Abstract
Objective
To determine whether pregnancy is an intrinsic motivator for drug abuse (DA) cessation.
Method
We conducted, in Swedish females born 1980–1990 who gave birth at ages 20–35 (N=149,512), prospective cohort, co-relative, co-spouse, and within-person analyses of registration for DA during pregnancy. DA was assessed from medical, criminal and pharmacy registries.
Results
In the population, rates of DA were lower during pregnancy (unadjusted OR=0.67, 95% CIs 0.60–0.74). Compared to population results, the negative association between pregnancy and DA was moderately stronger in cousins (OR=0.49, 0.39–0.62) and substantially stronger in siblings (OR=0.35, 0.24–0.51) discordant for pregnancy. The estimated OR for DA in pregnancy-discordant monozygotic twins was even stronger: 0.17 (0.10–0.31). Within-individuals, the OR for DA while pregnant compared to an equivalent pre-pregnancy interval was similar to that seen in pregnancy-discordant monozygotic twins: 0.22 (0.19–0.26). Compared to cohabiting fathers, mothers had a greater reduction in risk for DA during pregnancy (OR=0.40, 0.34–0.47). Pregnancy was more protective in those with low parental education and without a cohabiting actively drug abusing father. Compared to pre-pregnancy baseline, within-individual analyses indicate risk for DA is also substantially reduced post-partum e.g. day 0–242 OR=0.13 (0.11–0.16).
Conclusions
Risk for DA in women is substantially reduced during pregnancy. Multiple analyses suggest that this association is largely causal, suggesting that pregnancy is indeed a strong intrinsic motivator for DA cessation. Similar strong protective effects may be present immediately post-partum. Our results have implications for our etiologic models of DA and especially for contingency management programs seeking to reduce DA risk.
While major advances have been made in understanding the neurobiological basis of addiction (1–3), the etiologic importance of volitional and motivational components in drug abuse has been supported by the efficacy of contingency management in drug abuse treatment (4–6). Voucher-based rewards as extrinsic motivators for cessation are among the most effective psychosocial treatments for drug abuse (6).
In this report, we seek to expand our understanding of motivational factors in drug abuse by examining the unique natural experiment of pregnancy as a potential intrinsic motivator for reduction in drug abuse. The adverse impact of drug abuse on the developing fetus is well known (7–11), widely disseminated in modern Western cultures and typically reinforced when pregnant women interact with health care providers (12–14). Maternal prenatal attachment has been widely studied and, while variable, most drug using mothers are strongly motivated to protect the health of their fetus and, therefore, to reduce or cease their drug use (15–17). This desire is often supported by their social network (18;19). Thus, the degree to which rates of drug abuse decline during pregnancy can provide an estimate of the ability of motivated women to reduce levels of substance misuse.
The causal nature of the association between pregnancy and drug abuse is likely complex. A positive drug abuse → pregnancy pathway is suggested by evidence for an association between illicit substance use and teenage pregnancy (20). Drug abuse, and/or its risk factors, likely increase the chances of unplanned and early pregnancies (21). However, longitudinal analyses showed a reduction in use of tobacco, alcohol, cannabis, and cocaine during pregnancy in young adult women (22), and studies of clinical cohorts of pregnant women document a reduction in illicit substance use (23;24) suggesting a negative pregnancy → drug abuse pathway.
An optimal approach to clarifying the causal inter-relationships between pregnancy and drug abuse would require a large, representative, population-based cohort that could be studied longitudinally and where it would be possible to apply “natural” experimental methods to clarify causal pathways. We here report such a study.
We evaluate, using population-wide Swedish registry data, four complementary methods to elucidate the nature of the relationship between pregnancy and drug abuse risk:
Population-based analyses with covariates.
Co-relative designs with relative pairs discordant for pregnancy.
Within-individual longitudinal designs comparing pregnancy with preceding non-pregnant periods.
Co-spouse design comparing changes in drug abuse risk in pregnant women with that seen in the cohabiting fathers of their child.
We also explore whether the impact of pregnancy on risk for drug abuse varies as a function of parental education, prior deviant behaviors, school achievement, age, marital status, and drug abuse in the cohabiting father of the child. Finally, using within-individual analysis only, we examine whether the reduction in rates of drug abuse during pregnancy persist into the immediate post-partum period.
Methods
This study utilized several different Swedish population-based registers with national coverage, linking them using each person’s unique identification number. To preserve confidentiality, this ID number was replaced by a serial number. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (No. 2008/409). Drug abuse was identified in the Swedish medical registries by ICD codes (ICD9: Drug psychoses (292) and Drug dependence (304), Nondependent abuse of drugs (305; excluding 305.0); ICD10: Mental and behavioral disorders due to psychoactive substance use (F10-F19), except those due to alcohol (F10) or tobacco (F17)); in the Suspicion Register by codes 3070, 5010, 5011, and 5012, that reflect crimes related to drug abuse; and in the Crime Register by references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). Each individual could have several registrations in the criminal (Crime and Suspicion) and/or in the medical registers. In order not to double-count registrations we, within each type of register (Criminal and Medical), allowed for a 90-day period after each registration in which a new registration was not counted.
To study the association between pregnancy and drug abuse, we selected all females born in Sweden 1980–1990 who had at least one child registered in the Swedish multigenerational register where the mother was likely first aware of being pregnant between the ages of 20 and 35. We assumed an average of 280 days from the end of last menstrual period to birth, a 28-day menstrual cycle and the strong suspicion of pregnancy arising 10 days after the missed menstrual period. Therefore, we estimated women were aware of being pregnant 280-(28+10) or 242 days before birth.
