Abstract
Coronary artery disease (CAD) is the number one cause of death for men and women in the United States. Genetic predisposition and environmental factors lead to the development of atherosclerotic plaques in the vessel walls of the coronary arteries resulting in decreased myocardial perfusion. Treatment includes a combination of revascularization procedures and medical therapy. Due to the high surgical risk of many of the patients undergoing revascularization procedures, medical therapies to reduce ischemic disease is an area of active research. Small molecule, cytokine, endothelial progenitor cell, stem cell, gene and mechanical therapies show promise in increasing the collateral growth of blood vessels, thereby reducing myocardial ischemia.
Keywords: Coronary Artery Disease, Molecular Therapy
Introduction
Coronary artery disease (CAD) is the number one cause of death for men and women in the United States. 1,2 Genetic predisposition and environmental factors lead to the development of atherosclerotic plaques in the vessel walls of the coronary arteries resulting in decreased myocardial perfusion. Current diagnostic modalities for coronary artery disease start with noninvasive studies to determine myocardial function. If ischemic disease is suspected, coronary angiography is the current standard of care to evaluate the atherosclerotic disease. Treatment includes a combination of revascularization procedures and medical therapy. Due to the high surgical risk of many of the patients undergoing revascularization procedures, medical therapies to reduce ischemic disease is an area of active research.
Pathophysiology of Coronary Vascular Disease
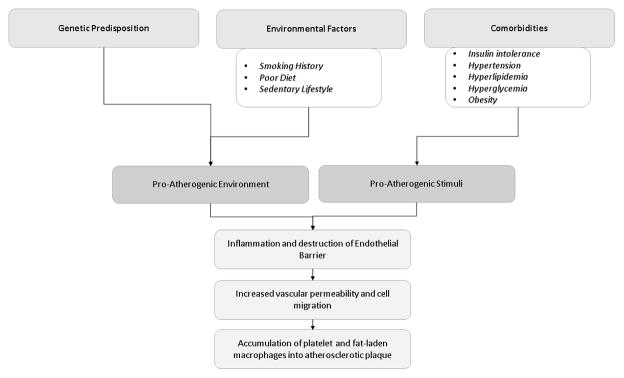
Genetic predisposition and environmental factors both contribute to a pro atherogenic environment leading to the development of coronary artery disease. 3 While many genes have been identified to be associated with ischemic heart disease, it is clear that genetic predisposition is not acting alone. Current research suggests the microbiome of the gastrointestinal tract may also play a role by breaking down certain cytokines. 3 Environmental factors that contribute to the development include smoking history, poor diet and sedentary lifestyle.
Patients with ischemia heart disease often have comorbidities that include insulin intolerance, hyperlipidemia, hypertension, hyperglycemia and obesity. Together, these comorbidities individually serve as proatherogenic stimuli and together are known as the pathology of metabolic syndrome. Proatherogenic stimuli induce inflammation and altered platelet function leading to abnormalities in the endothelial barrier which leads to increased vascular permeability. This increased vascular permeability facilitates trans endothelial migration of immune cells to the arterial intima and induction of vascular inflammation. This inflammation leads to destabilization of the cellular wall integrity, accumulation of platelet and fat laden macrophages resulting in the development of an atherosclerotic plaque in the vessel wall and decreased perfusion to the heart. 3–5
New blood vessel growth occurs via two different processes—angiogenesis and arteriogenesis. Angiogenesis, or capillary sprouting, results in high capillary density. Ischemia is a major stimulus for angiogenesis.6 Arteriogenesis, or rapid proliferation of collateral arteries, results in remodeling and growth of collateral arteries from a preexisting arteriolar network. 6,7 The development of functional arteriogenesis can alter the outcome of coronary and peripheral artery disease. [Figure 1]
Figure 1. Pathophysiology of Coronary Vascular Disease.
Flow diagram demonstrating factors that contribute to the development of coronary artery disease.
Arteriogenesis is mainly triggered by a number of different mechanisms including fluid shear stress, induced by the altered blood flow conditions after an arterial occlusion. Arteriogenesis involves activation of endothelial cells, degradation of the basal membrane, leukocyte invasion, vascular cell proliferation, neointima formation, changes to the extracellular matrix and cytokine production.8 A number of different cytokines that stimulate endothelial and smooth muscle cell proliferation, migration, recruitment and activation of monocytes and macrophages have been identified to stimulate angiogenesis and arteriogenesis including monocyte chemoattractant protein-1 (MCP-1), fibroblast growth factor 2 (FGF-2), transforming growth factor- beta (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), granulocyte macrophage stimulating factor (GM-CSF), and others. 6,9 These cytokines, endothelial progenitor cells, and certain genes are the target of current research aiming to alleviate the pathogenesis of coronary artery disease.
Significant Clinical Trial/Registry Data
Research in cardiovascular disease is at the forefront of the medical field. [Table 1] Breakthrough drugs such as clopidogrel have changed the longterm outcomes for patients with coronary artery disease. The prognostic significance of collateral circulation has been proven to be beneficial in recent clinical trials. Patients with high collateral blood flow had lower all-cause mortality and cardiac mortality compared to patients with low collateral blood flow. 10–16 Therapeutic angiogenesis refers to the concept that angiogenic growth factors can stimulate the development of collateral arteries and improve patient outcomes.
Table 1.
