Abstract
The studies reported here were motivated by the desire to probe the extent to which benzynes, one of the classical reactive intermediates in organic chemistry, would react in discriminating fashion with benzyne trapping reagents that contain multiple functional groups and, accordingly, possible modes and sites of reactivity. We deemed that natural products comprise a palette of potential multifunctional compounds with which to address this question. We show that benzynes produced by the hexadehydro-Diels-Alder (HDDA) reaction react with many secondary metabolites with a high preference for one among several potential pathways. Examples demonstrating such selectivity include reactions with: phenolics, through dearomatizing ortho-substitution; alkaloids, through Hofmann-type elimination; tropolone and furan, through cycloaddition; and alkaloids, through three-component fragmentation-coupling reactions. We also demonstrate that the cinchona alkaloids quinidine and quinine give rise to products, some in as few as three steps, that enable subsequent and rapid access to structurally diverse, polyheterocyclic compounds. The results show that benzynes are quite discriminating in their reactivity—a trait perhaps not broadly appreciated.
Nature provides countless examples of complex small molecules in the form of secondary metabolites collectively produced by living organisms. These natural products comprise a rich resource from the perspectives of both structural complexity and functional group diversity. Over 200,000 such substances have been characterized to date. Of growing importance are methods for chemically modifying natural products through a reaction that produces a relatively small change in structure (e.g., C–H hydroxylation1, hetero-Diels-Alder cycloaddition2, O–H functionalization3, arene fluorination4, arene bromination5, C–H carbenoid insertion6, C–H azidation,7 C–H amination8 ). The primary motivation for pursuing such methodology has often been to probe the structure-activity profile of a natural substance having a preexisting, valuable biological property. In contrast, chemical transformations that result in more substantial degrees of structural modification, via skeletal rearrangement and/or reorganization for example, are relatively rare9. With the goal of using benzynes that bring considerable structural complexity in their own right to effect such derivatizations, we set out to explore aspects of the reactivity of polyfunctional natural products with some of these reactive intermediates. Efficient processes that enable such substantial reorganization would allow rapid access to compounds having structural motifs that would be otherwise extremely challenging to synthesize. Additionally, we embarked on this study (i) to gain new insights about benzyne chemoselectivity and (ii) to discover new modes of benzyne reactivity. From the outset we viewed the natural product as the derivatizing agent (of the benzyne), rather than the agent being derivatized (by the benzyne). This contrasts with more common approaches in which the secondary metabolite is derivatized by the action of a derivatizing agent or process like those mentioned above1–8.
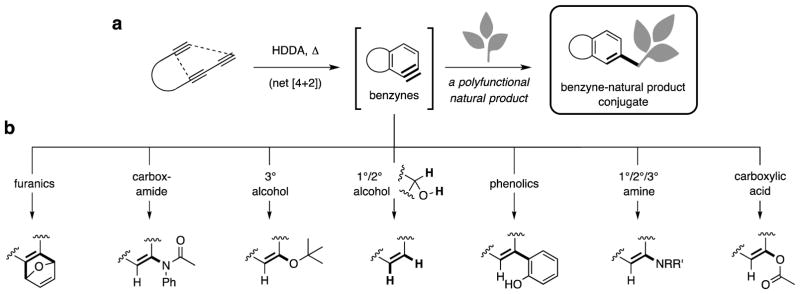
The hexadehydro-Diels–Alder (HDDA) cycloisomerization reaction of tri- or tetrayne substrates produces fused, bicyclic benzyne derivatives (brackets, Fig. 1a). Benzynes can be trapped in myriad ways, nearly all initiated through attack by a nucleophile at the more electrophilic benzyne carbon atom (Fig. 1b). Even though benzynes (and arynes more broadly) are highly reactive intermediates, such species can still exhibit a high degree of preference for one among several modes of reaction.10 Here we show that HDDA-generated benzynes11 can be captured by many structural classes of natural products in single-operation, reagent- and catalyst-free processes that produce conjugates having substantially increased structural complexity (Fig. 1a). We also show that, in many instances, these reactions proceed with surprisingly high degrees of chemoselectivity12.
Figure 1. The two-stage hexadehydro-Diels-Alder (HDDA) cascade.
Thermal activation of appropriate triyne substrates leads, through initial (and rate limiting) cycloisomerization, to highly reactive benzyne intermediates (stage I) that efficiently engage trapping agents (stage II) by reaction with a variety of common functional groups. a, This work: the benzyne trapping agents comprise various, multifunctional natural products that present multiple potential sites for reaction. b, Representative, known examples of trapping reactions of HDDA-benzynes11 with common functional groups.
We selected natural products that contained at least one principle functional group known to productively engage a HDDA benzyne. Our choice of benzynes was guided by two requirements: for any successful HDDA cascade, the trapping agent (i) must not react with the substrate triyne faster than its rate of cyclization to the benzyne and (ii) must engage the benzyne faster than the benzyne reacts with itself, solvent, or additional triyne substrate. To date, we have observed successful HDDA cascades with triyne (or tetrayne) substrates having over 20 different linkers that tether the 1,3-diyne and diynophile units. These fall into two broad classes differing in the nature of the tether—those with and those without a conjugated carbonyl functional group (ketone, ester, or amide). For example, compare 1 and 25 to 6 and 55. The HDDA cascades reported here are not meant to be exhaustive with the choice of either the benzyne or the NP; they only scratch the surface of possibilities.