We matched each mother to 5 non-related control females with the same year and month of birth. Furthermore, the control individual had to be alive and registered in Sweden at the time of the case’s pregnancy and not themselves registered as being a mother or having a child within 9 months after the date of birth of the case’s child. For all control individuals we studied drug abuse during the same period as the case individual (Ncases =149,512 and Ncontrols = 747,560). In the next step, we replicated the matching approach but instead of using non-related random individuals as controls, we matched on female cousins and full-siblings. In order to achieve a significant number of control individuals we allowed for up to three years’ age difference between the case and the relative control. We matched 58,640 control cousins to 50,317 cases and 19,812 control siblings to 19,115 cases. By matching on cousins and siblings, we account for a number of unmeasured genetic and environmental factors shared among cousins and siblings. Finally, we studied drug abuse using a within-individual model comparing a 242-day period prior to the pregnancy to the pregnancy period.
We used conditional logistic regression, with a separate stratum for each case and their control(s), in which we compare drug abuse in the case (i.e., drug abuse during pregnancy) with drug abuse in the controls (i.e., drug abuse during a non-pregnant period). Model 1 was only a crude model, whereas in model 2 we adjusted for mid-parent educational status [(1) <=9 years, (2) 10–11 years, (3) 12 years or more] and school achievement (SA) of the individual (see (25) for a definition of SA).
We then combined the population, full-sibling, and cousin datasets, and performed two co-relative analyses. The first allowed all coefficients for each sample to be independent. In the second we modeled the genetic resemblance assuming that it equaled: 0 for the population, +0.125 for cousins and +0.5 for full-siblings. We compared this model, using the AIC (26), with the previous model. If the second model fitted the data well, we obtained improved estimation of the drug abuse-pregnancy association among all types of relatives. In this model, we are also able to extrapolate an odds ratio for monozygotic twins (there was only one monozygotic twin registered for drug abuse while pregnant).
In additional analyses, we investigated if the association between pregnancy and drug abuse was moderated by the following variables; mid-parent educational status; SA of the individual; lifetime registration of drug abuse in parent (dichotomized into yes/no); drug abuse registration in the individual prior to the pregnancy period; registration for a psychiatric diagnosis in the in-patient or specialist registries; criminal registration in the individual prior to the pregnancy period (for a definition of Criminal registration see (27)); age at pregnancy (dichotomized into <=25 years and >25 years); marital status (dichotomized into married/not married; in the analyses of married people individuals that got married in the control or hazard period were excluded); drug abuse registration in the father to the child prior to the pregnancy period; drug abuse registration in the father to the child during the pregnancy period. These moderation analyses were performed only on the within-individual sample using an interaction term between the covariate of interest and drug abuse status in the mother. In the within-individual sample, we also tested if the decrease in drug abuse rates between control and pregnancy periods was the same for mothers and fathers. This was done by including an interaction term between a dummy variable indicating mother/father, and drug abuse status in mother and father.
Finally, we examined, using with-individual analyses only, the rates of drug abuse in the immediate post-partum period. Our control was the 242-day period prior to pregnancy and we examined three 242-day post-partum risk periods: 0–242, 243–484 and 485–726 days after childbirth. We eliminated from these analyses mothers where the child died during the risk-period. We included mothers no longer cohabiting with their child (n=249, 871 and 1,187 during these three periods) because these mothers had substantial elevations in drug abuse rates, suggesting that in some cases the child had been removed due to their problematic behavior. Excluding them would bias downward the post-partum drug abuse rates.
In all these models the within-individual and within-family clustering were taken into consideration. In models that included information on fathers, the father had to cohabit with the mother at the end of the year the child was born. All statistical analyses were performed using SAS 9.3 (28).
Results
Association between Pregnancy and Registration for Drug Abuse
In our general population sample examined without covariates, rates of drug abuse registration were moderately lowered during pregnancy (odds ratio=0.67, 95% CIs 0.60–0.74) (table 1). Adding parental education and subject educational achievement to the model substantially strengthened the association (odds ratio=0.41, 0.37–0.46) indicating that these factors acted as negative confounders attenuating the pregnancy-drug abuse relationship.
Table 1.
