Abstract
IMPORTANCE
In patients with intrahepatic cholangiocarcinoma (ICC), the oncologic benefit of surgery and perioperative outcomes for large multifocal tumors or tumors with contiguous organ involvement remain to be defined.
OBJECTIVES
To develop and externally validate a simplified prognostic score for ICC and to determine perioperative outcomes for large multifocal ICCs or tumors with contiguous organ involvement.
DESIGN, SETTING, AND PARTICIPANTS
This study of a contemporary cohort merged data from the California Cancer Registry (January 1, 2004, through December 31, 2011) and the Office of Statewide Health Planning and Development inpatient database. Clinicopathologic variables were compared between tumors that were intrahepatic, small (<7 cm), and solitary (ISS) and those that had extrahepatic extension and were large (≥7 cm) and multifocal (ELM). External validation of the prognostic model was performed using an independent data set from the National Cancer Institute’s Surveillance, Epidemiology, and End Results database from January 1, 2004, through December 31, 2013.
MAIN OUTCOMES AND MEASURES
Patient overall survival after hepatectomy.
RESULTS
A total of 275 patients (123 men [44.7%] and 152 women [55.3%]; median [interquartile range] age, 65 [55–72] years) met the inclusion criteria. No significant differences in overall complication rate (ISS, 48 [34.5%]; ELM, 37 [27.2%]; P = .19) and mortality rate (ISS, 10 [7.2%]; ELM, 6 [4.4%]; P = .32) were found. A multivariate Cox proportional hazards model demonstrated that multifocality, extrahepatic extension, grade, node positivity, and age greater than 60 years are independently associated with worse overall survival. These variables were used to develop the MEGNA prognostic score. The prognostic separation/discrimination index was improved with the MEGNA prognostic score (0.21; 95% CI, 0.11–0.33) compared with the staging systems of the American Joint Committee on Cancer sixth (0.17; 95% CI, 0.09–0.29) and seventh (0.18; 95% CI, 0.08–0.30) editions.
CONCLUSIONS AND RELEVANCE
The MEGNA prognostic score allows more accurate and superior estimation of patient survival after hepatectomy compared with current staging systems.
Intrahepatic cholangiocarcinomas (ICCs) are the second most common type of primary liver cancer.1 Approximately 10% of all cholangiocarcinomas are intrahepatic, amounting to 2000 to 3000 cases each year in the United States with an increasing incidence worldwide.2,3 Intrahepatic cholangiocarcinomas are resistant to chemotherapy and radiotherapy, and surgical resection is considered to be the mainstay of therapy, providing the only chance of cure. However, most patients have advanced disease at diagnosis and are not candidates for surgery. Resectability is defined by the ability to completely resect ICC while leaving an adequate liver remnant. Current consensus considers noncontiguous extrahepatic disease, multiple bilobar or multicentric tumors, or lymph node metastases beyond the primary echelon as contraindications to resection.4 The role of surgical resection for large or multifocal ICCs or those invading adjacent organs by direct extension remains unclear. The decision to pursue resection is complex because of unclear oncologic benefit and substantial rates of operative morbidity and mortality.
Data from specialized hepatobiliary centers5–8 have demonstrated improvement in hepatectomy outcomes during the past decade for major hepatic resections. These data suggest that resection of large or multifocal tumors is safe if an adequate liver remnant is preserved. Moreover, a few institutional studies9–11 have demonstrated that contiguous organ resection may be feasible with a higher but acceptable morbidity. Empirical evidence to support this practice has yet to be studied using cases derived from a population-based sample. We hypothesized that extended resections for ICC are safe, with acceptable morbidity in a population-based cohort. Furthermore, we sought to investigate predictors of morbidity and mortality after surgical resection in large or multifocal ICCs or those invading adjacent organs.
The decision to pursue surgery often hinges on the perceived oncologic benefit from, at times, extensive resections. To address this question, multiple prognostic nomograms have been developed to estimate survival after surgical resection by using data from institutional series.12,13 These nomograms are of limited clinical utility because they are complex and have not been validated in a population-based sample, and many of the variables used in the models are only available postoperatively. We therefore sought to identify the prognostic determinants of overall survival in patients with ICCs undergoing liver resection. Furthermore, using population-based data, we sought to develop and externally validate a simplified prognostic score that is easy to use in clinical practice, can be used in a preoperative setting, is accurate and discriminatory, and has a wide dynamic range.
Methods
Patients
In California, reporting of cancer care is mandatory, yielding a low rate of records missing or lost to follow-up. The California Cancer Registry (CCR) is one of the most complete registries in the nation.14 Cases of ICC identified in the CCR from January 1, 2004, through December 31, 2011, were linked to Patient Discharge Data (PDD) available from the California Office of Statewide Health, Planning, and Development by applying a probabilistic linking algorithm based on sex, date of birth, and social security number.15 The PDD files contain patient-level data for all general, acute-care, nonfederal hospitals in California. For each admission, the PDD files include principal diagnosis and as many as 24 secondary diagnoses coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-CM-9) format, the principal procedure, and as many as 20 secondary procedures. This information enables a more accurate assessment of patient comorbidities and more detailed information on surgical procedures than is currently available from cancer registry data alone. The study was approved by the institutional review boards of the State of California and City of Hope National Medical Center, Duarte, California, with a waiver of informed consent.
