Abstract
Curcuma longa L., commonly known as turmeric, is a rhizomatous herb of the family Zingiberaceae. It is mostly used as a spice, a coloring agent and broadly used in traditional medicine such as Ayurveda, Unani, etc., Turmeric rhizomes interact with a large numbers of rhizosphere-associated microbial species, and some enter the plant tissue and act as endophytes. Both rhizospheric and endophytic species are directly or indirectly involved in growth promotion and disease management in plants and also play an important role in the modulation of morphological growth, secondary metabolite production, curcumin content, antioxidant properties, etc. The present review focuses on the rhizobacterial and endophytic bacterial and fungal populations associated with the turmeric.
Keywords: Turmeric, Rhizobacteria, Endophyte, Inoculation, Growth, Curcumin
Introduction
Turmeric (Curcuma longa L.) is a rhizomatous herbaceous perennial plant belonging to the family Zingiberaceae, widely used as a spice and extensively applied in the traditional systems of Ayurveda, Unani, and Siddha, the various folk medicines (Amalraj et al. 2016). It is believed to be originated in south east countries like India, China, Bangladesh, but cultivated throughout the tropics and subtropics of the world. In north India, turmeric is popular with various vernacular names, such as “haldi”, and in the south it is called “manjal”. The word turmeric derived from the latin word-terra merita (meritorious earth) refers to the color of ground turmeric (Paramasivam et al. 2009; Prasad and Aggarwal 2011). The medicinal properties of this plant have been largely practiced for various diseases like asthma bronchial hyperactivity, rheumatism, diabetic wounds sinusitis smallpox, skin cancer, urinary tract infection, liver ailments (Dixit et al. 1988), jaundice, menstrual difficulties and abdominal pain (Bundy et al. 2004). Curcumin (orange-yellow crystalline substance) is one of the important ingredients of curcuminoids, which has different pharmacological activity such as anti-inflammatory, antioxidant, antimalerial, anticancerous, hypolipidemic, and immunoenhancer against life-threatening viral diseases (Ammon and Wahl 1990; Tonnesen 1992; Huang et al. 1994; Rao et al. 1995; Srimal 1997; Mukerjee and Vishwanatha 2009; Panahi et al. 2014). Demethoxycurcumin and ar-turmerone arrest cell division and induce apoptosis in various cell lines and become cytotoxic to the cancer cell lines (Mingjie et al. 2004), while curlone is known for antioxidant and antimutagenic properties (Osorio-Tobón et al. 2016).
Turmeric is a perennial therapeutic spice that can reach a height of about 1 m. Its palmate leaves are oblong in shape, alternate and arranged in two rows, which further divided into leaf sheath and later form false stem, petiole (50–115 cm long), and leaf blade (76–115 cm long). Plants have rough segmented, yellow to orange, cylindrical and aromatic rhizomes that measures 2.5–7.0 cm in length and nearly 2.5 cm in diameter (Prasad and Aggarwal 2011). Primary rhizome is mostly pear shaped known as “bulb” while secondary one is cylindrical (Ahmad et al. 2010). Its flowers are dull yellow, grouped together in a dense spike-like structure and 10–15 cm in length (http://www.b http://www.botanical.com).
The nutritional analysis showed that 100 g of turmeric contains protein (8 g), sugar (3 g), minerals (3.5 g), carbohydrates (69.9%), dietary fiber (21 g), moisture (13.1%) and significant amounts of vitamins (Balakrishnan 2007). Biochemical analysis of yellow rhizome revealed the presence of natural phenolic compounds like curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin), sesquiterpenoids (ar-turmerone, curlone, bisacumol, zingiberene, curcumene, germacrone, curcuminol, β-bsabolene, α and β termerones), and volatile oils like d-α-phellandrene, d-sabinene, cinol, borneol, zingiberene and sesquiterpenes (Ohshiro et al. 1990). Curcuminoids form a mixture of three components, curcumin (94%), demethoxycurcumin (6%), and bisdemethoxycurcumin (0.3%). The antioxidant and other pharmacological properties of curcumin have been elaborated in above lines. However, phenolic diketone like curcuminoids are responsible for the yellow color of turmeric, whereas the aroma is affiliated to turmerone, ar-tumerone and zingiberene (sesquiterpenoids) (Prasad and Aggarwal 2011).
Cultivation and agronomic aspects of turmeric
Morphological parameters of turmeric, such as leaves, shoots rhizomes and rhizomes yield, are affected by several factors like nutrition, cultivation practices, and plant genotype. Plantation time varies with climatic conditions, turmeric varieties and planting materials. Its requires a warm and humid climate and can be grown in diverse tropical conditions within temperature range of 20–30 °C and with a rainfall of 1500 mm or more per annum. Turmeric thrives in different soil types ranging from light black loam, red soils to clay loams and rich loamy soils having natural drainage and irrigation facilities. In India, turmeric cultivation season begins in the mid of April that continues up to the first week of July. It can be grown in rotation with sugarcane, chilli, onion, garlic, elephant foot yam, vegetables, pulses, wheat, ragi and maize. In some areas, turmeric is grown as an intercrop with mango, jack and litchi (Randhawa and Mahey 2002).