The Observed Association of First Pregnancy with Registration for Drug Abuse in the General Population, in Cousin and Sibling Pairs Discordant for Pregnancy and Within-Individuals
| N | Frequency of Drug Abuse Registration |
Odds Ratio and 95% Confidence Intervals | Cohen’s d# and 95% Confidence Intervals |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy | Control | Model 1* | Model 2** | Model 1* | |||||
| Population | 149,512 | 0.24% | 0.35% | 0.67 | 0.60; 0.74 | 0.41 | 0.37; 0.46 | 0.22 | 0.17; 0.28 |
| Cousins | 50,371 | 0.23% | 0.46% | 0.49 | 0.39; 0.62 | 0.33 | 0.26; 0.42 | 0.39 | 0.26; 0.52 |
| Siblings | 19,115 | 0.20% | 0.56% | 0.35 | 0.24; 0.51 | 0.58 | 0.37; 0.79 | ||
| Within Individual | 150,226 | 0.24% | 0.63% | 0.22 | 0.19; 0.26 | 0.83 | 0.74; 0.92 | ||
| Within Individual (2)1 | 150,226 | 0.24% | 0.61% | 0.25 | 0.21; 0.29 | 0.76 | 0.68; 0.86 | ||
| Within Individual (3)2 | 150,226 | 0.24% | 0.61% | 0.26 | 0.22; 0.31 | 0.74 | 0.64; 0.83 | ||
| Within Individual (2nd pregnancy) | 75,891 | 0.11% | 0.27% | 0.23 | 0.16; 0.33 | 0.81 | 0.61; 1.01 | ||
Unadjusted
Adjusted for, Mean Parental Education and School Achievement
Control period ends 6 months prior to start of pregnancy
Control period ends 12 months prior to start of pregnancy
(40)
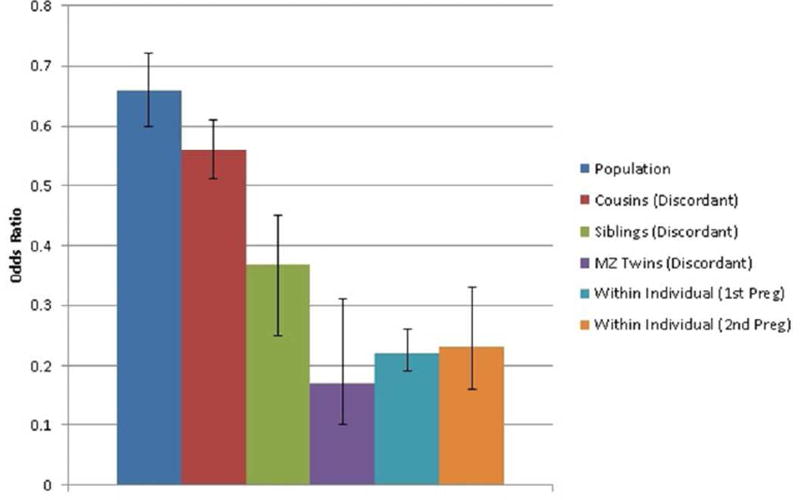
Compared to the general population examined without covariates, the negative association between pregnancy and drug abuse was stronger in cousins (odds ratio=0.49, 0.39–0.62) and especially sisters (odds ratio=0.35, 0.24–0.51) discordant for pregnancy. We then fitted our co-relative model to results from the general population and discordant cousin, sibling and monozygotic twin results. Our model fitted better than the raw results and estimated the odds ratio for drug abuse in pregnancy-discordant monozygotic pairs at 0.17 (0.10 – 0.31) (table 2).
Table 2.
The Association between Pregnancy and Drug Abuse assessed by an Odds Ratios in the General Population and in Cousins, Siblings and Monozygotic Twins Discordant for Pregnancy as Estimated from our Co-Relative Model
| Sample | Estimated Odds Ratios | 95% Confidence Intervals |
|---|---|---|
| Population | 0.66 | 0.60–0.72 |
| Discordant Cousins | 0.56 | 0.51–0.61 |
| Discordant Siblings | 0.37 | 0.25–0.45 |
| Discordant Monozygotic Twins | 0.17 | 0.10–0.31 |
| AIC* (Observed) | 660440.34 | |
| AIC* (Predicted) | 660438.78 |
Akaike’s information criterion (26)
We then examined our within-person model, first comparing rates of drug abuse during a first pregnancy with that observed in the comparable 242-day period immediately prior to the estimated date of impregnation. The estimated odds ratio for drug abuse using this within-person design was 0.22 (0.19–0.26) (table 1). Since drug use might be reduced for some women who are planning to become pregnant, we re-ran these analyses setting the pre-pregnancy period 6 and 12 months earlier. The odds ratios did not appreciably change and were estimated at, respectively, 0.25 (0.21–0.29) and 0.26 (0.22–0.31). The negative association between pregnancy and drug abuse registration for a second pregnancy (odds ratio = 0.23 (0.16–0.33)) did not differ significantly from that observed during the first pregnancy (p=0.12). The results from the population-based, co-relative and within-individual analyses are summarized in figure 1.
Figure 1.
The Association between Pregnancy and Risk for Drug Abuse – as assessed by an Odds Ratio (± 95% Confidence Intervals) – in the general population, cousins, siblings and monozygotic twins discordant for pregnancy, and within-individuals (comparing matched periods before and during pregnancy) for a first and second pregnancy. The odds ratios are all “raw’ – that is estimated from models without covariates.
Using within-person analyses, rates of drug abuse were significantly lower in the second than the first half of pregnancy: odds ratio=0.58 (0.46–0.74). To examine whether the pregnancy effect could be confounded with marital status, we re-ran these analyses excluding cases who changed marital status between the control and pregnancy periods. The odds ratio was unchanged: 0.22 (0.19–0.25).
In 130,368 cases, the mother was cohabiting with the father of her child at the end of the year the child was born. When examined together, the reduction in risk for drug abuse registration from the control to the pregnancy period was larger for the pregnant mother (odds ratio=0.22, 0.18–0.28) than for the father (odds ratio=0.62, 0.56–0.68). In a co-spouse design, the reduction in risk in the control versus pregnancy periods was much greater in the mother than the father: odds ratio=0.40 (0.34–0.47).