All adults 20 years or older with a histologic diagnosis of ICC who underwent surgical treatment were included. Patients undergoing transplant, biopsy, or palliative bypass were excluded, as were patients with metastatic disease. Patients with perihilar or distal extrahepatic cholangiocarcinomas were also excluded. Demographic data, tumor characteristics, and overall survival were obtained from cancer registry variables. Specifically, tumor size, tumor extent, lymph node status, and the number of lymph nodes assessed were obtained from the collaborative staging-related variables in the CCR. Major vascular invasion was defined as invasion of the branches of the main portal vein (right or left portal vein, not including sectoral or segmental branches) or as invasion of 1 or more of the 3 hepatic veins (right, middle, or left). Extrahepatic contiguous organ involvement was defined as any perforation of the visceral peritoneum, invasion of the hepatic artery or vena cava, and involvement of surrounding viscera, with the exception of the gallbladder. Tumors extending to the gallbladder were classified as intrahepatic tumors. The index surgery was identified as the first major liver resection to occur on or after the date of diagnosis, and any hospital readmissions within 90 days after the index surgery were identified. We used the Deyo modification of the Charlson Comorbidity Index (Charlson-Deyo comorbidity score; range, 0–25, with higher scores indicating more comorbidities), excluding any cancer-related comorbidities.16 Complications were identified by referencing surgery-related complications used in recent studies of the American College of Surgeons National Surgical Quality Improvement program data and by including anesthesia-related complications.17 A complete list of complication codes is provided in eTable 1 in the Supplement.
Statistical Analysis
Data were analyzed from January 1, 2004, through December 31, 2013. Tumors were classified into the following 2 groups: (1) intrahepatic, small (<7 cm), and solitary (ISS) or (2) extrahepatic extension, large (≥7 cm), and multifocal (ELM). A cut-off of 7 cm was established previously.5,12 Bivariate analysis of categorical variables was performed using the χ2 test. The Kruskal-Wallis rank test was used for comparison of nonparametric variable medians. Multivariate analysis of variables used to estimate morbidity or mortality was performed using stepwise logistic regression with backward elimination. Multivariate survival analysis was performed using a Cox proportional hazards model. Missing data were assumed to be at random, and multiple imputation was performed using multivariate normal regression.18,19 Evaluation of the proportional hazards assumption was assessed graphically using methods recommended by Hosmer and Lemeshow.20 For all statistical analyses, we used STATA/MP software (version 14.1; StataCorp) with assumption of 2-sided tests and a criterion for statistical significance set at α<.05.
The prognostic score was calculated by assigning 1 point to each of the significant variables identified through multivariable analysis (range, 0–5, with higher scores indicating worse estimated survival) The prognostic score was modeled using flexible parametric survival analysis as detailed by Royston and Sauebrei21 to allow estimation of survival function. To validate the prognostic model in an independent data set, we queried the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) database using data from non-Californian registries to avoid overlap of patients. The inclusion and exclusion criteria were the same between the estimation and validation data sets. Patients with missing prognostic factors were excluded from the validation data set.
Results
Demographics and Tumor Characteristics
A total of 275 patients (123 men [44.7%] and 152 women [55.3%]; median [interquartile range (IQR)] age, 65 [55–72] years) met inclusion criteria. The demographic and tumor characteristics are summarized in Table 1. The study population consisted of 139 patients (50.5%) with ISS tumors and 136 patients (49.5%) with ELM tumors. Most patients were older than 60 years (170 [61.8%]) and had at least 1 comorbidity (220 [80%]). The median tumor size was 5.5 (IQR, 3.5–8.0) cm. Most tumors were moderately differentiated (123 of 216 [56.9%]) and node negative (210 of 262 [80.2%]). Portal lymph node sampling was performed for 122 of 272 patients (44.8%). When portal lymphadenectomy was performed, a median of 4 lymph nodes were evaluated (IQR, 2–8). Approximately one-fifth of the tumors had major vascular invasion (58 of 267 [21.7%]). Adjuvant chemotherapy was used in 111 patients (40.4%), and radiotherapy was used in 44 patients (16.0%).
Table 1.
