Abstract
Background
There are limited data on vascular cognitive impairment (VCI) from low- and middle income countries where the stroke burden is burgeoning.
Objective
To characterize the burden, determinants, and effects on health-related quality of life, of VCI in sub-Saharan Africa (SSA).
Methods
From January 2015 to February 2016, we collected information on 147 consecutive stroke survivors (>45 years) seen at a tertiary hospital in Ghana and 49 demographically matched stroke-free controls. Data collected included demographics, clinical factors, health-related quality of life and presence of depression. Cognitive status was evaluated using a standard vascular neuropsychological battery which assessed memory, executive function/mental speed, language, and visuospatial/ visuoconstructive functioning. Expert VCI guideline and DSM IV criteria were used to classify stroke patients into no VCI, VCI but no dementia, and vascular dementia (VD).
Results
Mean ± SD age of stroke survivors was 59.9 ± 13.7 years of which 47.6% were women. Among the cohort, 77/147 (52.3%) had no VCI, 50/147 (34.0%) had VCI no dementia and 20/147 (13.6%) had VD. Three factors remained significantly associated with VCI: increasing age for each successive 10 year rise (OR 1.44, 95% CI: 1.03–2.02); lack of formal education (OR 5.26, 95% CI: 1.01–27.52); and worse functional disability on the modified Rankin scale (OR 2.46, 1.61–3.75). Patients with VD had the poorest health related quality of life.
Conclusion
Half of the Ghanaian stroke survivors encountered in this cross-sectional study had evidence of cognitive dysfunction. Future studies in SSA will need to identify strategies to address this immense burden.
Keywords: Vascular dementia, risk factors, quality of life, Ghana
INTRODUCTION
Stroke is a devastating medical disorder associated with significant morbidity and mortality particularly in Low-and-Middle Income Countries in sub-Saharan Africa (1–4). In addition to physical disability, stroke survivors often experience profound alterations in cognitive function as well as mental health impairments with adverse repercussions for stroke patients, their families and the society at large (5). Post-stroke neurocognitive dysfunction (PSNCD) is a multidomain impairment of cognitive ability with predilection for attention and concentration, executive function, memory, language and visuospatial domains of cognition (6–8). It is a spectrum that spans from mild impairments in single cognitive domains to post-stroke dementia with nearly 65% of stroke survivors estimated to suffer from cognitive impairments and about 30% developing dementia (9,10).
A myriad of factors has been posited to predispose to post-stroke vascular cognitive impairment including socio-demographic variables such as age, educational attainment, occupation and environmental enrichment; cardiovascular risk factor profile and stroke-related characteristics as well as neuroimaging correlates such as the number, size and sites of lesions, white matter changes, lacunar infarcts, strategic infarcts, cerebral microbleeds, medial temporal lobe atrophy and global cerebral atrophy. (11–16)
Characterizing the burden, spectrum, determinants and implications of post-stroke cognitive impairments in LMICs in sub-Saharan Africa (SSA) has received little attention largely due the perennial paucity of neurologists and mental health practitioners although stroke burden on the continent is enormous. Among the few studies conducted in SSA among Nigerian stroke survivors identified 40% with cognitive impairment without dementia and 8% with post-stroke dementia 3 months after stroke onset. (11) It remains unknown whether these findings are applicable to other African populations of diverse cultural backgrounds or subjects who have survived stroke for more than 1 year. Our objective for the present study is therefore to assess the burden, predictors and impact on a stroke specific, health-related quality of life of vascular cognitive impairment among a cross-section of Ghanaian stroke survivors attending a Neurology clinic in a tertiary Medical Center. The harmonized National Institute of Neurological Disorders and Stroke and Canadian Stroke Networks (NINDS-CSN) (9) established common standards for VCI assessments were used in the present study.
METHODS
Study design and setting
This cross-sectional study was approved by the Committee on Human Research Publication and Ethics (CHRPE) of the School of Medical Sciences, Kwame Nkrumah University of Science and Technology, and the Komfo Anokye Teaching Hospital, (KATH) Kumasi, Ghana. The study was conducted at the Neurology Clinic of the Komfo Anokye Teaching Hospital, a tertiary medical center in Kumasi, Ghana. Kumasi is the second largest city in Ghana with an estimated population of 4 million inhabitants. The Neurology clinic was instituted in 2011 and currently runs once a week providing care for adults >16 years with neurologic disorders from 6 out of the 10 administrative regions of Ghana and serves an estimated population of 10 million. (17)
Study Participants-Stroke subjects
Consecutive stroke survivors attending the Neurology service at KATH were approached for enrollment into the study after obtaining informed consent. Stroke subjects should have had stroke for at least 3 months to enable the resolution of acute post-stroke delirium in accordance with Desmond et al. (18) Stroke diagnosis and primary types were confirmed using a CT scan taken at onset of stroke in 125 out of 147 (85%) of stroke survivors due to the high cost of CT scans in the region with the WHO criteria used to classify the remainder.
We excluded (i) stroke subjects <45 years, (ii) stroke survivors on sedatives, (iii) those with profound aphasia without a proxy, (iv) those with significant physical illness and motor, visual or hearing impairments that precluded paper-based neuropsychological evaluations), (v) any co-morbid psychiatric or neurologic illness such as schizophrenia, manic-depressive disorder, major depression, Parkinson’s disease), (vi) any systemic disorders capable of impairing cognition such as chronic kidney disease and decompensated liver disease, and (vii) failure to give consent or complete the assessments. Recruitment of study participants was performed from January 2015 to February 2016.
Study Participants-Stroke-free subjects
Forty-nine stroke-free controls were recruited for comparison with the neuropsychological data from stroke survivors from communities in the Kumasi Metropolitan Assembly from an on-going epidemiological study on stroke in West Africa (19). Stroke-free status was ascertained using a pictorial version of a locally validated 8-item Questionnaire for Verifying Stroke Free Status (20). Control subjects were excluded if they had known background dementia (DSM IV criteria), psychiatric illnesses or were unable to provide consent or complete the evaluations required for the study. Controls were closely matched for age, gender and educational attainment.