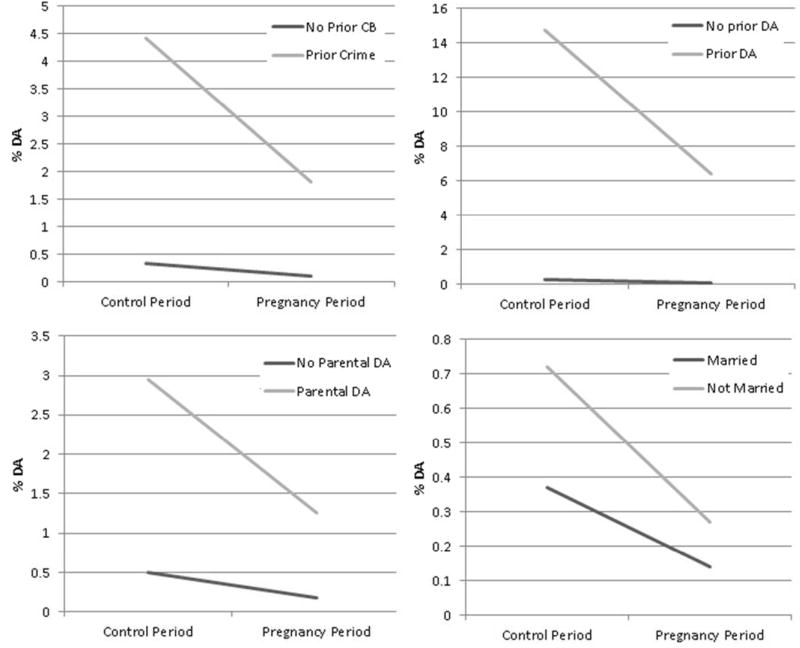
Finally, using within-individual analyses, we first examined, using a multiplicative model, a range of potential moderators of the protective effect of pregnancy on drug abuse risk (table 3). These moderators were tested one at a time, and their p values of the interaction increased moderately if consider all together. The protective effect of pregnancy was stronger with lower versus higher education for the mother’s parents but was unrelated to drug abuse in the parents or to six further features of the mother’s background including prior drug abuse registration. However, as illustrated in figure 2, while we found similar risk ratios for high and low risk groups, we also saw widely varying risk differences. Pregnancy was associated with a much larger absolute reduction in drug abuse caseness in the high versus low risk groups as reflected, for example, by prior histories of crime or drug abuse or single marital status. Then, we examined two features of drug abuse in the cohabiting spouse. The protective effect of pregnancy on drug abuse risk was modestly attenuated by a prior history of drug abuse in the spouse and strongly attenuated by spousal drug abuse during pregnancy.
Table 3.
Potential Moderators of the Effect of Pregnancy of Risk for Drug Abuse
| Absent or Present |
Sample Sizes |
Rates of Drug Abuse | Difference | Interaction p value |
Odds Ratio and 95% Confidence Intervals (95% CI) |
|||
|---|---|---|---|---|---|---|---|---|
| Pregnancy | Control | |||||||
| General Potential Moderators | ||||||||
| Parental Education* | Low | 43,458 | 0.32% | 0.84% | 0.52% | 0.0260 | 0.19 | 0.15; 0.23 |
| Mid | 83,428 | 0.22% | 0.61% | 0.39% | 0.25 | 0.21; 0.30 | ||
| High | 7,967 | 0.20% | 0.36% | 0.16% | 0.34 | 0.23; 0.49 | ||
| Drug Abuse in Parents of Mother | No | 142,592 | 0.18% | 0.50% | 0.32% | 0.8701 | 0.22 | 0.19; 0.26 |
| Yes | 7,634 | 1.26% | 2.95% | 1.69% | 0.23 | 0.17; 0.31 | ||
| School Achievement (SD units)*** | −2.5 – −1.5 | 15,853 | 1.07% | 2.79% | 1.72% | 0.2856 | 0.21 | 0.18; 0.25 |
| −1.5 – −0.5 | 38,047 | 0.30% | 0.81% | 0.51% | 0.23 | 0.20; 0.26 | ||
| −0.5 – 0.5 | 58,622 | 0.09% | 0.28% | 0.19% | 0.25 | 0.20; 0.31 | ||
| 0.5 – 1.5 | 33,225 | 0.05% | 0.08% | 0.03% | 0.26 | 0.19; 0.37 | ||
| 1.5 – 2.5 | 4,479 | 0.09% | 0.04% | −0.05% | 0.28 | 0.18; 0.45 | ||
| Prior Crime (in Mother) | No | 139,408 | 0.11% | 0.33% | 0.22% | 0.1944 | 0.24 | 0.20; 0.29 |
| Yes | 10,818 | 1.81% | 4.43% | 2.62% | 0.20 | 0.16; 0.25 | ||
| Prior Drug Abuse (in Mother) | No | 146,420 | 0.08% | 0.26% | 0.18% | 0.1194 | 0.24 | 0.21; 0.30 |
| Yes | 3,806 | 6.38% | 14.74% | 8.36% | 0.20 | 0.16; 0.24 | ||
| Prior Psychiatric Diagnosis | No | 298,494 | 0.23% | 0.62% | 0.39% | 0.1501 | 0.23 | 0.20; 0.26 |
| Yes | 979 | 0.72% | 2.45% | 1.73% | 0.06 | 0.01; 0.38 | ||
| Age at Pregnancy | −25 | 73,828 | 0.34% | 0.97% | 0.63% | 0.1165 | 0.21 | 0.18; 0.25 |
| 25+ | 76,398 | 0.14% | 0.30% | 0.16% | 0.27 | 0.21; 0.35 | ||
| Married** | No | 119,325 | 0.27% | 0.72% | 0.45% | 0.6519 | 0.22 | 0.19; 0.26 |
| Yes | 14,870 | 0.14% | 0.37% | 0.23% | 0.19 | 0.10; 0.36 | ||
| Features of Cohabiting Biological Father’s Drug Abuse as Potential Moderators | ||||||||
| Prior Drug Abuse in Father | No | 124,408 | 0.03% | 0.13% | 0.10% | 0.0973 | 0.16 | 0.11; 0.23 |
| Yes | 5,960 | 1.90% | 4.68% | 2.78% | 0.23 | 0.18; 0.30 | ||
| Drug Abuse during Pregnancy in All Fathers | No | 129,700 | 0.05% | 0.19% | 0.14% | <0.0001 | 0.17 | 0.13; 0.22 |
| Yes | 668 | 12.13% | 19.11% | 6.98% | 0.35 | 0.26; 0.46 | ||
Parental education was used as a continuous term in the models, hence only one interaction term. The odds ratios in the far right column are an illustration of the odds ratios at different levels of parental education.