Demographic and Tumor Characteristics of the Study Population and ISS vs ELM Subgroups
| Characteristic | Subgroupa | P Value | ||
|---|---|---|---|---|
| All (N = 275) |
ISS (n = 139) |
ELM (n = 136) |
||
| Age, median (IQR) | 65 (55–72) | 65 (55–73) | 65 (55–72) | .80 |
| Age group, y | ||||
| 18–60 | 105 (38.2) | 54 (38.8) | 51 (37.5) | .82 |
| >60 | 170 (61.8) | 85 (61.2) | 85 (62.5) | |
| Race/ethnicity | ||||
| Non-Hispanic white | 143 (52.0) | 68 (48.9) | 75 (55.1) | .76 |
| Asian or Pacific Islander | 68 (24.7) | 38 (27.3) | 30 (22.1) | |
| Hispanic white | 47 (17.1) | 24 (17.3) | 23 (16.9) | |
| Black | 9 (3.3) | 4 (2.9) | 5 (3.7) | |
| Other | 8 (2.9) | 5 (3.6) | 3 (2.2) | |
| Sex | ||||
| Male | 123 (44.7) | 68 (48.9) | 55 (40.4) | .16 |
| Female | 152 (55.3) | 71 (51.1) | 81 (59.6) | |
| Year of diagnosis | ||||
| 2004–2006 | 81 (29.4) | 35 (25.2) | 46 (33.8) | .16 |
| 2007–2009 | 105 (38.2) | 60 (43.2) | 45 (33.1) | |
| 2010–2012 | 89 (32.4) | 44 (31.7) | 45 (33.1) | |
| Insurance | ||||
| Government | 152 (55.3) | 76 (54.7) | 76 (55.9) | .84 |
| Private | 123 (44.7) | 63 (45.3) | 60 (44.1) | |
| NCI-designated cancer center | ||||
| No | 209 (76.0) | 110 (79.1) | 99 (72.8) | .22 |
| Yes | 66 (24.0) | 29 (20.9) | 37 (27.2) | |
| Charlson-Deyo comorbidity score16,b | ||||
| 0 | 55 (20.0) | 27 (19.4) | 28 (20.6) | .62 |
| 1 | 143 (52.0) | 74 (53.2) | 69 (50.7) | |
| 2 | 48 (17.5) | 21 (15.1) | 27 (19.8) | |
| ≥3 | 29 (10.5) | 17 (12.2) | 12 (8.8) | |
| Tumor size, median (IQR), cm | 5.5 (3.5–8.0) | 4.0 (3.0–5.5) | 7.9 (5.35–10.0) | <.001 |
| Grade | ||||
| Well differentiated | 28 (13.0) | 13 (12.3) | 15 (13.6) | .60 |
| Moderately differentiated | 123 (56.9) | 64 (60.4) | 59 (53.6) | |
| Poorly differentiated | 65 (30.1) | 29 (27.4) | 36 (32.7) | |
| Node positivity | ||||
| No | 210 (80.2) | 110 (84.6) | 100 (75.8) | .07 |
| Yes | 52 (19.8) | 20 (15.4) | 32 (24.2) | |
| No. of nodes examined, median (IQR) | 4 (2–8) | 4 (2–8) | 3 (2–7) | .52 |
| Extent of hepatectomy | ||||
| Ablation only | 28 (10.3) | 14 (10.3) | 14 (10.4) | .68 |
| Partial hemihepatectomy | 81 (29.9) | 43 (31.6) | 38 (28.1) | |
| Hemihepatectomy | 102 (37.6) | 53 (39.0) | 49 (36.3) | |
| Extended hemihepatectomy | 60 (22.1) | 26 (19.1) | 34 (25.2) | |
| Portal lymphadenectomy | ||||
| No | 150 (55.1) | 82 (60.3) | 68 (50.0) | .09 |
| Yes | 122 (44.9) | 54 (39.7) | 68 (50.0) | |
| Vascular invasion | ||||
| No | 209 (78.3) | 110 (83.3) | 99 (73.3) | .048 |
| Yes | 58 (21.7) | 22 (16.7) | 36 (26.7) | |
| Bilobar tumor | ||||
| No | 169 (85.8) | 97 (98.0) | 72 (73.5) | <.001 |
| Yes | 28 (14.2) | 2 (2.0) | 26 (26.5) | |
| Adjuvant chemotherapy | ||||
| No | 164 (59.6) | 86 (61.9) | 78 (57.3) | .44 |
| Yes | 111 (40.4) | 53 (38.1) | 58 (42.6) | |
| Adjuvant radiotherapy | ||||
| No | 231 (84.0) | 118 (84.9) | 113 (83.1) | .68 |
| Yes | 44 (16.0) | 21 (15.1) | 23 (16.9) | |
Abbreviations: ELM, extrahepatic extension, large (≥7cm), and multifocal; IQR, interquartile range; ISS, intrahepatic, small (<7cm), and solitary; NCI, National Cancer Institute.
Data are presented as number (percentage) of patients unless otherwise indicated. Missing data are not included in the calculation of percentages. Percentages have been rounded and may not total 100.
This score is the Deyo modification of the Charlson Comorbidity Index. Scores range from 0 to 25, with higher scores indicating more comorbidities.