Evaluation of study subjects
We first collected demographic information including age, gender, marital, educational and occupational status as well as location of residence. Vascular risk factor profile was assessed for stroke survivors based on self-report, use of relevant medications and review of medical records for evidence of hypertension, diabetes mellitus, dyslipidemia, atrial fibrillation or other cardiac disorders, cigarette smoking and alcohol use. The following criteria were used to assess vascular risk factor status
The weight of study subjects was measured in kilograms using a scale with patient standing at the anatomical position on a scale and the height in centimeters was measured using a stadiometer with patient standing at the anatomical position in front of the stadiometer. The weight and height measurements were used to calculate the body mass index (BMI). Subjects with BMI ≥ 30kg/m2 were classified as obese.
Blood pressure was measured thrice on the upper left arm using a validated automatic sphygmomanometer, after at least 5 minutes of rest and the second and third readings were averaged for analysis. Hypertension was diagnosed if the patient was on antihypertensive medications over the last 15 consecutive days or if the patient had a systolic and/or diastolic blood pressure of 140 / 90 mmHg.
Participants were considered to have diabetes mellitus if they were on hypoglycemic medications or if their fasting blood glucose levels were > 126mg/dl and/or HbA1C >6.5%.
Dyslipidemia was defined as a high total cholesterol > 200mg/dl or LDL-cholesterol > 130mg/dl, triglyceride > 150mg/dl or HDL-cholesterol <40mg/dl for women and <50mg/dl for men or previous use of statin for dyslipidemia.
Cardiac disease including myocardial infarction, rheumatic valvular heart disease, and prosthetic heart valve, atrial fibrillation or flutter was based on self-reported history, clinical examination, review of baseline ECG and/or echocardiography result at enrollment into care at the neurology clinic.
Current smoking status and alcohol intake status was ascertained from either the patient or a reliable relative. A high alcohol intake was defined as ≥ 14 U per week for women, ≥ 21 U per week for men.
Physical activity status of participants was assessed using the International Physical Activity Questionnaire. Responders who reported spending more than half the day on their feet or reported daily exercises were classified as physically active. Those who spent less than half of the day on their feet or led a sedentary life were classed as physically inactive.
Stroke type was defined radiologically into ischemic and hemorrhagic based on cranial CT scan done at onset of stroke symptoms for 85% of study subjects who had information in their medical records. Stroke severity was assessed using National Institute of Health Stroke Scale (NIHSS) (21), and functional status assessed using the Modified Rankin scale (22) were collected by two trained Research Assistants through review of medical charts and interview of stroke survivors and/or their proxy. The 20-item Center for Epidemiologic Studies Depression Scale (23) was used to screen for depressive symptoms among stroke survivors. The Health-Related Quality of Life in Stroke Patients questionnaire24 is a multidimensional instrument which assesses the physical, psycho-emotional, cognitive and socio-economic domains of well being was used to assess the quality of life of each subject. The physical, psycho-emotional, and socio-economic domains of the HRQOLISP has 7 items each with a minimum and maximum scores of 7 and 35 respectively, while the cognitive domain has 5 items with minimum and maximum scores of 5 and 25 respectively. Higher scores on the HRQOLISP indicate a better quality of life and vice versa.
Neurocognitive Assessment
Neurocognitive assessments were performed by two experienced Research Officers who received four weeks of training on the study instruments until proficiency was attained with inter-rater agreement of >95% among hospital-based volunteers. The cognitive evaluations comprised of the Montreal Cognitive Assessment (MOCA) (25) and the Vascular Neuropsychological Battery (V-NB) (9). While the MOCA is considered a test of general cognitive functioning, the V-NB is comprised of a battery of tests which evaluates the functions of specific cognitive domains and was patterned after the NINDS-CSN Harmonization Standards 60-minute neuropsychological protocol (9). Specifically, the V-NB assesses 4 key domains namely executive function, memory/learning, language and visuospatial/visuoconstructive skills using validated test items.
Executive/activation and mental speed were assessed using the category (animal) fluency test, (26) verbal and visual reasoning tests adapted from the Cambridge Cognitive Examination (CAMCOG) battery.
Language was evaluated using the 15-item Boston Naming Test and memory/learning was assessed with the 10-item word list learning test and delayed recall of stick design as previously described (26,27). The word list learning consists of a 3-trials of a 10-item list with recall taken after each learning trial and after a brief delay. The total number of words recalled across the three trials totals a score range of 0–30, with higher scores indicative of better performance.
The visuospatial / visuoconstructive domain was assessed using a non-graphomotor test called the Stick Design Test (27). This test which is particularly useful in older adults with limited formal education requires the respondent to use 4 match sticks to reproduce four different shapes with attention to the correctness of the relative positions of the match heads without any cues to assist.
An individual subject is deemed to have failed a test item if the mean score was at least 1.5 standard deviations below the mean score of the control group. Impairment in a domain is defined as failure on at least 50% of the tests examining that domain. (6) Vascular Cognitive Impairment was defined as impairment in at least 1 cognitive domain (memory/learning, executive domain, visuospatial/visuoconstructive skills and language) and normal or mild impairment of activities of daily living independent of motor/sensory symptoms according to the American Stroke Association/American Heart Association Vascular Cognitive Impairment Guideline (6). Post stroke dementia was defined according to the DSM IV criteria as impairment in > 2 cognitive domains of sufficient severity to affect the individuals ability to perform activities of daily living independent of motor or sensory symptoms (6). Functional impairment was defined as a Barthel Index score of less than 75% (28).