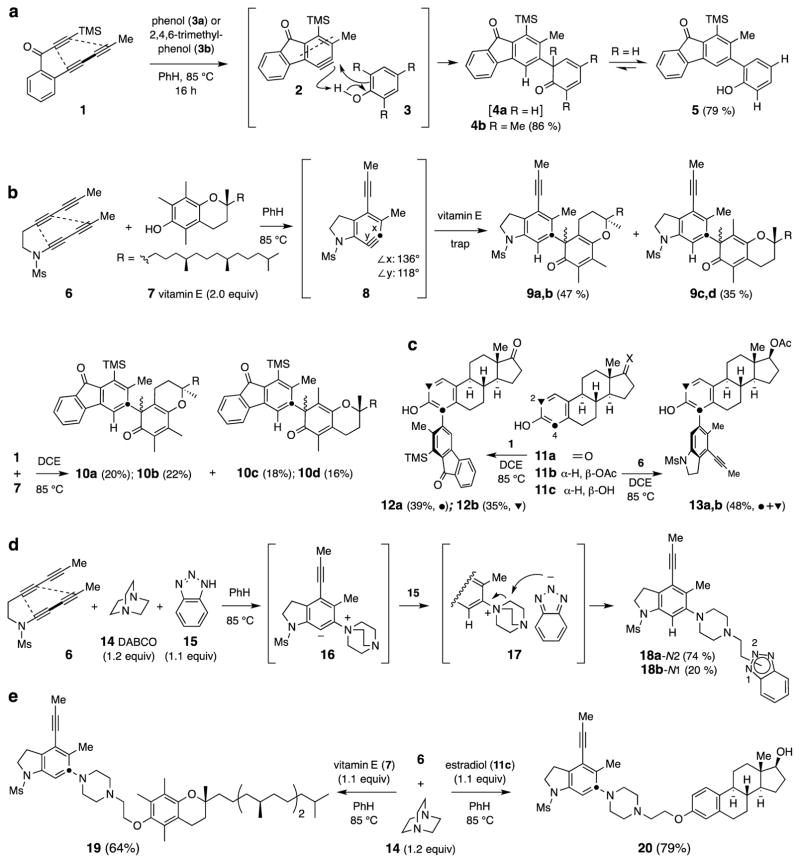
Trapping with phenolics (Fig. 2)
Figure 2. Different modes of reaction between a benzyne (2 or 8) and various phenolics.
Phenols react with HDDA benzynes by either a phenol-ene type of process16 or, in the presence of a tertiary amine that outcompetes this ene reaction, by providing a proton that then leads to a three-component reaction. a, Phenol (3a) or 2,4,6-trimethylphenol (3b) is trapped by benzyne 2 through a phenol-ene-like reaction. b, Vitamin E (7) is engaged by both benzynes 8 and 2 in the same manner; the internal bond angles x and y at the sp-hybridized carbon atoms in benzyne 8 were computed by density functional theory (DFT, M06-2X/6-31+G**). c, Estradiol derivatives 11a and 11b are trapped by 2 and 8, respectively, to produce phenolic ene adducts 12 and 13. d, In a three-component process initiated by attack of 1,4-diazabicyclo[2.2.2]octane (DABCO, 14), zwitterion 16 is protonated by, in this case, benzotriazole (15); ring-opening within the ion pair 17 gives 18. e, In the presence of DABCO, vitamin E (7) [or estradiol (11c)] plays a new role, that of proton-donor and nucleophile to capture the more rapidly formed zwitterion 16; ring-cleavage of the quaternary ammonium ion (cf. 17) by the phenoxide counterion then gives 19 (or 20).
Because HDDA-derived benzynes are produced thermally, they engage in their subsequent trapping reactions in the absence of the reagents and byproducts that are necessarily associated with the generation of benzynes by classical methods13,11. This circumstance can result in new modes of trapping reactions compared with those seen in traditional benzyne chemistry11,14,15. One example is the trapping by neutral phenols11,16. Under classical conditions, often in the presence of basic reagents, phenols typically form adducts with benzynes by nucleophilic attack through the oxygen atom to produce phenoxy ether products17 and only rarely by predominant functionalization at the ortho carbon atom18. Phenol (3a) traps the thermally generated benzyne 2 (derived from heating triyne 1) in a fashion we reported previously11—namely, by adding through its ortho-carbon atom to efficiently give the adduct 5 (after rearomatization of the dienone intermediate 4a, Fig. 2a). Consistent with a mechanistic formulation that involves a phenolic-ene type process (cf. 2 + 3), trapping by 2,4,6-trimethylphenol (3b) leads in high yield to a non-enolizable dienone, 4b—an unprecedented type of transformation. Yield data, throughout, represent the amount of product obtained following chromatographic purification.
The tetrayne 6 undergoes HDDA cyclization highly regioselectively19 to produce only the benzyne 8 (Fig. 2b). Because the HDDA reaction to form the benzyne is the rate limiting step in all HDDA cascades, the conditions for all of the reactions using tetrayne 6 as substrate (Figs. 2b–d and 3–6) were identical (see Methods Summary below). We have computed [density functional theory (DFT), M06-2X/6-31+G**] an optimized geometry for 8 and observed it to have a distorted geometry, as indicated by the angles shown in Fig. 2b20,21. Accordingly, we were not surprised to observe that trimethylphenol (3b) had engaged the more electrophilic carbon atom in benzyne 8 (•) to give a single 2,4,6-trimethylcyclohexadienone-bearing adduct (71%, see 103 in SI).