Patients with ELM and ISS tumors were comparable with respect to age, race/ethnicity, sex, year of diagnosis, insurance status, comorbidity score, grade, extent of hepatectomy, and use of adjuvant chemotherapy and radiotherapy. Tumors classified as ELM were more likely to have vascular invasion (36 of 135 [26.7%] vs 22 of 132 [16.7%]; P = .048) or to be bilobar (26 of 98 [26.5%] vs 2 of 99 [2.0%]; P < .001) when compared with ISS tumors. Although not statistically significant, ELM tumors had more frequent node-positive findings than ISS tumors (32 of 132 [24.2%] vs 20 of 130 [15.4%]; P = .07) and more frequently underwent portal lymphadenectomy (68 of 136 [50.0%] vs 54 of 136 [39.7%]; P = .09).
Morbidity and Mortality
Overall 90-day morbidity rates were 30.9% (85 patients), and mortality was 5.8%) (16 patients). We found no difference in the morbidity (ISS, 48 [34.5%]; ELM, 37 [27.2%]; P = .19) or mortality (ISS, 10 [7.2%]; ELM, 6 [4.4%]; P = .32) between patients with ISS or ELM tumors. The most common surgical complication was hemorrhage or the need for blood transfusion (43 patients [15.6%]). Respiratory complications were the most common postoperative medical morbidity in 32 patients (11.6%). We found no differences in the pattern or the frequency of complications in ELM and ISS subgroups (eTable 2 in the Supplement). Bivariate and multivariate analysis of predictors of morbidity and mortality in ELM tumors are presented in eTable 3 in the Supplement. Charlson-Deyo comorbidity scores of 2 (odds ratio [OR], 3.02; 95% CI, 1.12–8.17) and 3 or greater (OR, 5.25; 95% CI, 1.32–20.9) and extent of surgical resection (OR, 3.93; 95% CI, 1.50–10.30) were independently associated with morbidity and mortality in logistic regression models of ELM tumors.
Long-term Outcomes
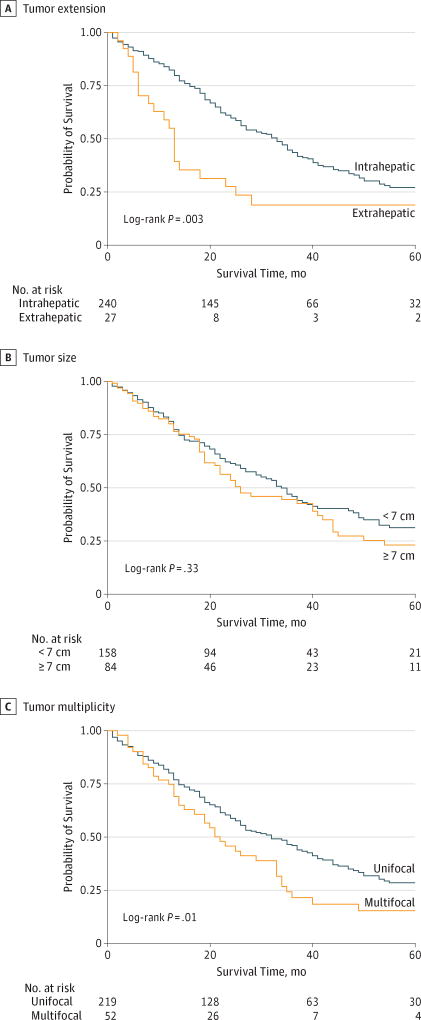
Median follow-up for all patients was 23 months (IQR, 13–40 months). Median follow-up for surviving patients was 35 months (IQR, 20–73 months). One hundred seventy-nine deaths occurred in the entire cohort at the end of the study period. Kaplan-Meier survival estimates for tumors with extrahepatic extension vs intrahepatic tumors (Figure 1A), large (≥7-cm) vs small (<7-cm) tumors (Figure 1B), and multifocal vs unifocal tumors (Figure 1C) are shown. Overall survival at 1, 3, and 5 years among patients with large (≥7-cm) ICCs (79.8%, 44.3%, and 23.0%, respectively) was comparable to that among patients with small (<7-cm) ICCs (81%, 45.8%, and 31.0%, respectively; log-rank P = .33). However, the overall survival at 1, 3, and 5 years among patients with multifocal tumors (75.0%, 21.6%, and 15.5%, respectively) was significantly lower compared with that among patients with unifocal ICCs (80.3%, 46.1%, and 28.5%, respectively; log-rank P = .01). Similarly, overall survival was worse at 1, 3, and 5 years was noted for ICCs with contiguous organ involvement (55.1%, 18.9%, and 18.9%, respectively) vs ICCs without contiguous organ involvement (82.5%, 43.7%, and 27.2%, respectively; log-rank P = .003).
Figure 1.
Kaplan-Meier Overall Survival Estimates by Tumor Characteristics
Survival is estimated by tumor extension, size, and multiplicity.
