Abstract
Background
Diabetes is independently associated with increased risk of sudden cardiac arrest (SCA), with a need to identify novel methods for risk stratification. Diabetics can develop autonomic dysfunction which has been associated with increased risk of ventricular arrhythmogenesis, and manifests as reduced heart rate variability (HRV). However, previously published studies have not accounted for resting heart rate (HR), important from both pathophysiologic and prognosticating standpoints.
Objective
We sought to evaluate autonomic remodeling of the sinus node response in SCA and diabetes while accounting for HR.
Methods
We performed a case-control study in which SCA cases (age 35–59, 2002–2014) from the ongoing Oregon Sudden Unexpected Death Study (catchment population 1 million), and archived 12-lead ECGs performed prior to the SCA event were compared to geographical controls. Short-term HRV was calculated from digitized 10-second ECGs using established methods. We analyzed 313 subjects (mean age 52.4±5.3; 70.4% male) and compared 4 groups: 111 diabetics (49 cases, 62 controls) and 202 nondiabetics (80 cases, 122 controls).
Results
Covariance analysis showed an absence of the expected interaction between HRV and HR (HRV-HR) in diabetic patients with SCA (regression slope −0.008, CI −0.023 to 0.0071, p=0.26). This finding, unique to this population of diabetic SCA cases, was not detected using traditional HRV measures.
Conclusion
By incorporating resting heart rate in this analysis, we observed that this population of diabetics with SCA had loss of the expected HRV-HR relationship. This potentially novel, non-invasive risk measurement warrants further investigation, especially at the level of the individual patient.
Keywords: Sudden cardiac arrest, autonomic nervous system, diabetes, electrocardiogram, ventricular fibrillation, risk stratification
INTRODUCTION
Sudden cardiac arrest (SCA) remains a major cause of mortality, resulting in an estimated 300,000 to 350,000 deaths each year in the United States1. Hence, risk identification and prevention are the cornerstones of SCA management, especially in middle aged subjects who are most likely to suffer these lethal events as their first and final manifestation of heart disease.2 Diabetes is a major, independent risk factor for SCA3–5. One of the complications of diabetes is cardiac autonomic neuropathy (CAN)6, which is a potential mediator of the increased risk of SCA associated with diabetes7. Therefore, the potential role of CAN-related abnormalities in risk stratification of SCA needs focused evaluation.
Several heart rate variability (HRV) measures have been used to assess cardiac autonomic dysfunction and to diagnose CAN7. However, in normal populations HRV increases as the baseline heart rate slows. This association between baseline heart rate and HRV, which for convenience we can term HRV-HR, has been largely ignored in previous analysis8,9. Moreover, it is well-established that elevated resting heart rates are associated with increased all-cause mortality and SCA3,10,11, making it difficult to evaluate the effect of HRV on SCA without examining the relationship between HRV and heart rate9,12.
Reduced HRV is a predictor of ventricular arrhythmias and SCA, but also non-arrhythmic mortality in various subgroups of patients5,13–16; and it is not presently recommended as a tool for SCA risk stratification17. Traditionally, HRV is quantified using spectral analysis from long-term ECG-monitor data providing measures of both sympathetic and parasympathetic contributions to HRV.18 However, it is difficult to obtain such measurements in feasible numbers of patients who suffer SCA, an unexpected condition that often occurs without warning. Consequently, HRV from the standard 10-seconds electrocardiogram (ECG) was suggested to be a practical substitute for the assessment of cardiac vagal tone, which is the major contributor to the high-frequency component of HRV.19
To analyze the risk of SCA associated with this novel marker we compared HRV-HR obtained from a standard 12-lead ECG in diabetic SCA cases, non-diabetic SCA cases, as well as diabetic and non-diabetic control subjects.