Trials in Coronary Angiogenesis
| Multicenter Clinical Trial | Enrollment | Dates | Drug/Device | Outcome |
|---|---|---|---|---|
| AGENT | 79 | 10/2001–11/2008 | Ad5FGF-4 | Results show evidence of favorable anti-ischemic effects with Ad5-FGF4 compared with placebo, and it appears to be safe. Angiogenic gene transfer with Ad5-FGF4 shows promise as a new therapeutic approach to the treatment of angina pectoris |
| FIRST | 337 | 10 years | FGF-2 | A single intracoronary infusion of rFGF2 does not improve exercise tolerance or myocardial perfusion but does show trends toward symptomatic improvement at 90 (but not 180) days |
| C-CURE | 319 | 12/2008–1/2012 | Mesenchymal stem cells | Cardiopoietic stem cell therapy was found feasible and safe with signs of benefit in chronic heart failure, meriting definitive clinical evaluation |
| CHART-1 | 484 | 11/2012–7/2017 | Mesenchymal stem cells | Primary outcome neutral; exploratory analyses suggested a benefit of cell treatment on the primary composite in patients with baseline left ventricular end-diastolic volume 200–370 mL |
The FGF Initiation Revascularization Trial (FIRST) evaluated the efficacy and safety of recombinant FGF-2 as a single bolus intracoronary administration to improve symptoms and myocardial function. The study found that a single intracoronary infusion of FGF2 does not improve exercise tolerance or myocardial perfusion but does show trends toward symptomatic improvement at 90 (but not 180) days. 17 The angiogenic gene therapy (AGENT) trial evaluated the safety and anti-ischemic effects of 5 doses of adenovirus containing a human fibroblast growth factor gene in patients with stable angina pectoris. Patients who received the treatment had greater improvements in exercise time at 4 weeks and no adverse reactions were seen. The study determined that adenovirus containing a human fibroblast growth factor gene is shown to be effective and safe as a therapy. 18
Stem cell therapy is a promising approach to patients with coronary artery disease. 19,20 However, a Cochrane review performed in 2012 of the effectiveness of adult bone marrow-derived stem cells to treat acute myocardial infarction concluded that only moderate improvement in global heart function is significant and sustainable on a long-term basis. Furthermore, there was a high degree of heterogeneity between studies. 21 Current stem cell research focuses on optimization of cell therapy by improving cellular homing, combining cell regimens and using resident cell populations. Cardiopoietic cells are produced through cardiogenic conditioning of patient- derived mesenchymal cells resulting in stem cells that have enhanced cardioreparative functionality. 22,23 Pre-clinical ischemic heart failure models suggest that cardiopoietic stem cell therapy improves left ventricular functioning and improves myocardial remodeling. The Cardiopoietic stem Cell therapy in heart failure (C-CURE) evaluated the feasibility and safety of cardiopoietic cell therapy in patients which ischemic heart failure and concluded that cardiopoietic stem cell therapy merited definiti9ve clinical evaluation. 23 The Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial evaluated the efficacy of cardiopoietic cell biotherapy (versus a sham procedure) in patients with advanced ischaemic heart failure and determined that there was no difference in Finkelstein-Schoenfeld hierarchical composite (all cause mortality, worsening heart failure, Minnesota Living with Heart Failure Questionnaire score, 6-min walk distance, left ventricular end-systolic volume, and ejection fraction) at 39 weeks. Further analysis suggested that there is a benefit of cardiopoietic cell therapy in patients with elevated baseline left ventricular end-diastolic volume (200–379mL). There was no difference in adverse effects. The review concluded that the evaluation of cardiopoietic cell therapy in patients with elevated end-diastolic volume as a result of ischemic disease. should be further studied. 22
To date, there have been no successful therapeutic angiogenesis clinical trials to induce functional collateral vessel growth which is associated with improved myocardial function.
Diagnostic Strategies
There are extensive ACC/AHA Guidelines on appropriate diagnostic, interventional and medical treatment of patients with coronary artery disease. Regarding the strength of recommendation there are five classes: I (strong), IIa (moderate), IIb (weak), III No benefit (Moderate) and III Harm (strong). The current guidelines for diagnostic and therapeutic strategies will be briefly discussed.
Noninvasive Diagnostic Testing
The National Institute for Health and Care Excellences (NICE) Clinical Guideline assessed the performance and cost utility of different non-invasive imaging strategies in patients presenting with chest pain and determined that the low cost and high sensitivity of the cardiac CT makes it the non-invasive test of choice. 24 Other modalities for non-diagnostic workup include rest echocardiography, electrocardiography and stress testing.
Invasive Diagnostic Testing
Coronary angiography is considered the gold standard for diagnosis of CAD. However its major shortcoming is that it cannot assess the physiological significant of lesions or the stability of the plaque. Additionally, interventional studies carry 1.5% incidence of procedural complications including death, stroke, bleeding, infection, myocardial infarction, anaphylactic reactions, nephropathy, vascular damage, arrhythmias and need for immediate revascularization. Despite these shortcomings, coronary angiography is useful in patients with presumed stable ischemic heart disease (SIHD) who have unacceptable ischemic symptoms despite guideline-directed medical treatment (GDMT) and who are amenable to, and candidates for, coronary revascularization (Class I). 25 Coronary angiography is also useful when noninvasive tests yield ambiguous or indeterminate results.
The International Study of Comparative Health Effectiveness With Medical And Invasive Approaches (ISCHEMIA) trial is currently randomizing patients with moderate ischemia on stress testing to a strategy of optimal medical therapy alone (with coronary angiography reserved for failure of medical therapy) or routing cardiac catheterization followed by revascularization (when appropriate) plus optical medical therapy. Before randomization, patients with normal renal function undergo a blinded computed tomography angiography to exclude them if significant left main CAD or no significant CAD is present. The ISCHEMIA trial will provide evidence about optimal strategy for managing patients with non-left main SIHD and moderate to severe ischemia. 25
Therapeutic Strategies
Current Revascularization Therapy
In the setting of severe ischemia, disease revascularization has been proven to improve survival compared with medical therapy alone. 26 Depending on the extent of their disease patients will undergo a coronary artery bypass grafting (CABG) alone, a CABG and percutaneous intervention (PCI), PCI alone or no revascularization procedure at all. Regardless of revascularization procedure performed, all patients need medical therapy.