Prognostic Score
Univariate and multivariate analyses to identify prognosticators of overall survival using the estimation data set are shown in Table 2. Age greater than 60 years (HR, 1.87; 95% CI, 1.35–2.59; P < .001), high-grade tumors (HR, 1.88; 95% CI, 1.28–2.75; P = .002), node positivity (HR, 2.35; 95% CI, 1.60–3.47; P < .001), multifocality (HR, 1.57; 95% CI, 1.08–2.28; P = .02), and extrahepatic extension to contiguous organs (HR, 1.91;95% CI, 1.13–3.20; P = .007) were independently associated with lower overall survival. Tumor size, extent of surgery, vascular invasion, adjuvant chemotherapy or radiotherapy, and surgical complications were not associated with overall survival. Although node positivity was independently associated with lower overall survival, performing portal lymphadenectomy did not affect overall survival in the multivariate model (HR, 1.08; 95% CI, 0.80–1.46; P = .61).
Table 2.
Variables Associated With Overall Survival Using Cox Proportional Hazards Model
| Variable | Cox Proportional Hazards Analysis (n = 275) |
|||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, continuous | 1.02 (1.01–1.03) | .001 | NA | NA |
| Age, y | ||||
| 18–60 | 1 [Reference] | NA | NA | NA |
| >60 | 1.76 (1.28–2.43) | <.001 | 1.87 (1.35–2.59) | <.001 |
| Race/ethnicity | ||||
| Non-Hispanic white | 1 [Reference] | NA | NA | NA |
| Asian or Pacific Islander | 0.99 (0.69–1.41) | .94 | NA | NA |
| Hispanic white | 1.34 (0.94–2.04) | .10 | NA | NA |
| Black | 0.74 (0.27–2.02) | .56 | NA | NA |
| Other | 1.17 (0.48–2.89) | .73 | NA | NA |
| Sex | ||||
| Male | 1 [Reference] | NA | NA | NA |
| Female | 0.93 (0.70–1.25) | .66 | NA | NA |
| Year of diagnosis | ||||
| 2004–2006 | 1 [Reference] | NA | NA | NA |
| 2007–2009 | 1.34 (0.94–1.92) | .11 | NA | NA |
| 2010–2012 | 1.52 (0.99–2.35) | .06 | NA | NA |
| Insurance | ||||
| Government | 1 [Reference] | NA | NA | NA |
| Private | 0.66 (0.49–0.90) | .009 | NA | NA |
| NCI-designated cancer center | 0.68 (0.48–0.98) | .04 | NA | NA |
| Charlson-Deyo comorbidity score16,a | ||||
| 0 | 1 [Reference] | NA | NA | NA |
| 1 | 0.73 (0.50–1.08) | .12 | NA | NA |
| 2 | 0.84 (0.52–1.36) | .48 | NA | NA |
| ≥3 | 1.42 (0.84–2.39) | .19 | NA | NA |
| Tumor size, continuous | 1.04 (0.99–1.10) | .09 | NA | NA |
| Tumor size, cm | ||||
| <7 | 1 [Reference] | NA | NA | NA |
| ≥7 | 1.17 (0.86–1.61) | .32 | NA | NA |
| Grade | ||||
| Low to moderate | 1 [Reference] | NA | NA | NA |
| High | 1.83 (1.27–2.64) | .001 | 1.88 (1.28–2.75) | .001 |
| Node positivity | 2.44 (1.69–3.51) | <.001 | 2.35 (1.60–3.47) | <.001 |
| No. of nodes examined, continuous | 1.00 (0.99–1.01) | .89 | NA | NA |
| Resection | ||||
| Partial hepatectomy | 1 [Reference] | NA | NA | NA |
| Hemihepatectomy or extended | 1.13 (0.83–1.53) | .43 | NA | NA |
| Portal lymphadenectomy | 1.08 (0.80–1.46) | .61 | NA | NA |
| Vascular invasion | 1.31 (0.93–1.85) | .12 | NA | NA |
| Bilobar | 1.69 (1.05–2.72) | .03 | NA | NA |
| Multifocal | 1.60 (1.13–2.28) | .009 | 1.57 (1.08–2.28) | .02 |
| Extrahepatic extension | 1.94 (1.21–3.10) | .006 | 1.91 (1.13–3.20) | .02 |
| Adjuvant chemotherapy | 0.92 (0.68–1.24) | .57 | NA | NA |
| Adjuvant radiotherapy | 1.04 (0.70–1.54) | .84 | NA | NA |
| Postoperative complication | 1.13 (0.83–1.53) | .43 | NA | NA |
Abbreviations: HR, hazard ratio; NA, not applicable; NCI, National Cancer Institute.
This score is the Deyo modification of the Charlson Comorbidity Index. Scores range from 0 to 25, with higher scores indicating more comorbidities.