In the analyses of married people individuals that got married in the control or hazard period were excluded (for these individuals we could not separate if the effect is due to marriage or pregnancy)
SA was used as a continuous term in the models, hence only one interaction term. The odds ratios in the far right column are an illustration of the odds ratios at different levels of school achievement (−2, −1, 0, 1, 2).
Figure 2.
Estimated Risk for Drug Abuse (DA) During the Control Periods and Pregnancy as a Function of Four Potential Moderators: Prior Crime, Parental Drug Abuse, Prior Drug Abuse (in the Pregnant mother) and Marital Status. These estimates are from logistic regression models with interaction terms (as seen in table 3).
Risk for Registration for Drug Abuse in the Immediate Post-Partum Period
To examine whether the protective effect of pregnancy on drug abuse extended into the post-partum period, we conducted within-individual analyses using as baseline the rates of drug abuse in the 242-day pre-pregnancy period. As shown in table 4, rates of drug abuse registration were even lower in the immediate post-partum period (0–242 days) than during pregnancy (odds ratio=0.13 (0.11–0.16)). The rates of drug abuse rose modestly in subsequent periods (odds ratio = 0.15 (0.10–0.18) 243–484 days after childbirth and odds ratio=0.23 (0.19–0.28) 485–726 days after childbirth) so that by two-years post-partum, rates of drug abuse in these new mothers were similar to those seen during their pregnancy.
Table 4.
Within-Individual Analyses of Rates of Drug Abuse in the Pre-Pregnancy and Post-Partum Periods
| Risk Period | N | Prevalence of Drug Abuse during the |
Odds Ratio (95% Confidence Interval) |
|
|---|---|---|---|---|
| Control Period |
Risk Period |
|||
| 0–242 days after childbirth | 126,586 | 0.65% | 0.14% | 0.13 (0.11–0.16) |
| 243–484 days after childbirth | 110, 067 | 0.66% | 0.16% | 0.15 (0.10–0.18) |
| 485–726 days after childbirth | 93,799 | 0.66% | 0.22% | 0.23 (0.19–0.28) |
All control periods − 242 days prior to birth
DISCUSSION
We utilized multiple approaches to clarify the magnitude and causal nature of the association between pregnancy and drug abuse in Swedish women. We addressed 5 specific questions and review these results in turn.
First, consistent with prior studies (20;23;24;29), we showed a moderate cross-sectional inverse association between pregnancy and rates of drug abuse. When controlling for parental education and subject school achievement, this association substantially strengthened, suggesting inverse confounding. With these covariates, rates of drug abuse were reduced by 60% during pregnancy.
Second, we applied a co-relative design examining risk for drug abuse in pairs of related women discordant for pregnancy. As expected given inverse familial confounding, the pregnancy-drug abuse association became progressively stronger the more closely related the relative pair. Using a model that fitted the data well, we could predict a reduction in risk for drug abuse of 83% in a pregnant woman compared to her non-pregnant monozygotic co-twin.
Third, because pregnancy is episodic and discrete, we could conduct within-individual analyses with subjects acting as their own control. Compared to the immediately preceding equivalent time period, rates of drug abuse declined 78% during pregnancy – nearly identical to that estimated in pregnancy discordant monozygotic twins. We also showed that the protective effect of pregnancy was not affected by changing marital status, was similar in first and second pregnancies, and was stronger in the second than the first half of pregnancy. While conclusions from observational data are always tentative, our results from population findings with covariates, and co-relative, and within-person analyses are consistent in suggesting that the large majority of the inverse association between pregnancy and drug abuse is causal in nature.
These results can be usefully compare to several prior studies. In longitudinal analyses of the Monitoring the Future Study, 4% of pregnant women reported using cannabis in the last 30 days, a level one-third that of matched non-pregnant women (22). Multivariate analyses found that most of this effect was independent of other predictors including marital status. Analyses of recent US national survey data showed, in cross-sectional analyses, that recent cannabis use in the second and third trimester of pregnancy among women aged 12–44 was 3.6 and 1.7%, versus in a matched group of non-pregnant women of 7.6% (29). In a sample of 1,492 consecutive prenatal care patients from four urban US clinics, 55.6% of mothers using illicit drugs reported cessation during pregnancy (23). In a sample of 1,336 mothers from the Baltimore-Washington infant study, 54.6% of mothers who reported illicit drug use quit during pregnancy (24). Finally, nurse-midwife reports of smoking rates in pregnant women in Sweden show a decline in any and heavy smoking from 21.9%/12.3% prior to pregnancy to 10.1%/3.3% at week 30–32 (30). The validity of these smoking measures is supported by much higher risk of COPD in women still smoking in their third trimester (30).