Current Medical Therapy
Class 1 recommendation states that patients with coronary artery disease should be on aspirin (100 mg to 325 mg daily), beta blockers, angiotensin converting enzyme inhibitors and angiotensin receptor blockers and smoking cessation. 27 Class II recommendation suggests patients with CAD and acute coronary syndrome (ACS) benefit from statin use. Patients with a history of ACS greater than 1 year prior who have remained free of recurrent ACS are considered to have transitioned to stable ischemic heart disease (SIHD). 28
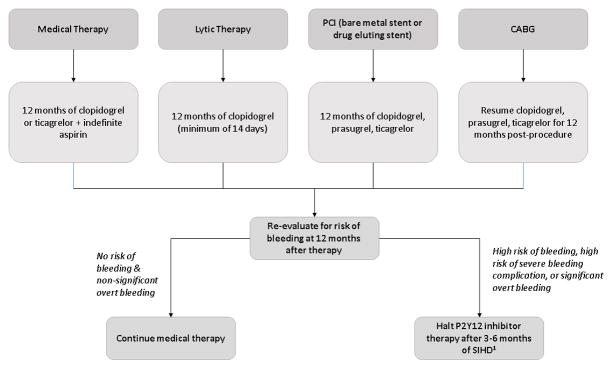
Patients with acute disease should be treated in one of 4 ways: 1) with medical therapy, 2) with lytic therapy, 3) with percutaneous coronary intervention (PCI) (bare metal stent (BMS) or drug-eluting stent (DES), or 4) with CABG. Class 1 evidence suggests that patients treated with medical therapy should get at least 12 months of clopidogrel or ticagrelor in addition to indefinite aspirin. Patients treated with lytic treatment should get a minimum of 14 days and ideally at least 12 months of clopidogrel. Patients treated with PCI should get at least 12 months of clopidogrel, prasugrel or ticagrelor. Patients treated with CABG should resume the P2Y12 inhibitor (clopidogrel, prasugel or ticagrelor) to complete 1 year. At 12 months, all patients should be evaluated for risk of bleeding. Class IIb evidence suggests that patients at no risk of bleeding and nonsignificant overt bleeding while on therapy can continue medical therapy. In patients treated with a PY212 inhibitor after DES implantation who develop a high risk of bleeding, are at high risk of severe bleeding complication, or develop significant overt bleeding, the P2Y12 inhibitor maybe stopped after 3 months of SIHD or after 6 months for ACS. 29
Patients with stable ischemic heart disease can be split into three categories: 1) patients with no past history of MI, PCI or CABG (within 12 months), 2) patients s/p PCI and 3) patient’s s/p CABG. Class III evidence suggest patients with no history of MI, PCI or recent CABG receive no benefit from P2Y12 inhibitor therapy. Class 1 evidence suggests that patient’s s/p PCI with BMS should receive at least 1 month of Clopidogrel and with DES should receive at least 6 months of Clopidogrel. At 6 months, all patients should be evaluated for risk of bleeding. Class IIb evidence suggests that patients at no risk of bleeding and nonsignificant overt bleeding while on therapy can continue medical therapy for an additional 1 month (BMS) or 6 months (DES). Class IIB evidence suggest that patients s/p CABG may be treated with 12 months of clopidogrel. Clopidogrel is the only currently used P2Y12 inhibitor studied in patients with SIHD undergoing PCI. 29 [Figure 2]
Figure 2. Treatment algorithm of patients with acute disease.
1. P2Y12 inhibitor may be stopped after 3 months of SIHD if no ACS, or 6 months of SIHD in event of ACS.
Research for Novel Therapeutic Angiogenesis Strategies
Therapeutic angiogenesis is a popular area of research due to the large patient population that suffers from and the high mortality of rate of patients with coronary vascular disease. Many of the clinical trials involving drugs that were successful in animal models fail to be successful in phase I clinical trials. One reason for this failure to translate from animal models to human populations is that most animal models induce coronary ischemia alone. However, it is well known that patients who suffer from coronary vascular disease also suffer from metabolic syndrome. The individual aspects of metabolic syndrome contribute to endothelial dysfunction and intensify the coronary disease present in these patients. Therefore, an ideal animal model will induce not only coronary vascular disease but also metabolic syndrome.