METHODS
Study population
The Oregon Sudden Unexpected Death Study (Oregon-SUDS) is an ongoing, prospective, population-based study of SCA in the Portland, Oregon metropolitan region (regional population approximately 1 million). Detailed descriptions of subject recruitment and methodologies have been described in previous publications20,21. In brief, patients with out-of-hospital cardiac arrests in the Portland Oregon metropolitan region were identified through the emergency medical services system, the state medical examiner’s office, and 16 hospital emergency rooms in the region. All potential SCA cases were then adjudicated by three physicians based on complete medical records including pre-hospital and in-hospital records. SCA was defined as a sudden, unexpected pulseless state due to likely cardiac causes. Patients with unwitnessed arrests must have been seen in their usual state of health within 24 hours of the event and individuals with likely non-cardiac causes of death such as trauma, chronic terminal illness or drug overdose were excluded. Concurrently, control subjects were selected from the same geographic location. A majority of control subjects had prevalent coronary artery disease (CAD), since prior studies have shown that CAD is responsible for a large majority of SCA in both diabetic and non-diabetic patients.22 Control subjects with documented CAD were selected from all potential eligible patients who visited cardiology outpatient clinics or who had coronary angiography at a regional healthcare system, or (from 2003 – 2006) were transported by emergency medical services due to complaints of angina. All eligible CAD controls who consented were enrolled, regardless of age or sex. In addition, control subjects with and without prevalent CAD were enrolled (2009-present) from a randomly-selected list of members in a large health maintenance organization, frequency-matched by age and sex to the case population. CAD was defined by ≥50% stenosis in a large coronary vessel, or history of prior coronary artery bypass graft, myocardial infarction or percutaneous coronary intervention. All available medical records were reviewed in detail for the presence of diabetes or diabetic medications and each patient adjudicated for presence of diabetes, defined as specific documentation of diabetes in the medical record or the use of insulin or other hypoglycemic agents. Patients with prior ventricular arrhythmias or SCA were excluded from the control population.
Subjects included in the current analysis were aged 35–59 with a sinus rhythm ECG prior to arrest available, and all clinical information was gathered from comprehensive and systematic review of medical records. For patients with multiple prior ECGs, the one closest to and prior to cardiac arrest for cases, and closest to day of ascertainment for controls were used. Diabetes was adjudicated if there was a documentation of diabetes in the medical records or the use of insulin or other hypoglycemic agents. Obesity was defined as body-mass index (BMI) ≥30 kg/m2. The institutional review boards of Cedars-Sinai Medical Center, Oregon Health and Science University, and all other relevant health systems approved the study.
ECG Analysis
Twelve-lead ECG tracings at 25mm/s paper speed and 10mm/mV amplitude performed during routine clinical practice closest prior to SCA were scanned with a minimum resolution of 300 dots per inch and tracings digitized using ECGScan (Amps LLC). For controls, ECGs were done at the time of enrollment in the study or were obtained from the closest prior routine clinical visit. The QT-interval was measured manually and corrected for heart rate according to the Bazett formula23. Digitized tracings were reviewed by a trained, blinded physician to ensure accuracy and then imported into Matlab version R2013a for further analysis. R waves were automatically detected using an open-source, wavelet-based detection algorithm and then manually verified by another trained, blinded physician.24,25 R-R intervals were calculated based on the continuous lead with the largest amplitude R-wave deflection. Baseline heart rate was calculated from the average of all detected RR intervals. ECGs were required to have at least 6.67 seconds of consecutive sinus rhythm without premature ventricular or premature atrial contractions to be included in the final analysis. Cutoff time of 6.67 seconds was chosen to include all signals from the high frequency domain in HRV spectral analysis which spans from 0.4 to 0.15Hz (6.67 seconds = 1/.15Hz). ECGs were excluded if they didn’t have a continuous rhythm strip due to concerns for inconsistent RR interval measures between leads.
Definitions of standard time-domain measures of HRV
Standard Deviation of all R-R intervals (SDNN): Computed standard deviation of all RR intervals in the recording.26 Root-mean square differences of successive R-R intervals (rMSSD): The square root of the mean of sum of the squares of differences between adjacent R-R intervals in the recording.26 Maximal difference (Max diff): Absolute difference between the longest and shortest RR interval measured in the duration.26
Normalizing HRV for baseline heart rate
As baseline heart rate has been shown to account for up to 30% of heart rate variability, we applied previously published heart-rate normalizing techniques to account for our statistically significant differences in baseline heart rate between cases and controls.8 By dividing each variability measure by RRMean and RRMean2 we sought to eliminate all correlation between heart rate and HRV.