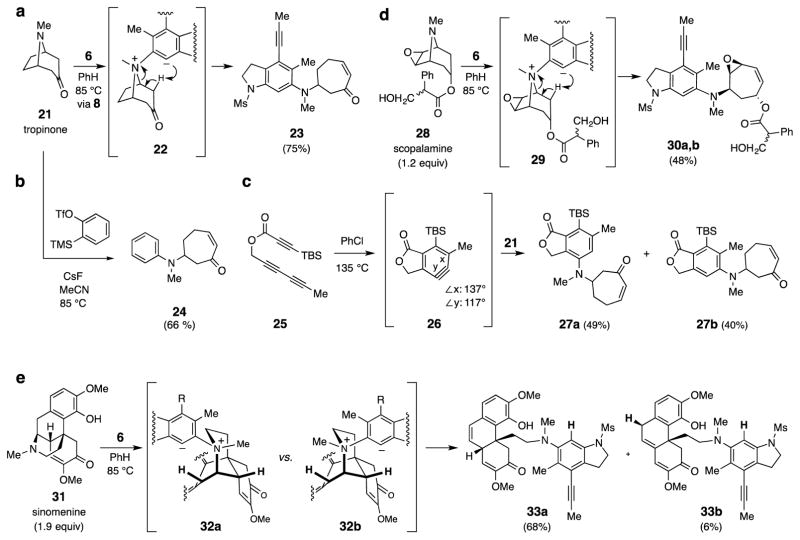
Figure 3. Reactions with tertiary amines.
Alkaloids with tertiary amines are fragmented via Hofmann-type elimination reactions. a, b, c, Tropinone (21) reacts with 6 (via 8), o-benzyne, or 25 (via 26) to give adduct 23, 24, or 27a–b; each of these products contains a substituted cycloheptenone, a relatively rare substructural moiety, that is functionalized in a manner that allows for subsequent chemical modification. d, Scopolamine (28) and 8 give diastereomeric adducts 30a and 30b (ca. 1:1) via zwitterion 29; this shows the compatibility of alcohols as well as vinylic epoxides in the reaction medium. e, Sinomenine (31) and 8 give the isomeric dienone adducts 33a and 33b via the diastereomeric 1,3-zwitterions 32a and 32b, respectively.
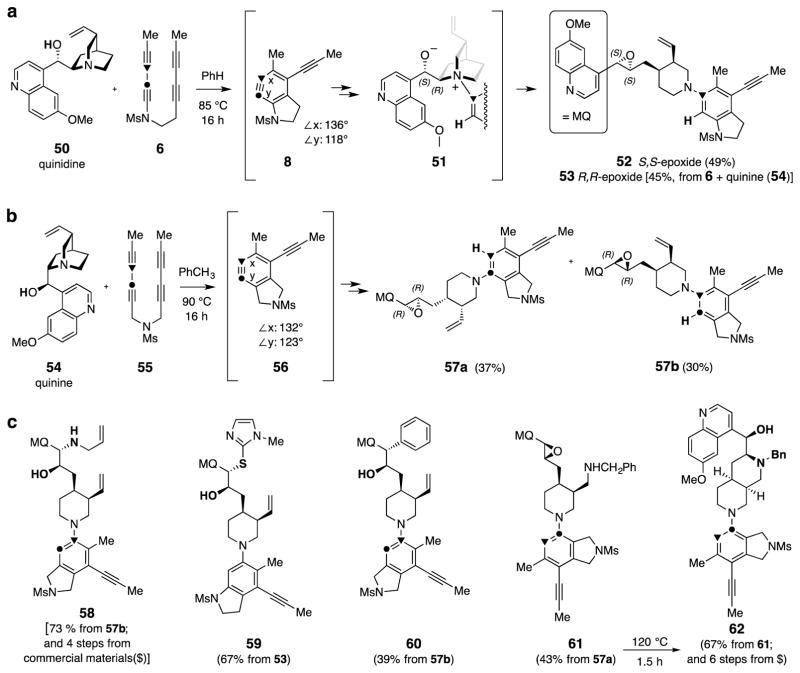
Figure 6. Quinidine (50) and quinine (54) derivatizations via benzynes 8 and 56.
Cinchona alkaloids present at least six different sites for reaction with an HDDA benzyne, so it is remarkable that they react largely through a single mode. a, Quinidine (50) is trapped by the distorted benzyne 8 to give the epoxide 52 as the predominant product, presumably through the intermediate 51; similarly quinine (54) leads to the bis-epimeric epoxide 53 (not explicitly shown, see SI). b, Quinine traps the less distorted benzyne 56 competitively at both benzyne carbon atoms to give the (easily separable) constitutional isomers 57a and 57b. c, Examples of products having diverse substructural units that arise from straightforward modification of 57b (58 and 60), 53 (59), or 57a (61 and 62).