Small Molecule Therapy
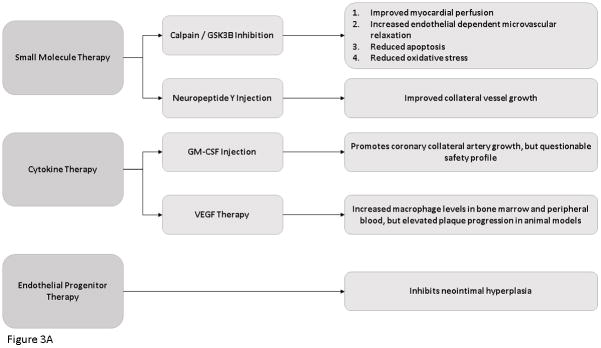
Recently, there have been a number of small molecular which have been successfully shown to improve a functional neovasculature in the setting of ischemia. 30,31 In a pig model of chronic myocardial ischemia in the setting of diet-induced metabolic syndrome, calpain inhibition has been shown to improve myocardial perfusion, increase endothelial dependent microvascular relaxation, decrease apoptosis and improve oxidative stress in ischemic myocardial tissue.32–34 In the same pig model, a downstream molecular target of calpain, glycogen synthase kinase-3B, inhibition was found to have the same beneficial effects. 35
Neuropeptide Y serves as a neurotransmitter that is associated with stress, diet and fat accumulation. In a pig model of diet-induced metabolic syndrome and chronic coronary ischemia, neuropeptide-Y injection into the area of ischemia was found to improve collateral vessel growth and increase angiogenesis and myocardial perfusion and function. 36
Cytokine Therapy
Monocyte activation plays a major role in angiogenesis and collateral artery growth via FGF and VEGF mediated pathways. 37–40 Monocyte chemoattractant protein-1 expression on leukocytes, predominately macrophages, increases the progression of atherosclerosis by increased macrophage numbers and oxidized lipid accumulation. 41 Granulocyte macrophage colony stimulating factor injection into patients with patients with both chronic stable and stenotic coronary artery disease has been found to be effective in promoting coronary collateral artery growth and improving myocardial function. 16,42–44 However, granulocyte macrophage colony stimulating factor was also found to induce development of acute coronary syndrome making its safety questionable. VEGF therapy has been found to increase macrophage levels in bone marrow and peripheral blood, but was also shown to increase plaque progression in cholesterol-fed mice and rabbits, making its efficacy for clinical use questionable.45
Endothelial Progenitor Cell Therapy
Endothelial progenitor cells (EPCs) are thought to serve as a biological marker for vascular function and cumulative cardiovascular risk. 46 Endothelial cell injection has been shown to inhibit neo intimal hyperplasia after arterial injury and to mobilize EPCs to the neo-endothelium. 47 In a mouse model of left anterior descending coronary artery ligation, samples of sites of myocardial infarction demonstrate endothelial progenitor cell incorporation in foci of neovascularization at the border of the infarct. These findings suggest that postnatal neovascularization does not solely rely on sprouting from preexisting blood vessels but also stems from endothelial progenitor cells which circulate from bone marrow to incorporate into and contribute to neovascularization. 48 Endothelial progenitor cells are found at sites of tissue injury and differentiate into mature endothelial cells. Spleen-derived EPCs from healthy rats injected into rats with inflammatory mediated cardiomyopathy resulted in functional improvement in cardiac muscle, reduced scar tissue and thickened ventricular walls in rats receiving the injection as compared with untreated animals. 49 Vascular endothelial growth factor modulates postnatal endothelial progenitor cell kinetics. 50 Patients with unstable angina and no evidence of cardiac stenosis and patients with stable angina were evaluated for circulating EPC numbers. The patients in the experimental group showed an increased number of circulating EPCs. 51
Stem Cell Therapy
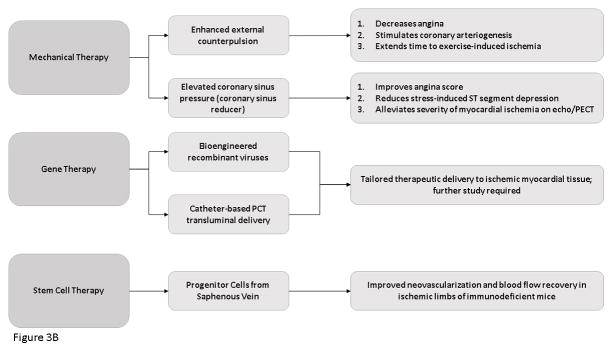
Stem cell therapy is a promising therapeutic approach to reduce myocardial ischemia. Progenitor cells from the saphenous vein of patients undergoing coronary artery bypass surgery established N-cadherin-mediated physical contact with capillary endothelium and improved neovascularization and blood flow recovery in ischemic limbs of immunodeficient mice. 52
Autologous transplantation to grow an artificial blood conduit of any required length and diameter from the cells of the host for autologous transplantation is currently under research. Silastic tubing is inserted into the peritoneal cavity of rats or rabbits. The tubing becomes covered in myofibroblasts, collagen matrix and mesothelium. The silastic tubing is then removed and the implants are inverted so the inner lining consists of a nonthrombotic mesothelial cell “intima” with a new myofibroblast like “media” and a collagen and elastin outer “adventitia” layer. The new vessel is then anastomosed to the carotid artery or abdominal aorta of the same animal in which they were grown and remain patent for at least 4 months. These neo-vessels develop structures resembling elastic lamellae. The myofibroblasts increase in counts and contract. 53 Further research is needed to determine the long term efficacy of these neo vessels.
A Cochrane Review found evaluating the effectiveness of adult bone marrow derived stem cells (BMSC) to treat acute myocardial ischemia infarction found despite the high degree of heterogeneity between studies, BMSC are effective at inducing improvement in global heart function. However a larger number of participants is required. 21
Gene Therapy
Cardiac gene therapy is a promising technique that will allow effective clinical delivery of gene therapy to the heart. The development of bioengineered recombinant viruses that specifically target the myocardium in addition to advances in selection and design of the genetic payload are currently being tested in preclinical models of heart disease. 54–61 Catheter based percutaneous transluminal gene delivery into the coronary artery has been used in pig models to present gene therapy to ischemic myocardial tissue via retrograde delivery into the anterior cardiac vein. 59,62,63 Kaminksy et al. studied the safety profile of an Ad5 vector expression the cDNA/genomic hybrid of vascular endothelial growthfactor (expressing isoforms, 121, 165 and 189) (AdVEGF-AII6A+) in an adult rat ischemia heart model to support the initiation of a clinical study to treat humans with diffuse coronary artery disease. The study concluded that the safety profile of AdVEGF-AII6A+. was safe to proceed to clinical trial. 64 The Phase I Study of Direct Administration of a Replication Deficient Adenovirus Vector (AdGVVEGF121.10) Containing the VEGF121 cDNA to the Ischemic Myocardium of Individuals with Diffuse Coronary Artery Disease was and the Phase I Study of Direct Administration of a Replication Deficient Adenovirus Vector (AdGVVEGF121.10) Containing the VEGF121 cDNA to the Ischemic Myocardium of Individuals with Diffuse Coronary Artery Disease Via Minimally Invasive Surgery were started in2010. The study is closed but the results have not yet been reported. 65 Gene therapy is a promising new approach to ischemic disease. 66 However, future work is needed to determine the most appropriate route of delivery and the best gene to target.