Statistical Analysis
Means and medians were compared using the Student’s t-test or Mann-Whitney’s U-test for paired comparisons and ANOVA or Kruskal Wallis tests for three or more groups comparisons. Proportions were compared using the Chi-squared test with Yates correction for proportions with subgroupings <5. A multivariate logistic regression analysis was performed to identify independent predictors of Vascular Cognitive Impairment. In all analysis, two-tailed p-values <0.05 were considered statistically significant with no adjustments for multiple comparisons. Statistical analysis was performed using SPSS version 19 and GraphPad Prism version 7.
RESULTS
Study Population
249 subjects comprising of 200 stroke survivors and 49 community controls were enrolled into the study. Among the stroke survivors, 53 were excluded from further analysis due moderate-to-severe aphasia without a proxy, n=34; restlessness /tiredness by subjects who could not complete the study, n=17, severe visual defects n=1, and hearing impairment, n=1. Thus 147 stroke survivors met the selection criteria for further analysis.
Demographic and clinical characteristics of Stroke survivors & controls
The mean ± SD age of stroke cases of 59.9 ± 13.7 years was not significantly different from that of control subjects of 60.3 ± 15.5 years. Stroke cases were less likely than controls to be currently employed and dwell in urban locations but the two groups had similar educational attainment and marital status. (Table 1). Waist-to-hip ratio, body mass indices, systolic blood pressures were not dissimilar in the two groups however mean diastolic blood pressure of 91.6 ± 15.9mmHg was significantly higher among stroke survivors compared with 82.8 ± 17.9mmHg for controls.
TABLE 1.
Comparison of socio-demographic and clinical characteristics of Study Subjects.
| CHARACTERISTIC | Stroke Cases N = 147 |
Control Subjects n=49 |
P-value | |
|---|---|---|---|---|
| Age, mean ± SD | 59.9 ± 13.7 | 60.3 ± 15.5 | 0.86 | |
| Gender, Female, n (%) | 70 (47.6) | 27 (55.1) | 0.41 | |
| Educational Status | ||||
| None | 22 (15.0) | 11 (22.4) | 0.66 | |
| Primary | 42 (28.6) | 14 (28.6) | ||
| Secondary | 60 (40.8) | 17 (34.7) | ||
| Tertiary | 23 (15.6) | 7 (14.3) | ||
| Location of domicile | ||||
| Rural | 5 (3.4) | 1 (2.0) | 0.01 | |
| Semi-urban | 42 (28.6) | 4 (8.2) | ||
| Urban | 100 (68.0) | 44 (89.8) | ||
| Marital Status | ||||
| Married | 100 (68.0) | 33 (67.3) | 0.46 | |
| Divorced/single | 23 (15.6) | 5 (10.2) | ||
| Widow | 24 (16.3) | 11 (22.4) | ||
| Occupational Status | ||||
| Employed | 108 (73.5) | 44 (89.8) | 0.02 | |
| Unemployed | 39 (16.5) | 5 (10.2) | ||
| BMI, mean ± SD | 27.4 ± 5.5 | 28.1 ± 8.3 | 0.86 | |
| WHR, mean ± SD | 0.92 ± 0.09 | 0.93 ± 0.16 | 0.5 | |
| Systolic BP, mean ± SD | 147.1 ± 48.9 | 145.5 ± 25.1 | 0.83 | |
| Diastolic BP, mean ± SD | 91.6 ± 15.9 | 82.8 ± 17.9 | 0.001 | |
| Montreal Cognitive | ||||
| Assessment, mean ± SD | 16.4 ± 8.4 | 20.0 ± 7.9 | 0.01 | |
Profile and Patterns of cognitive performance
The mean ± (SD) scores on the MOCA test among stroke survivors was 16.4 ± 8.4 compared with 20.0 ± 7.9 among controls, p=0.01. Using a conservative cut-off score of 23/30 for the MOCA test with adjustment for years of education attained, 27 out of 49 (55.1%) of control subjects and 107 out of 147 (72.8%) stroke subjects, p=0.001 demonstrated cognitive impairments on screening with this instrument.
Further interrogation using the validated Neuropsychiatric battery which assessed executive function/activation and mental speed, memory/learning, language and visuospatial skills domains revealed 77/147 (52.3%) had no VCI, 50/147 (34.0%) had VCI no dementia and 20/147 (13.6%) had post-stroke dementia (PSD). Among subjects with VCI no dementia, 21/50 (42.0%) had single domain non-amnestic, 7/50 (14.0%) had multiple domain non-amnestic, 7/50 (14.0%) had single domain amnestic and 15/50 (30.0%) had multiple domain amnestic impairments. The sub-group with single domain non-amnestic VCI comprised of 11/21 with visuospatial impairment, 6/21 with executive dysfunction and 4/21 with language dysfunction.
Among stroke survivors, those with no VCI were significantly younger, had higher attainment of educational status, better ability to perform activities of daily living with lower NIHSS and modified Rankin scores respectively compared with those with Vascular cognitive impairment or post-stroke dementia. (Table 2) There were no significant differences between the Vascular Cognitive Impairment without dementia group compared with those with Post-stroke dementia except for severity of neurologic impairment assessed using the NIHSS and functional status. Cardiac disease was more commonly associated with post-stroke dementia.
TABLE 2.