Statistical Analysis
Case-control comparisons of continuous and categorical clinical variables and HRV measures were made using independent t-test and chi-squared tests, respectively. Fisher’s exact test was utilized for comparisons for variables with small counts. Changes in HRV-HR, the inverse association between heart rate and HRV, were detected using analysis of covariance (ANCOVA). The relationship between two variables in ANCOVA is quantified as the linear regression slope between the two variables. Comparisons between different linear regression lines and their respective slopes were made using the F-statistic, with a larger F-statistic indicating a statistically significant change in covariance between two groups. A two-tailed p value of < 0.05 was used as the cutoff for statistical significance. Residuals were examined to ensure homoscedasticity. All analysis was performed in Matlab version 2013a (Mathworks Inc).
RESULTS
Study population
Of 355 total subjects aged 35–59 with available ECGs, 313 (88.1%) met inclusion criteria for the study, 20 (5.6%) were excluded due to lack of continuous ECG leads, while an additional 22 (6.2%) did not have at least 6.67 seconds of sinus rhythm. The final analysis included 111 diabetics (49 cases, 62 controls) and 202 non-diabetic (80 cases, 122 controls) patients. For cases ECGs were obtained a median of 0.72 years prior to sudden cardiac arrest. Baseline characteristics of diabetic and non-diabetic cases and controls are presented in Table 1. Diabetic cases were more likely than diabetic controls to have chronic renal insufficiency (49.0% vs 12.9%, p<0.001). On the reviews of ECGs, diabetic cases had faster heart rates (mean RR interval 779 ± 148 ms vs 842 ± 175 ms, p=0.047) and longer QTc intervals (457 ms vs 437 ms, p<0.001) when compared with diabetic controls. However, notably diabetic SCA and non-diabetic SCA groups had similar QTc intervals (457ms vs 445, p = 0.067).
Table 1.
Baseline characteristics of subjects
| DM Cases (n=49) |
DM Controls (n=62) |
Non-Dm Cases (n=80) |
Non-DM Controls (n=122) |
P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| DM Cases vs Controls |
Non-DM Cases vs Controls |
|||||
| Age (years) | 51.3±5.8 | 53.0±5.0 | 51.2±5.7 | 52.3±5.4 | 0.11 | 0.20 |
| Male | 61.2% | 61.3% | 76.3% | 70.5% | 0.99 | 0.37 |
| CAD | 59.2% | 88.7% | 47.5% | 73.8% | 0.0002 | 0.0003 |
| CRI | 49.0% | 12.9% | 8.8% | 3.3% | <0.0001 | 0.12 |
| Sleep apnea | 24.5% | 25.8% | 7.5% | 5.7% | 0.88 | 0.57 |
| Obesity | 55.5% | 70.5% | 42.1% | 40.8% | 0.12 | 0.86 |
| BMI (kg/m2) | 34.1±12.4 | 34.1±7.5 | 31.3±12 | 29.7±6.8 | 0.98 | 0.28 |
| HbA1C (%) | 8.1±2.2 | 8.4±2.8 | 5.5±0.4 | 5.6±0.3 | 0.63 | 0.40 |
| Hypertension | 83.7% | 69.4% | 47.5% | 56.6% | 0.08 | 0.21 |
| Beta Blocker | 56.3% | 70.7% | 39.4% | 48.6% | 0.13 | 0.24 |
| Mean HR (bpm) | 80±15 | 74±15 | 79±18 | 67±14 | 0.056 | <0.0001 |
| Mean RR (ms) | 779±148 | 842±175 | 800±200 | 930±183 | 0.047 | <0.0001 |
| QTc (ms) | 457±33 | 437±35 | 445±39 | 414±29 | 0.003 | <0.0001 |
| Median time ECG obtained prior to SCA (years) | 0.72±1.26 | N/A | 0.82±2.15 | N/A | N/A | N/A |
Plus-minus values are means ± SD.
BMI – body mass index, CAD – Documented coronary artery disease, CRI – chronic renal insufficiency, DM – diabetes mellitus, HR – heart rate
Traditional HRV measurements in cases and controls
Initial analysis using traditional time-domain measures of HRV such as standard deviation for all RR intervals (SDNN)26, root mean of sum of the square differences (rMSSD)26, and their log-transforms yielded no statistically significant differences between diabetic cases and controls. (Table 2). Diabetic cases and controls appeared to have similar SDNN (17.2 ms vs 21.9 ms, p=0.27), rMSSD (20.8 ms vs 27.0 ms, p=0.31) and maximal difference (54.8 ms vs 68.9 ms, p=0.30). Their log-transforms yielded similar results (Table 2).