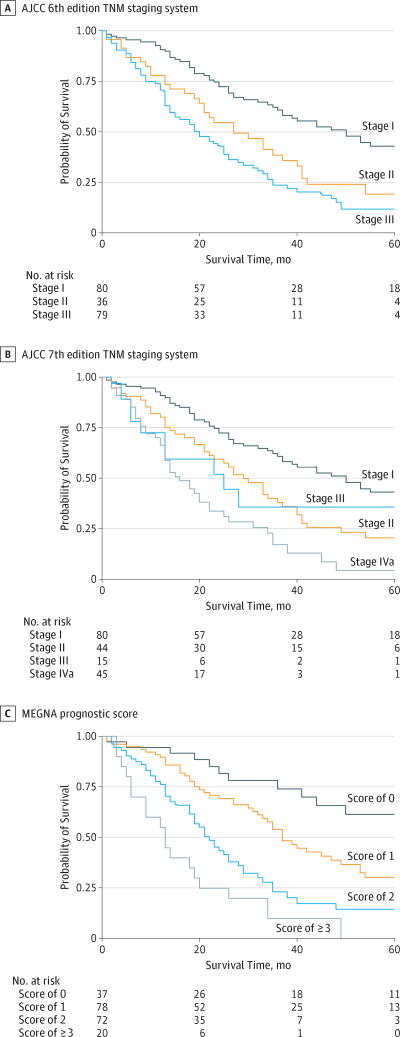
Based on the multivariate analysis, a simplified prognostic score (MEGNA prognostic score) was calculated by assigning 1 point each for the presence of multifocality, extrahepatic contiguous organ involvement, grade (high), node positivity, and age older than 60 years. The Kaplan-Meier overall survival estimates for MEGNA prognostic categories are shown compared with those of the American Joint Committee on Cancer (AJCC) sixth22 and seventh23 edition TNM staging systems (Figure 2). We tested performance of the MEGNA prognostic score in an independent data set.
Figure 2.
Kaplan-Meier Overall Survival Estimates of California Cancer Registry Patients by Prognostic System
AJCC indicates American Joint Committee on Cancer; MEGNA, multifocality, extrahepatic extension, grade, node positivity, and age older than 60 years. The MEGNA prognostic score assigns 1 point to each significant variable. Scores range from 0 to 5, with higher scores indicating lower estimated survival.
To validate the MEGNA prognostic score, a total of 261 patients were identified from the non-Californian SEER registries that met the inclusion criteria. The baseline survivor function of the estimation and validation set was not significantly different (eFigure in the Supplement) (median overall survival in CCR vs SEER: 32 months vs 29 months; log-rank P = .99). Distribution of prognostic variables was similar in the estimation and validation set (eTable4 in the Supplement). The MEGNA prognostic score demonstrated excellent predictive accuracy for probability of survival in all prognostic groups when tested in an independent validation data set (Table 3). In addition, MEGNA offered an improved prognostic separation and discrimination index21 (0.21; 95% CI, 0.11–0.33) compared with the AJCC seventh (0.18; 95% CI, 0.08–0.30) and sixth (0.17; 95% CI, 0.09–0.29) edition staging systems. Dynamic range in median and overall survival was also improved in the MEGNA prognostic score compared with the AJCC seventh and sixth edition TNM staging systems (Table 3).
Table 3.
A Comparison of Proposed and Existing Prognostic Models for ICC
| Model | Group, by Score or Stage (Predictive Accuracy, R2)a |
Discriminatory Capacity Index, R2 (95% CI)b |
Dynamic Rangec | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Median OS, mo | 5-y OS, % | ||||||||
|
|
|
|
|
||||||
| 1 | 2 | 3 | 4 | Estimation Cohort |
Validation Cohort |
Estimation Cohort |
Validation Cohort |
||
| MEGNA prognostic score | 0 (0.93) | 1 (0.97) | 2 (0.97) | ≥3 (0.96) | 0.21 (0.11–0.33) | >48 | >47 | 36.1 | 61.5 |
|
| |||||||||
| AJCC seventh edition TNM staging systemd |
I (0.96) | II (0.93) | III (0.81) | IVa (0.99) | 0.18 (0.08–0.30) | 32 | 35 | 33.0 | 42.2 |
|
| |||||||||
| AJCC sixth edition TNM staging systeme |
I (0.96) | II (0.92) | III (0.99) | NA | 0.17 (0.09–0.29) | 27 | 34 | 21.0 | 36.4 |
Abbreviations: AJCC, American Joint Committee on Cancer; ICC, intrahepatic cholangiocarcinoma; MEGNA, multifocality, extrahepatic extension, grade, node positivity, and age older than 60 years; NA, not applicable; OS, overall survival.
Indicates observed vs predicted probability of survival.
Calculated using the measure of prognostic separation with the bootstrap method with 5000 replications of Royston and Sauebrei.21
Indicates the best group minus the worst group.