Vitamin E (7) is a naturally occurring phenol that lacks aromatic hydrogens at positions ortho to the hydroxyl. When 7 was heated with 6 (Fig. 2b), an analogous process led to the adducts 9a–d in a combined yield of 82%. This reaction was not unique to this pair of reactants. The triyne 1, via benzyne 2, also efficiently engaged vitamin E (7) to give the four separable isomers of ene products 10a–d (76%). Estrone (11a), having the unsymmetrical C3-hydroxylated A-ring, was trapped by benzyne 2 to give the constitutionally isomeric biaryls substituted at C4 (12a, ❻, 39%) and C2 [12b, ❼, not shown (see SI), 35%]. Estradiol-17O-acetate (11b) reacted with benzyne 8 in a similar fashion to produce the adducts 13a (❻) and 13b [❼, not shown, (see SI)] in a combined yield of 48%. In contrast, reaction between vitamin E (7) and o-benzyne [1.5 equiv, generated from 2-(trimethylsilyl)phenyl triflate and CsF22 ] gave a complex mixture of at least five products, most incorporating two benzyne-derived subunits (LCMS). A different mode of reaction was seen when estrone (11a) was reacted with o-benzyne—namely, estrone phenyl ether (see 104, Supplementary Information) was cleanly produced (92%). These outcomes serve to emphasize the differences in reaction pathways that can be seen with benzynes used under basic (F−) vs. neutral (HDDA) conditions.
In a different vein and prior to studying reactions of HDDA-derived benzynes with alkaloids containing bridgehead nitrogen atoms, we observed that 1,4-diazabicyclo[2.2.2]octane (DABCO, 14), in the presence of a sufficiently acidic third reactant such as benzotriazole (15), will engage benzyne 8 in a three-component coupling reaction (Fig. 2d). When 6 was heated in the presence of only a slight excess of both 14 and 15, the piperazine-containing adducts 18a and 18b were formed in good yield. This presumably arises by way of sequential formation of the 1,3-zwitterion 16 and ammonium-benzotriazolide ion pair 17, which finally undergoes nucleophilic opening of one of its three equivalent bridges. This can occur through the N1- or N2-position of the benzotriazolide anion, which gives rise to the isomers 18a or 18b, respectively.
We then explored an analogous three-component reaction involving phenolic natural products. When tetrayne 6 was heated in the presence of DABCO (14) and vitamin E (7) (Fig. 2e, left) 19 was formed in good yield. Estradiol (11c), a substrate with both phenol and alcohol hydroxyl groups, was also examined. It formed only the 1:1:1 adduct 20 in a highly chemoselective reaction. It is notable that the formation of the three-component products 18a–b, 19, and 20 (Figs. 2d and 2e) is efficient even when a nearly stoichiometrically balanced set of the three reactants is used. Many multicomponent reactions require the use of at least one reactant in substantial excess, precluding application to trios of reactants where each is precious.
Trapping with alkaloids via Hofmann elimination (Fig. 3)
The ready participation of DABCO in these processes encouraged us to explore alkaloidal natural products having tertiary basic amine groups. When tropinone (21, 1.5 equivalents, Fig. 3a) and tetrayne 6 were heated, the 1:1 adduct (±)-23 was efficiently formed (75%). This presumably arises through the intermediacy of zwitterion 22, which undergoes a Hofmann-type fragmentation to give the cycloheptenone 23. This mode of reactivity is not unique to HDDA generated benzynes. When o-benzyne22 was heated in the presence of tropinone (21), the analogous 1:1 adduct (±)-24 was cleanly formed (66%).
In the course of this work, we observed that trapping of HDDA precursors containing electrophilic ynones and ynoates with tertiary amines is sometimes inefficient, presumably due to deleterious events initiated by conjugate addition pathways. However, by a slight redesign of the HDDA substrate, this problem can be mitigated. For example, the triyne 25 contains a bulky tert-butyldimethylsilyl (TBS) substituent at the terminal carbon of the ynoate, which renders it compatible with such amines. Thus, when 25 was heated in the presence of tropinone (21), the constitutional isomers 27a (49%) and 27b (40%) were formed. The near absence of regioselectivity is surprising based on the extent of distortion; benzynes 26 and 8 each show a similarly large deviation of their internal bond angles x and y (DFT). The different outcomes (i.e., a single isomer in Fig. 3a vs. ca. 1:1 ratio of isomers in Fig. 3c) are likely due to the difference in size of the flanking substituents in 8 vs. 26, having large methanesulfonamide vs. small methylene groups, respectively.
The acidity of the protons alpha to the ketone in 21 is not a requirement for this Hofmann-like elimination. Thus, scopolamine (28, Fig. 3d) gave adducts 30a and 30b (via 29) as a 1:1 mixture of separable diastereomers. The epoxide, even with its allylic activation; the primary alcohol15; and the ester functionality all remain intact in the product 30. The more complex alkaloid sinomenine (31) was trapped by benzyne 8 to give the separable isomeric dienones 33a and 33b in a combined yield of 74% (Fig. 3e), likely via fragmentation of epimers 32a and 32b, respectively23,24.