Table 2.
HRV comparisons between subgroups
| DM Cases (n=49) |
DM Controls (n=62) |
Non-DM Cases (n=80) |
Non-DM Controls (n=122) |
P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| DM Case vs DM Control |
Non-DM cases vs controls |
|||||
| SDNN (ms) | 17.2±16.4 | 21.9±26 | 29.4±30.1 | 27.7±21.6 | 0.27 | 0.65 |
| Log (SDNN) | 2.54±0.75 | 2.70±0.82 | 2.91±0.96 | 3.07±0.71 | 0.29 | 0.18 |
| rMSSD (ms) | 20.8±18.6 | 27.0±38.7 | 34.9±44.1 | 29.5±23.4 | 0.31 | 0.27 |
| Log (rMSSD) | 2.73±0.75 | 2.86±0.83 | 3.00±0.99 | 3.14±0.70 | 0.42 | 0.24 |
| Max diff (ms) | 54.8±51.2 | 68.9±82.1 | 92.0±97.1 | 80.7±58.6 | 0.30 | 0.30 |
| Log (max diff) | 3.71±0.74 | 3.88±0.76 | 4.08±0.92 | 4.17±0.68 | 0.24 | 0.15 |
| SDNN/RR | 22.3±22 | 25.7±30.6 | 35.1±37.8 | 29.2±21.3 | 0.52 | 0.13 |
| SDNN/RR^2 | 30.0±31 | 30.8±36.3 | 44.1±50.3 | 32.0±23.4 | 0.89 | 0.06 |
Plus-minus values are means ± SD. SDNN – standard deviation for all RR intervals, rMSSD – Root-mean square differences of successive R-R intervals, DM – diabetes mellitus, Max diff – maximal difference
Although there were initially statistically significant differences between diabetics and non-diabetics in our observation, these differences disappeared with simple adjustments of baseline heart rate. Additional analysis using rate-normalized measures of HRV found no significant difference between diabetic cases and diabetic controls, but did reach significance when comparing pooled cases and controls for SDNN (Table 2).
HRV-HR in diabetes and sudden cardiac arrest
Increased HRV at slower baseline heart rates, a widely validated finding we are referring to as HRV-HR, can be expressed mathematically as the linear regression slope between the two variables. The inverse association between HRV and heart rate is represented by a negative regression slope. Three subgroups had negative regression slopes for HRV-HR (diabetic controls slope: −0.025, CI −0.037 to −0.013, p<0.0001; non-diabetic controls slope: −0.025 CI −0.032 to −0.018, p<0.0001; non-diabetic cases slope: −0.028 CI −0.038 to −0.018, p<0.0001).
However, the regression slope in diabetic cases did not significantly differ from zero (regression slope −0.008, CI −0.023 to 0.0071, p=0.26), indicating that decreasing HR did not increase HRV and that this population of diabetic SCA cases lost expected HRV-HR (Figure 1, Figure 2). These findings were consistent across different measures of HRV including SDNN and rMSSD (Table 3).
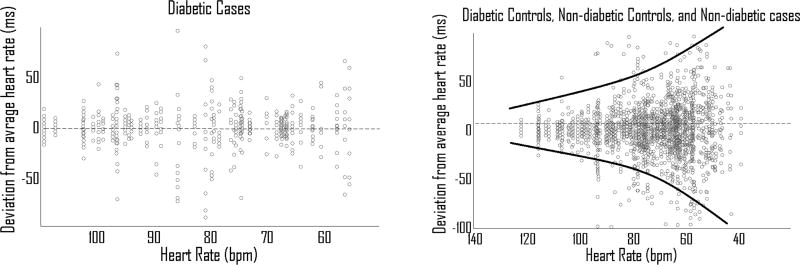
Figure 1. Diabetic cases lack HRV-HR.
HRV-HR is the consistent inverse relationship between HR and HRV in normal physiology which manifests as a negative linear regression slope in ANCOVA. Diabetic controls, non-diabetic controls and non-diabetic cases all have negative regression slopes. However, diabetic cases have a regression slope not significantly different from zero (p = .26), and is significantly different from the other subgroups (P=0.02).
