Abstract
Premenstrual dysphoric disorder (PMDD) symptoms are eliminated by ovarian suppression, stimulated by administration of ovarian steroids, yet appear in the context of levels of ovarian steroids indistinguishable from those in women without PMDD. Thus PMDD symptoms could be precipitated by either an acute change in the levels of ovarian steroids, or that stable levels of ovarian steroids above a critical threshold play a permissive role in the expression of an underlying infradian affective “pacemaker.” In this study, we attempted to define the kinetics of the ovarian steroid event relevant to triggering of PMDD symptoms.
We studied 22 women with PMDD, aged 30 to 50 years. Twelve women who experienced symptom remission after 2–3 months of GnRH agonist-induced ovarian suppression (leuprolide) then received one month of single-blind(participant only) placebo and then three months of continuous combined estradiol/progesterone. Primary outcome measure was the Rating for Premenstrual Tension observer rater- and self-ratings completed every two weeks during clinic visits. Multivariate repeated measure ANOVA for mixed models was employed.
Both Premenstrual Tension -self and –rater scores were significantly increased (more symptomatic) during the first month of combined estradiol/progesterone compared with all other months (i.e., last month of leuprolide alone (p=.0003,p<.0001, respectively), placebo(p=.0015,p=.0013, respectively), and 2nd month of estradiol/progesterone (p=.0014,p<.0001, respectively), and 3rd month of estradiol/progesterone (p=.0006,p<.0001, respectively). There were no significant differences in symptom severity scores (Premenstrual Tension -self, –rater)between the last month of leuprolide alone and the placebo month(p=.609, p=.106, respectively), 2nd month of estradiol/progesterone (p=.639, p=.524, respectively) and 3rd month of estradiol/progesterone (p=.99, p=.812, respectively). Finally, Premenstrual Tension scores in the 2nd and 3rd estradiol/progesterone months did not significantly differ.
We demonstrated that the change in levels of estradiol/progesterone from low to high, and not the steady state levels, was associated with the onset of PMDD symptoms. Therapeutic efforts to modulate the change in steroid levels proximate to ovulation merit further study.
Introduction
Premenstrual dysphoric disorder (PMDD) is characterized by distressing mood and behavioral symptoms during the luteal phase of the normal menstrual cycle which disappear within a few days after menses begin (1;2). No abnormalities of ovarian hormone levels have been consistently identified that distinguish women with PMDD from those women who experience no mood or behavioral symptoms during the luteal phase (3). Nonetheless, a critical role for ovarian steroids in the expression of PMDD symptoms is suggested by the following: First, both GnRH agonist-induced ovarian suppression and ovariectomy eliminate symptoms in the majority of women with PMDD (4;4–15). Second, re-exposure to physiologic doses of either estradiol or progesterone (but not placebo) for four weeks duration resulted in a recurrence of PMDD symptoms after 2–3 weeks of exposure in women with PMDD whose symptoms remitted after GnRH agonist treatment (controls who participated in an identical hormone manipulation study were not symptomatic) (14). Finally, inhibition of the luteal phase increase in the progesterone metabolite allopregnanolone with dutasteride, a 5alpha-reductase inhibitor, mitigated symptom emergence in PMDD (16).
Reproductive steroids, therefore, appear to play a role in PMDD. What remains unclear is the nature of the ovarian steroid symptom-trigger in PMDD. Evidence to date cannot disambiguate the effects of an acute change in the level of ovarian steroid (or metabolite) from those of continuous exposure to elevated levels of ovarian steroids. Preclinical studies suggest that the short term exposure or withdrawal of progesterone could impact CNS function to induce affective symptoms in an otherwise vulnerable woman. Both increases and decreases in progesterone (and its neurosteroid metabolites) induce changes in the alpha 4 subunit conformation of the GABAA R sufficient to produce anxiety-like behaviors (17–19). Alternatively, studies in rodents demonstrate that estradiol is proconvulsant and accelerates the acquisition of amygdalar kindled seizures (20;21). Thus, ovarian steroids can modulate intrinsic neuronal excitation, lower thresholds for neuronal firing, and potentially impact the set-points for certain behavioral states. If PMDD is associated with an abnormal infradian zeitgeber, the expression of which is dependent on a critical threshold of exposure to ovarian steroids, then ovarian steroids could play a permissive role in the expression of abnormal neuronal activity within the affective circuits involved with PMDD, thus leading to symptom onset.
In this study, we attempted to define the kinetics of the ovarian steroid event relevant to triggering PMDD symptoms. We selected women with PMDD who responded to treatment with GnRH agonist-induced ovarian suppression (i.e., PMDD symptom remission), who then were exposed to three months of combined continuous estradiol and progesterone treatment. If the change in hormone level is critical, then we would expect the initial recurrence of PMDD symptoms in the first month of ovarian steroid exposure followed by a remission of PMDD symptoms once ovarian steroid levels were stable and maintained during months 2–3 of hormone treatment. Alternatively, if the ovarian steroid exposure above threshold levels is the key physiologic event to permit an infradian pacemaker to produce episodic cyclic symptoms during the luteal phase, then we would predict recurrent episodes of affective symptoms reminiscent of luteal phase-related symptom cyclicity in the context of stable hormone levels during each of the three months of ovarian steroid exposures.
Methods
Participant Selection
We studied 22 women, aged 30 to 50 years, all of whom met study criteria for PMDD, which are based on requirements outlined in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (1). All women were free of medical illness, had regular menstrual cycles (i.e., 21–35 days in duration) and were medication-free (including oral contraceptives and any hormonal therapy). All women enrolled had normal physical findings and laboratory tests, were not pregnant and agreed to use barrier contraception throughout the study. None of the women with PMDD had any Axis I psychiatric illness currently or within the previous two years as determined by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (fourth edition) (DSM-IV) (1).
Before study enrollment, women prospectively confirmed the timing and severity of their mood symptoms by rating themselves daily for three months using a four-item visual-analogue scale that confirmed the timing and severity of their menstrually-related mood symptoms (irritability, sadness, anxiety, and mood swings) as described previously (14;22). The mean score of at least one of these self-rated negative mood symptoms had to be at least 30% higher (relative to the range of the scale used by each woman) in the week before menstruation compared to the week after the cessation of menstruation in at least two of the three cycles assessed.
Functional impairment was assessed through self-reports of distress and functional impairment on the Daily Rating Form (23). Daily ratings and the results of both a semi-structured interview and a self-report questionnaire were employed to confirm that all women met the required number of symptoms specified in the DSM criteria for PMDD. Women with significant negative mood symptoms occurring during the follicular phase of the menstrual cycle (on the Daily Rating Form) were excluded. Thus, in this study, the diagnostic criteria for PMDD were augmented by the prospectively confirmed severity criterion of a 30% increase in mean negative mood during the week before menses compared with the week after menses, a more stringent criterion than that of DSM- 4 or 5 (1;2).