Mechanical Therapy
The introduction of mechanical therapy to induce collateral vessel growth is a recent area of research. Transmyocardial laser revascularization (TMLR) has been proposed to improve symptoms of chronic angina in patients with advanced coronary artery disease. Transmyocardial laser revascularization is a surgical intervention which uses a laser device directly on the heart surface to reestablish blood flow in certain areas of the heart. Its intended use in on patients who have severe coronary artery disease with poor quality of life who are not candidates for revascularization procedures. A Cochrane Review published in February of 2015 assess the effects of TMLR versus optimal medical treatment in people with refractory angina who are not candidate for revascularization procedures to alleviate angina severity, reduce mortality and improve ejection fraction. The review established that there was no difference in survival between groups and that there was a significant increase in postoperative mortality and other safety outcomes after TMLR therapy versus the control. Therefore, the review concluded that the risks of TMLR therapy outweigh the potential clinical benefits. 67
New devices to mechanically induce collateral growth are currently under investigation. Enhanced External Counterpulsation treatment has been found to reduce angina, stimulate coronary arteriogenesis and extend time to exercise induced ischemia in patients with symptomatic CAD. 13,68–70 Additionally, increased coronary sinus pressure can reduce myocardial ischemia by redistribution of blood from nonischemic to ischemic territories. A Coronary Sinus Reducer is a percutaneous implantable device designed to establish coronary sinus narrowing and to elevate coronary sinus pressure. It has been found to improve angina score, reduce stress induced ST-segment depression and alleviate the extent and severity of myocardial ischemia by dobutamine echocardiography and by thallium single-photon emission computed tomography. 71 [Figure 3]
Figure 3. Novel Molecular Therapies for The Treatment of Ischemic Heart Disease.
Flow Diagram demonstrating the new molecular therapies
Conclusion
There have been huge breakthroughs in cardiovascular treatment in the last decade both with interventional and medical therapies. However, due to large number of people who suffer from cardiovascular disease and the high morbidity and mortality associated with it, new medical therapies to reduce ischemic disease will continue to change the way we treat patients. [Table 2] Therapeutic angiogenesis is an active area of research. Small molecule, cytokine, endothelial progenitor cell, stem cell, gene and mechanical therapies show promise in increasing the collateral growth of blood vessels, thereby reducing myocardial ischemia.
Table 2.
Potential Angiogenic Therapy
| Potential Angiogenic Therapy | Proposed Mechanism of Action | Planned Clinical Trials (Y/N) |
|---|---|---|
| Calpain/GSK3B inhibition | Improved myocardial perfusion, reduced apoptosis, increased microvascular relaxation, reduced oxidative stress | Y |
| Neuropeptide Y injection | Improved collateral vessel growth | Y |
| GM-CSF injection | Increased coronary collateral arterial growth | Y |
| VEGF Therapy | Increased macrophage levels in bone marrow and peripheral blood | Y |
| Endothelial progenitor therapy | Inhibits neointimal hyperplasia | Y |
| Stem Cell Therapy | Improved neovascularization and blood-flow recovery in ischemic myocardial tissue | Y |
Acknowledgments
This work was supported in part by the National Heart, Lung, and Blood Institute (RO1HL128831-01A1 (Sellke, Usheva-Simdjuyska)); NIH/NIGMS GM1P20GM103652 (Project-3) and American Heart Association Grant-in-Aid 14GRNT20460291 (Abid); NIH/NIGMS Training Grant 2T32 GM065085-12 (Potz).
References
- 1.Hartiala J, Schwartzman WS, Gabbay J, Ghazalpour A, Bennett BJ, Allayee H. The Genetic Architecture of Coronary Artery Disease: Current Knowledge and Future Opportunities. Curr Atheroscler Rep [Internet] 2017;19:6. doi: 10.1007/s11883-017-0641-6. Available from: http://link.springer.com/10.1007/s11883-017-0641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, De Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, MacKey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Executive summary: Heart disease and stroke statistics-2016 update: A Report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 3.Hartiala J, Schwartzman WS, Gabbay J, Ghazalpour A, Bennett BJ, Allayee H. The Genetic Architecture of Coronary Artery Disease: Current Knowledge and Future Opportunities. Curr Atheroscler Rep. 2017;19:6. doi: 10.1007/s11883-017-0641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verrier ED, Boyle EM., Jr Endothelial cell injury in cardiovascular surgery. Ann Thorac Surg. 1996;62:915–922. doi: 10.1016/s0003-4975(96)00528-0. [DOI] [PubMed] [Google Scholar]
- 5.Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial Barrier and Its Abnormalities in Cardiovascular Disease. Front Physiol [Internet] 2015;6:1–11. doi: 10.3389/fphys.2015.00365. Available from: http://journal.frontiersin.org/article/10.3389/fphys.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buschmann I, Heil M, Jost M, Schaper W. Influence of inflammatory cytokines on arteriogenesis. Microcirculation. 2003;10:371–379. doi: 10.1038/sj.mn.7800199. [DOI] [PubMed] [Google Scholar]
- 7.Buschmann I, Schaper W. Arteriogenesis Versus Angiogenesis: Two Mechanisms of Vessel Growth. News Physiol Sci [Internet] 1999;14:121–125. doi: 10.1152/physiologyonline.1999.14.3.121. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11390835. [DOI] [PubMed] [Google Scholar]
- 8.Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai) 2008;40:681–692. [PubMed] [Google Scholar]