Comparison of demographic and clinical features of Stroke survivors according to Vascular cognitive Impairment status.
| VARIABLES | Stroke with no VCI, n=77 |
VCI no dementia, n=50 |
Post-stroke Dementia, n=20 |
P-value | A vs B | A vs C | B vs C | A vs B+C | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 55.5 ± 11.8 | 63.5 ± 14.5 | 67.8 ± 12.8 | <0.0001 | 0.001 | <0.0001 | 0.26 | <0.0001 | |
| Female gender | 30 (39.0) | 25 (50.0) | 11 (55.0) | 0.29 | 0.22 | 0.2 | 0.71 | 0.13 | |
| Educational Status | |||||||||
| None | 3 (3.9) | 13 (26.0) | 6 (30.0) | 0.0015 | 0.0017 | 0.001 | 0.15 | 0.0007 | |
| Primary | 25 (32.5) | 9 (18.0) | 8 (40.0) | ||||||
| Secondary | 38 (49.4) | 19 (38.0) | 3 (15.0) | ||||||
| Tertiary | 11 (14.3) | 8 (16.0) | 3 (15.0) | ||||||
| Location of residence | 0.9 | 0.98 | 0.61 | 0.58 | 0.94 | ||||
| Rural | 3 (3.9) | 2 (4.0) | 0 (0.0) | ||||||
| Semi-urban | 22 (28.6) | 15 (30.0) | 5 (25.0) | ||||||
| Urban | 52 (67.5) | 33 (66.0) | 15 (75.0) | ||||||
| Stroke type | 0.43 | 0.48 | 0.37 | 0.32 | 0.52 | ||||
| Ischemic | 49 (63.6) | 32 (64.0) | 16 (80.0) | ||||||
| Hemorrhagic | 19 (24.7) | 9 (18.0) | 3 (15.0) | ||||||
| Unknown | 9 (11.7) | 9 (18.0) | 1 (5.0) | ||||||
| Duration of stroke (years) | |||||||||
| mean ± SD | 2.7 ± 2.1 | 3.7 ± 4.3 | 1.7 ± 4.3 | 0.06 | 0.08 | 0.14 | 0.09 | 0.31 | |
| NIHSS, mean ± SD | 3.9 ± 4.9 | 6.9 ± 5.7 | 14.6 ± 7.8 | <0.0001 | 0.003 | <0.0001 | <0.0001 | <0.0001 | |
| MRS, mean ± SD | 1.5 ± 1.0 | 2.5 ± 1.2 | 4.2 ± 0.5 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Barthels Index | 92.7 ± 15.8 | 83.1 ± 18.0 | 39.5 ± 15.2 | <0.0001 | 0.002 | <0.0001 | <0.0001 | <0.0001 | |
| CES-D score | 18.7 ± 7.3 | 20.3 ± 5.8 | 19.5 ± 4.8 | 0.41 | 0.2 | 0.66 | 0.34 | 0.21 | |
| Vascular Risk Factors | |||||||||
| Hypertension | 71 (92.2) | 49 (98.0) | 18 (90.0) | 0.31 | 0.16 | 0.75 | 0.14 | 0.38 | |
| Diabetes Mellitus | 17 (22.1) | 16 (32.0) | 8 (40.0) | 0.2 | 0.22 | 0.1 | 0.52 | 0.1 | |
| Dyslipidemia | 37 (48.1) | 18 (36.0) | 10 (50.0) | 0.35 | 0.18 | 0.88 | 0.28 | 0.03 | |
| Alcohol intake | 25 (32.5) | 10 (20.0) | 1 (5.0) | 0.03 | 0.12 | 0.01 | 0.12 | 0.06 | |
| Cigarette smoking | 5 (6.5) | 3 (6.0) | 0 (0.0) | 0.51 | 0.91 | 0.24 | 0.26 | 0.47 | |
| Physical inactivity | 33 (42.9) | 16 (32.0) | 3 (15.0) | 0.06 | 0.22 | 0.02 | 0.15 | 0.05 | |
| Heart disease | 0 (0.0) | 0 (0.0) | 3 (15.0) | <0.0001 | 1 | 0.0006 | 0.005 | 0.07 | |
| BMI | 26.7 ± 8.0 | 27.5 ± 4.9 | 25.6 ± 4.8 | 0.60 | 0.90 | 0.14 | 0.15 | 0.60 | |
| HRQOLISP | |||||||||
| Physical domain | 18.9 ± 3.5 | 18.5 ± 3.3 | 17.2 ± 3.5 | 0.13 | 0.51 | 0.05 | 0.13 | 0.16 | |
| Psychosocial domain | 23.6 ± 3.9 | 21.3 ± 4.5 | 18.2 ± 3.4 | <0.0001 | 0.001 | <0.0001 | 0.001 | <0.0001 | |
| Cognitive domain | 21.1 ± 3.6 | 18.8 ± 3.8 | 14.7 ± 5.0 | <0.0001 | 0.001 | <0.0001 | 0.0003 | <0.0001 | |
| Eco-social domain | 29.2 ± 4.3 | 25.5 ± 4.0 | 21.7 ± 4.4 | <0.0001 | <0.0001 | <0.0001 | 0.0007 | <0.0001 | |
A = Stroke with no VCI, B = VCI no dementia, C = Post-stroke Dementia, VCI = Vascular Cognitive Impairment, NIHSS
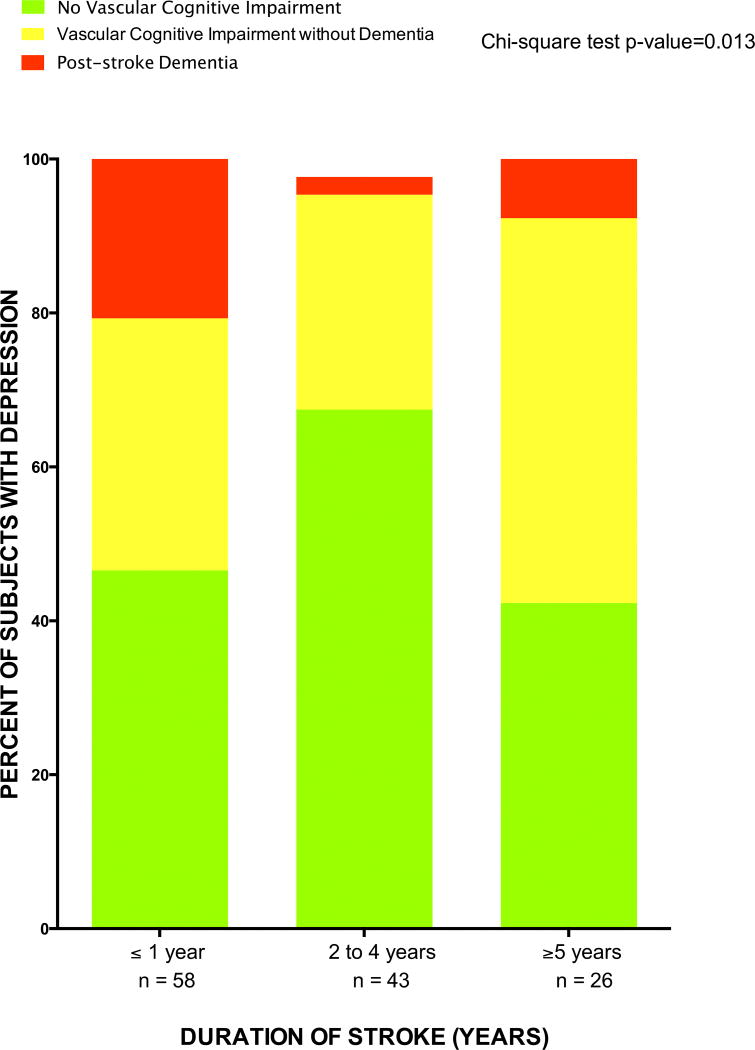
Duration of stroke and frequency of vascular cognitive impairment
Duration of stroke diagnosis is associated with vascular cognitive dysfunction as shown in Figure 1. For instance, the rates of PSD were 20.7%, 2.3%, and 7.7% among stroke survivors within year 1, between 2–4 years and ≥ 5 years after stroke. The proportions with vascular cognitive impairment no dementia were 32.8%, 27.9% and 50.0% among stroke survivors within year 1, between 2–4 years and ≥ 5 years after stroke respectively, p=0.01.