Currently, India is the world’s largest producer, consumer and exporter of turmeric followed by China and several other subcontinent countries (Nisar et al. 2015). Many local cultivars of turmeric are available that are known mostly by the name of the locality, where they are cultivated. The cultivated varieties show significant variation in size and color of the rhizomes and curcumin content. The two leading varieties of turmeric predominating in the world market are “Madras” and “Alleppey”. The Patna variety is notorious for its deep color. Out of the two types cultivated in Maharashtra, “‘Lokhandi” contains bright colored hard rhizomes while the other has light-colored soft rhizomes. “Duggirala” and “Tekurpeta” are the prominent commercial varieties of turmeric in Andhra Pradesh, which have long, stout, smooth and hard fingers. Likewise “Kasturi Pasupa” of the Godavari Delta, the “Armoor” type of the Nizamabad area and the “Chaya Pasupa” are other important varieties of Andhra Pradesh. In Orissa, important varieties cultivated are Roma, Suroma, Ranga and Rasmi. The “Lakadong” variety of ginger is grown in Meghalaya which is popular for high curcumin content (5–5.05%) (http://iisr.agropedias.iitk.ac.in/).
At the time of turmeric planting through the rhizome, powdered neem cake (25 g) mixed well with the soil applied in each pit at a spacing of 20–25 cm within and between the rows. Seed rhizomes can be put in shallow pits and covered with well rotten cattle manure or compost mixed with Trichoderma (10 g compost inoculated with Tricoderma). A seed rate of 1000 kg rhizomes is required for planting in one acre of land while for intercropping in a fruit-garden, seed rate may be as low as 125–200 kg per acre.
Biotechnological aspects of turmeric
Generally, conventional turmeric plantation experiences rhizome degeneration after long-term propagation. As a result, propagation through seed offers challenges because of poor flowering and seed setting (Zhao 2002). Therefore, an alternate method is required for the multiplication of turmeric. Plant tissue culture is a novel biotechnological approach, which is widely gaining momentum for the successful propagation of plants but have slow growth and multiplication rate (Kartha 1985). In vitro culture techniques provide alternative tools for crop improvement and can be commercially utilized in herbaceous crops like banana, cardamom, and orchids, etc., to efficiently multiply and conserve the rich germplasm of these species. However, limited information of turmeric multiplication is available (Nadgauda et al. 1978). Now in vitro formation of storage organs such as bulbs, corms, tubers, and rhizomes in turmeric came into existence because these kinds of propagules can be directly transferred to the field without undergoing acclimatization or hardening (Nayak 2000). Moreover, the work of Niedz and Evens (2007) concluded that rhizomatous bud of turmeric can be used as source of explants for shoot induction on MS medium supplemented with different concentrations of BAP.
Micropropagation is an important technique for the propagation of selected genotypes using in vitro culture techniques (Debergh and Read 1991). Some successful micropropagation of turmeric reports are available (Nadgauda et al. 1978; Meenakshi et al. 2001; Salvi et al. 2003; Ghosh et al. 2013). Variants with high curcumin content were reported from tissue-cultured plantlets (Nadgauda et al. 1982). Furthermore, researches are continuing to achieve organogenesis and plantlet formation via callus cultures of turmeric (Salvi et al. 2000). The micro-propagated plants showed significant increase in shoot length, number of tillers, number and length of leaves, number of fingers, and total fresh rhizome weight per plant compared with conventionally propagated plants (Salvi et al. 2002). Balachandran et al. (1990) reported the methods of rapid clonal multiplication and short-term germplasm conservation of turmeric, referred as in vitro microrhizome production that seems to be advantageous over in vitro plantlet production in following ways: (a) microrhizomes can be planted directly in the soil without acclimatization and (b) storage and transport of microrhizomes are easier, may facilitate germplasm exchange across national borders (Shirgurkar et al. 2001). It is also considered as an ideal method for the production of disease-free planting material. In contrast, Nirmal babu et al. (2003) from their study revealed least variation in the variants developed through in vitro microrhizome plants. Furthermore, some molecular studies also proposed the molecular phylogeny of zingiberaceae family based on DNA sequence of nuclear internal transcribed spacer (ITS) and plasmid K regions. Study revealed that curcuma is paraphyletic with Hitchenia, Stahlianthus, and Smithatris. Assessment of genetic diversity using ISSR and RAPD markers were carried out among C.longa and other 14 species of curcuma and placed them into seven groups which are congruent with the morphological characters-based classification (Syamkumar and Sasikumar 2007). Recent progress in molecular markers was the development and characterization of EST-derived and genomic microsatellites which can be used for the diversity analysis of turmeric (Joshi et al. 2010; Senan et al. 2013).