- 9.Deroanne CF, Hajitou A, Calberg-Bacq CM, Nusgens BV, Lapiere CM. Angiogenesis by fibroblast growth factor 4 is mediated through an autocrine up-regulation of vascular endothelial growth factor expression. Cancer Res. 1997;57:5590–5597. [PubMed] [Google Scholar]
- 10.Billinger M, Kloos P, Eberli FR, Windecker S, Meier B, Seiler C. Physiologically assessed coronary collateral flow and adverse cardiac ischemic events: A follow-up study in 403 patients with coronary artery disease. J Am Coll Cardiol. 2002;40:1545–1550. doi: 10.1016/s0735-1097(02)02378-1. [DOI] [PubMed] [Google Scholar]
- 11.Meier P, Gloekler S, Zbinden R, Beckh S, De Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: A 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation. 2007;116:975–983. doi: 10.1161/CIRCULATIONAHA.107.703959. [DOI] [PubMed] [Google Scholar]
- 12.Planat-Benard V. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells: Physiological and Therapeutic Perspectives. Circulation [Internet] 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. Available from: http://circ.ahajournals.org/cgi/doi/10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 13.Buschmann EE, Utz W, Pagonas N, Schulz-Menger J, Busjahn A, Monti J, Maerz W, Le Noble F, Thierfelder L, Dietz R, Klauss V, Gross M, Buschmann IR. Improvement of fractional flow reserve and collateral flow by treatment with external counterpulsation (Art.Net.-2 Trial) Eur J Clin Invest. 2009;39:866–875. doi: 10.1111/j.1365-2362.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 14.Schaper W. Collateral circulation. Past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamping KG, Zheng W, Xing D, Christensen LP, Martins J, Tomanek RJ. Bradycardia stimulates vascular growth during gradual coronary occlusion. Arterioscler Thromb Vasc Biol. 2005;25:2122–2127. doi: 10.1161/01.ATV.0000179598.57819.77. [DOI] [PubMed] [Google Scholar]
- 16.Schirmer SH, van Nooijen FC, Piek JJ, van Royen N. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart [Internet] 2009;95:191–7. doi: 10.1136/hrt.2007.136119. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19144878. [DOI] [PubMed] [Google Scholar]
- 17.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, Moon T, Chronos NA. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: Double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 18.Grines CL. Angiogenic Gene Therapy (AGENT) Trial in Patients With Stable Angina Pectoris. Circulation [Internet] 2002;105:1291–1297. doi: 10.1161/hc1102.105595. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-0037133624&partnerID=tZOtx3y1. [DOI] [PubMed] [Google Scholar]
- 19.Janssens Stefan, MD, Dubois Christophe, MD, Bogaert Jan, MD, Theunissen Koen, MD, Deroose Christophe, MD, Desmet Walter, MD, Kalantzi Maria, MD, Herbots Lieven, MD, Sinnaeve Peter, MD, Dens Joseph, MD, Maertens Johan, MD, Rademakers Frank, MD, Steven DM. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:P113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 20.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: Final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 21.Clifford DMM, Fisher SAA, Brunskill SJJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane database Syst Rev [Internet] 2012;2:CD006536. doi: 10.1002/14651858.CD006536.pub3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22336818. [DOI] [PubMed] [Google Scholar]
- 22.Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz-Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guédès A, Heyse A, Moccetti T, Fernandez-Aviles F, Jimenez-Quevedo P, Bayes-Genis A, Hernandez-Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W. Cardiopoietic cell therapy for advanced ischemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J [Internet] 2016:ehw543. doi: 10.1093/eurheartj/ehw543. Available from: https://academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehw543. [DOI] [PMC free article] [PubMed]
- 23.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: The C-CURE (cardiopoietic stem cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 24.Moss AJ, Williams MC, Newby DE, Nicol ED. The Updated NICE Guidelines: Cardiac CT as the First-Line Test for Coronary Artery Disease. Curr Cardiovasc Imaging Rep [Internet] 2017;10:15. doi: 10.1007/s12410-017-9412-6. Available from: http://link.springer.com/10.1007/s12410-017-9412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Thomas M, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Cardiovascul. 2014 [Google Scholar]
- 26.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH, Jacobs AK, Albert N, Creager MA, Ettinger SM, Halperin JL, Hochman JS, Kushner FG, Magnus Ohman E, Stevenson W, Yancy CW. ACCF/AHA/SCAI guideline for percutaneous coronary intervention a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;2011:124. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 27.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, DiSesa VJ, Hiratzka LF, Hutter AM, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD, Jacobs AK, Albert N, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: Executive summary. J Am Coll Cardiol [Internet] 2011;58:2584–2614. Available from: http://dx.doi.org/10.1016/j.jacc.2011.08.008. [Google Scholar]
- 28.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK, Smith SC. ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of th…. Circulation [Internet] 2016 doi: 10.1016/j.jtcvs.2016.07.044. [cited 2017 Apr 27];134. Available from: http://circ.ahajournals.org/content/134/10/e123#T3. [DOI] [PubMed]