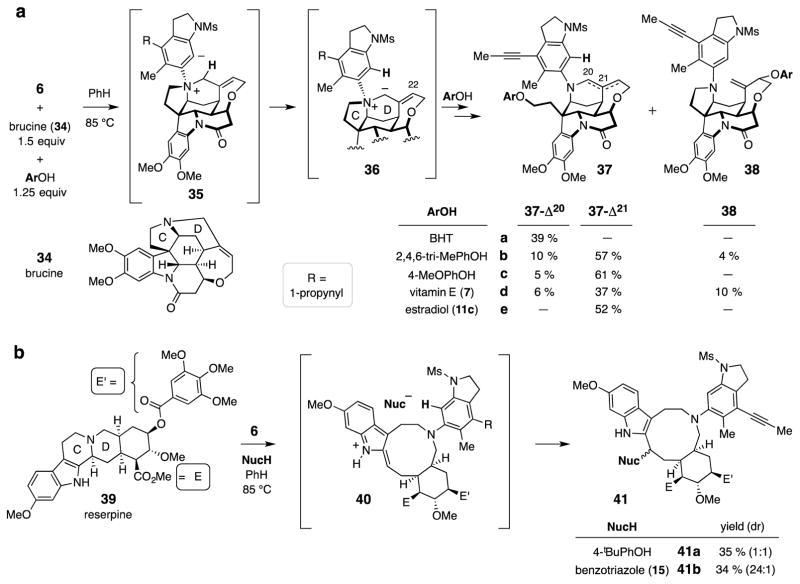
Trapping with brucine and reserpine (Fig. 4)
Figure 4. Three-component reactions involving complex alkaloids.
Three component reactions similar to those described above for DABCO (Fig. 2d and 2e) can be extended to structurally complex tertiary amine nitrogen centers such as those present in brucine (34) and reserpine (39). a, Brucine (34) and one of various phenols give adducts 37-Δ20 and/or 37-Δ21 (and 38) via 35 and the allylic aza-ylide 36. b, The HDDA-benzyne activates the CD-fusion bond in reserpine (39) toward cleavage; the iminium ion 40 is then trapped by the in situ-generated Nuc− counterion from a phenol or benzotriazole to produce the azecane derivative 41.
The proclivity of zwitterionic intermediates such as those in Fig. 3 to preferentially undergo Hofmann-type fragmentations led us to explore alkaloid substrates containing a bridgehead nitrogen. That arrangement should preclude the elimination pathway, thereby affording the opportunity for three-component couplings. When tetrayne 6 was heated in the presence of both brucine (34, 1.5 equiv, Fig. 4a) and 3,5-bis-tert-butyl-4-hydroxytoluene (BHT, 1.25 equiv), a single three-component adduct 37a-Δ20 was isolated. To our surprise, alkene migration had occurred25; presumably the initial zwitterion 35 had undergone intramolecular proton transfer to form the unusual aza-allyl ylide 36, which was then protonated by the hindered BHT at its more sterically accessible C22. The resulting ion pair then cleaved the C-ring C–N bond. Use of less hindered phenols in place of BHT produced varying amounts of the isomeric alkenes 37-Δ20 and 37-Δ21. In the case of 2,4,6-trimethylphenol, a small amount of the allylic aryl ether 38b, arising from SN2′ opening of ring-D, was observed. Finally, two different natural products, brucine and a phenol derivative, could be amalgamated into a single three-component product: vitamin E (7) gave rise to 37d-Δ20, 37d-Δ21, and 38d while estradiol (11c) afforded only 37e-Δ21.
We next considered members of the yohimbans family—another well-known class of alkaloids that contain a bridgehead nitrogen atom. For example, when the benzyne precursor 6 was heated in the presence of both reserpine (39, 1.5 equiv) and 4-tert-butylphenol (1.25 equiv), a mixture of diastereomeric indoloazocines 41a was produced in a combined yield of 35% (Fig. 4b). These presumably arose via electrophilic activation of the basic nitrogen atom followed by cleavage of the C/D ring fusion bond26,27. The iminium ion 40 was then trapped by the phenoxide counterion. Use of benzotriazole as the Nu–H component resulted in formation of the indole/indoline/triazole/azecane-containing adduct 41b (34%). This reaction proceeded with a very high level of diastereoselectivity; trapping of iminium ion 40 is presumably sensitive to the steric bulk of the trapping nucleophile.
The use of near equimolar loading for each of the seven three-component reactions presented in Fig. 4 is noteworthy. These examples demonstrate the enabling power of using a HDDA cascade to fuse three separate molecules, each of considerable structural complexity.
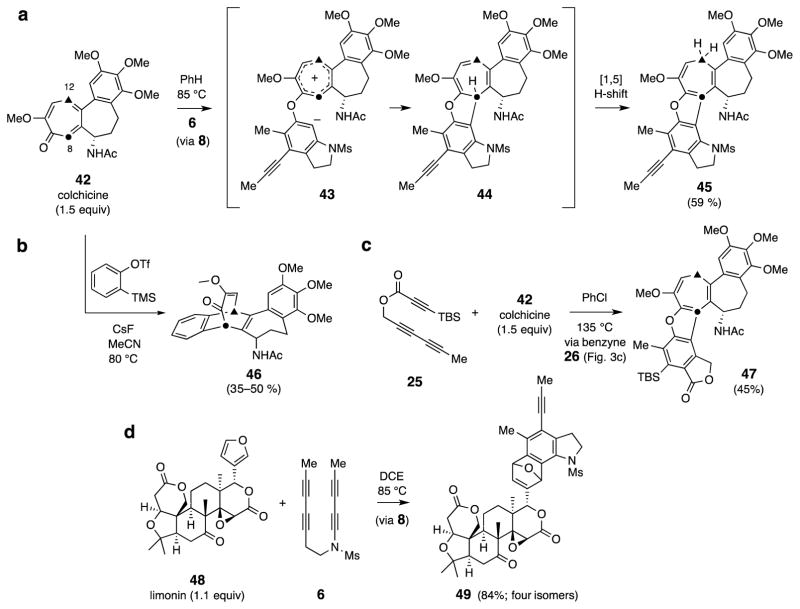
Trapping via [8+2] or [4+2] cycloaddition (Fig. 5)
Figure 5. Net cycloaddition reactions between benzynes and conjugated π-systems.