Besides molecular markers, efficient and stable genetic transformations in turmeric have been documented using particle bombardment on callus culture (Shirgurkar et al. 2006a, b). Through PCR and GUS assay, some herbicide (glufosinate) resistant plantlets were developed (Mahadtanapuk et al. 2006). Presently, advances in turmeric research likely to attain the core of molecular biology and targeted to achieve the primary goal of enhance plant fitness, yield and metabolite content against stress conditions.
Plant growth promoting rhizobacteria (PGPR) of turmeric
Turmeric harbors myriad of diverse microbes in the rhizosphere. The release of rhizodeposition or carbon compounds from the root favors the interactions of high Gram-negative microbial populations in the rhizosphere compared to the bulk soil (Ahmad et al. 2008; Ambardar and Vakhlu 2013; Kumar et al. 2015, 2016b). Extensive work of Kumar et al. (2016b) detected dominant PGPR groups associated with the turmeric rhizosphere belong to three subdivisions—α-Proteobacteria, β-Proteobacteria and γ-Proteobacteria in which Pseudomonas, Klebsiella, Agrobacterium, Azotobacter and Burkholderia found to be dominant species, contributing nearly two-third of all bacterial isolates under Proteobacteria and the rest 30% are constituted by Firmicutes (Bacillus sp.,).
Various bacterial species such as Pseudomonas, Azospirillum, Azotobacter, Klebsiella, Enterobacter, Alcaligenes, Arthrobacter, Burkholderia, Bacillus and Serratia noted for plant growth promotion activity have been assigned as plant growth promoting bacteria (PGPB) (Joseph et al. 2007; Jasim et al. 2014; Kumar et al. 2014, 2016b). Several PGPR inoculants currently commercialized for the enhancement of plant growth either of the widely reported mechanisms like the suppression of plant diseases (as biocontrol), improved nutrient acquisition (biofertilizers) or phytohormone production (biostimulants). PGPR–plant interactions are widely attracting researchers to identify fate of associations and theirs applications in plant growth and medicinal importance of plant including turmeric.
Endophytes of turmeric
Endophytes are defined as the microorganisms colonizing intercellular and intracellular regions of plant tissues without causing apparent diseases and remain unobtrusive in nature (Schulz and Boyle 2006; Kumar et al. 2016b). They have been well recorded by some researchers from turmeric plants (Table 1). Paenibacillus sp. is reported as endophyte having indole 3 acetic acid-producing property (Aswathy et al. 2013), while Klebsiella sp. is documented for plant growth promotion activity (Anisha et al. 2013) associated with turmeric rhizome. In another study, Kumar et al. (2016a) reported six endophytic bacterial strains, i.e., Bacillus cereus, B. thuringiensis, Bacillus sp., B. pumilis, Pseudomonas putida and Clavibacter michiganensis belonging to Firmicutes, γ-Protobacteria and Bacteroidates groups, respectively, from the turmeric rhizome having multi-plant growth promoting properties. Apart from that, some fungal endophytes have also been noted from the turmeric plant. Bustanussalam et al. (2015) reported 44 fungi out of which 6 fungi showed antioxidant activities over 65%, while Jalgaonwala and Mahajan (2014) and Singh et al. (2013), respectively, reported Eurotium sp., and Pencillium strains as fungal endophytes correspondingly associated with the turmeric rhizome and leaves.
Table 1.
Beneficial microbes associated with turmeric and their plant growth promoting properties
| Bacterial strains | Source | Plant growth promoting properties | References |
|---|---|---|---|
| Klebsiella sp. | Turmeric rhizome | IAA production, phosphate solubilization and ACC deaminase enzyme production | Anisha et al. (2013) |
| Penibacillus sp. | Turmeric rhizome | IAA production | Aswathy et al. (2013) |
| AM fungi + PGPR | Turmeric rhizosphere | Enhancement in antioxidant properties, flavonoids and total phenol content | Dutta and Neog (2016) |
| Eurotium sp. | Turmeric rhizome | Asparaginase enzyme production | Jalgaonwala and Mahajan (2014) |
| Bacillus cereus, Bacillus thuringiensis, Bacillus sp., Bacillus pumilis, Pseudomonas putida and Clavibacter michiganensis | Turmeric rhizome | Antifungal activity, IAA production and phosphate solubilization | Kumar et al. (2016a) |