Figure 1.
Frequency of Vascular Cognitive Impairment categories among Ghanaians according to duration of stroke.
Predictors of Vascular Cognitive Impairment
On bivariate analyses, increasing age, female gender, lower educational attainment, duration of stroke, severity of neurologic deficits on the NIHSS scale, functional limitation, alcohol intake and physical activity were all significantly associated with vascular cognitive dysfunction compared with stroke subjects without vascular cognitive dysfunction. (Table 3). Upon adjustment for confounding variables, three factors remained significantly associated with vascular cognitive dysfunction with accompanying adjusted ORs (95% CI) namely: increasing age, 1.44 (1.03–2.02) for each 10 year increase; no formal education, 5.26 (1.01–27.52); and modified Rankin scale, 2.46 (1.61–3.75).
TABLE 3.
Predictors of Vascular Cognitive Impairment
| Unadjusted OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| Age, each 10-year increase | 1.75 (1.32–2.33) | 0.0001 | 1.44 (1.03–2.02) | 0.04 | |
| Female gender | 2.09 (1.08–4.04) | 0.03 | 0.99 (0.38–2.62) | 0.99 | |
| Educational status | |||||
| None | 9.19 (2.58–32.68) | 0.0006 | 5.26 (1.01–27.52) | 0.05 | |
| Some education | 1 | 1 | |||
| Stroke type | |||||
| Ischemic | 1 | ||||
| Hemorrhagic | 0.64 (0.28–1.47) | 0.3 | |||
| Undetermined | 1.13 (0.42–3.04) | 0.8 | |||
| Duration of stroke | |||||
| <1 year | 2.56 (1.11–5.89) | 0.03 | 1.94 (0.40–9.41) | 0.41 | |
| 2–4 years | 1 | ||||
| >5 years | 3.04 (1.10–8.41) | 0.03 | 1.52 (0.49–4.71) | 0.47 | |
| NIHSS | 1.16 (1.09–1.24) | <0.0000 | 1.00 (0.91–1.10) | 0.92 | |
| MRS | 2.84 (1.95–4.16) | <0.0000 | 2.46 (1.61–3.75) | <0.0000 | |
| CESD | 1.01 (0.95–1.07) | 0.81 | |||
| Vascular Risk Factors | |||||
| Hypertension | 1.56 (0.30–8.13) | 0.6 | |||
| Diabetes Mellitus | 2.21 (0.95–5.11) | 0.07 | |||
| Dyslipidemia | 0.54 (0.24–1.21) | 0.13 | |||
| Alcohol intake | 0.17 (0.05–0.62) | 0.007 | 0.74 (0.25–2.19) | 0.52 | |
| Cigarette smoking | 0.38 (0.04–3.36) | 0.38 | |||
| Physical activity | 0.29 (0.11–0.74) | 0.01 | 0.79 (0.32–1.99) | 0.59 | |
| BMI | 0.95 (0.89–1.02) | 0.17 | |||
Vascular Cognitive Impairment and Quality of life
Overall, health related quality of life was lowest among post-stroke dementia subjects followed by vascular cognitive impairment without dementia and then stroke survivors without vascular cognitive impairment. This trend was most obvious in the psycho-social, cognitive and ecosocial domains of the HRQOLISP questionnaire (table 2) but not in the physical domain. There were also no observed associations between the three categories of vascular cognitive impairment and risk of depression assessed using the CES-D questionnaire.
DISCUSSION
Approximately 50% of Ghanaian stroke survivors experience vascular cognitive impairment after an average of 2 years of stroke onset. 34.0% of stroke subjects had VCI without dementia and 13.6% had post-stroke dementia (PSD) with profound diminution in quality of life observed in proportion with severity of cognitive categories. The prevalence of VCI without dementia of 34.0% among this cohort from Kumasi, Ghana is comparable with reports from Ibadan, Nigeria, 39.9% (11), Sydney, Australia, 39.4% (29), Santiago, Chile, 39.0% (30), Chong-qing, China, 37.1% (31) and Newcastle, UK, 32% (32) but lower than 55.0% from Lisbon, Portugal, (33) 54.8% from Singapore, (34) and 49.9% from Korea (35). However some previous such as the South London Stroke Register cohort found a lower rate of 22%, (36) as well as 21.8% from a study in Hong Kong, China (37). Post-stroke dementia occurred a frequency of 13.6% which is slightly higher than 8.4% found in Ibadan, Nigeria, (11) and 8.6% found in Newcastle (38) but within the pooled prevalence of 7.4% to 41.3%. (10) Overall, the prevalence of Post-stroke vascular cognitive impairment reported in our study concurs with the body of literature.