All women received payment for participation according to the NIH Healthy Volunteer Office guidelines. The study protocol was reviewed and approved by the NIMH Institutional Review Board, and all women provided written consent to study participation.
Study Design (Figure 1)
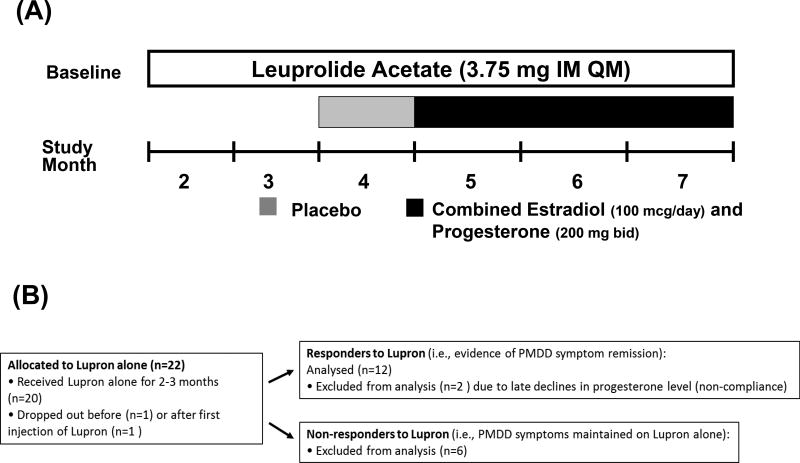
Figure 1.
(A) Study Design Schematic: After a baseline cycle in which the diagnosis of PMDD was established all women received open label leuprolide. Between 2 and 6 days after onset of menses, women received six monthly intramuscular injections of 3.75 mg leuprolide After two - three months of leuprolide alone, those women whose PMDD symptoms were in remission (responders to leuprolide) (i.e., self-reported improvement confirmed by Premenstrual Tension scores <5 and the absence of symptom cyclicity on Daily Rating Form (i.e., weekly [7-day] average daily scores for irritability, sadness or anxiety of < 3 (indicating less than moderate severity of a particular symptom) (52;53) were selected to continue on leuprolide for an additional 4 months. Women not meeting these criteria were considered non-responders and were not included in the statistical analysis. Responders to leuprolide continued to receive monthly leuprolide injections for another four months and received one month of single blind (to participant only) placebo (patch and suppository) followed by three months of combined estradiol (100 ug daily by skin patch) and progesterone (200 mg vaginal suppository twice daily) replacement.
Functional impairment was assessed through self-reports of distress and functional impairment on the Daily Rating Form (23). The Daily Rating Form criteria for functional impairment were as follows: a score of 2 (minimal) or higher on one of 4 questions related to functional impairment (i.e., stayed at home or avoided social activities, had conflicts or problems with people, symptoms interfered with relationships at work or home, or symptoms interfered with work productivity) in at least 3 days out of 7 days pre-menses.
(B) Study Patient Flow Chart
Between 2 and 6 days after onset of menses, women with PMDD received six or seven monthly intramuscular injections of 3.75 mg leuprolide , which after an initial stimulation suppresses ovarian function. Clinic visits occurred every 2 weeks. Plasma FSH, LH, estradiol, and progesterone levels were measured at each visit to confirm ovarian suppression. Following two-three months of leuprolide alone, those women whose PMDD symptoms were in remission (i.e., responders to leuprolide: self-reported improvement confirmed by Rating for Premenstrual Tension scale scores <5 and the absence of symptom cyclicity on Daily Rating Form) were selected to continue in the study. Five women with PMDD reported either the experience of distressing life events or a partial symptom response after their second month of leuprolide, and these five subjects were therefore maintained for a third month to evaluate if symptom remission could be demonstrated in each woman. The responders to leuprolide continued to receive monthly leuprolide for another four months and received one month of single blind (to participant only) placebo (patch and suppository) followed by three months of combined estradiol and progesterone replacement. [see Figure 1 for details]All women used a patch and a suppository each day during the hormonal add-back to maintain the patency of the blind.
Symptom Rating Scales
Our primary outcome measure was the Rating for Premenstrual Tension observer (rater)-ratings and self-ratings (24) completed every two weeks during regularly scheduled clinic visits to assess the presence (or absence) of PMDD symptoms. Additionally, Daily Rating Form scores for the core PMDD symptoms of irritability, sadness, and anxiety (2) were evaluated throughout the study. The Daily Rating Form is a six point Likert-type scale. Scores of 1= symptoms absent; 6= symptoms present in the extreme and measures reported symptoms of PMDD. Responders to leuprolide were defined by the Premenstrual Tension scores ≤ 5 during the last month of leuprolide alone as well as the absence of a weekly average daily rating score for irritability, sadness or anxiety ≥ 3. Women not meeting these criteria were considered non-responders and were not included in the statistical analysis.
Statistical Analysis
Only those women with PMDD who met criteria for symptom remission during the last month of leuprolide alone were continued on to the estradiol/progesterone and placebo addback phases of this protocol. Henceforth, month 1 thru 7 are as follows: Month 1 = baseline prior to receiving leuprolide (not employed in analysis), Month 2 = first month of leuprolide (not employed in analysis), Month 3 = last month of leuprolide, Month 4 = placebo, Month 5 = first month of estradiol/progesterone, Month 6 = second month of estradiol/progesterone, and Month 7 = third (last) month of estradiol/progesterone. The principal comparisons of our analyses were twofold: first, to examine PMDD symptom severity during each of the three months of estradiol/progesterone addback compared with the last month of leuprolide alone when PMDD symptoms were in remission (Month 5 v 3, 6 v 3 and 7 v 3), and, second, to look for potential differences in PMDD symptom severity during the first month of addback (initial change in estradiol/progesterone levels) compared with symptoms during the subsequent months of addback (when stable levels of estradiol/progesterone had been established) (Month 5 v 6 and 5 v 7). Additionally, to evaluate the presence or absence of placebo effects on PMDD symptoms, we compared PMDD symptom severity between the one month of single-blind placebo (Month 4) with both the first month of estradiol/progesterone addback (Month 5), and the last month of leuprolide alone (Month 3). Finally, we compared the last two months of estradiol/progesterone replacement to examine potential differences in PMDD symptom severity during the months when stable levels of estradiol/progesterone had been established (Month 6 v Month 7). Since the focus of this study was to examine the pattern of PMDD symptom “recurrence” during estradiol/progesterone addback, we did not include in the statistical analyses either the average rating scale scores for baseline pre-leuprolide(Month 1) or for the first month of leuprolide alone (Month 2) when in many women estradiol/progesterone levels will vary secondary to the flare of ovarian function induced by the first injection of leuprolide.