| Azotobacter chroococcum | Turmeric rhizosphere | Enhancement in curcumin content | Kumar et al. (2014) |
| Bacillus subtilis, Bacillus sp., Burkholderia thailandensis, Agrobacterium tumefaciens, Klebsiella sp., Bacillus cereus, Pseudomonas putida, Pseudomonas fluorescens | Turmeric rhizosphere | Antibacterial activity, antifungal activity, IAA production and phosphate solubilization | Kumar et al. (2016b) |
| Penicillium sp. | Turmeric leaves | Nanoparticle-mediated antibacterial activity | Singh et al. (2013) |
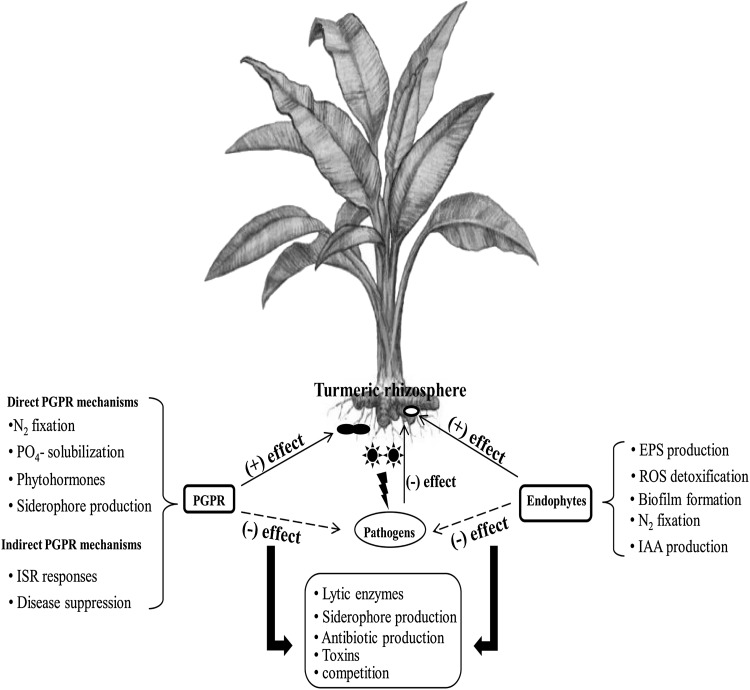
Endophytes commonly enter plant tissue primarily through the root zone; however, aerial portions of plants such as flowers, stems, and cotyledons are the other sites for the same (Kobayashi and Palumbo 2000). They may either become localized at the point of entry or get spread throughout the plant tissue (Reinhold-Hurek and Hurek 1998). It is reported that endophytes confer beneficial effects to the plant (Hardoim et al. 2015). Certain endophytic bacteria have been documented to enhance growth of plant and also increase resistance to pathogens, drought, heavy metals and even herbivores, and their commercial potential has received much attention from last few years(Lee et al. 2004; Ryan et al. 2008). Similar to PGPR, endophytes too promote plant growth by a numbers of mechanisms including phosphate solubilization activities, indole acetic acid production and the production of a siderophores (Fig. 1).
Fig. 1.
Overview of pivotal microbial interactions with turmeric plant
Turmeric response to PGPR inoculation
PGPR efficacy is dependent on establishing an effective population density of microbial active cells in the plant rhizosphere. Broadly, PGPR bacterial suspensions are prepared at densities of 10−8–10−9 CFU ml−1 for root dipping and soil inoculations (Martinez-Viveros et al. 2010). After inoculation, the cell number may undergo rapid decline depending on whether or not the soil has been sterilized. Inoculation efficacy also depends on the competence of the rhizobacteria for the particular plant (Zehnder et al. 2001).
In case of turmeric plant, different bacterial strains had been inoculated in the rhizome for the modulation of growth and metabolite synthesis. Inoculation of diazotroph bacterial suspension (1:1 ratio of Pseudomonas and Bacillus sp.,) showed significant enhancement in rhizome yield (21%), plant height (5%) rhizome weight (60%) and soil microbial population over respective controls (Suryadevara and Ponmurugan 2012). Boominathan and Sivakumaar (2012) reported the efficiency of Pseudomonas fluorescens and Bacillus megaterium strains used for the improvement of curcumin content. Kumar et al. (2014) inoculated Azotobacter chroococcum in the turmeric rhizome and observed the enhancement of leaves number, shoot height, shoot biomass, rhizome biomass and curcumin content. In a follow up study, Kumar et al. (2016b) reported the enhancement of morphological yields and curcumin content of turmeric followed by P. fluorescens inoculation. Similarly, Dutta and Neog (2016) co-cultivated mycorrhizal fungi and plant growth promoting bacteria in the turmeric rhizome and reported significant increase in the antioxidant activity, flavanoids, phenol, and curcumin contents. Other studies were also congruent to the above reported works (Karnwal and Guleria 2011).
Literature survey revealed the effects of chemical fertilizer application (N, K, Mn and P) either singly or in combination on the growth and yield of turmeric. Akamine et al. (2007) observed that separate application of P and K could not increase the growth and yield of turmeric, whereas N alone application enhanced growth and yield both. Furthermore, this study revealed the combined application of either N and K (NK) or N, P and K (NPK) enhanced greater shoot biomass (4–6 times) and higher yield (8–9 times). Other studies followed the similar trend, suggesting that application of N alone or in combination either with P or K stimulates the growth and yield (Rao and Reddy 1977). Overall, continuous and inappropriate application of chemical fertilizers adversely affects the texture and productivity of soil and also production of turmeric. In this regard, the use of PGPB seems highly beneficial for both the turmeric yield and growth and the soil.