The cycloheptatrienone in colchicine (42) reacts in an unexpected fashion with a HDDA benzyne, whereas the furan in limonin (48) behaves in a well-precedented29 mode. a, Tropolone-containing colchicine (42) and the HDDA-benzyne 8 gives the all-fused hexacycle 45. b, Colchicine and o-benzyne [via Kobayashi generation22] leads, instead, to a product, 46, having a bridged motif. c, Colchicine and triyne 25 gives the benzofuran 47 (via benzyne 26). d, The furan in limonin (48) is efficiently trapped by the benzyne 8 to give the DA cycloadducts 49.
We have also explored some net cycloaddition reactions (Fig. 5). Colchicine (42) is known to undergo [4+2] cycloaddition reactions with many dienophiles across the 1,3-diene subunit terminated by carbons C8 and C12 2,28. However, when heated in the presence of the HDDA substrate 6, colchicine (1.5 equiv) was engaged by benzyne 8 in a new type of tropolone cycloaddition, giving rise to the benzofuran derivative 45 in good yield (59%, Fig. 5a). This is a formal 8π + 2π cycloadduct that can be viewed as proceeding either by a discrete tropylium-containing zwitterion 43 or via an asynchronous concerted process, presumably with a transition state geometry having that character. The primary adduct 44 has undergone rearrangement via [1,5]-hydrogen atom shift en route to the more stable fused benzofuran 45. We also examined the reaction of 42 with (symmetrical) o-benzyne itself22 and, interestingly, observed an entirely different reaction course—namely, the formation of the [4+2] adduct 46 (Fig. 5b). The contrasting behavior for these two benzynes perhaps results from the unpolarized, symmetrical nature of the latter vs. the polarized, distorted character of 8. This difference in the course of the reaction vis-à-vis o-benzyne is not unique to 8. When triyne 25 was heated in the presence of colchicine (1.5 equiv), again, a benzofuran adduct, 47, was formed from benzyne 26 (Fig. 5c). Finally, furans are classic dienes used for aryne trapping29. Heating 6 in the presence of the naturally occurring furan limonin (48, 1.1 equiv, Fig. 5d) gave, in excellent overall yield (84%), the mixture of isomeric [4+2] adducts 49.
Trapping with quinidine and quinine (Fig. 6)
The significant degree of selectivity observed for many of the reactions already described encouraged us to explore a substrate possessing an even greater number of potential sites and/or modes of reactivity. Quinidine (50) and quinine (54) both meet this criterion. Engagement with a benzyne could occur via: the quinoline nitrogen30, insertion into the hydroxylic O–H bond15, dihydrogen transfer from either the secondary alcohol or one of the (four) pairs of eclipsed H–CC–H units15, Alder ene reaction of the alkene31, 4π+2π cycloaddition with the anisole-like moiety32, or nucleophilic attack by the bridgehead tertiary amine (Figs. 2–4). When 6 was heated in the presence of quinidine (1.5 equiv), remarkably, even with this wide array of possibilities, trapping proceeded to generate a single isolable material, the epoxide 52 in 49% yield (Fig 6a)33. This presumably arose via initial attack on the benzyne 8 by the tertiary amine in 50 followed by proton transfer, now intramolecularly from the alcohol hydroxyl group, to give the zwitterion 51. Collapse of this β-oxido ammonium ion through C–N bond cleavage led to the trans-substituted [Jvic-epox = 1.8 Hz34 ], S,S-epoxide 52. Likewise, quinine gave the analogous R,R-epoxide 53 (45%, Fig. 6a; 2.5 mmol scale).
The degree of selectivity exhibited by the transformations shown in Fig. 6a leads us to suggest that benzynes should be considered as one of the more discriminating of all reactive intermediates. That is, they can preferentially exit even a complex reaction manifold of possible trapping events largely via one channel. Indeed, this feature may be responsible for the remarkable versatility (and preparative value) that has been a hallmark of aryne chemistry from its outset35,36.
With utility in diversity-oriented synthesis approaches in mind, we also examined the reaction of quinine (54) with the symmetrical tetrayne 55, a substrate that can be prepared in two reactions. The derived benzyne 56 often undergoes nucleophilic capture competitively at both benzyne carbon atoms,19 consistent with the relatively small difference between its two internal bond angles (DFT)37. When 55 (1.2 g, 5 mmol) was heated in toluene with 54 (1.5 equiv), indeed, a (separable) mixture of adducts 57a and 57b was cleanly formed (67% combined, Fig. 6b). Although the details are beyond the scope of what is presented here, the benzyne 56 was reacted with most of the natural products described in Figs. 2–5. It consistently engaged in the same mode of reactivity, but, as with the outcome to produce both 57a and 57b, led to a mixture of various ratios of constitutional isomers because of the inherently low degree of selectivity in the trapping of 56. o-Benzyne itself reacts with members of the quinine family in the same fashion. For example, hydroquinine produced the N-phenyldihydro analog of 57 in good yield (76%, see 107 in SI).
