older groups than for the younger group. In contrast, N1 peaks appeared later and with poorer morphology for younger and older normal-hearing groups relative to the older group with hearing impairment. While a P2 morphology effect related to age is not well understood, an enhanced N1 in older hearing-impaired individuals may be a result of release from inhibition with these participants [19].
Robust effects of SNR found here, especially for the continuous noise, replicate our previous findings [2,12]. The novelty of this experiment was the combination of noise type and group variables manipulated along with SNR, thereby allowing a more thorough analysis of interaction effects to be completed.
Noise Type × SNR Interaction
The effect of noise type on CAEP varied as a function of SNR. This interaction was explored by reducing the SNR variable to a difference between the extreme SNRs (i.e., 9 and −3) and comparing noise conditions, revealing differences in the SNR effect between continuous noise and modulated or babble noise. This interaction between noise type and SNR is evident in Figure 2 where the SNR slope in the continuous noise condition for both CAEP latency and amplitude was more systematic and followed a linear trend, while the modulated and babble noises showed shallower SNR slopes. For the babble, the shallowness of the slope may have been a function of the relatively weak neural response for all SNR conditions (see Figure 1), which may have produced a floor effect. More favorable SNRs may have revealed a more systematic SNR effect. The lack of an SNR effect in the modulated noise condition may have been due to the large gaps in the one-talker-modulated noise; that is, because the gaps were so long relative to the somewhat short syllable, it appears that large portions of the signal were audible regardless of the level of the modulated noise.
Figure 2.
SNR growth functions as a function of noise type for CAEP latency, amplitude, and area. Slopes of functions are steepest for continuous noise.
Noise Type × Group Interaction
The noise types used in this study provide an examination of various masking effects. While energetic masking produces a predictable interaction between overlapping signal and noise in the cochlea, informational masking causes disruptive interactions that go beyond peripheral interactions alone [5]. A physiological release from energetic masking is best demonstrated in this dataset by comparing responses recorded in continuous noise to those recorded in modulated noise conditions; whereas, informational-like masking may be demonstrated by comparing responses recorded in babble to those recorded in continuous noise. Both masking effects are relatively limited in this dataset. Interestingly, the earlier and more exogenous N1 wave was sensitive to the more peripheral release-from-masking effects and present only as a function of hearing status; whereas, the later P2 wave was sensitive only to higher-level informational-like masking, whose magnitude varied as a function of age. These results are in agreement to some extent with our understanding of N1 and P2 generators. That is, N1 is thought to be primarily an obligatory stimulus-driven response representing the acoustics of the stimulus. In contrast, P2 has been shown to be sensitive to higher-level processing such as those associated with stimulus exposure and learning [20].
Conclusion
Effects of noise type, SNR, age, and hearing status on CAEPS are all demonstrated in this study and are important factors that modulate our listening in noise ability. This study also provides evidence that the SNR effect varies as a function of noise type and is most systematic for continuous noise. Effects of age and hearing loss were limited to CAEP latency and were differentially modulated depending on the noise types that were compared. It is clear that the spectrotemporal characteristics of signals and noises, as well as participant factors such as age and hearing status, contribute to the ability to listen in noise.
Research highlights.
Spectrotemporal content of background noise determines the effect of SNR on CAEPs.
Age and hearing status interact to affect N1 and P2 latency.
Acknowledgments
This work was presented, in part, at the 2014 Society for Neuroscience meeting and was supported by the United States (U.S.) National Institutes of Health (NIDCD-R03DC10914) and by the U.S. Department of Veterans Affairs (RR&D-C8006W). The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government. The authors would also like to acknowledge the efforts and support of Tina Penman, Paul Pendergraft, and Brandon Madsen.