In leuprolide-responders both Premenstrual Tension and daily data involved repeated measures on the same woman during five hormone conditions (i.e., months). Premenstrual Tension symptom ratings were analyzed as two-week measures, and daily symptom ratings were analyzed as weekly averages. Multivariate repeated measures analyses were done with SAS Version 9.2 software (PROC MIXED, SAS Institute, Inc, Cary, NC). Separately, for each of the 9 symptom ratings, the predictor variable of interest, study month, instead of two-week or weekly time points, was used because the ratings within each month (weeks 2 and 4 of each month on the Premenstrual Tension-rater and –self, and weeks 1–4 of the Daily Rating Form ratings) showed no significant main or interactive effects of visit within each month or week in these symptom ratings, respectively. We used the Kenward and Roger method for computing the degrees of freedom for tests of fixed effects. The value of the least square means, associated standard errors, and P values are reported. Eight post hoc pairwise comparisons of least square means were compared among hormone conditions using t-tests. To adjust for multiple comparisons, results with p-values less than 0.005 (instead of 0.05) are considered statistically significant. Values above this threshold are reported but are considered not significant. This is an informal multiplicity adjustment given the exploratory nature of this study. Formally adjusting p-values in exploratory studies, e.g. Bonferroni-adjusted p-values, is not universally accepted because it reduces Type I errors at the expense of increasing Type II errors (25;26).
Clinical characteristics in women who did and did not meet criteria for response to leuprolide were compared with Fisher’s exact test for categorical variables and Student’s t-tests for continuous variables (Table1).
Table 1.
Demographics and clinical characteristics of women with PMDD treated with leuprolide.
| Leuprolide | Leuprolide | ||||
|---|---|---|---|---|---|
| Responders (n=12) |
Non-Responders (n=6) |
P value# | |||
| Age (years), mean SD | 38.4 | 7.4 | 40.8 | 3.4 | 0.56 |
| BMI (kg/m2), mean SD | 31.9 | 10.8 | 29.4 | 4.4 | 0.77 |
| Past Axis I Psychiatric Illness, numbers of women (%) | 3 (25) | 4 (67) | 0.14 | ||
| Duration of PMDD, years, mean (SD) | 19.0 | 10.5 | 16.8 | 8.1 | 0.66 |
| Age of onset of PMDD, years, mean (SD) | 19.0 | 7.1 | 23.2 | 6.8 | 0.25 |
| Current Medications + | 1 | 0 | |||
| Worse symptom (number of women)* | |||||
| Irritability | 6 | 4 | |||
| Sadness | 2 | 0 | |||
| Anxiety | 1 | 1 | |||
| Mood swings | 2 | 1 | |||
One woman received synthroid on a regular basis prior to the study
One women in the leuprolide responders group self-reported fatigue as her worst symptom
All P values represent the results of Students t-tests except Past Axis 1 Psychiatric illness in which the P value is from Fisher’s exact test.
Legend for table 1: There were no significant differences in age, BMI, age of onset of PMDD, and duration of PMDD between the women with PMDD who responded to GnRH agonist-induced ovarian suppression and those women who continued to exhibit symptoms during ovarian suppression.
Results
Participant characteristics
There were no significant differences in age, BMI, age of onset of PMDD, and duration of PMDD between the women with PMDD who responded to leuprolide and those women who continued to exhibit symptoms during ovarian suppression (Table 1). Twenty-two women with PMDD were enrolled and commenced leuprolide; two women dropped out early in the study, one before and one after the first injection of leuprolide, due to unexpected scheduling conflicts with their work. Thus twenty women received two to three months of leuprolide, six of whom did not respond to leuprolide with PMDD symptom improvement/remission, and fourteen women who met criteria for response to leuprolide and were then continued on leuprolide plus placebo, then estradiol/progesterone addback. Two of these fourteen women were not compliant with the estradiol/progesterone addback regimen as determined by plasma hormone levels (and subsequent self-report) and, therefore, were not included in the final analyses. (Figure 1b)
Symptom ratings
Rating for Premenstrual Tension scores
Both Premenstrual Tension -self and –rater scores were significantly increased (more symptomatic) during the first month of estradiol/progesterone addback (Month 5) compared with all of the other months (month 5 v 3: p =0.0003 and <0.0001, respectively; month 5 v 4: p=0.0015 and 0.0013, respectively; month 5 v 6: p=0.0014 and <0.0001, respectively; month 5 v 7: p= 0.0006 and <0.0001, respectively) (tables 2 and 3, figure 2). In contrast, there were no significant differences in symptom severity scores in either Premenstrual Tension -self or –rater scores between the last month of leuprolide alone (Month 3) and scores during placebo, second and third month of estradiol/progesterone addback. Finally, Premenstrual Tension scores in the second and third months of estradiol/progesterone addback also were not significantly different. This pattern of between month differences in symptom severity reflected the presence of significantly increased Premenstrual Tension-self and –rater scores during the first month of estradiol/progesterone compared with all other months (i.e. symptom recurrence in estradiol/progesterone addback month 1 only)
Table 2.
Differences in least squares mean (Δ LSM) and standard error of difference (SE) in women with PMDD who responded to Leuprolide (n=12) and then received combined estradiol and progesterone addback: PMDD symptom severity during each of the three months of E/P addback compared with the last month of Leuprolide alone when PMDD symptoms were in remission
| Study Month (M): | M3 V M5 | M3 V M6 | M3 V M7 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Symptom Rating Scale | leuprolide - E/P(1) | leuprolide - E/P(2) | leuprolide - E/P(3) | ||||||
| Δ LSM | SE | p value | Δ LSM | SE | p value | Δ LSM | SE | p value | |
| PMTS (self) (p = 0.0013) | −3.792 | 0.971 | 0.0003 | −0.458 | 0.971 | 0.6392 | −0.009 | 1.03 | 0.9928 |
| PMTS (rater) (p < 0.0001) | −4.625 | 0.907 | <0.0001 | −0.5833 | 0.907 | 0.5236 | 0.223 | 0.932 | 0.8119 |
| Daily Rating Form | |||||||||
| Sadness (p = 0.0158) | −0.272 | 0.09 | 0.0036 | −0.148 | 0.09 | 0.1051 | −0.004 | 0.091 | 0.9684 |
| Irritability (p = 0.0017) | −0.394 | 0.112 | 0.0008 | −0.212 | 0.112 | 0.0634 | 0.027 | 0.114 | 0.8132 |
| Anxiety (p = 0.3851) | −0.196 | 0.116 | 0.0969 | −0.189 | 0.116 | 0.1085 | −0.058 | 0.118 | 0.6266 |
| Mood Swings (p = 0.5370) | −0.116 | 0.075 | 0.1272 | −0.073 | 0.075 | 0.3351 | −0.077 | 0.076 | 0.3114 |
| Cravings (p = 0.1893) | −0.181 | 0.09 | 0.0484 | −0.038 | 0.09 | 0.6768 | 0.022 | 0.092 | 0.8138 |
| Bloating (p = 0.0445) | −0.306 | 0.131 | 0.0224 | −0.164 | 0.131 | 0.2145 | −0.397 | 0.133 | 0.004 |
| Breast Pain (p = 0.2697) | −0.225 | 0.153 | 0.1484 | −0.255 | 0.153 | 0.102 | −0.324 | 0.154 | 0.0405 |
| Plasma Hormone levels | |||||||||
| Estradiol (pg/ml) (p < 0.0001) | −45.854 | 12.04 | 0.0004 | −52.571 | 12.04 | <.0001 | −72.718 | 12.352 | <.0001 |
| Progesterone (ng/ml) (p < 0.0001) | −12.021 | 1.529 | <.0001 | −12.617 | 1.529 | <.0001 | −12.392 | 1.571 | <.0001 |
Abbreviations: E/P = combined estradiol and progesterone replacement; PMTS = Rating for Premenstrual Tension
Legend to Table 2:
PMDD symptom severity was compared during each of the three months of E/P addback with symptom severity during the last month of Leuprolide alone when PMDD symptoms were in remission. To adjust for multiple comparisons, results with p-values less than 0.005 are considered statistically significant. This is an informal adjustment given the exploratory nature of this study. Values above this threshold are reported but were considered not significant.