The array of orthogonally reactive functional groups in the readily available adducts 52, 53, and 57 makes each well suited for additional diversifying derivatizations. Examples of such transformations are shown in Fig. 6c. These demonstrate: (i) epoxide ring-opening by nucleophiles (58, 59, and 60), (ii) successive oxidative cleavage and reductive amination of the vinyl group (61), (iii) installation of an additional heterocyclic appendage (59), and (iv) de novo construction of a new heterocycle (61 to 62). The structures represented in Fig. 6c are reminiscent of the multiply linked, mono- and bi-heterocyclic motifs found in many kinase inhibitor lead compounds and drugs.38 Myriad variations and combinations of these themes can be imagined.
Conclusion
By way of example, we have shown that HDDA-derived benzynes can be captured by complex reaction partners belonging to a variety of different classes of secondary metabolites. These trapping agents typically present multiple potential reaction sites or modes, yet the processes often occurred with remarkable levels of selectivity. This underscores the fact that reactive intermediates of the aryne family are quite discriminating.10 These reactions proceed under mild conditions, often with close to equimolar loading and perfect atom economy, and give natural product derivatives that have been extensively modified in their topology. We have also demonstrated that the products of some of these HDDA cascades can be modified in straightforward fashion to achieve further diversification. The majority of all the new compounds reported here were produced in just 3–4 chemical transformations (and none more than 6) from commercially available chemicals. We also note that numerous other triyne HDDA-substrates, differing in the constitution of the linker atoms that tether the 1,3-dyne and diynophile subunit11,37, could be used to confer additional variety to the molecular structures arising from this approach. Overall, this work provides a new strategy for capitalizing upon nature’s bounty of structurally fascinating and complex secondary metabolites.
Methods
General procedure for the HDDA-trapping reactions of natural products
An oven-dried culture tube containing the tetrayne precursor in organic solvent (initial concentration of 0.02–0.03 M) and the indicated number of equivalents of trapping component(s) was closed with a Teflon-lined cap and the solution was heated at 85–90 °C for 16 h. The half-life for conversion of each of the polyynes used here to the corresponding benzyne was ca. 3–4 h. The product(s) was(were) separated/purified by chromatography on silica gel. Full experimental details and characterization data for all new compounds (polyyne HDDA substrates and products) and a description of the computational methods and results are provided in the Supplementary Information.
Supplementary Material
Acknowledgments
These studies were supported by the U.S. Department of Health and Human Services [National Institute of General Medical Sciences (GM-65597) and National Cancer Institute (CA-76497)]. The computational portions were carried out using software and hardware provided by the University of Minnesota Supercomputing Institute (MSI). NMR spectra were obtained using an instrument purchased with a grant from the NIH Shared Instrumentation Grant program (S10OD011952).
Footnotes
Author contributions
S.R. and T.H. conceived the experiments, interpreted the data, co-wrote the manuscript, and agreed to the content of the final submission. S.R. executed the experiments and collected the data.
Additional information
Supplementary information is available in the online version of the paper.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Chen MS, White MC. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science. 2007;318:783–787. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 2.Li F, et al. Iminonitroso Diels–Alder reactions for efficient derivatization and functionalization of complex diene-containing natural products. Org Lett. 2007;9:2923–2926. doi: 10.1021/ol071322b. [DOI] [PubMed] [Google Scholar]
- 3.Peddibhotla S, Dang Y, Liu JO, Romo D. Simultaneous arming and structure/activity studies of natural products employing O–H insertions: An expedient and versatile strategy for natural products-based chemical genetics. J Am Chem Soc. 2007;129:12222–12231. doi: 10.1021/ja0733686. [DOI] [PubMed] [Google Scholar]
- 4.Tang P, Furuya T, Ritter T. Silver-catalyzed late-stage fluorination. J Am Chem Soc. 2010;132:12150–12154. doi: 10.1021/ja105834t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathak TP, Miller SJ. Site-selective bromination of vancomycin. J Am Chem Soc. 2012;134:6120–6123. doi: 10.1021/ja301566t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He J, Hamann LG, Davies HML, Beckwith REJ. Late-stage C–H functionalization of complex alkaloids and drug molecules via intermolecular rhodium-carbenoid insertion. Nature Commun. 2015;6:1–9. doi: 10.1038/ncomms6943. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Hartwig JF. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature. 2015;517:600–604. doi: 10.1038/nature14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Cisar JS, Zhou C-Y, Vera B, Williams H, Rodríguez AD, Cravatt BF, Romo D. Simultaneous structure–activity studies and arming of natural products by C–H amination reveal cellular targets of eupalmerin acetate. Nature Chem. 2013;5:510–517. doi: 10.1038/nchem.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huigens RW, III, et al. A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products. Nature Chem. 2013;5:195–202. doi: 10.1038/nchem.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayr H, Ofial AR. The reactivity-selectivity principle: An imperishable myth in organic chemistry. Angew Chem Int Ed. 2006;45:1844–1854. doi: 10.1002/anie.200503273. [DOI] [PubMed] [Google Scholar]