It is important to note that a majority of cited studies have either assessed vascular cognitive dysfunction between 3 and 12 months after stroke (11,29–35) or have focused on individuals with mild strokes or TIA (29). The present study however provides data from a cross-section of stroke survivors with varying duration of stroke onset and severity. We show that the rates and categories of vascular cognitive dysfunction may vary depending on duration of stroke symptoms which could account for the variance in prevalence of VCI reported in literature to date. For instance, we observed that the overall frequency of VCI was higher within the first year after stroke being 53.5%, but it dropped to 33.6% among individuals 2 to 4 years after stroke onset and peaked at 57.7% among individuals who had survived stroke for 5 or more years, p=0.013 (Figure 1). However, whereas the highest rates of post-stroke dementia were noted among stroke survivors within the first year of stroke where functional limitations are preponderant, vascular cognitive impairment without dementia was most prevalent among study subjects with ≥5 years of stroke symptoms. These findings may suggest that cognitive impairment after stroke may be persistent or progressive even with resolution of physical deficits/impairments and better adaptation for activities of daily living. And although it was not feasible, due to the cross-sectional study design, to examine the trajectory of vascular cognitive dysfunction from our study, data from pooled prospective cohorts suggest a linear increase in Post-stroke dementia rates of 3% and 1.7% per year in hospital- and community-based studies respectively (10). More recently, it has been postulated that stroke survivors may exhibit different cognitive trajectories and that these trajectories could change over time (39). A host of variables including demographic factors (such as age, educational level), pre-stroke factors (such as physical impairment, cognitive impairment), index stroke factors (including hemorrhagic stroke, recurrent strokes), post-stroke factors (such as infection, delirium, early seizures) and neuroimaging predictors (example cerebral small-vessel disease, cortical atrophy) may all conspire to differentially influence the trajectory of post-stroke dementia. (10,40)
We identified a number of demographic and clinical features associated with vascular cognitive dysfunction in this cohort in bivariate analysis but only three predictors namely age, educational attainment and functional status remained significantly associated after adjustment for confounders. Increasing age has been consistently (5,10,40,41–43) identified as a risk factor for vascular cognitive impairment although our cohort is relatively young with a mean age of 60 years. For each 10-year increase in age, risk of VCI increased by 44% in this African population possibly implicating a toxic interaction between neurodegeneration and vascular insults from stroke in culminating in cognitive impairment and dementia. Indeed stroke among Africans affects a younger population (1,4,44) as captured in the present study and the high prevalence of VCI in this young population would add to the weight of evidence implicating negroids with a higher proclivity towards dementia. (41) Lower educational attainment, a surrogate of cognitive reserve was strongly associated with VCI. It has been shown that educational attainment has been found to be protective against cognitive impairment in vascular dementia, Alzheimer’s disease and Mild cognitive impairment (45,46). Occupational complexity and social engagement together with educational attainment together appears to reduce the long-term risk of dementia (47–49). We found functional impairment after stroke to be the most potent risk factor for VCI in the present cohort which as expected would be a corollary of severity of neurologic deficit from vascular insults from stroke but could also stem from sub-optimal acute stroke care and rehabilitation as well as risk factor control. A recent study among Ghanaian stroke survivors identified up to 35% with less than optimal blood pressure control within the first year of stroke. (50) However, none of the traditional vascular risk factors were independently associated with VCI in this Ghanaian population, although regular physical activity seemed to confer some protection against VCI in unadjusted analysis. Interestingly regular physical activity which protects against stroke occurrence (44) has also been shown to be protective against VCI (51) potentially via enhanced cerebral perfusion and the elaboration of growth factors (51).
Our study demonstrates a substantive impact of VCI on health related quality of life among African stroke survivors. With the notable exception of the physical domain, a stratified decrement in cognitive, psycho-social and eco-social domains of quality of life according to cognitive categories was observed. A previous study among Korean study participants also documented a graded decline in HRQoL among subjects with VCI no dementia and PSD compared with age-and sex-matched subjects with normal cognition using EQ-5Dindex questionnaire (52). Although the low HRQoL observed among subjects with dementia has been suggested to be associated with depression in these patients, we did not observe such as association (Table 2). In fact, it is increasingly becoming apparent that depression and HRQoL among subjects with dementia or cognitive impairment may represent different constructs (53,54). The implication is that vascular cognitive dysfunction may exert its effect on quality of life independently of depression and this awaits further studies preferably of prospective design.
Quite apart from the modest sample size of the study, there are other limitations worth noting. As a cross-sectional study, causal inferences cannot be drawn between the risk factors identified and vascular cognitive impairment among African. Although a head CT scan was performed at onset of stroke for stroke diagnosis and stroke type information for nearly 90% of study subjects, most subjects nor hospital facilities did not have electronic copies of CT scan for analysis as part of this study. We did not assess pre-stroke cognitive status hence we would not assess its impact on post-stroke cognitive impairment. These limitations notwithstanding, this study is among the few conducted in Africa to assess vascular cognitive impairment/dementia in long-term stroke survivors. We have highlighted a heavy burden of VCI in this population with a need to identify, evaluate and fashion interventions that may alter the trajectory of VCI. In this direction further studies aimed at defining the trajectory of VCI, its biomarkers and pharmacological interventions for its prevention are urgently needed.
In conclusion, nearly 50% of Ghanaian stroke survivors experience vascular cognitive dysfunction most of which have never been screened as part of routine care. With the growing population of stroke survivors in LMICs, attention should be focused on addressing the unmet need of vascular cognitive impairment which is the second leading cause of dementia globally.