Abbreviations
- CAEP
cortical auditory evoked potentials
- SNR
signal-to-noise ratio
- SSC
continuous noise
- 1TM
one-talker-modulated noise
- 4TB
four-talker babble noise
- YNH
younger-normal hearing individuals
- ONH
older-normal hearing individuals
- OHI
older-hearing impaired individuals
Appendix A
The mean and standard deviation of the CAEP latency and amplitude in all conditions for all subject groups
| Stimulus condition |
GROUP | SNR | CAEP Latency | CAEP Amplitude | GFP Area |
||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| P1 | N1 | P2 | P1 | N1 | P2 | ||||
|
| |||||||||
| In quiet | YNH | 61 (6.9) | 110 (6.7) | 195 (11.2) | 0.60 (0.6) | −2.57 (1.3) | 1.98 (1.0) | 118 (57.4) | |
| ONH | 54 (12.6) | 111 (7.4) | 204 (8.7) | 0.92 (0.6) | −2.66 (0.7) | 1.40 (0.6) | 114 (39.3) | ||
| OHI | 55 (6.9) | 117 (6.8) | 216 (35.0) | 0.74 (0.5) | −3.34 (1.0) | 1.45 (0.7) | 143 (60.4) | ||
|
| |||||||||
| In continuous noise | YNH | −3 | 114 (22.2) | 176 (10.5) | 270 (24.3) | 0.38 (0.2) | −0.77 (0.6) | 0.94 (0.5) | 43 (18.1) |
| +3 | 85 (15.5) | 148 (13.8) | 235 (23.3) | 0.53 (0.4) | −1.31 (0.8) | 1.42 (0.6) | 77 (27.8) | ||
| +9 | 80 (13.3) | 135 (13.7) | 214 (28.8) | 0.48 (0.5) | −1.75 (0.9) | 1.68 (0.8) | 92 (36.4) | ||
| ONH | −3 | 105 (23.4) | 181 (10.7) | 290 (24.0) | 0.32 (0.2) | −1.05 (0.4) | 0.62 (0.4) | 43 (18.7) | |
| +3 | 86 (10.3) | 151 (10.4) | 227 (26.9) | 0.45 (0.2) | −1.84 (0.4) | 0.87 (0.5) | 74 (24.7) | ||
| +9 | 69 (14.4) | 133 (13.1) | 218 (20.7) | 0.68 (0.3) | −2.20 (0.5) | 1.22 (0.5) | 92 (29.0) | ||
| OHI | −3 | 94 (21.7) | 168 (14.8) | 302 (28.3) | 0.38 (0.3) | −1.39 (0.7) | 0.66 (0.3) | 57 (24.8) | |
| +3 | 67 (15.5) | 136 (7.7) | 251 (42.0) | 0.56 (0.2) | −2.20 (0.7) | 0.96 (0.4) | 95 (32.0) | ||
| +9 | 67 (9.3) | 127 (8.4) | 222 (31.3) | 0.61 (0.5) | −2.65 (0.7) | 1.16 (0.6) | 112 (37.9) | ||
|
| |||||||||
| In modulated noise | YNH | −3 | 85 (28.9) | 155 (24.2) | 250 (19.2) | 0.43 (0.5) | −1.22 (0.7) | 0.89 (0.8) | 52 (28.4) |
| +3 | 74 (7.4) | 152 (12.5) | 234 (15.7) | 0.44 (0.3) | −1.47 (0.6) | 0.69 (0.6) | 60 (27.2) | ||
| +9 | 69 (16.5) | 149 (11.3) | 228 (10.6) | 0.38 (0.2) | −1.47 (0.8) | 1.07 (0.8) | 70 (32.6) | ||
| ONH | −3 | 81 (7.9) | 148 (16.3) | 260 (34.0) | 0.36 (0.2) | −1.21 (0.4) | 0.42 (0.3) | 48 (15.2) | |
| +3 | 74 (10.3) | 146 (12.8) | 243 (35.5) | 0.55 (0.3) | −1.39 (0.3) | 0.65 (0.3) | 62 (34.5) | ||
| +9 | 4 (10.2) | 135 (16.5) | 226 (22.6) | 0.58 (0.4) | −1.62 (0.4) | 0.73 (0.3) | 70 (22.4) | ||
| OHI | −3 | 74 (13.6) | 164 (11.1) | 301 (35.4) | 0.43 (0.2) | −1.32 (0.5) | 0.44 (0.2) | 52 (20.6) | |
| +3 | 74 (14.8) | 150 (13.0) | 266 (34.1) | 0.58 (0.3) | −1.58 (0.5) | 0.41 (0.4) | 63 (21.6) | ||
| +9 | 64 (14.6) | 139 (9.4) | 245 (36.0) | 0.62 (0.4) | −1.92 (0.5) | 0.76 (0.5) | 82 (25.8) | ||
|
| |||||||||
| In babble | YNH | −3 | 106 (20.4) | 89 (11.3) | 283 (37.6) | 0.52 (0.5) | −0.35 (0.2) | 0.22 (0.2) | 30 (34.3) |
| +3 | 105 (19.3) | 174 (13.9) | 277 (42.9) | 0.44 (0.3) | −0.49 (0.4) | 0.36 (0.2) | 25 (12.5) | ||
| +9 | 89 (11.3) | 177 (23.9) | 275 (40.0) | 0.60 (0.4) | −0.87 (0.4) | 0.29 (0.4) | 36 (16.0) | ||
| ONH | −3 | 87 (14.0) | 171 (17.9) | 233 (21.4) | 0.45 (0.3) | −0.47 (0.2) | 0.29 (0.2) | 21 (12.2) | |
| +3 | 83 (13.9) | 175 (15.5) | 243 (27.8) | 0.54 (0.3) | −0.58 (0.3) | 0.35 (0.3) | 21 (12.7) | ||
| +9 | 76 (12.9) | 170 (14.8) | 245 (33.9) | 0.49 (0.2) | −0.75 (0.3) | 0.39 (0.2) | 27 (12.5) | ||
| OHI | −3 | 87 (12.7) | 150 (25.8) | 256 (31.1) | 0.45 (0.3) | −0.62 (0.4) | 0.38 (0.3) | 22 (8.2) | |
| +3 | 73 (11.0) | 162 (27.8) | 292 (21.6) | 0.61 (0.3) | −0.96 (0.3) | 0.29 (0.4) | 39 (13.9) | ||
| +9 | 66 (12.3) | 140 (8.1) | 255 (45.7) | 0.48 (0.5) | −1.37 (0.5) | 0.44 (0.3) | 60 (28.1) | ||