Figure 2. HRV-HR in the different groups of patients.
Beat-to-beat variability comparison between (A) Diabetic cases (B) Diabetic controls, non-diabetic controls and non-diabetic cases. Note the presence of HRV-HR in all other subgroups as visualized by increasing variability at lower HR. This is not seen in diabetic cases.
Table 3.
Regression slope representing the relationship between heart rate and HRV in diabetic cases vs non-diabetic cases and controls in ANCOVA.
| Regression Slope | P for different slope vs diabetic cases |
P slope = 0 | |
|---|---|---|---|
| Regression using Log(SDNN) vs heart rate | |||
| Diabetic Cases | −0.008 | N/A | 0.26 |
| Diabetic Controls | −0.025 | 0.04 | <0.0001 |
| Non-Diabetic Cases | −0.028 | 0.03 | <0.0001 |
| Non-Diabetic Controls | −0.024 | 0.05 | <0.0001 |
| Regression using Log(rMSSD) vs heart rate | |||
| Diabetic Cases | −0.005 | N/A | 0.44 |
| Diabetic Controls | −0.03 | 0.01 | <0.0001 |
| Non-Diabetic Cases | −0.029 | 0.01 | <0.0001 |
| Non-Diabetic Controls | −0.027 | 0.02 | <0.0001 |
When comparing the slopes between diabetic cases and each other subgroup, there were statistically significant differences between diabetic cases and all three subgroups (diabetic controls p= 0.04, non-diabetic cases p = 0.03, and non-diabetic controls p= 0.05) (Table 3).
Effects of other variables on HRV-HR
The median time of ECG prior to SCA was 265 days for diabetic cases and 301 days for non-diabetic cases. There was no significant difference in HRV-HR when comparing ECGs collected less than 1 year prior SCA vs more than 1 year prior (diabetic cases: slope −0.0037 vs. −0.014, p=0.27, non-diabetic cases: slope = −0.025 vs −0.037, p=0.11). Beta-blocker therapy did not significantly impact HRV-HR in diabetic cases (slope −0.004 vs 0.008, p=0.59). Covariance analysis of HRV-HR incorporating QTc did not significantly change HRV-HR in any subgroup including diabetic cases (slope −0.0087, CI 0.010 to −0.027, p=0.25).
DISCUSSION
We sought to examine the largely overlooked, but well-described relationship between HRV and heart rate, which we refer to as HRV-HR, in diabetic SCA. Importantly this group of diabetic SCA cases lost any association between HR and HRV as a whole, while all other control groups retained the expected association. Interestingly, this loss of HRV-HR appears unique to populations of diabetics with SCA, since non-diabetics who suffered SCA and diabetics who did not suffer SCA still exhibit this phenomenon. Changes in HRV-HR were not readily apparent when HRV and heart rate were analyzed separately. Isolated HRV analysis using Fourier transforms and de-trended frequency analysis were unable to examine the readily apparent relationship between important covariates such as HRV and heart rate27. Subsequently, joint covariance analysis, as our findings indicated, may play an important role in HRV analysis, and may prove to have value in risk stratification for SCA in diabetic patients.
One important distinction must be made between the relationship of HRV-HR in individuals versus populations. This study focused on the HRV-HR relationship for pooled patient populations as sufficient data for each individual patient was not readily available. The inverse correlation of HRV-HR has been confirmed in populations of cells, isolated hearts, and mammals in three different species. However, for individual humans there is ample evidence in literature of a quadratic relationship where after reaching a peak HRV begins to decrease at extreme low heart rates (RR interval > 1500ms in human subjects achieved under intense vagal stimulation)28. Given such differences between individual HRV-HR and population HRV-HR, which we report here, caution must be used before attempting to apply the same conclusions on an individual level.