- 29.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O’Gara PT, Sabatine MS, Smith PK, Smith SC. ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of th…. Circulation. 2016;2016:134. doi: 10.1016/j.jtcvs.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 30.Hoang MV, Nagy Ja, Fox JEB, Senger DR. Moderation of calpain activity promotes neovascular integration and lumen formation during VEGF-induced pathological angiogenesis. PLoS One. 2010:5. doi: 10.1371/journal.pone.0013612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoang MV, Smith LESD. Calpain inhibitors reduce retinal hypoxia in ischemic retinopathy by improving neovascular architecture and functional perfusion. Biochim Biophys Acta [Internet] 2011;1812:997–1549. doi: 10.1016/j.bbadis.2010.08.008. –557003. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3005970/pdf/nihms233182.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potz Brittany A, Sabe Ashraf A, MD, Elmadhun Nassrene Y, MD, Feng Jun, MD/PhD, Clements Richard T, PhD, Ruhul Abid M, MD/PhD, Sellke Frank WM. Calpain Inhibition Modulates GSK-3β Pathways in A Swine Model of Chronic Myocardial Ischemia in the Setting of Metabolic Syndrome: A Proteomic and Mechanistic Analysis. J Thorac Cardiovasc Surg. 2017;153:342–357. doi: 10.1016/j.jtcvs.2016.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potz BA, Sabe AA, Elmadhun NY, Feng J, Liu Y, Mitchell H, Quesenberry P, Abid MR, Sellke FW. Calpain inhibition decreases myocardial apoptosis in a swine model of chronic myocardial ischemia. Surgery [Internet] 2015;158:445–452. doi: 10.1016/j.surg.2015.03.034. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0039606015002391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabe AA, Potz BA, Elmadhun NY, Liu Y, Feng J, Abid MR, Abbott JD, Senger DR, Sellke FW. Calpain Inhibition Improves Collateral Dependent Perfusion in a Hypercholesterolemic Swine Model of Chronic Myocardial Ischemia. J Thorac Cardiovasc Surg [Internet] 2016;151:245–52. doi: 10.1016/j.jtcvs.2015.08.101. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0022522315015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potz Brittany A, Sabe Ashraf A, MD, Elmadhun Nassrene Y, MD, Feng Jun, MD, PhD, Clements Richard T, PhD, Ruhul Abid M, MD, PhD, Sellke Frank W., M Glycogen Synthase Kinase 3B Inhibition Improves Myocardial Angiogenesis and Collateral-dependent Perfusion in a Swine Model of Metabolic Syndrome. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003694. pii: e003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matyal R, Chu L, Mahmood F, Robich MP, Wang A, Hess PE, Shahul S, Pinto DS, Khabbaz K, Sellke FW. Neuropeptide Y improves myocardial perfusion and function in a swine model of hypercholesterolemia and chronic myocardial ischemia. J Mol Cell Cardiol. 2012;53:891–898. doi: 10.1016/j.yjmcc.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 37.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujii T, Yonemitsu Y, Onimaru M, Tanii M, Nakano T, Egashira K, Takehara T, Inoue M, Hasegawa M, Kuwano H, Sueishi K. Nonendothelial mesenchymal cell-derived MCP-1 is required for FGF-2-mediated therapeutic neovascularization: Critical role of the inflammatory/arteriogenic pathway. Arterioscler Thromb Vasc Biol. 2006;26:2483–2489. doi: 10.1161/01.ATV.0000244684.23499.bf. [DOI] [PubMed] [Google Scholar]
- 39.Hoefer IE, Van Royen N, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–617. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- 40.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012 Sep 3; doi: 10.3389/fphys.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Natoli E, Rollins BJ, Milos PM. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol [Internet] 1999;19:1518–1525. doi: 10.1161/01.atv.19.6.1518. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10364084%5Cnhttp://atvb.ahajournals.org/content/19/6/1518.full.pdf. [DOI] [PubMed] [Google Scholar]
- 42.Zbinden S, Zbinden R, Meier P, Windecker S, Seiler C. Safety and efficacy of subcutaneous-only granulocyte-macrophage colony-stimulating factor for collateral growth promotion in patients with coronary artery disease. J Am Coll Cardiol. 2005;46:1636–1642. doi: 10.1016/j.jacc.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 43.Seiler C, Pohl T, Wustmann K, Hutter D, Nicolet PA, Windecker S, Eberli FR, Meier B. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation [Internet] 2001;104:2012–7. doi: 10.1161/hc4201.097835. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11673338. [DOI] [PubMed] [Google Scholar]
- 44.Meier P, Gloekler S, De Marchi SF, Indermuehle A, Rutz T, Traupe T, Steck H, Vogel R, Seiler C. Myocardial salvage through coronary collateral growth by granulocyte colony-stimulating factor in chronic coronary artery disease: A controlled randomized trial. Circulation. 2009;120:1355–1363. doi: 10.1161/CIRCULATIONAHA.109.866269. [DOI] [PubMed] [Google Scholar]
- 45.Celletti FL, Waugh JM, Amabile PG, Brendolan A, Hilfiker PR, Dake MD. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med [Internet] 2001;7:425–9. doi: 10.1038/86490. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11283668. [DOI] [PubMed] [Google Scholar]
- 46.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AAFT. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. Nejm. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 47.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, Matsunaga S, Tateishi K, Nomura T, Takahashi T, Tatsumi T, Matsubara H. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 48.Boden W, O’Rourke R. Optimal medical therapy with or without PCI for stable coronary disease (COURAGE TRIAL) N Engl J Med [Internet] 2007:1503–1516. doi: 10.1056/NEJMoa070829. Available from: http://www.nejm.org/doi/full/10.1056/NEJMoa070829. [DOI] [PubMed]
- 49.Werner L, Deutsch V, Barshack I, Miller H, Keren G, George J, et al. Transfer of endothelial progenitor cells improves myocardial performance in rats with dilated cardiomyopathy induced following experimental myocarditis. J Mol Cell Cardiol [Internet] 2005;39:691–7. doi: 10.1016/j.yjmcc.2005.06.015. [cited 2017 Mar 26] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16125196. [DOI] [PubMed] [Google Scholar]