Future prospective
Turmeric is also called “golden spice” due to its everyday importance from kitchen to pharmaceutical industries. Researches carried out from last few decades precisely indicated the potential antioxidant property of curcumin compared to the α-tocopherol. Such property is widely employed as an inhibitor of atherosclerosis, growth of viruses and bacteria, as well as chemopreventive agent for the variety of cancers including colon, breast, prostrate, esophagus lung and oral. Other cucurminoids compounds, such as demethoxycurcumin and bisdemethoxycurcumin, have been also found to be cytotoxic, antioxidant with anti-inflammatory effect on the cancer cell lines. The contents of such medicinal important compounds are highly beneficial for the pharmaceutical industries principally as medicine.
Sustainable production of turmeric is one of the future challenges in the present scenario. The use of chemical fertilizers for the enhancement of curcuminoids and sesquiterpenoids is very common agronomic practices that supposed to break microbial community structures and functional attributes. PGPR applications not only reduce the negative impact of synthetic chemicals on plant and soil, but also induce the eco-friendly environment between plant–microbes. Ongoing researches on plant–microbe interactions are receiving tremendous momentum. Developments in high-throughput technologies, such as next-generation sequencing, may explore complex microbiomes, which will facilitate larger sample sizes and encourage deeper analyses of microbial communities. The new “omics” approaches are valuable tools for exploring, identifying, and characterizing the contributions of genetic and metabolic elements involved in the interactions between host plants and endophytes. For example, metagenome sequencing has revealed important functions required for survival of bacterial endophytes inside the host plants, and established beneficial effects on the primary metabolites of plants. Coupled cultivation-independent and improved cultivation technologies will permit the exploration of uncultured groups thriving in association with plants (Van Overbeek and van Elsas 2008). Moreover, the presence of endophytes in different compartments of plant is controversial; nevertheless, in situ probe analyses may provide insight information about their exact localization within plant tissues and the physical connections between different microbial groups. Certainly, this approach may figure out some hidden facts and technical modernizations in microbial revelation and will surpassingly refine our concept of endophytes as living organisms colonizing internally the plant compartments.
Acknowledgements
The authors thank the University Grants Commission and CSIR, New Delhi for granting fellowship in the form of JRF and SRF, Head, Centre of Advanced Study in Botany, Banaras Hindu University for providing the laboratory facilities.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Ajay Kumar and Amit Kishore Singh share equal contribution.
References
- Ahmad F, Ahmad I, Khan MS. Screening of free-living rhizosphereric bacteria for their multiple growth promoting activities. Microbiol Res. 2008;163:173–181. doi: 10.1016/j.micres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ahmad W, Hassan A, Ansari A, Tarannum T. Cucurma longa L.—a review. Hippocratic J Unani Med. 2010;5:179–190. [Google Scholar]
- Akamine H, Hossain MA, Ishimine Y, Yogi K, Hokama K, Iraha Y, Aniya Y. Effects of application of N, P and K alone and in combination on growth, yield and curcumin content of turmeric (Curcuma longa L.) Plant Prod Sci. 2007;10:151–154. doi: 10.1626/pps.10.151. [DOI] [Google Scholar]
- Amalraj A, Piusb A, Gopib S, Gopia S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J Tradit Complement Med. 2016 doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambardar S, Vakhlu J. Plant growth promoting bacteria from Crocus sativus rhizosphere. World J Microbiol Biotechnol. 2013;29:2271–2279. doi: 10.1007/s11274-013-1393-2. [DOI] [PubMed] [Google Scholar]
- Ammon HPT, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1990;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- Anisha C, Mathew J, Radhakrishnan EK. Plant growth promoting properties of endophytic Klebsiella sp. isolated from Curcuma longa. IJBPAS. 2013;2(3):593–601. [Google Scholar]