Month 3 is the last month of leuprolide alone, month 5 is the first month of combined estradiol and progesterone replacement E/P(1), and months 6 and 7 represent the second and third months of E/P(2) and E/P(3) (i.e., stable estradiol/progesterone levels).
Both PMTS-self and –rater scores were significantly increased (more symptomatic) during the first month of E/P addback (month 5) compared with leuprolide alone, whereas there were no significant differences in symptom severity scores in either PMTS-self or –rater scores between the last month of leuprolide (month 3) and scores during the second-third months of E/P (month 6 and month 7).
DRF scores: A pattern similar to that observed in PMTS scale scores was observed for the DRF symptom of irritability. Irritability scores during the first month of E/P addback were significantly increased compared with leuprolide alone, whereas there were no significant differences in symptom severity scores in irritability between the last month of leuprolide (month 3) and scores during the second and third months of E/P (month 6 and month 7). Daily Rating Form severity scores for sadness during the first month of E/P addback were significantly increased compared with the last month of leuprolide. There were no significant differences in the severity scores of any month for the symptoms of anxiety, mood swings, bloating (except between E/P addback month 3 and last month of leuprolide), breast pain and cravings.
Plasma progesterone and estradiol levels were measured by electrochemiluminescence immunoassay at the NIH Clinical Center Department of Laboratory Medicine. The lower limits of detectability for the progesterone and estradiol assays were 0.03 – 0.2 ng/ml and 5.0 – 10.0 pg/ml, respectively.
The Rating Scale for Premenstrual Tension (PMTS) scale consists of both an observer/rater-completed and a self-report rating that measures mood, behavior and physical symptoms on a 36-point scale, with scores > 10 consistent with PMDD symptoms and scores < 5 consistent with the absence of significant PMDD symptoms (51).
Daily Rating Form scores for the core PMDD symptoms of irritability, sadness, and anxiety were evaluated throughout the study. Each evening during the 2–3 months of baseline and during the three menstrual cycles on treatment, all women completed the daily rating form. All women were instructed that the ratings should represent a composite score for the previous 12 hours.
Table 3.
Differences in least squares mean (Δ LSM) and standard error of difference (SE) in women with PMDD: PMDD symptom severity during first month of estradiol and progesterone addback (E/P 1) compared with placebo addback, and the second and third months of estradiol and progesterone addback; leuprolide alone compared with placebo addback; and differences between PMDD symptom severity between the second and third months of estradiol and progesterone addback.
| Study Month (M): | M4 V M5 | M3 V M4 | M5 V M6 | M5 V M7 | M6 V M7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom Rating Scale | Placebo - E/P(1) | leuprolide - Placebo | E/P(1) - E/P(2) | E/P(1) - E/P(3) | E/P(2) - E/P(3) | ||||||||||
| Δ LSM | SE | p value | Δ LSM | SE | p value | Δ LSM | SE | p value | Δ LSM | SE | p value | Δ LSM | SE | p value | |
| PMTS (self) (p = 0.0013) | −3.292 | 0.971 | 0.0015 | −0.500 | 0.971 | 0.6092 | 3.333 | 0.971 | 0.0014 | 3.782 | 1.03 | 0.0006 | 0.449 | 1.03 | 0.6649 |
| PMTS (rater) (p < 0.0001) | −3.125 | 0.907 | 0.0013 | −1.500 | 0.907 | 0.1055 | 4.042 | 0.907 | <0.0001 | 4.848 | 0.932 | <0.0001 | 0.806 | 0.932 | 0.3915 |
| Daily Rating Form | |||||||||||||||
| Sadness (p = 0.0158) | −0.100 | 0.09 | 0.2723 | −0.172 | 0.09 | 0.0604 | 0.125 | 0.09 | 0.1702 | 0.268 | 0.091 | 0.0046 | 0.144 | 0.091 | 0.1200 |
| Irritability (p = 0.0017) | −0.347 | 0.113 | 0.0031 | −0.047 | 0.113 | 0.6773 | 0.182 | 0.112 | 0.1105 | 0.421 | 0.114 | 0.0005 | 0.239 | 0.114 | 0.0397 |
| Anxiety (p = 0.3851) | −0.099 | 0.117 | 0.3981 | −0.097 | 0.117 | 0.4105 | 0.007 | 0.116 | 0.9547 | 0.138 | 0.118 | 0.2441 | 0.132 | 0.118 | 0.2671 |
| Mood Swings (p = 0.5370) | −0.0004 | 0.077 | 0.9955 | −0.115 | 0.076 | 0.1356 | 0.043 | 0.075 | 0.5671 | 0.039 | 0.076 | 0.6142 | −0.005 | 0.076 | 0.9511 |
| Cravings (p = 0.1893) | −0.167 | 0.09 | 0.0704 | −0.015 | 0.09 | 0.8725 | 0.143 | 0.09 | 0.1157 | 0.203 | 0.091 | 0.0304 | 0.059 | 0.091 | 0.5195 |
| Bloating (p = 0.0445) | −0.120 | 0.131 | 0.3633 | −0.186 | 0.131 | 0.1599 | 0.143 | 0.131 | 0.2797 | −0.091 | 0.133 | 0.4977 | −0.233 | 0.133 | 0.084 |
| Breast Pain (p = 0.2697) | −0.095 | 0.153 | 0.5392 | −0.130 | 0.153 | 0.4005 | −0.030 | 0.153 | 0.8435 | −0.099 | 0.154 | 0.5216 | −0.069 | 0.154 | 0.6559 |
| Plasma Hormone levels | |||||||||||||||
| Estradiol (pg/ml) (p < 0.0001) | −44.204 | 12.045 | 0.0007 | −1.650 | 12.045 | 0.8917 | −6.717 | 12.045 | 0.5800 | −26.864 | 12.352 | 0.0351 | −20.147 | 12.352 | 0.1101 |
| Progesterone (ng/ml) (p < 0.0001) | −12.063 | 1.529 | <.0001 | 0.042 | 1.529 | 0.9784 | −0.596 | 1.529 | 0.6987 | −0.371 | 1.5701 | 0.8142 | 0.225 | 1.571 | 0.8870 |
Abbreviations: E/P = combined estradiol and progesterone replacement; PMTS = Rating for Premenstrual Tension
Legend to Table 3:
To evaluate the presence or absence of placebo effects on PMDD symptoms, we compared PMDD symptom severity between the one month of single-blind placebo with both the first month of E/P addback (Month 4 v 5), and the last month of leuprolide alone (Month 3 v 4). Additionally, we evaluated differences in PMDD symptom severity during the first month of addback (initial change in E/P levels) compared with symptoms during the subsequent months of addback (when stable levels of E/P had been established) (Month 5 v 6 and 5 v 7). Finally, we compared the last two months of E/P replacement to examine potential differences in PMDD symptom severity during the months when stable levels of E/P had been established (Month 6 v 7). To adjust for multiple comparisons, results with p-values less than 0.005 are considered statistically significant. This is an informal adjustment given the exploratory nature of this study. Values above this threshold are reported but were considered not significant.