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnstone PM, Litovsky RY. Effect of masker type and age on speech intelligibility and spatial release from masking in children and adults. J. Acoust. Soc. Am. 2006;120:2177. doi: 10.1121/1.2225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billings CJ, Tremblay KL, Stecker GC, Tolin WM. Human evoked cortical activity to signal-to-noise ratio and absolute signal level. Hear. Res. 2009;254:15–24. doi: 10.1016/j.heares.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Androulidakis S. AG and Jones, Detection of signals in modulated and unmodulated noise observed using auditory evoked potentials. Clin. Neurophysiol. 2006;117:1783–1793. doi: 10.1016/j.clinph.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Bennett KO, Billings CJ, Molis MR, Leek MR. Neural Encoding and Perception of Speech Signals in Informational Masking. Ear Hear. 2012;33:349–366. doi: 10.1097/AUD.0b013e3182597b2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Festen JM, Plomp R. Effects of fluctuating noise and interfering speech on the speech-reception threshold for impaired and normal hearing. J. Acoust. Soc. Am. 1990;88:1725–1736. doi: 10.1121/1.400247. [DOI] [PubMed] [Google Scholar]
- 6.Stanley R, Tun PA, Brownell H, Wingfield A. Hidden costs of effortful listening on speech comprehension. J. Commun. Res. 2012;4:157–180. [Google Scholar]
- 7.Helfer KS, Freyman RL. Aging and speech-on-speech masking. Ear Hear. 2008;29:87–98. doi: 10.1097/AUD.0b013e31815d638b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summers V, Molis MR. Speech recognition in fluctuating and continuous maskers: effects of hearing loss and presentation level. J. Speech. Lang. Hear. Res. 2004;47:245–56. doi: 10.1044/1092-4388(2004/020). [DOI] [PubMed] [Google Scholar]
- 9.Willot J. Aging and the auditory system: Anatomy, physiology and psychophysics, 1991 [Google Scholar]
- 10.Cranford JL, Martin DR. Age-related changes in binaural processing:I. Evoked potential findings. Am. J. Otol. 1991;12:357–364. [PubMed] [Google Scholar]
- 11.Bertoli S, Smurzynski J, Probst R. Effects of Age, Age-Related Hearing Loss, and Contralateral Cafeteria Noise on the Discrimination of Small Frequency Changes: Psychoacoustic and Electrophysiological Measures. Jaro. 2005;222:207–222. doi: 10.1007/s10162-005-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billings CJ, Penman TM, McMillan GP, Ellis EM. Electrophysiology and Perception of Speech in Noise in Older Listeners: Effects of Hearing Impairment and Age. Ear Hear. 2015;36:710–22. doi: 10.1097/AUD.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salisbury DF, Desantis MA, Shenton ME, McCarley RW. The effect of background noise on P300 to suprathreshold stimuli. Psychophysiology. 2002;39:111–5. doi: 10.1017/S0048577202010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billings CJ, Bennett KO, Molis MR, Leek MR. Cortical encoding of signals in noise: effects of stimulus type and recording paradigm. Ear Hear. 2011;32:53–60. doi: 10.1097/AUD.0b013e3181ec5c46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billings CJ, McMillan GP, Penman TM, Gille SM. Predicting Perception in Noise Using Cortical Auditory Evoked Potentials. J. Assoc. Res. Otolaryngol. 2013;14:891–903. doi: 10.1007/s10162-013-0415-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett K, Billings C, Molis M, Leek M. Neural encoding and perception of speech signals in informational masking. Ear Hear. 2012:349–366. doi: 10.1097/AUD.0b013e31823173fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tremblay KL, Billings C, Rohila N. Speech evoked cortical potentials: Effects of age and stimulus presentation rate. J. Am. Acad. Audiol. 2004;15:226–237. doi: 10.3766/jaaa.15.3.5. [DOI] [PubMed] [Google Scholar]
- 18.Neuroscan, SCAN 4.4 - Vol. I. I. Offline Analysis of Acquired Data (Document Number 2203, Revision E) Charlotte, NC: Compumedics Neuroscan; 2007. pp. 141–148. [Google Scholar]
- 19.Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J. Neurosci. 2005;25:3908–18. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tremblay KL, Ross B, Inoue K, McClannahan K, Collet G. Is the auditory evoked P2 response a biomarker of learning? Frontiers Neurosci. 2015 [Google Scholar]