- Aswathy AJ, Jasim B, Jyothis M, Radhakrishnan EK. Identification of two strains of Paenibacillus sp. as indole 3 acetic acid-producing rhizome-associated endophytic bacteria from Curcuma longa. 3 Biotech. 2013;3(3):219–224. doi: 10.1007/s13205-012-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran SM, Bhat SR, Chandel KPS. In vitro clonal multiplication of turmeric (Curcuma spp.) and ginger (Zingiber officinale Rosc.) Plant Cell Rep. 1990;8:521–524. doi: 10.1007/BF00820200. [DOI] [PubMed] [Google Scholar]
- Balakrishnan KV. Postharvest technology and processing of turmeric. In: Ravindran PN, Nirmal Babu K, Sivaraman K, editors. Turmeric: the genus Curcuma. Boca Raton: CRC Press; 2007. [Google Scholar]
- Boominathan U, Sivakumaar PK. A liquid chromatography method for the determination of curcumin in PGPR inoculants Curcuma longa L. plant. Int J Pharm Sci Res. 2012;3(11):4438–4441. [Google Scholar]
- Bundy R, Walker AF, Middleton RW, Booth J. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J Altern Complement Med. 2004;10:1015–1018. doi: 10.1089/acm.2004.10.1015. [DOI] [PubMed] [Google Scholar]
- Bustanussalam RF, Septiana E, Lekatompessy SJ, Widowati T, Sukiman HI, Simanjuntak P. Screening for endophytic fungi from turmeric Plant (Curcuma longa L.) of Sukabumi and Cibinong with potency as antioxidant compounds producer. Pak J Biol Sci. 2015;18(1):42–45. doi: 10.3923/pjbs.2015.42.45. [DOI] [PubMed] [Google Scholar]
- Debergh PC, Read PE. Micropropagation. In: Debergh PC, Zimmerman RH, editors. Micropropagation: technology and application. Dordrecht: Kluwer Academic Publication; 1991. pp. 1–13. [Google Scholar]
- Dixit VP, Jain P, Joshi SC. Hypolipidaemic effects of Curcuma longa L. and Nardostachys jatamansi, DC in triton-induced hyperlipidaemic rats. Indian J Physiol Pharmacol. 1988;32:299–304. [PubMed] [Google Scholar]
- Dutta SC, Neog B. Accumulation of secondary metabolites in responsible to antioxidant activity of Turmeric rhizomes co-inoculated with native arbuscular mycorrhizal fungi and plant growth promoting bacteria. Sci Hortic. 2016;204:179–184. doi: 10.1016/j.scienta.2016.03.028. [DOI] [Google Scholar]
- Ghosh A, Chatterjee P, Ghosh P. A protocol for rapid propagation of genetically true to type Indian turmeric (Curcuma longa L.) through in vitro culture technique. Adv Appl Sci Res. 2013;4:39–45. [Google Scholar]
- Hardoim PR, van Overbeek LS, Berg G, Pirttila AM, Compant S, Campisano A, Doing M, Sessitch A. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev. 2015;79:293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MT, Lou YR, Ma W, Newmark HL, Reuhl KR, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–5847. [PubMed] [Google Scholar]
- Jalgaonwala RE, Mahajan RT. Production of anticancer enzyme asparaginase from endophytic Eurotium sp. isolated from rhizomes of Curcuma longa. European. J Exp Biol. 2014;4(3):36–43. [Google Scholar]
- Jasim B, Joseph AA, John CJ, Mathew J, Radhakrishnan EK. Isolation and characterization of plant growth promoting endophytic bacteria from the rhizome of Zingiber officinale. 3 Biotech. 2014;4(2):197–204. doi: 10.1007/s13205-013-0143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B, Patra RR, Lawrence R. Characterization of plant growth promoting Rhizobacteria associated with chickpea (Cicer arietinum L) Int J Plant Prod. 2007;1(Suppl 2):141–152. [Google Scholar]
- Joshi RK, Kuanar A, Mohanty S, Subudhi E, Nayak S. Mining and characterization of EST derived microsatellites in Curcuma longa L. Bioinformation. 2010;5(3):128. doi: 10.6026/97320630005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnwal A, Guleria M (2011) Plant growth promoting activity of Bacillus sp. on turmeric. The Annals of “VALAHIA” University of Targoviste, pp 34–37
- Kartha KK. Cryopreservation of plant cells and organs. Boca Raton: CRC Press Inc; 1985. pp. 115–134. [Google Scholar]
- Kobayashi DY, Palumbo JD. Bacterial endophytes and their effects on plants and uses in agriculture. In: Bacon CW, White JF, editors. Microbial endophytes. New York: Marcel Dekker, Inc; 2000. pp. 199–233. [Google Scholar]
- Kumar A, Singh R, Giri DD, Singh PK, Pandey KD. Effect of Azotobacter chroococcum CL13 inoculation on growth and curcumin content of turmeric (Curcuma longa L.) Int J Curr Microbiol App Sci. 2014;3(9):275–283. [Google Scholar]