Both Premenstrual Tension-self and –rater (PMTS-self and PMTS-rater) scores were significantly increased (more symptomatic) during the first month of E/P addback (month 5) compared with placebo addback (Month 4), as well as with scores during the second and third months of addback (Months 6 and 7). There were no significant differences in symptom severity scores in either PMTS-self or –rater scores between the last month of leuprolide (month 3) and scores during placebo (month 4), and the second-third months of E/P (month 6 and month 7). Finally, PTMS scores in the second month of E/P addback (month 6) and the third month of E/P addback (month 7) also were not significantly different.
Daily Rating Form scores: A pattern similar to that observed in PMTS scale scores was observed for the symptom of irritability. Irritability scores during the first month of E/P addback were significantly increased (more symptomatic) during the first month of E/P addback (month 5) compared with placebo addback (Month 4), as well as with scores during the third month of addback (Month 7). There were no significant differences in irritability symptom severity scores between the last month of leuprolide (month 3) and scores during placebo (month 4), and the second-third months of E/P (month 6 and month 7). Daily severity scores for sadness during the first month of E/P addback were significantly increased compared with the third month of E/P addback (Month 5 v 7). Symptom severity scores in no other months differed significantly.
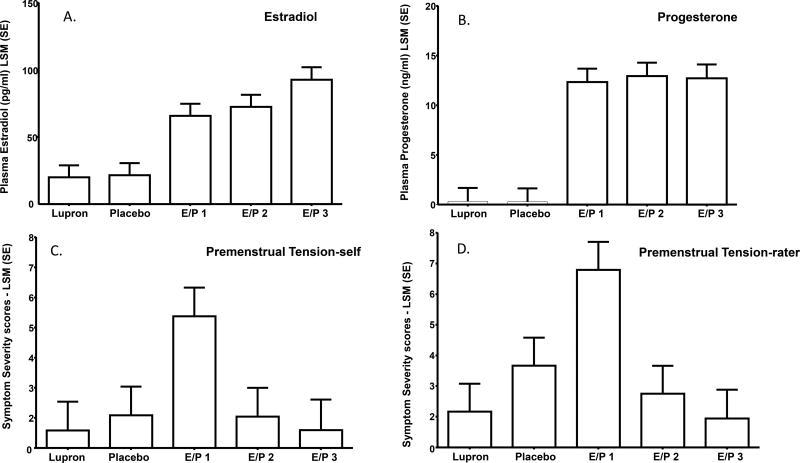
Figure 2.
Upper Panel: Plasma estradiol (A) and progesterone (B) were significantly increased in the three months of estradiol/progesterone addback compared with the last month of leuprolide and the month of single-blind placebo. There were no significant differences in plasma levels between the first month of estradiol/progesterone addback compared with the second and third months of estradiol/progesterone addback.
Lower Panel: The pattern of between month differences in symptom severity reflects the presence of significantly increased Premenstrual Tension-self (C) and –rater (D) scores during the first month of estradiol/progesterone (E/P in figure) addback (Month 5) compared with all other months (i.e., last month of leuprolide alone, placebo, and the second and third months of estradiol/progesterone addback). In contrast, there were no significant differences in symptom severity scores in either Premenstrual Tension-self or –rater scores between the last month of leuprolide alone (Month 3) and scores during placebo, second and third month of estradiol/progesterone addback. Finally, Premenstrual Tension scores in the second and third months of estradiol/progesterone addback also were not significantly different.
Daily Rating Form scores
A pattern similar to that observed in Premenstrual Tension scale scores was observed for the daily symptom of irritability. Irritability scores during the first month of estradiol/progesterone addback were significantly increased compared with all other months with the exception of scores during the second month of estradiol/progesterone addback (i.e., last month of leuprolide alone [Month 5 v 3, p=.0008], placebo [Month 5 v 4, p=.0031], and third month of estradiol/progesterone addback [Month 5 v 7, p=.0005]) (Tables 2 and 3). Daily irritability scores remained higher during the second month of estradiol/progesterone, reflecting non-significantly higher scores of irritability in the first month of estradiol/progesterone addback that carried over to month 2 of estradiol/progesterone addback and then remitted during the third month of estradiol/progesterone addback. There were no significant differences in symptom scores between the other months.
Daily symptom severity scores for sadness during the first month of estradiol/progesterone were significantly increased compared with the last month of leuprolide alone (Month 5 v 3, p=.0036) and the third month of estradiol/progesterone addback (Month 5 v 7, p=.0046). There were no significant differences in the severity scores of any month for the symptoms of anxiety, mood swings, bloating (except between estradiol/progesterone month 3 and last month of leuprolide [p=.004]), breast pain and cravings (Tables 2 and 3).
We inquired whether each woman believed they were on placebo or estradiol/progesterone replacement. After the placebo month, in the 12 women included in the analysis, 3 women felt they were on placebo, 5 thought they were on active estradiol/progesterone, and 4 did not know what they had received. Most of the women based their beliefs on either the severity of their hot flushes or their mood state. Additionally, 10 of these 12 women also reported break through menstrual bleeding during active addback but none during placebo addback, bleeding ranged from occasional spotting in most women to reports of full menses in two women. Menstrual bleeding if it did occur ranged from 2–5 days and occurred in all three months, albeit not in the same individual, of estradiol/progesterone addback.
Plasma estradiol and progesterone levels were significantly increased in estradiol/progesterone addback months 1–3 compared with the last month of leuprolide and the month of single-blind placebo. There were no significant differences between estradiol/progesterone month 1 compared with values during estradiol/progesterone months 2 and 3 (Tables 2 and 3, Figure 2).