From Edge et al.23
From Greene et al.22
Discussion
Liver resection outcomes have improved tremendously during the past 2 decades.7,8 What were once considered to be operations with a high risk for morbidity are now routinely performed with acceptable morbidity and very low mortality rates at high-volume centers owing to improvement in critical care, anesthetic management, and most important, better patient selection.6,24
For ICC, the safety of resecting ELM tumors remains to be defined. For instance, in a retrospective analysis, Yamamoto et al11 demonstrated that poor oncologic outcomes and complications preclude vessel resection and/or pancreatic resection in patients with ICC. On the contrary, Lang et al9 found that contiguous organ and vessel resection in the context of an extended resection is feasible with good oncologic outcomes, provided a negative margin is achieved. Data from Bergeat et al10 suggest that extended resections for large tumors are more likely to require contiguous resections and to have a higher morbidity, but the oncologic outcomes are comparable to those of nonextended resections. Recently, a multi-institutional study of specialized hepatobiliary centers5 demonstrated that resection of large ICCs (≥7 cm) or multifocal tumors has acceptable morbidity and mortality. Together these studies highlight the evolving role of surgical resection irrespective of tumor size, multifocality, or contiguous organ involvement in specialized hepatobiliary centers. Beyond the experience of single institutions and highly specialized hepatobiliary centers, the data presented in this study demonstrate the overall safety of surgical resections in patients with ELM tumors in the general population. Consistent with prior studies, we note the extent that liver resection and comorbidities are independently associated with worse outcome in this subset of patients.
In regard to oncologic outcomes, multiple prognostic nomograms have been developed in recent years to assess survival after surgical resection. The AJCC seventh edition TNM staging system is largely based on the National Cancer Institute’s SEER data set, which identified multifocality, vascular invasion, contiguous organ extension, and regional lymph node involvement as poor prognostic factors. This system was further validated in a multi-institutional cohort study.25 Additional analysis of this multi-institutional cohort led to the development of the nomogram of Hyder et al,12 which includes tumor size, age, and cirrhosis, in addition to the prognostic variables identified in AJCC seventh edition TNM staging system but excludes contiguous organ extension. Wang et al26 proposed a nomogram based on analysis of a cohort of Chinese patients that included carcinoembryonic antigen level, cancer antigen 19–9 level, vascular invasion, lymph node metastases, contiguous organ involvement, mutifocality, and tumor size as important prognostic determinants. The 2 nomograms have been noted to be superior to the AJCC seventh edition TNM staging system and have been evaluated in an independent cohort of patients.27 However, these prognostic models have limited external validity because they have not been validated in a US population-based cohort. Review of extant literature reveals a remarkable heterogeneity in the clinicopathologic and genomic characteristics of ICCs based on geography.28,29 Therefore, the performance of these models should be externally validated before widespread acceptance by clinicians. Second, the complexity of these prognostic models limits their utility and their widespread use in patient care, even if they are validated. These limitations of prognostic models are described by Wyatt et al.13 In the present study, we propose a simplified, easy-to-use prognostic score. We also demonstrate evidence that the MEGNA prognostic score is accurate and effective and can be generalized to the US population. We find this score to be superior to the AJCC sixth and seventh edition TNM staging systems in terms of accuracy, discriminatory capacity, and dynamic range. Although the MEGNA prognostic score was developed using data from patients who underwent surgical resection, the MEGNA score can be derived preoperatively and can inform decisions regarding surgery and adjuvant therapy. For instance, additional imaging with positron emission tomography may be warranted in patients with a high MEGNA prognostic score to rule out distant disease.
Limitations
Some of the study limitations are inherent to using a reimbursement database to study complications because the application of ICD-9-CM codes is not independently verified by medical record review, and the severity of complications cannot be accurately graded. However, this bias should not have affected our comparison of ISS and ELM tumors, given that the same methods were used to capture complications in both groups. Moreover, the complications seen in this study are comparable to those observed in previous institutional reports.5 In regard to the development of a prognostic model, our results could be influenced by missing data. We note that missing data were infrequent and were statistically addressed by multiple imputations to minimize bias as best as possible. We were also limited in comparing the MEGNA prognostic score with the nomogram of Hyder et al12 or Wang et al26 because the variables used in those nomograms were not available in population data sets. Although the MEGNA prognostic score uses variables that can be ascertained preoperatively, this study validates its use only in the postoperative context. Additonal studies are needed to assess its utility preoperatively.
Conclusions
Multifocality, large size, or contiguous organ involvement alone are not contraindications to resection in ICC. The decision to operate must be weighed in the context of adequate functional liver remnant, extent of liver resection, and patient comorbidities. Our MEGNA prognostic score is an easy-to-use tool that allows accurate estimation of patient survival after hepatectomy. Additional prospective studies are needed to evaluate its use in the preoperative context.
Supplementary Material
Key Points.
Question
What are the prognostic determinants of overall survival among patients with intrahepatic cholangiocarcinoma undergoing liver resection?