- Kumar A, Vandana RS, Yadav A, Giri DD, Singh PK, Pandey KD. Rhizosphere and their role in plant–microbe interaction. In: Chaudhary KK, Dhar DW, editors. Microbes in soil and their agricultural prospects. New York: Nova Science Publisher, Inc; 2015. pp. 83–97. [Google Scholar]
- Kumar A, Singh R, Yadav A, Giri DD, Singh PK, Pandey KD. Isolation and characterization of bacterial endophytes of Curcuma longa L. 3 Biotech. 2016;6:60. doi: 10.1007/s13205-016-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Vandana RS, Singh M, Singh PP, Singh SK, Singh PK, Pandey KD. Isolation of plant growth promoting rhizobacteria and their impact on growth and curcumin content in Curcuma longa L. Biocatal Agric Biotechnol. 2016;8:1–7. [Google Scholar]
- Lee S, Flores-ncarnacion M, Contreras-Zentella M, Garcia-Flores L, Escamilla JE, Kennedy C. Indole-3-acetic acid biosynthesis is deficient in Gluconacetobacter diazotrophicus strains with mutations in cytochrome C biogenesis genes. J Bacteriol. 2004;186:5384–5391. doi: 10.1128/JB.186.16.5384-5391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadtanapuk S, Anuntalabhochai S, Handa T, Sanguansermsri M, Topoonyanont N. Genetic transformation of Curcuma alismatifolia Gagnep. using retarded shoots. Plant Biotechnol. 2006;23:233–237. doi: 10.5511/plantbiotechnology.23.233. [DOI] [Google Scholar]
- Martinez-Viveros O, Jorquera MA, Crowley DE, Gajardo G, Mora ML. Mechanisms and practical considerations involved in plant growth promotion by rhizobacteria. J Soil Sci Plant Nutr. 2010;10(3):293–319. doi: 10.4067/S0718-95162010000100006. [DOI] [Google Scholar]
- Meenakshi N, Suliker GS, Krishnamoorthy V, Hegde RV. Standardization of chemical environment for multiple shoot induction of turmeric (Curcuma longa L.) for in vitro clonal propagation. Crop Res Hissar. 2001;22:449–453. [Google Scholar]
- Mingjie J, Choi V, Lee J, Lee V. Induction of apoptosis by ar-turmerone on various cell lines. Int J Mol Med. 2004;14(2):253–256. [PubMed] [Google Scholar]
- Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–3875. [PubMed] [Google Scholar]
- Nadgauda RS, Mascarenhas AF, Hendre RR, Jagannathan V. Rapid clonal multiplication of turmeric Curcuma longa L. plants by tissue culture. Ind J Exp Biol. 1978;16:120–122. [Google Scholar]
- Nadgauda RS, Khuspe SS, Mascarenhas AF. Isolation of high curcumin varieties of turmeric from tissue culture. In: Iyer RD, editor. Proceedings V annual symposium on plantation crops. Kasargod: CPCRI; 1982. pp. 143–144. [Google Scholar]
- Nayak S. In vitro multiplication and microrhizome induction in Curcuma aromatica Salisb. Plant Growth Regul. 2000;32:41–47. doi: 10.1023/A:1006307316393. [DOI] [Google Scholar]
- Niedz RP, Evens TJ. Regulating plant tissue growth by mineral nutrition. In vitro Cell Dev Biol Plant. 2007;43:370–381. doi: 10.1007/s11627-007-9062-5. [DOI] [Google Scholar]
- Nirmal Babu K, Ravindran PN, Sasikumar B (2003) Field evaluation of tissue cultured plants of spices and assessment of their genetic stability using molecular markers. Final report submitted to Department of Biotechnology, Government of India
- Nisar T, Iqbal M, Raza A. Turmeric: a promising spice for phytochemical and antimicrobial activities. Am Eurasian J Agric Environ Sci. 2015;15:1278–1288. [Google Scholar]
- Ohshiro M, Kuroyanag M, Keno A. Structures of sesquiterpenes from Curcuma longa. Phytochem. 1990;29:2201–2205. doi: 10.1016/0031-9422(90)83038-3. [DOI] [Google Scholar]
- Osorio-Tobón JF, Carvalho PIN, Barbero GF, Nogueira GC, Rostagno MA, de Almeida Meireles MA. Fast analysis of curcuminoids from turmeric (Curcuma longa L.) by high-performance liquid chromatography using a fused-core column. Food Chem. 2016;200:167–174. doi: 10.1016/j.foodchem.2016.01.021. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Saadat A, Beiraghdar F, Nouzari SMH, Jalalian HR, Sahebkar A. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: a randomized double blind placebo-controlled trial. J Funct Foods. 2014;6:615–622. doi: 10.1016/j.jff.2013.12.008. [DOI] [Google Scholar]
- Paramasivam M, Poi R, Banerjee H, Bandyopadhyay A. High performance thin layer chromatographic method for quantitative determination of curcuminoids in Curcuma longa germplasm. Food Chem. 2009;113:640–644. doi: 10.1016/j.foodchem.2008.07.051. [DOI] [Google Scholar]
- Prasad S, Aggarwal BB. Turmeric, the Golden spice from traditional medicine to modern medicine. In: Benzie IFF, Wachtel-Galor S, editors. Herbal medicine. Biomolecular and clinical aspects. 2. Boca Raton: CRC Press; 2011. pp. 1–26. [PubMed] [Google Scholar]