Acknowledgments
Funding: Grant R21 NS094033 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, et al. Global burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:1205–6. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owolabi MO, Arulogun O, Melikam S, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr. 2015;26(2 sUPPL 1):S27–38. doi: 10.5830/CVJA-2015-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adeloye D. An estimate of the incidence and prevalence of stroke in Africa: a systematic review and meta-analysis. PLoS One. 2014;9:e100724. doi: 10.1371/journal.pone.0100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarfo FS, Akassi J, Awuah D, Adamu S, Nkyi C, Owolabi M, et al. Trends in stroke admission and mortality rates from 1983 to 2013 in central Ghana. J Neurol Sci. 2015;357(1–2):240–5. doi: 10.1016/j.jns.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 5.Pendlebury ST. Stroke-related dementia: rates, risk factors and implications for future research. Maturitas. 2009;64:165–71. doi: 10.1016/j.maturitas.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professional from the American Heart Association/America Stroke Association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henon H, Pasquier F, Leys D. Poststroke dementia. Cerebrovasc Dis. 2006;22:61–70. doi: 10.1159/000092923. [DOI] [PubMed] [Google Scholar]
- 8.Ballard C, Rowan E, Stephen S, Kalaria R, Kenny RA. Prospective follow-up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia-free stroke survivors >75 years of age. Stroke. 2003;34:2440–4. doi: 10.1161/01.STR.0000089923.29724.CE. [DOI] [PubMed] [Google Scholar]
- 9.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 10.Pendlebury ST, Rothwell PM. Prevalence, incidence, and risk factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–18. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 11.Akinyemi RO, Allan L, Owolabi MO, Akinyemi JO, Ogbole G, Ajani A, et al. Profile and determinants of vascular cognitive impairment in African stroke survivors: The CogFAST Nigeria Study. Journal of Neurological Sciences. 2014;346:241–249. doi: 10.1016/j.jns.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Akinyemi RO, Mukaetova-Ladinska EB, Attems J, Ihara M, Kalaria RN. Vascular risk factors and neurodegeneration in ageing related dementias: Alzheimer’s diseases and vascular dementia. Curr Alzheimer Res. 2013;10:642–53. doi: 10.2174/15672050113109990037. [DOI] [PubMed] [Google Scholar]
- 13.Allan LM, Rowan EN, Firbank MJ, Thomas AJ, Parry SW, Polvikoski TM, et al. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134:3716–27. doi: 10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottesman R, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischemic stroke. Lancet Neurol. 2010;9:895–905. doi: 10.1016/S1474-4422(10)70164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton EJ, Kenny RA, O’Brien J, Stephens S, Bradybury M, Rowan E, et al. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke. 2004;35:1270–5. doi: 10.1161/01.STR.0000126041.99024.86. [DOI] [PubMed] [Google Scholar]
- 16.Firbank MJ, Burton EJ, Barber R, Stephen S, Bradbury M, Rowan E, et al. Medial temporal atrophy rather than white matter hyperintensities predict cognitive decline in stroke survivors. Neurobiol Aging. 2007;28:1664–9. doi: 10.1016/j.neurobiolaging.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Sarfo FS, Akassi J, Badu E, Okorozo A, Ovbiagele B, Akpalu A. Profile of neurological disorders in an sdult neurology clinic in Kumasi, Ghana. eNeurologicalSci. 2016;3:69–74. doi: 10.1016/j.ensci.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmond DWMJ, Sano M, Stern Y. Recovery of cognitive function after stroke. Stroke. 1996;27:1978–803. doi: 10.1161/01.str.27.10.1798. [DOI] [PubMed] [Google Scholar]
- 19.Akpalu A, Sarfo FS, Ovbiagele B, Akinyemi R, Gebregziabher M, Obiako R, et al. Phenotyping Stroke in sub-Saharan Africa: Stroke Investigative Research and Education Network (SIREN) Phenomics protocol. Neuroepidemiology. 2015;45(2):73–82. doi: 10.1159/000437372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarfo F, Gegrebziabher M, Ovbiagele B, Akinyemi R, Owolabi L, Obiako R, et al. Multiligual validation of the Questionnaire for Verifying stroke-free status in West Africa. Stroke. 2016;47(1):167–72. doi: 10.1161/STROKEAHA.115.010374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyden PD, Lu M, Levine S, Brott TG, Broderick J. A Modified National Institutes of Health Stroke Scale for Use in Stroke Clinical Trials. Preliminary Reliability and Validity. Stroke. 2001;32:1310–1317. doi: 10.1161/01.str.32.6.1310. [DOI] [PubMed] [Google Scholar]
- 22.Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002 Sep.33(9):2243–6. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 23.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 24.Ojo Owolabi M. HRQoLISP-26: A concise, multiculturally valid, multidimensional, flexible, and reliable stroke-specific measure. ISRN Neurol. 2011;2011:295096. doi: 10.5402/2011/295096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MOCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 26.Guruje O, Unverzargt FW, Osuntokun BO, Hendrie HC, Baiyewu O, Ogunniyi A, et al. The CERAD Neuropsychological Test Battery: norms from a Yoruba-speaking Nigerian sample. West Afr J Med. 1995;14:29–33. [PubMed] [Google Scholar]
- 27.Baiyewu O, Unverzagt FW, Lane KA, Gureje O, Ogunniyi M, Musick B, et al. The Stick design Test: a new measure of visuoconstructional ability. J Int Neuropsycholol Sco. 2005;11:598–605. doi: 10.1017/S135561770505071X. [DOI] [PubMed] [Google Scholar]