Findings
In this evaluation of the California Cancer Registry and Office of Statewide Health, Planning, and Development database, records of 275 patients undergoing resection for intrahepatic cholangiocarcinoma were analyzed. A prognostic model based on the independent association of multifocality, extrahepatic extension, grade, nodal status, and age with overall survival was developed and validated.
Meaning
The prognostic score is an easy-to-use tool that allows accurate estimation of patient survival after hepatectomy for intrahepatic cholangiocarcinoma.
Acknowledgments
Funding/Support: This study was supported by grant 5K12CA001727-20 from the National Cancer Institute of the National Institutes of Health (Dr Melstrom).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Raoof had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: Raoof, Dumitra, Melstrom, Warner, Fong, Singh.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Raoof, Dumitra, Melstrom, Warner, Singh.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Raoof, Ituarte.
Administrative, technical, or material support: Raoof, Singh.
Study supervision: Fong, Singh.
Conflict of Interest Disclosures: None reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208(1):134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Chang KY, Chang JY, Yen Y. Increasing incidence of intrahepatic cholangiocarcinoma and its relationship to chronic viral hepatitis. J Natl Compr Canc Netw. 2009;7(4):423–427. doi: 10.6004/jnccn.2009.0030. [DOI] [PubMed] [Google Scholar]
- 4.Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17(8):669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spolverato G, Kim Y, Alexandrescu S, et al. Is hepatic resection for large or multifocal intrahepatic cholangiocarcinoma justified? results from a multi-institutional collaboration. Ann Surg Oncol. 2015;22(7):2218–2225. doi: 10.1245/s10434-014-4223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoof M, Aloia TA, Vauthey JN, Curley SA. Morbidity and mortality in 1174 patients undergoing hepatic parenchymal transection using a stapler device. Ann Surg Oncol. 2014;21(3):995–1001. doi: 10.1245/s10434-013-3331-9. [DOI] [PubMed] [Google Scholar]
- 7.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138(11):1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 8.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191(1):38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 9.Lang H, Sotiropoulos GC, Frühauf NR, et al. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg. 2005;241(1):134–143. doi: 10.1097/01.sla.0000149426.08580.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergeat D, Sulpice L, Rayar M, et al. Extended liver resections for intrahepatic cholangiocarcinoma: friend or foe? Surgery. 2015;157(4):656–665. doi: 10.1016/j.surg.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Takasaki K, Yoshikawa T. Extended resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat Surg. 1999;6(2):117–121. doi: 10.1007/s005340050093. [DOI] [PubMed] [Google Scholar]
- 12.Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg. 2014;149(5):432–438. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt JC, Altman DG. Prognostic models: clinically useful or quickly forgotten? BMJ. 1995;311(7019):1539–1541. [Google Scholar]
- 14.North American Association of Central Cancer Registries. US registries certified in 2013 for 2010 incidence data. [Accessed February 7, 2017];2017 http://www.naaccr.org/Certification/USCert2010.aspx.
- 15.Zingmond DS, Ye Z, Ettner SL, Liu H. Linking hospital discharge and death records—accuracy and sources of bias. J Clin Epidemiol. 2004;57(1):21–29. doi: 10.1016/S0895-4356(03)00250-6. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973–981. doi: 10.1097/SLA.0b013e31826b4c4f. [DOI] [PubMed] [Google Scholar]
- 18.Allison PD. Missing Data. Vol. 136. Thousand Oaks, CA: Sage Publications; 2001. [Google Scholar]
- 19.Schafer JL. Analysis of Incomplete Multivariate Data. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- 20.Hosmer DW, Jr, Lemeshow S. Applied Survival Analysis: Time-to-Event. Vol. 317. Hoboken, NJ: Wiley-Interscience; 1999. [Google Scholar]
- 21.Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med. 2004;23(5):723–748. doi: 10.1002/sim.1621. [DOI] [PubMed] [Google Scholar]
- 22.Greene FL, Page DL, Fleming ID, et al. In: American Joint Committee on Cancer. 6. Manual CS, editor. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. In: American Joint Committee on Cancer. 7. Manual CS, editor. New York, NY: Springer; 2010. [Google Scholar]
- 24.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236(4):397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farges O, Fuks D, Le Treut YP, et al. AFC-IHCC-2009 Study Group. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma. Cancer. 2011;117(10):2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 27.Doussot A, Groot-Koerkamp B, Wiggers JK, et al. Outcomes after resection of intrahepatic cholangiocarcinoma: external validation and comparison of prognostic models. J Am Coll Surg. 2015;221(2):452–461. doi: 10.1016/j.jamcollsurg.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandi G, Farioli A, Astolfi A, et al. Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies. Oncotarget. doi: 10.18632/oncotarget.4539. [published online June, 20, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bragazzi MC, Cardinale V, Carpino G, et al. Cholangiocarcinoma: epidemiology and risk factors. Transl Gastrointest Cancer. 2011;1(1):21–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.