- 50.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G. Circulating endothelial progenitor cells in patients with unstable angina: Association with systemic inflammation. Eur Heart J. 2004;25:1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 52.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human Adult Vena Saphena Contains Perivascular Progenitor Cells Endowed With Clonogenic and Proangiogenic Potential. Circulation [Internet] 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20368523%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC2917746%0Ahttp://circ.ahajournals.org/cgi/doi/10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell JH, Efendy JL, Campbell GR. Novel vascular graft grown within recipient’s own peritoneal cavity. Circ Res. 1999;85:1173–1178. doi: 10.1161/01.res.85.12.1173. [DOI] [PubMed] [Google Scholar]
- 54.ARL, MS, RJH, RJS, SEH Gene therapy: Targeting the myocardium [Internet] Heart. 2008;94:89–99. doi: 10.1136/hrt.2007.116483. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed8&NEWS=N&AN=2008011030. [DOI] [PubMed] [Google Scholar]
- 55.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, Isner JM. Clinical Investigation and Reports Constitutive Expression of phVEGF 165 After Intramuscular Gene Transfer Promotes Collateral Vessel Development in Patients With Critical Limb Ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 56.Logeart D, Hatem SN, Rücker-Martin C, Chossat N, Névo N, Haddada H, Heimburger M, Perricaudet M, Mercadier JJ. Highly efficient adenovirus-mediated gene transfer to cardiac myocytes after single-pass coronary delivery. Hum Gene Ther [Internet] 2000;11:1015–22. doi: 10.1089/10430340050015329. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10811230. [DOI] [PubMed] [Google Scholar]
- 57.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsurumi Y, Kearney M, Chen D, Silver M, Takeshita S, Yang J, Symes J, Isner JM. Treatment of acute limb ischemia by intramuscular injection of vascular endothelial growth factor gene. Circulation. 1997;96:II–8. [PubMed] [Google Scholar]
- 59.Boekstegers P, von Degenfeld G, Giehrl W, Heinrich D, Hullin R, Kupatt C, Steinbeck G, Baretton G, Middeler G, Katus H, Franz WM. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther [Internet] 2000;7:232–40. doi: 10.1038/sj.gt.3301079. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10694800. [DOI] [PubMed] [Google Scholar]
- 60.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K, Isner JM. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation [Internet] 1998;98:2800–4. doi: 10.1161/01.cir.98.25.2800. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9860779. [DOI] [PubMed] [Google Scholar]
- 61.Rubanyi GM. Mechanistic, Technical, and Clinical Perspectives in Therapeutic Stimulation of Coronary Collateral Development by Angiogenic Growth Factors. Mol Ther. 2013;21:725–738. doi: 10.1038/mt.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi W, Schmarkey LS, Jiang R, Bone CC, Condit ME, Dillehay DL, Engler RL, Rubanyi GM, Vinten-Johansen J. Ischemia-reperfusion increases transfection efficiency of intracoronary adenovirus type 5 in pig heart in situ. Hum Gene Ther Methods [Internet] 2012;23:204–212. doi: 10.1089/hgtb.2012.048. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22816318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasano T, Kikuchi K, McDonald AD, Lai S, Donahue JK. Targeted high-efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007;42:954–961. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaminsky SM, Quach L, Chen S, Pierre-Destine L, Van de Graaf B, Monette S, Rosenberg JB, De BP, Sondhi D, Hackett NR, Mezey JG, Rosengart TKCR. Safety of direct cardiac administration of AdVEGF-All6A+, a replication-deficient adenovirus vector cDNA/genomic hybrid expressing all three major isoforms of human vascular endothelial growth factor, to the ischemic myocardium of rats. Hum Gene Ther Methods. 2013;24(1):38–46. doi: 10.1089/humc.2013.054. [DOI] [PubMed] [Google Scholar]
- 65.University SWMC of CUCSB. Post Ten Year Follow up Assessment of a Phase I Trial of Angiogenic Gene Therapy [Internet] Clin. Trials.gov Identifier NCT01174095. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01174095?term=VEGF+and+coronary+artery+disease&rank=3.
- 66.Donahue JK, Kikkawa K, Johns DC, Marban E, Lawrence JH. Ultrarapid, highly efficient viral gene transfer to the heart. Proc Natl Acad Sci U S A [Internet] 1997;94:4664–8. doi: 10.1073/pnas.94.9.4664. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=20781&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Briones E, Lacalle JR, Marin-Leon IRJ. Transmyocardial laser revascularization versus medical therapy for refractory angina. Cochrane Database Syst Rev [Internet] 2015:2. doi: 10.1002/14651858.CD003712.pub3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21755257. [DOI] [PMC free article] [PubMed]
- 68.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): Effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–1840. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 69.Braverman DL. Enhanced external counterpulsation: an innovative physical therapy for refractory angina. PM R [Internet] 2009;1:268–76. doi: 10.1016/j.pmrj.2008.12.002. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19627906. [DOI] [PubMed] [Google Scholar]
- 70.Gloekler S, Meier P, de Marchi SF, Rutz T, Traupe T, Rimoldi SF, Wustmann K, Steck H, Cook S, Vogel R, Togni M, Seiler C. Coronary collateral growth by external counterpulsation: a randomised controlled trial. Heart [Internet] 2010;96:202–7. doi: 10.1136/hrt.2009.184507. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19897461. [DOI] [PubMed] [Google Scholar]
- 71.Banai S, Ben Muvhar S, Parikh KH, Medina A, Sievert H, Seth A, Tsehori J, Paz Y, Sheinfeld A, Keren G. Coronary Sinus Reducer Stent for the Treatment of Chronic Refractory Angina Pectoris. A Prospective, Open-Label, Multicenter, Safety Feasibility First-in-Man Study. J Am Coll Cardiol. 2007;49:1783–1789. doi: 10.1016/j.jacc.2007.01.061. [DOI] [PubMed] [Google Scholar]