Discussion
Apart from the ostensible linkage of PMDD symptoms to the luteal phase of the menstrual cycle, the pathophysiologic role of ovarian steroids in this condition is suggested by observations that the short term addback of either estradiol or progesterone is sufficient to result in a recrudescence of symptoms in women with PMDD whose symptoms remitted during ovarian suppression (14;27). These observations left open the possibility that either dynamic hormonal events (i.e., changing ovarian hormone levels) during the menstrual cycle or the prolonged exposure to a threshold level of ovarian steroids were critical to the triggering of PMDD symptoms. In this study, we demonstrated that it was the changes in levels of estradiol and progesterone from low to high levels, and not the steady state levels, that were associated with the onset of PMDD symptoms. We observed that compared with leuprolide alone conditions, several symptom rating scores significantly increased during the first month of ovarian steroid add-back but not during the second and third months of ovarian steroid addback when plasma levels of estradiol and progesterone were stable. Additionally, we observed no significant changes in symptoms during single-blind placebo addback. Thus our findings demonstrate that the change in level of ovarian steroids from low to high triggers the onset of a negative affective state in women with PMDD. Our results are consistent with several previous publications (14;27;28) that have demonstrated an increase in symptoms during the initial ovarian steroid addback in women whose PMDD responded to ovarian suppression. Interestingly, in the study by Segebladh (28), symptom recurrence was mainly observed upon addback of estradiol/progesterone compared with low dose estradiol alone, suggesting that either progesterone is a critical component of the symptom-triggering hormone event or that levels of ovarian steroids need to reach a specific threshold to trigger symptoms.
The appearance of symptoms in those women who experienced a recurrence of PMDD after the initial addback was time-limited in all women, and symptoms remitted during the second and third months of hormone addback when plasma levels of estradiol and progesterone were relatively stable. Thus, our findings suggest that there is a “half-life” of the affective state that is triggered, following which it remits. The nature of the “switch-out” or termination of the symptomatic state in PMDD remains to be characterized.
The mechanism whereby a change in ovarian steroid levels induces a recurrence of symptoms in women with a history of PMDD is unclear. Steroid nuclear receptor signaling provides for a wide range of time- and rate-dependent regulatory mechanisms whereby a change in steroid level could impart differential cellular effects compared with those caused by steady state levels (29). For example, basic science studies suggest that the initial change (i.e., increase or decrease) in progesterone levels can induce alterations in GABA receptor subunit conformation and induce paradoxical anxiety-like behavior in rodents (17–19), and an initial pulse of progesterone activates different transcriptional co-regulators from those seen after exposure to stable levels (30). Indeed, the timing and pulsatility of a hormone signal may have physiologic relevance in the biology of several clinical phenomena including the function of the hypothalamic-pituitary-gonadal axis, the stress response, growth and development, and circadian rhythms (31–40). Interestingly, symptoms recurred during the third month of addback in two women with PMDD proximate to observed declines in progesterone levels. This anecdotal observation suggests that both acute increases and declines in ovarian steroids may trigger a transition from the asymptomatic to the symptomatic state, consistent with the observations of Gulinello in rodents (17–19). Regardless of the mechanism underlying the steroid-induced recurrence of symptoms in PMDD, our findings provide a major clue to help decode the process by which clearly defined biological signals are, in susceptible individuals, translated into what is likely network-based affective dysregulation.
These findings have implications for the treatment of women with PMDD. First, a continuous exposure to hormones reminiscent of pregnancy could be an effective treatment for some women with PMDD (41). Studies using oral contraceptives have confirmed their efficacy in some women with PMDD (42–47). However, our data would suggest that continuous versus interrupted oral contraceptive would be more effective since the latter regimen would recapitulate changes in estrogen and progestin secretion that could induce symptoms, but perhaps at different times in the 28 day cycle than would occur across the natural menstrual cycle. Certainly women who are being treated with either leuprolide with the continuous addback or those commencing oral contraceptives should be warned about the possible recurrence of symptoms during the first phase of the addback. Additionally it is possible given the findings by Segebladh (28) that low dose estradiol alone could be employed for some women with PMDD with proper monitoring of the endometrium. Finally, preliminary findings from a small trial of the 5-alpha reductase inhibitor dutasteride suggested that preventing the luteal phase increase in allopregnanolone levels mitigates symptoms in PMDD (16). Thus, treatment strategies to attenuate or eliminate the change in estradiol and progesterone (or their metabolites) could effectively target the hormonal trigger in this condition.
Challenges and limitations of this study include the successful maintenance of the blind during active and placebo addbacks and the small sample size, which limits generalizability of our findings, respectively. First, certainly the presence or absence of hot flushes or menstrual bleeding could have suggested the presence of placebo or combined active addback. Nonetheless, this was not a traditional clinical trial design to contrast ovarian steroid add-back with placebo addback. Our main contrast was between the first month of active hormone add-back and months 2 and 3 of active addback. Consequently, even if participants correctly inferred when they received active versus placebo add-back (which was often not the case), the presence of PMDD symptoms during month 1 of estradiol/progesterone compared with months 2 and 3 confirms the study’s hypothesis. Second, although we observed a recurrence of symptoms in the daily ratings for irritability and sadness, it is possible that had we employed a larger sample, we would have identified significant changes in a broader range of PMDD symptoms. Additionally, our observed response rates are similar to those reported in several previous studies by our group (14) and others (4;5;9;48;49), and suggests that ovarian suppression is not uniformly effective in PMDD. These observations suggest that additional clinical characteristics of women with PMDD could militate against the beneficial effects of ovarian suppression in PMDD. For example, Pincus et al (50) suggested the pattern of symptom variability in some women with PMDD was predictive of response to leuprolide. Our approach was to test a specific hypothesis about the role of ovarian steroids in a phenomenon that we identified in a prior publication, (14) and a homogeneous sample of women with PMDD with evidence of hormone sensitivity (confirmed by their response to leuprolide) was necessary to achieve this study goal. Indeed, in addition to identifying the change in ovarian steroids as the relevant symptom-producing stimulus, our findings also emphasize the heterogeneity and complexity of the effects of hormone change in PMDD. Approximately two thirds of the women with PMDD showed symptom suppression on leuprolide, and of those 60% (7 of 12) showed symptom provocation when receiving estradiol or progesterone addback. Our findings, therefore, apply only to a sub-group of women with PMDD. Most importantly, these findings advance our understanding of the effects of ovarian steroids in the pathophysiology of PMDD and related conditions.