Both sympathetic and parasympathetic tones contribute to beat-to-beat heart rate changes and may be the dominant mechanism responsible for HRV-HR. Given the lack of impact beta-blockade has on HRV-HR in our population, parasympathetic tone likely dominates our measurement. However, the balance between the two phenomena is disrupted in diabetes through the unique process of CAN. CAN begins to manifest with pathologic injury to the vagus nerve responsible for the parasympathetic stimulation of the heart resulting in reduced HRV and resting tachycardia from unchecked sympathetic tone29,30. Although CAN has been associated with increased mortality31, attempts to link it to SCA have been limited by small sample size.32 It is clear from prior studies that the balance of autonomic tone between vagal and sympathetic is altered in CAN29,30, and alterations in autonomic tone through pharmacologic blockade have been shown to alter the correlation of HRV-HR33,34. It is thus possible that our finding of loss of HRV-HR in this population of diabetic SCA cases is the result of more extensive CAN, which may have an independent contribution to ventricular arrhythmogenesis or serve as a marker of more advanced disease. However, based on these findings it is difficult to determine whether this autonomic remodeling in diabetic SCA is stable over time or is a dynamic phenomenon. In the first scenario, the phenomenon would contribute to an abnormal substrate, but in the latter situation this could function as a trigger for arrhythmias. It is possible that these effects could be teased out in larger cohort studies of diabetics where temporal trends can be evaluated from multiple ECGs, as well as in animal models of diabetes.
Previously described HRV measures required long-term ambulatory monitors ranging from 5-minutes to 24-hours in order to evaluate both sympathetic and parasympathetic tone, recent studies have shown that SDNN and rMSSD from a 10-second ECG can predict 87.9%, 92.9%, and 95.8% of variability in cardiac vagal tone as measured from 5-minute Holter monitors.6 Also, short-term beat-to-beat variation is largely mediated by the baroreflex through the vagus nerve.4 This is particularly important for investigation of SCA since this condition is an unexpected event making long term ambulatory recordings rare, but many will undergo a 12-lead ECG prior, and unrelated to the SCA event. By utilizing the more common 10-second ECG as a substitute for more difficult to obtain longer recordings, we sought to evaluate a feasible number of SCA cases and control subjects.
Other limitations need to be considered while interpreting these results. Our analysis was restricted to patients with available ECGs prior and unrelated to SCA. Few patients had ECGs available immediately prior to arrest, with a median time of 0.72 years prior to cardiac arrest. The lag time is relatively short when compared to contemporary cohort studies of SCA. This limitation is inherent in a community-based design for investigation of SCA since a large proportion will present with SCA as their first manifestation, without having had a reason for a health care provider to obtain a 12-lead ECG. Conversely, the advantage over cohort designs is that feasible numbers are made available for analysis. Given the incident rates of SCA in the general population, even cohorts as large as 10,000 would yield only 5–10 SCAs per year, with even smaller numbers of diabetics with SCA. The small sample size of this study is an unfortunate reality for contemporary studies of diabetic SCA. An analysis of diabetic SCA in the ARIC study consisting of 15,792 men and women followed for an average of 12.4 years yielded only 69 cases35, comparable to our n of 49. Although we show a statistically significant difference in slopes between diabetic cases and other subgroups, we cannot rule out the possibility that a non-negative slope could be a result of the study being underpowered. Although an observational study such as ours cannot definitively identify advanced diabetic CAN as a cause for sudden cardiac death, it certainly warrants further investigation. Since distinction between type 1 and type 2 diabetes, glycemic control, or duration of diabetes could not be reliably made for a large proportion of study subjects, we cannot estimate their contribution to the present findings.
In conclusion we demonstrate that in our case-control study that this population of diabetics who suffered sudden cardiac death had novel autonomic remodeling with unique loss of HRV-HR, a normal physiologic interaction between HRV and heart rate. The mechanisms underlying this phenomenon need further investigation, especially at the individual level. This widely-available and easily obtained measure warrants further evaluation as a risk factor for SCA in diabetes.
Acknowledgments
The authors would like to acknowledge the significant contribution of American Medical Response, Portland/Gresham fire departments and the Oregon State Medical Examiner’s office.