We examined an array of variables that might moderate the impact of pregnancy on drug abuse. For most of them, including prior crime or drug abuse (which strongly predicted risk for drug abuse during pregnancy), the protective effect of pregnancy (as indexed by the odds ratio) was similar in those at low or high baseline risk. However, as evidence in figure 2, taking a risk difference approach shows that pregnancy reduces a far greater number of drug abuse episodes in those at high than low prior risk. Features of drug abuse in the cohabiting father of the child weakened the protective effect of pregnancy, particularly when the father was abusing drugs during the pregnancy.
Fourth, studying multiple pregnancies and patterns of drug abuse in the cohabiting child’s father provide some insights into the mechanism of the drug abuse-pregnancy association. If driven largely by long-term changes in attitudes towards drug use, we would expect an attenuation of the protective effect over multiple pregnancies. If, by contrast, the reduction results from a time-limited desire to reduce fetal exposure, then the effect might be more similar across pregnancies. Our results that show similar protective effects for a first and second pregnancy supports the latter hypothesis. The large reduction in rates of drug abuse in the pregnant woman compared to the cohabiting father allows us to isolate the impact of her direct motivation to reduce exposure to her child from more general social pressures on the couple. However, the cohabiting father does reduce his rate of drug abuse modestly during his partner’s pregnancy and fathers who desist likely encourage the mothers’ desistance. Indeed, the protective effect of pregnancy on drug abuse risk in mothers weakened when the husband abused drugs during the pregnancy.
Finally, we examined, using within-individual analyses, whether the large reduction in risk for drug abuse during pregnancy persisted into the immediate post-partum period. We found that it did and indeed during the first year post-partum, rates of drug abuse fell even lower – to 84% below their pre-pregnancy rates. They then climbed gradually so that by approximately two years after birth, rates of drug abuse were similar to those observed during pregnancy.
Whereas post-partum drug abuse relapse is common in the USA, ranging from 27% for cocaine, 41% for marijuana (31) and up to 85% for cigarettes (32), our study showed, after birth, a further decrease in drug abuse below those observed in pregnancy. Higher rates of breastfeeding among Swedish women (33–35) may play a role in this, as breastfeeding appears to be protective against relapse to alcohol (36) and cigarette smoking (37). Klee has previously reviewed the wide range of factors that might contribute to a protective effect of parenting on drug abuse (38). More specifically, and consistent with our findings, Bachman et al, using longitudinal data from the Monitoring the Future study, observed among both women who remained married and those who remained single, considerable reductions in cannabis and cocaine use associated with the transition into parenthood (39).
Recent meta-analysis of controlled trials of contingency management in the treatment of drug abuse estimated effect sizes by Cohen’s d (40) of 0.32 (5), 0.42 (4), and 0.58 (6). Using our within-person analyses, the effect size for pregnancy and early parenthood on drug abuse registration were larger (0.83, 0.74–0.92) and (1.12, 1.01–1.21), respectively, suggesting strong maternal motivation to protect her developing child and young infant from the direct and indirect effects of drug exposure.
While pregnancy intrinsically motivates many women to discontinue drug abuse, others do not. For them, pregnancy provides OB practitioners and counselors with a “teachable moment” to kindle maternal motivation to abstain from drug use (41). Research finds contingency management can further encourage prenatal care (42) and counseling session attendance (43;44). Despite some evidence that extrinsic motivation interferes with intrinsic motivation to stop smoking (45), integrated therapies for drug abuse are gaining support, often pairing interventions that promote intrinsic motivation, such as Motivational Interviewing, with extrinsic contingency motivation (e.g., (46).
Limitations
These results should be interpreted in the context of five potential methodological limitations. First, our results are limited to the Swedish population and may not extrapolate to other countries. Second, drug abuse was ascertained using medical and criminal records which are not dependent on subject cooperation or accurate recall. Compared to interviews, these methods likely generate both false positive and, particularly, false negative diagnoses. While large interview-based studies of drug abuse prevalence do not exist in Sweden, lifetime prevalence of drug abuse/dependence in near-by Norway is only slightly higher than the estimates we obtain using our methods in Sweden (47). Third, we cannot rule out additional factors such as counseling from nurse mid-wives or threats of child removal influencing pregnant women’s decisions to cease drug abuse. However, removal of children from mothers is very rare in Sweden and in a recent year involved only 0.08% of children aged 0–3 (48).
Fourth, because of frequent amenorrhea, drug-abusing women may have a delayed recognition of pregnancy. We therefore re-ran our within-person analyses assuming the women did not recognize their pregnancy until 32 days after what would have typically been their last menstrual period. The resulting odds ratio for drug abuse during pregnancy (0.23 (0.19–0.27)) was nearly identical to that found in our main analyses.
Fifth, pregnancy could impact on our methods of drug abuse ascertainment. To examine this, we repeated the within-person analyses for drug abuse based on our two most common forms of ascertainment. The protective effect of pregnancy on drug abuse was clearly seen but was slightly weaker from medical (odds ratio=0.32, 0.26–0.40) than from criminal registration (odds ratio=0.20, 0.16–0.25). A similar but more striking difference was seen in the immediate post-partum period where the protective effects were weaker from medical (odds ratio=0.19 (0.15; 0.25)) than from criminal registration for drug abuse (odds ratio=0.09 (0.07; 0.13)).
Conclusions
While definitive resolutions of causal questions in observational data is impossible, multiple lines of evidence suggest that women voluntarily decrease their levels of drug abuse during pregnancy and early parenthood. Whereas we have made major advances in the understanding the biological basis of addiction (1–3), there are limitations to an exclusively brain-based view of drug abuse. Consistent with prior studies of contingency management (4–6), this study suggests that volitional and motivational components can interact with biological vulnerabilities and neurobiological changes to alter the course of drug abuse. Understanding the strong and often effective motivation demonstrated by pregnant women and young mothers to stop their drug abuse might help us improve our contingency management treatment methods.