In conclusion, our findings confirm that the change in ovarian steroids contributes to the onset of negative affective symptoms in women with PMDD. We did not distinguish between the effects of estradiol and progesterone on symptom onset since we did not administer and withdraw these hormones separately. Indeed, in our previous study (14) we observed PMDD symptom recurrence after 2–3 weeks of either estradiol or progesterone, suggesting both hormones have the capacity to induce symptoms, whereas findings by Segebladh (28) suggest that PMDD recurrence is limited to combined estradiol and progesterone and not induced by low dose estradiol. These issues remain to be clarified in future studies. What also remains to be determined is why PMDD symptom recurrence is self-limited and symptoms remit despite continuing stable ovarian steroid levels. Presumably homeostatic mechanisms are activated related to either the presence of the negative affective state or the presence of stable levels of ovarian steroids. The latter possibility has been described by Smith in rodents, with alterations in GABA-A subunit conformations occurring after increases or decreases in progesterone or its neurosteroid metabolite allopregnanolone, but with conformations returning to normal during stable levels of these hormones (17–19). Although the mechanisms underlying the mood destabilizing effects of ovarian steroids in PMDD remain to be better characterized, as does the source of susceptibility to this trigger, our findings provide a new target on which interventions could be focused. Specifically, therapeutic efforts to inhibit the change in steroid levels proximate to ovulation, similar to that reported by Martinez, (16) merit further study.
Acknowledgments
This work was written as part of Peter J. Schmidt’s official duties as a Government employee. The views expressed in this article do not necessarily represent the views of the NIMH, NIH, HHS, or the United States Government.
This research was supported by the Intramural Research Program of the NIMH, NIH; NIMH Protocol 00-M-0103, NIMH Project # MH002865.
Funding:
The Intramural Research Program (IRP) of the NIMH, NIH supported this study; however, the NIMH IRP was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosures:
The Authors have no potential conflicts of interest or financial support regarding this manuscript.
Dr. Schmidt had full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Schmidt, Wakim, and Koziol performed data analyses for this study.
Clinical trial registration number: ClinicalTrials.gov Identifier: NCT00005011
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.American Psychiatric Association Fifth Edition . American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: 2013. [Google Scholar]
- 3.Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Frontiers in Neuroendocrinology. 2006;27:210–216. doi: 10.1016/j.yfrne.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Brown CS, Ling FW, Andersen RN, Farmer RG, Arheart KL. Efficacy of depot leuprolide in premenstrual syndrome: effect of symptom severity and type in a controlled trial. Obstetrics and Gynecology. 1994;84:779–786. [PubMed] [Google Scholar]
- 5.Helvacioglu A, Yeoman RR, Hazelton JM, Aksel S. Premenstrual syndrome and related hormonal changes: long-acting gonadotropin releasing hormone agonist treatment. Journal of Reproductive Medicine. 1993;38:864–870. [PubMed] [Google Scholar]
- 6.Hammarback S, Backstrom T. Induced anovulation as a treatment of premenstrual tension syndrome: a double-blind cross-over study with GnRH-agonist versus placebo. Acta Obstetricia et Gynecologica Scandinavica. 1988;67:159–166. doi: 10.3109/00016348809004191. [DOI] [PubMed] [Google Scholar]
- 7.Mezrow G, Shoupe D, Spicer D, Lobo R, Leung B, Pike M. Depot leuprolide acetate with estrogen and progestin add-back for long-term treatment of premenstrual syndrome. Fertility and Sterility. 1994;62:932–937. [PubMed] [Google Scholar]
- 8.Bancroft J, Boyle H, Warner P, Fraser HM. The use of an LHRH agonist, buserelin, in the long-term management of premenstrual syndromes. Clinical Endocrinology. 1987;27:171–182. doi: 10.1111/j.1365-2265.1987.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 9.West CP, Hillier H. Ovarian suppression with the gonadotrophin-releasing hormone agonist goserelin (Zoladex) in management of the premenstrual tension syndrome. Human Reproduction. 1994;9:1058–1063. doi: 10.1093/oxfordjournals.humrep.a138633. [DOI] [PubMed] [Google Scholar]
- 10.Muse KN, Cetel NS, Futterman LA, Yen SSC. The premenstrual syndrome: effects of “medical ovariectomy”. New England Journal of Medicine. 1984;311:1345–1349. doi: 10.1056/NEJM198411223112104. [DOI] [PubMed] [Google Scholar]
- 11.Casson P, Hahn PM, VanVugt DA, Reid RL. Lasting response to ovariectomy in severe intractable premenstrual syndrome. American Journal of Obstetrics and Gynecology. 1990;162:99–105. doi: 10.1016/0002-9378(90)90830-z. [DOI] [PubMed] [Google Scholar]
- 12.Casper RF, Hearn MT. The effect of hysterectomy and bilateral oophorectomy in women with severe premenstrual syndrome. American Journal of Obstetrics and Gynecology. 1990;162:105–109. doi: 10.1016/0002-9378(90)90831-q. [DOI] [PubMed] [Google Scholar]
- 13.Mortola JF, Girton L, Fischer U. Successful treatment of severe premenstrual syndrome by combined use of gonadotropin-releasing hormone agonist and estrogen/progestin. Journal of Clinical Endocrinology and Metabolism. 1991;72:252A–252F. doi: 10.1210/jcem-72-2-252. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. New England Journal of Medicine. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt KM, Dimmock PW, Ismail KMK, Jones PW, O’Brien PMS. The effectiveness of GnRHa with and without “add-back” therapy in treating premenstrual syndrome: a meta analysis. British Journal of Obstetrics and Gynaecology. 2004;111:585–593. doi: 10.1111/j.1471-0528.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 16.Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, Cintron D, Thompson KD, Khine KK, Schmidt PJ. 5alpha-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder. Neuropsychopharmacology. 2016 Apr 4;4:1093–1102. doi: 10.1038/npp.2015.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. Journal of Pharmacology and Experimental Therapeutics. 2003;305(2):541–548. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- 18.Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases a4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Research. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buterbaugh GG. Acquisition of amygdala-kindled seizures in female rats: relationship between the effect of estradiol and intra-amygdaloid electrode location. Pharmacology Biochemistry and Behavior. 1987;28(2):291–297. doi: 10.1016/0091-3057(87)90227-9. [DOI] [PubMed] [Google Scholar]
- 21.Hom AC, Buterbaugh GG. Estrogen alters the acquisition of seizures kindled by repeated amygdala stimulation or pentylenetetrazol administration in ovariectomized female rats. Epilepsia. 1986;27:103–108. doi: 10.1111/j.1528-1157.1986.tb03510.x. [DOI] [PubMed] [Google Scholar]