- 28.Uyttenboogaart M, Stewart RE, Vroomen PC, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the Barthel Index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke. 2005;36:1984–7. doi: 10.1161/01.STR.0000177872.87960.61. [DOI] [PubMed] [Google Scholar]
- 29.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JCL, Wen W, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–9. doi: 10.1212/01.wnl.0000115108.65264.4b. [DOI] [PubMed] [Google Scholar]
- 30.Delgado C, Donoso A, Orellana P, Vasquez C, Diaz V, Behrens MI. Frequency and determinants of poststroke cognitive impairment at three and twelve months in Chile. Dement Geriatr Cogn Disord. 2010;29:397–405. doi: 10.1159/000305097. [DOI] [PubMed] [Google Scholar]
- 31.Zhou DH, Wang JY, Li J, Deng J, Gao C, Chen M. Study on frequency and predictors of dementia after ischemic stroke: the Chongqing stroke study. J Neurol. 2004;251:421–7. doi: 10.1007/s00415-004-0337-z. [DOI] [PubMed] [Google Scholar]
- 32.Ballard C, Stephen S, McLaren A, Wesnes K, Kenny RA, Burton E, et al. Neuropsychological deficits in older stroke patients. Ann N Y Acad Sci. 2002;977:179–82. doi: 10.1111/j.1749-6632.2002.tb04815.x. [DOI] [PubMed] [Google Scholar]
- 33.Madureira S, Guerreiro M, Ferro JM. Dementia and cognitive impairment three months after stroke. Eur J Neurol. 8:621–7. doi: 10.1046/j.1468-1331.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- 34.Dong Y, Venketasubramanian N, Chn BP, Sharma VK, Slavin MJ, Collinson SL, et al. Brief screening tests during acute admission in patients with mild stroke are predictive of vascular cognitive impairment 3–6 months after stroke. J Neurol Neurosurg Psychiatry. 2012;83:580–5. doi: 10.1136/jnnp-2011-302070. [DOI] [PubMed] [Google Scholar]
- 35.Yu KH, Cho SJ, Oh MS, Jung S, Lee JH, Shin JH, et al. Cognitive impairment evaluated with vascular cognitive impairment harmonization standards in a multicenter prospective stroke cohort in Korea. Stroke. 2013;44:786–8. doi: 10.1161/STROKEAHA.112.668343. [DOI] [PubMed] [Google Scholar]
- 36.Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke. 2013;44:138–45. doi: 10.1161/STROKEAHA.112.670844. [DOI] [PubMed] [Google Scholar]
- 37.Tang WK, Chan SS, Chiu HF, Ungvari GS, Wong KS, Kwok TC, et al. Frequency and determinants of poststroke dementia in Chinese. Stroke. 2004;35:930–5. doi: 10.1161/01.STR.0000119752.74880.5B. [DOI] [PubMed] [Google Scholar]
- 38.Stephen S, Kenny RA, Rowan E, Allan L, Kalaria RN, Bradbury M, et al. Neuropsychological characteristics of mild vascular cognitive impairment and dementia after stroke. Int J Geriatr Psychiatry. 2004;19:1053–7. doi: 10.1002/gps.1209. [DOI] [PubMed] [Google Scholar]
- 39.Mijajlovic MD, Pavlovic A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen H, et al. Post-stroke dementia- a comprehensive review. BMC Med. 2017;15(1):11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasquier F, Henon H, Leys D. Risk factors and mechanisms of post-stroke dementia. Rev Neurol. 1999;155(9):749–53. [PubMed] [Google Scholar]
- 41.Kalaria RN. Risk factors and neurodegenerative mechanisms in stroke related dementia. Panminerva Med. 2012;54:139–48. [PubMed] [Google Scholar]
- 42.Patel M, Coshall C, Rudd AG, Wolfe CD. Natural history of cognitive impairment after stroke and factors associated with its recovery. Clin Rehabil. 2003;17:158–66. doi: 10.1191/0269215503cr596oa. [DOI] [PubMed] [Google Scholar]
- 43.Das S, Paul N, Hazra A, Ghosal M, Ray BK, Banerjee TK, et al. Cognitive dysfunction in stroke survivors: a community-based prospective study from Kolkata, India. J Stroke Cerebrovasc Dis. 2013;22:1233–42. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potential modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388(10046):761–75. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 45.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–12. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valenzuela M, Brayne C, Sachev P, Wilcock G, Matthews F. Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol. 2011;173:1004–12. doi: 10.1093/aje/kwq476. [DOI] [PubMed] [Google Scholar]
- 48.Valenzuela MJ, Matthews FE, Brayne C, Ince P, Halliday G, Krill JJ, et al. Multiple biological pathways link cognitive lifestyle to protection from dementia. Biol Psychiatry. 2012;71:783–91. doi: 10.1016/j.biopsych.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 49.Valenzuela MJ, Sachdev P. Brain reserve and cognitive decline: a non-parametric systematic review. Psychol Med. 2006;36:1065–73. doi: 10.1017/S0033291706007744. [DOI] [PubMed] [Google Scholar]
- 50.Sarfo FS, Kyem G, Ovbiagele B, Akassi J, Sarfo-Kantanka O, Agyei M, et al. One-year rates and determinants of post-stroke systolic blood pressure control among Ghanaians. J Stroke Cerebrovasc Dis. 2017;26(1):78–86. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzhemiers Dement. 2007;3:S30–7. doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 52.Park JH, Kim BJ, Bae HJ, Lee J, Lee J, Han MK, et al. Impact of post-stroke cognitive impairment with no dementia on health-related quality of life. J Stroke. 2013;15(1):49–56. doi: 10.5853/jos.2013.15.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriatr Psychiatry. 2009;(2491):15–24. doi: 10.1002/gps.2090. [DOI] [PubMed] [Google Scholar]
- 54.Banerjee S, Smith SC, Lamoing DL, Harwood RH, Foley B, Smith P, et al. Quality of life in dementia: more than just cognition. An analysis of associations with quality of life in dementia. J Neurol Neurosurg Psychiatry. 2006;77:146–148. doi: 10.1136/jnnp.2005.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]