Acknowledgments
Disclosures and Acknowledgements: This project was supported by grants R01DA030005 and UG1DA013034 from the National Institutes of Health, the Swedish Research Council (K2012-70X-15428-08-3), the Swedish Research Council for Health, Working Life and Welfare (In Swedish: Forte; Reg.nr: 2013–1836), the Swedish Research Council (2012–2378; 2014–10134) and FORTE (2014–0804) as well as ALF funding from Region Skåne awarded.
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest to declare.
Where was the work performed: Analyses – Malmö Sweden; Writing - Richmond USA and Malmö Sweden.
Ethical Standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (No. 2008/409).
References
- 1.Leshner AI. Addiction is a brain disease, and it matters. Science. 1997 Oct 3;278(5335):45–7. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Le Moal M. Neurobiology of Addiction. London, UK: Elsevier, Inc - Academic Press; 2006. [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010 Jan;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006 Nov;101(11):1546–60. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 5.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006 Feb;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 6.Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008 Feb;165(2):179–87. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 7.Gunn JK, Rosales CB, Center KE, Nunez A, Gibson SJ, Christ C, Ehiri JE. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e009986. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cain MA, Bornick P, Whiteman V. The maternal, fetal, and neonatal effects of cocaine exposure in pregnancy. Clin Obstet Gynecol. 2013 Mar;56(1):124–32. doi: 10.1097/GRF.0b013e31827ae167. [DOI] [PubMed] [Google Scholar]
- 9.Richardson GA, Goldschmidt L, Larkby C, Day NL. Effects of prenatal cocaine exposure on adolescent development. Neurotoxicol Teratol. 2015 May;49:41–8. doi: 10.1016/j.ntt.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwiatkowski MA, Roos A, Stein DJ, Thomas KG, Donald K. Effects of prenatal methamphetamine exposure: a review of cognitive and neuroimaging studies. Metab Brain Dis. 2014 Jun;29(2):245–54. doi: 10.1007/s11011-013-9470-7. [DOI] [PubMed] [Google Scholar]
- 11.Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta-analysis. BMC Psychiatry. 2014;14:104. doi: 10.1186/1471-244X-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith E, Kamel I, Mastrogiannis DS. Drug Addiction and Pregnancy: Part 2 - Management of Pregnancy Complicated by Substance Abuse. J Postgrad Gynecol Obs. 2014;34(22):1–7. [Google Scholar]
- 13.ACOG Committee on Health Care for Underserved Women, American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070–6. doi: 10.1097/AOG.0b013e318256496e. [DOI] [PubMed] [Google Scholar]
- 14.Haug NA, Duffy M, McCaul ME. Substance abuse treatment services for pregnant women: psychosocial and behavioral approaches. Obstet Gynecol Clin North Am. 2014 Jun;41(2):267–96. doi: 10.1016/j.ogc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Brandon AR, Pitts S, Denton WH, Stringer CA, Evans HM. A HISTORY OF THE THEORY OF PRENATAL ATTACHMENT. J Prenat Perinat Psychol Health. 2009;23(4):201–22. [PMC free article] [PubMed] [Google Scholar]
- 16.Cannella BL. Maternal-fetal attachment: an integrative review. J Adv Nurs. 2005 Apr;50(1):60–8. doi: 10.1111/j.1365-2648.2004.03349.x. [DOI] [PubMed] [Google Scholar]
- 17.Yarcheski A, Mahon NE, Yarcheski TJ, Hanks MM, Cannella BL. A meta-analytic study of predictors of maternal-fetal attachment. Int J Nurs Stud. 2009 May;46(5):708–15. doi: 10.1016/j.ijnurstu.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Hutchins E, DiPietro J. Psychosocial risk factors associated with cocaine use during pregnancy: a case-control study. Obstet Gynecol. 1997 Jul;90(1):142–7. doi: 10.1016/S0029-7844(97)00181-6. [DOI] [PubMed] [Google Scholar]
- 19.Gatny H, Barber J, Kusunoki Y. Young Women and Substance Use during Pregnancy: The Role of Social Support. Population Studies Center, University of Michigan, Institute for Social Research; 2013. Report No.: Population Studies Center Research Report 13–788. 2013 Apr [Google Scholar]
- 20.Salas-Wright CP, Vaughn MG, Ugalde J, Todic J. Substance use and teen pregnancy in the United States: evidence from the NSDUH 2002–2012. Addict Behav. 2015 Jun;45:218–25. doi: 10.1016/j.addbeh.2015.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby D. Looking for reasons why: The antecedents of adolescent sexual risk-taking, pregnancy, and child-bearing. Washington, D.C: National Campaign to Prevent Teen Pregnancy; 1999. [Google Scholar]
- 22.Bachman JG, Wadsworth KN, O’Malley PM, Johnston LD, Schulenberg JE. Smoking, drinking, and drug use in young adulthood: The impacts of new freedoms and new responsibilities. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- 23.Harrison PA, Sidebottom AC. Alcohol and drug use before and during pregnancy: an examination of use patterns and predictors of cessation. Matern Child Health J. 2009 May;13(3):386–94. doi: 10.1007/s10995-008-0355-z. [DOI] [PubMed] [Google Scholar]