- 22.Rubinow DR, Roy-Byrne PP, Hoban MC, Gold PW, Post RM. Prospective assessment of menstrually related mood disorders. American Journal of Psychiatry. 1984;141:684–686. doi: 10.1176/ajp.141.5.684. [DOI] [PubMed] [Google Scholar]
- 23.Endicott J, Nee J, Cohen J, Halbreich U. Premenstrual changes: patterns and correlates of daily ratings. Journal of Affective Disorders. 1986;10:127–135. doi: 10.1016/0165-0327(86)90035-2. [DOI] [PubMed] [Google Scholar]
- 24.Steiner M, Haskett RF, Carroll BJ. Premenstrual tension syndrome: the development of research diagnostic criteria and new rating scales. Acta Psychiatrica Scandinavica. 1980;62:177–190. doi: 10.1111/j.1600-0447.1980.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ. Six persistent research misconceptions. Journal of General Internal Medicine. 2014;29(7):1060–1064. doi: 10.1007/s11606-013-2755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streiner DL. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. American Journal of Clinical Nutrition. 2015;102(4):721–728. doi: 10.3945/ajcn.115.113548. [DOI] [PubMed] [Google Scholar]
- 27.Henshaw C, Foreman D, Belcher J, Cox J, O’Brien S. Can one induce premenstrual symptomatology in women with prior hysterectomy and bilateral oophorectomy? Journal of Psychosomatic Obstetrics and Gynecology. 1996;17:21–28. doi: 10.3109/01674829609025660. [DOI] [PubMed] [Google Scholar]
- 28.Segebladh B, Borgstrom A, Nyberg S, Bixo M, Sundstrom-Poromaa I. Evaluation of different add-back estradiol and progesterone treatments to gonadotropin-releasing hormone agonist treatment in patients with premenstrual dysphoric disorder. American Journal of Obstetrics and Gynecology. 2009;201(2):139–8. doi: 10.1016/j.ajog.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol.Cell Endocrinol. 2012 Jun 24;357(1–2):18–29. doi: 10.1016/j.mce.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, Sclafani RA, Lange CA, Horwitz KB. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27Kip1. Molecular Endocrinology. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 31.Hauffa BP. Clinical implications of pulsatile hormone signals. Growth Horm.IGF.Res. 2001;11(Suppl A):S1–S8. doi: 10.1016/s1096-6374(01)80002-2. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138(3):1224–1231. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- 33.Veldhuis JD, Liu PY, Takahashi PY, Weist SM, Wigham JR. Analysis of the impact of intravenous LH pulses versus continuous LH infusion on testosterone secretion during GnRH-receptor blockade. Am.J.Physiol Regul.Integr.Comp Physiol. 2012 Nov 15;303(10):R994–R1002. doi: 10.1152/ajpregu.00314.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarabdjitsingh RA, Joels M, De Kloet ER. Glucocorticoid pulsatility and rapid corticosteroid actions in the central stress response. Physiol Behav. 2012 Apr 12;106(1):73–80. doi: 10.1016/j.physbeh.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA, McKenna MA, Spiga F, Wood SA, Conway-Campbell BL. The significance of glucocorticoid pulsatility. European Journal of Pharmacology. 2008 Apr 7;583(2–3):255–262. doi: 10.1016/j.ejphar.2007.11.073. [DOI] [PubMed] [Google Scholar]
- 36.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14(4):398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonnefont X. Circadian timekeeping and multiple timescale neuroendocrine rhythms. Journal of Neuroendocrinology. 2010;22(3):209–216. doi: 10.1111/j.1365-2826.2010.01955.x. [DOI] [PubMed] [Google Scholar]
- 38.Singh KB. Persistent estrus rat models of polycystic ovary disease: an update. Fertility and Sterility. 2005;84(Suppl 2):1228–1234. doi: 10.1016/j.fertnstert.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Ortega HH, Lorente JA, Mira GA, Baravalle C, Salvetti NR. Constant light exposure causes dissociation in gonadotrophin secretion and inhibits partially neuroendocrine differentiation of Leydig cells in adult rats. Reprod.Domest.Anim. 2004;39(6):417–423. doi: 10.1111/j.1439-0531.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 40.Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocrine Reviews. 2006;27(2):101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- 41.Schiller CE, Johnson AL, Abate AC, Rubinow DR, Schmidt PJ. Reproductive steroid regulation of mood and behavior. Comprehensive Physiology. 2016 doi: 10.1002/cphy.c150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72:414–421. doi: 10.1016/j.contraception.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Freeman EW, Kroll R, Rapkin A, Pearlstein T, Brown C, Parsey K, Zhang P, Patel H, Foegh M. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. Journal of Women’s Health & Gender-Based Medicine. 2001;10:561–569. doi: 10.1089/15246090152543148. [DOI] [PubMed] [Google Scholar]
- 44.Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstetrics and Gynecology. 2005;106:492–501. doi: 10.1097/01.AOG.0000175834.77215.2e. [DOI] [PubMed] [Google Scholar]
- 45.Lopez LM, Kaptein A, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome (review) The Cochrane Library. 2012;4:1–58. doi: 10.1002/14651858.CD006586.pub4. [DOI] [PubMed] [Google Scholar]
- 46.Halbreich U, Freeman EW, Rapkin AJ, Cohen LS, Grubb GS, Bergeron R, Smith L, Mirkin S, Constantine GD. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85(1):19–27. doi: 10.1016/j.contraception.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Freeman EW, Halbreich U, Grubb GS, Rapkin AJ, Skouby SO, Smith L, Mirkin S, Constantine GD. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. 2012;85(5):437–445. doi: 10.1016/j.contraception.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Freeman EW, Sondheimer SJ, Rickels K, Albert J. Gonadotropin-releasing hormone agonist in treatment of premenstrual symptoms: with and without comorbidity of depression: a pilot study. Journal of Clinical Psychiatry. 1993;54:192–195. [PubMed] [Google Scholar]
- 49.Hussain SY, Massil JH, Matta WH, Shaw RW, O’Brien PMS. Buserelin in premenstrual syndrome. Gynecological Endocrinology. 1992;6:57–64. doi: 10.3109/09513599209081007. [DOI] [PubMed] [Google Scholar]
- 50.Pincus SM, Alam S, Rubinow DR, Bhuvaneswar CG, Schmidt PJ. Predicting response to leuprolide of women with premenstrual dysphoric disorder by daily mood rating dynamics. Journal of Psychiatric Research. 2011;45(3):386–394. doi: 10.1016/j.jpsychires.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steiner M, Haskett RF, Osmun JN, Carroll BJ. Treatment of premenstrual tension with lithium carbonate. A pilot study. Acta Psychiatrica Scandinavica. 1980;61:96–102. doi: 10.1111/j.1600-0447.1980.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 52.Halbreich U, Backstrom T, Eriksson E, O’Brien S, Calil H, Ceskova E, Dennerstein L, Douki S, Freeman E, Genazzani A, Heuser I, Kadri N, Rapkin A, Steiner M, Wittchen H-U, Yonkers K. Clinical diagnosis criteria for premenstrual syndrome and guidelines for their quantification for research studies. Gynecological Endocrinology. 2007;23:123–130. doi: 10.1080/09513590601167969. [DOI] [PubMed] [Google Scholar]
- 53.Halbreich U. The diagnosis of premenstrual syndromes and premenstrual dysphoric disorder - clinical procedures and research perspectives. Gynecological Endocrinology. 2004;19:320–334. doi: 10.1080/0951590400018215. [DOI] [PubMed] [Google Scholar]