- Randhawa GS and Mahey RK (2002) Advances in agronomy and production of turmeric in India. In: Cracker LE, Simon JE (Ed) Herbs spices and medicinal plants—recent advances in botany, horticulture and pharmacology, volume 3 (Indian reprint). CBS Publishers and Distributers, Darya Ganj, New Delhi, pp 71–101
- Rao MR, Reddy VR. Effect of different levels of nitrogen, phosphorus and potassium on yield of turmeric (Curcuma longa L.) J Plant Crops. 1977;5:60–63. [Google Scholar]
- Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- Reinhold-Hurek B, Hurek T. Interactions of gramineous plants with Azoarcus spp. and other diazotrophs: identification, localization and perspectives to study their function. Crit Rev Plant Sci. 1998;17:29–54. doi: 10.1016/S0735-2689(98)00355-4. [DOI] [Google Scholar]
- Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN. Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Salvi ND, George L, Eapen S. Direct regeneration of shoots from immature infl orescence cultures of turmeric. Plant Cell Tissue Organ Cult. 2000;62:235–238. doi: 10.1023/A:1006459822879. [DOI] [Google Scholar]
- Salvi ND, George L, Eapen S. Micropropagation and field evaluation of micropropagated plants of turmeric. Plant Cell Tissue Organ Cult. 2002;68:143–151. doi: 10.1023/A:1013889119887. [DOI] [Google Scholar]
- Salvi ND, Eapen S, George L. Biotechnological studies of turmeric (Curcuma longa L.) andginger (Zingiber officinale Roscoe) Adv Agric Biotechnol. 2003;1:11–32. [Google Scholar]
- Schulz B, Boyle C. What are endophytes? In: Schulz BJE, Boyle CJC, Sieber TN, editors. Microbial root endophytes. Berlin: Springer; 2006. pp. 1–13. [Google Scholar]
- Senan S, Kizhakayil D, Sheeja TE, Sasikumar B, Bhat AI, Parthasarathy VA. Novel polymorphic microsatellite markers from turmeric, Curcuma longa L. (Zingiberaceae) Acta Bot Croat. 2013;72:407–412. [Google Scholar]
- Shirgurkar M, John CK, Nadgauda RS. Factors affecting in vitro microrhizome production in turmeric. Plant Cell Tissue Organ Cult. 2001;64:5–11. doi: 10.1023/A:1010645624618. [DOI] [Google Scholar]
- Shirgurkar MV, Naik VB, von Arnold S, Nadgauda RS, Clapham D. An efficient protocol for genetic transformation and shoot regeneration of turmeric (Curcuma longa L.) via particle bombardment. Plant Cell Rep. 2006;25:112–116. doi: 10.1007/s00299-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Shirgurkar MV, Naik VB, Arnold S, Nadgauda RS, Clapham D. An effi cient protocol for genetic transformation and shoot regeneration of turmeric (Curcuma longa L.) via particle bombardment. Plant Cell Rep. 2006;25(2):112–116. doi: 10.1007/s00299-005-0033-1. [DOI] [PubMed] [Google Scholar]
- Singh D, Rathod V, Niganagouda S, Herimath J, Kulkarni P. Biosynthesis of Silver nanoparticle by endophytic fungi Pencillum sp. isolated from Curcuma longa (turmeric) and its antibacterial activity against pathogenic gram negative bacteria. J Pharm Res. 2013;7:448–453. [Google Scholar]
- Srimal RC. Turmeric: a brief review of medicinal properties. Fitoterapia. 1997;68:483–493. [Google Scholar]
- Suryadevara N, Ponmurugan P. Response of turmeric to plant growth promoting rhizobacteria (pgpr) inoculation under different levels of nitrogen. Int J Biol Technol. 2012;3(1):39–44. [Google Scholar]
- Syamkumar S, Sasikumar B. Molecular marker based genetic diversity analysis of Curcuma species from India. Sci Hortic. 2007;112:235–241. doi: 10.1016/j.scienta.2006.12.021. [DOI] [Google Scholar]
- Tonnesen HH (1992) Chemistry of curcumin and curcuminoids. In: Ho C-T, Lee CY and Huang, M.-T (eds), Phenolic compounds in food and their effect on health. I: analysis, occurrence and chemistry. ACS symposium series no. 506, American Chemical Society, Washington, DC, pp 143–153
- Van Overbeek L, van Elsas JD. Effects of plant genotype and growth stage on the structure of bacterial communities associated with potato (Solanum tuberosum L.) FEMS Microbiol Ecol. 2008;64:283–296. doi: 10.1111/j.1574-6941.2008.00469.x. [DOI] [PubMed] [Google Scholar]
- Zehnder GW, Murphy JF, Sikora EJ, Kloepper JW. Application of rhizobacteria for induced resistance. Eur J Plant Pathol. 2001;107:39–50. doi: 10.1023/A:1008732400383. [DOI] [Google Scholar]
- Zhao DW. High quality and production of ginger–theory and technology. Beijing: China Agricultural Publishing Company; 2002. pp. 10–30. [Google Scholar]