- 24.Johnson SF, McCarter RJ, Ferencz C. Changes in alcohol, cigarette, and recreational drug use during pregnancy: implications for intervention. Am J Epidemiol. 1987 Oct;126(4):695–702. doi: 10.1093/oxfordjournals.aje.a114709. [DOI] [PubMed] [Google Scholar]
- 25.Kendler KS, Ohlsson H, Mezuk B, Sundquist K, Sundquist J. A Swedish National Prospective and Co-relative Study of School Achievement at Age 16, and Risk for Schizophrenia, Other Nonaffective Psychosis, and Bipolar Illness. Schizophr Bull. 2015 Jul 31;42(1):77–86. doi: 10.1093/schbul/sbv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–32. [Google Scholar]
- 27.Kendler KS, Larsson Lönn S, Morris NA, Sundquist J, Långström N, Sundquist K. A Swedish National Adoption Study of Criminality. Psychol Med. 2014;44(9):1913–25. doi: 10.1017/S0033291713002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SAS Institute I. SAS/STAT User’s Guide, Version 9.3. Cary, NC: SAS Institute Inc, SAS Institute Inc; 2011. [Google Scholar]
- 29.Alshaarawy O, Anthony JC. Cannabis use during pregnancy for the United States, 2005–2013. 2016 [Google Scholar]
- 30.Kendler KS, Lonn SL, Sundquist J, Sundquist K. Smoking and Schizophrenia in Population Cohorts of Swedish Women and Men: A Prospective Co-Relative Control Study. Am J Psychiatry. 2015 Jun 5;172(11):1092–100. doi: 10.1176/appi.ajp.2015.15010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forray A, Merry B, Lin H, Ruger JP, Yonkers KA. Perinatal substance use: a prospective evaluation of abstinence and relapse. Drug Alcohol Depend. 2015 May 1;150:147–55. doi: 10.1016/j.drugalcdep.2015.02.027. May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang WL, Goldstein AO, Butzen AY, Hartsock SA, Hartmann KE, Helton M, Lohr JA. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004 Jul;17(4):264–75. doi: 10.3122/jabfm.17.4.264. [DOI] [PubMed] [Google Scholar]
- 33.Socialstyrelsen. Statistics on Breastfeeding 2014. Sweden: Socialstyrelsen The National Board of Health and Welfare; 2016. Report No.: Art.no: 2016-9-20. [Google Scholar]
- 34.Yngve A, Sjostrom M. Breastfeeding in countries of the European Union and EFTA: current and proposed recommendations, rationale, prevalence, duration and trends. Public Health Nutr. 2001 Apr 4;(2B):631–45. doi: 10.1079/phn2001147. [DOI] [PubMed] [Google Scholar]
- 35.Callen J, Pinelli J. Incidence and duration of breastfeeding for term infants in Canada, United States, Europe, and Australia: a literature review. Birth. 2004 Dec;31(4):285–92. doi: 10.1111/j.0730-7659.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 36.Giglia R, Binns C. Alcohol and lactation: A systematic review. Nutr Diet. 2006;63(0):103–16. [Google Scholar]
- 37.Forray A. Substance use during pregnancy. F1000Res. 2016 May 13;5 doi: 10.12688/f1000research.7645.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klee H. Drugs and Parenting. In: Klee H, Jackson M, Lewis S, editors. Drug Misuse and Motherhood. London: Routledge; 2002. [Google Scholar]
- 39.Bachman JG, Wadsworth KN, O’Malley PM, Scuhlenberg J, Maggs JL. Marriage, divorce, and parenthood during the transition to young adulthood: Impacts on drug use and abuse. In: Schulenberg J, Maggs JL, editors. Health Risks and developmental transitions during adolescence. Cambridge, UK: The Cambridge University Press; 1997. [Google Scholar]
- 40.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Orlando, FL: Academic Press, Inc; 1977. [Google Scholar]
- 41.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003 Apr;18(2):156–70. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- 42.Elk R, Mangus L, Rhoades H, Andres R, Grabowski J. Cessation of cocaine use during pregnancy: effects of contingency management interventions on maintaining abstinence and complying with prenatal care. Addict Behav. 1998 Jan;23(1):57–64. doi: 10.1016/s0306-4603(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 43.Svikis DS, Silverman K, Haug NA, Stitzer M, Keyser-Marcus L. Behavioral strategies to improve treatment participation and retention by pregnant drug-dependent women. Subst Use Misuse. 2007;42(10):1527–35. doi: 10.1080/10826080701212121. [DOI] [PubMed] [Google Scholar]
- 44.Jones HE, Haug N, Silverman K, Stitzer M, Svikis D. The effectiveness of incentives in enhancing treatment attendance and drug abstinence in methadone-maintained pregnant women. Drug Alcohol Depend. 2001 Feb 1;61(3):297–306. doi: 10.1016/s0376-8716(00)00152-6. [DOI] [PubMed] [Google Scholar]
- 45.Curry SJ, Wagner EH, Grothaus LC. Evaluation of intrinsic and extrinsic motivation interventions with a self-help smoking cessation program. J Consult Clin Psychol. 1991 Apr;59(2):318–24. doi: 10.1037//0022-006x.59.2.318. [DOI] [PubMed] [Google Scholar]
- 46.Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000 Dec;68(6):1051–61. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- 47.Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. Am J Psychiatry. 2001 Jul;158(7):1091–8. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]
- 48.Socialstyrelsen. Statistik om socialtjänstinsatser till barn och unga 2014. Sweden: Socialstyrelsen: The National Board of Health and Welfare: Ministry of Health and Social Affairs; 2015. Report No.: Art.nr:2015-12-33. [Google Scholar]