- 11.Hoye TR, Baire B, Niu D, Willoughby PH, Woods BP. The hexadehydro-Diels-Alder reaction. Nature. 2012;490:208–212. doi: 10.1038/nature11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trost BM. Sculpting horizons in organic chemistry. Science. 1985;227:908–916. doi: 10.1126/science.3969569. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura T. Synthetic methods for the generation and preparative application of benzyne. Aust J Chem. 2010;63:987–1001. [Google Scholar]
- 14.Niu D, Willoughby PH, Woods BP, Baire B, Hoye TR. Alkane desaturation by concerted double hydrogen atom transfer to benzyne. Nature. 2013;501:531–534. doi: 10.1038/nature12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willoughby PH, et al. Mechanism of the reactions of alcohols with o-benzynes. J Am Chem Soc. 2014;136:13657–13665. doi: 10.1021/ja502595m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Niu D, Brinker VA, Hoye TR. The phenol-ene reaction: Biaryl synthesis via trapping reactions between HDDA-generated benzynes and phenolics. Org Lett. 2016;18:5596–5599. doi: 10.1021/acs.orglett.6b02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, Larock RC. Facile O-arylation of phenols and carboxylic acids. Org Lett. 2004;6:99–102. doi: 10.1021/ol0361406. [DOI] [PubMed] [Google Scholar]
- 18.Truong T, Daugulis O. Divergent reaction pathways for phenol arylation by arynes: Synthesis of helicenes and 2-arylphenols. Chem Sci. 2013;4:531–535. doi: 10.1039/c2sc21288a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karmakar R, Yun SY, Wang KP, Lee D. Regioselectivity in the nucleophile trapping of arynes: The electronic and steric effects of nucleophiles and substituents. Org Lett. 2014;16:6–9. doi: 10.1021/ol403237z. [DOI] [PubMed] [Google Scholar]
- 20.Garr AN, et al. Experimental and theoretical investigations into the unusual regioselectivity of 4,5-, 5,6-, and 6,7-indole aryne cycloadditions. Org Lett. 2010;12:96–99. doi: 10.1021/ol902415s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetz AE, et al. An efficient computational model to predict the synthetic utility of heterocyclic arynes. Angew Chem Int Ed. 2012;51:2758–2762. doi: 10.1002/anie.201108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himeshima Y, Sonoda T, Kobayashi H. Fluoride-induced 1, 2-elimination of o-trimethylsilylphenyl triflate to benzyne under mild conditions. Chemistry Letters. 1983;12:1211–1214. [Google Scholar]
- 23.Goto K, Takubo K. Sinomenine and disinomenine. XXV On three different sinomenine-methines. Bull Chem Soc Jpn. 1931;6:79–87. [Google Scholar]
- 24.Garcia A, Drown BS, Hergenrother PJ. Access to a structurally complex compound collection via ring distortion of the alkaloid sinomenine. Org Lett. 2016;18:4852–4855. doi: 10.1021/acs.orglett.6b02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravarti RN, Robinson R. 16 Strychnine and brucine Part XLVI The preparation of neostrychnine and neobrucine. J Chem Soc. 1947;1947:78–80. doi: 10.1039/jr9470000078. [DOI] [PubMed] [Google Scholar]
- 26.Wenkert E, Liu LH. The constitution of the alloyohimbanes. Experientia. 1955;11:302–303. doi: 10.1007/BF02158385. [DOI] [PubMed] [Google Scholar]
- 27.Gaskell AJ, Joule JA. The zinc-acetic acid reduction of reserpine and other tetrahydro-beta-carboline alkaloids. Tetrahedron. 1968;24:5115–5122. doi: 10.1016/s0040-4020(01)88422-2. [DOI] [PubMed] [Google Scholar]
- 28.Brecht R, et al. Positional and facial selectivity in Diels-Alder reactions of (−)-(aS, 7S)-colchicine: Synthesis of novel analogues of the alkaloid. Liebigs Ann/Recueil. 1997;1997:851–857. [Google Scholar]
- 29.Wittig G, Pohmer L. Intermediäre Bildung von Dehydrobenzol (Cyclohexadienin) Angew Chem. 1955;67:348–348. [Google Scholar]
- 30.Bhunia A, Porwal D, Gonnade RG, Biju AT. Multicomponent reactions involving arynes, quinolines, and aldehydes. Org Lett. 2013;15:4620–4623. doi: 10.1021/ol4023134. [DOI] [PubMed] [Google Scholar]
- 31.Karmakar R, Mamidipalli P, Yun SY, Lee D. Alder-ene reactions of arynes. Org Lett. 2013;15:1938–1941. doi: 10.1021/ol4005905. [DOI] [PubMed] [Google Scholar]
- 32.Tabushi I, Yamada H, Yoshida Z, Oda R. Reactions of benzyne with substituted benzenes. Bull Chem Soc Jpn. 1977;50:285–290. [Google Scholar]
- 33.Paramjit S, Geeta A. Organic synthesis under PTC: Part 6 - preparation and decomposition of chiral phase transfer catalysts derived from quinine. Indian J Chem B. 1986;25:1034–1037. [Google Scholar]
- 34.Gui Y, et al. A New Chiral Organosulfur Catalyst for Highly Stereoselective Synthesis of Epoxides. Adv Synth Catal. 2008;350:2483–2487. [Google Scholar]
- 35.Hoffmann RW. Organic Chemistry, A Series of Monographs. Vol. 11 Academic Press; New York: 1967. Dehydrobenzene and Cycloalkynes. [Google Scholar]
- 36.Tadross PM, Stoltz BM. A comprehensive history of arynes in natural product total synthesis. Chem Rev. 2012;112:3550–3577. doi: 10.1021/cr200478h. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Palani V, Hoye TR. Reactions of HDDA-derived benzynes with sulfides: Mechanism, modes, and three-component reactions. J Am Chem Soc. 2016;138:4318–4321. doi: 10.1021/jacs.6b01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.