FUNDING SOURCES: Funded in part by National Heart Lung and Blood Institute grants R01HL122492 and R01HL126938 to Dr Chugh. Dr Chugh holds the Pauline and Harold Price Chair in Cardiac Electrophysiology at Cedars-Sinai, Los Angeles. Dr Aro is funded by grants from the Finnish Foundation for Cardiovascular Research, the Paavo Nurmi Foundation, and the Orion Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
DISCLOSURES: None
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update A Report From the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000350. CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Noheria A, Teodorescu C, Uy-Evanado A, Reinier K, Mariani R, Gunson K, Jui J, Chugh SS. Distinctive profile of sudden cardiac arrest in middle-aged vs. older adults: a community-based study. Int J Cardiol. 2013;168:3495–3499. doi: 10.1016/j.ijcard.2013.04.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jouven X, Lemaître RN, Rea TD, Sotoodehnia N, Empana J-P, Siscovick DS. Diabetes, glucose level, and risk of sudden cardiac death. Eur Heart J. 2005;26:2142–2147. doi: 10.1093/eurheartj/ehi376. [DOI] [PubMed] [Google Scholar]
- 4.Balkau B, Jouven X, Ducimetière P, Eschwège E. Diabetes as a risk factor for sudden death. Lancet Lond Engl. 1999;354:1968–1969. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 5.Eranti A, Kerola T, Aro AL, Tikkanen JT, Rissanen HA, Anttonen O, Junttila MJ, Knekt P, Huikuri HV. Diabetes, glucose tolerance, and the risk of sudden cardiac death. BMC Cardiovasc Disord. 2016;16:51. doi: 10.1186/s12872-016-0231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes. 2014;5:17–39. doi: 10.4239/wjd.v5.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8:405–416. doi: 10.1038/nrendo.2012.21. [DOI] [PubMed] [Google Scholar]
- 8.Sacha J, Sobon J, Sacha K, Barabach S. Heart rate impact on the reproducibility of heart rate variability analysis. Int J Cardiol. 2013;168:4257–4259. doi: 10.1016/j.ijcard.2013.04.160. [DOI] [PubMed] [Google Scholar]
- 9.Monfredi O, Lyashkov AE, Johnsen A-B, Inada S, Schneider H, Wang R, Nirmalan M, Wisloff U, Maltsev VA, Lakatta EG, Zhang H, Boyett MR. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64:1334–1343. doi: 10.1161/HYPERTENSIONAHA.114.03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teodorescu C, Reinier K, Uy-Evanado A, Gunson K, Jui J, Chugh SS. Resting heart rate and risk of sudden cardiac death in the general population: influence of left ventricular systolic dysfunction and heart rate-modulating drugs. Heart Rhythm. 2013;10:1153–1158. doi: 10.1016/j.hrthm.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaper AG, Wannamethee G, Macfarlane PW, Walker M. Heart rate, ischaemic heart disease, and sudden cardiac death in middle-aged British men. Br Heart J. 1993;70:49–55. doi: 10.1136/hrt.70.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazmi SZH, Zhang H, Aziz W, Monfredi O, Abbas SA, Shah SA, Kazmi SSH, Butt WH. Inverse Correlation between Heart Rate Variability and Heart Rate Demonstrated by Linear and Nonlinear Analysis. PLOS ONE. 2016;11:e0157557. doi: 10.1371/journal.pone.0157557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC Study. Atherosclerosis Risk In Communities. Circulation. 2000;102:1239–1244. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 14.Mäkikallio TH, Huikuri HV, Mäkikallio A, Sourander LB, Mitrani RD, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J Am Coll Cardiol. 2001;37:1395–1402. doi: 10.1016/s0735-1097(01)01171-8. [DOI] [PubMed] [Google Scholar]
- 15.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 16.Huikuri HV, Raatikainen MJP, Moerch-Joergensen R, Hartikainen J, Virtanen V, Boland J, Anttonen O, Hoest N, Boersma LVA, Platou ES, Messier MD, Bloch-Thomsen P-E. Prediction of fatal or near-fatal cardiac arrhythmia events in patients with depressed left ventricular function after an acute myocardial infarction†. Eur Heart J. 2009;30:689–698. doi: 10.1093/eurheartj/ehn537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, Maron BJ, Page RL, Passman RS, Siscovick D, Stevenson WG, Zipes DP. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific Statement on Noninvasive Risk Stratification Techniques for Identifying Patients at Risk for Sudden Cardiac DeathA Scientific Statement From the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J Am Coll Cardiol. 2008;52:1179–1199. doi: 10.1016/j.jacc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton RM, McKechnie PS, Macfarlane PW. Can cardiac vagal tone be estimated from the 10-second ECG? Int J Cardiol. 2004;95:109–115. doi: 10.1016/j.ijcard.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–670. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. doi: 10.1161/CIRCULATIONAHA.111.023846. [DOI] [PubMed] [Google Scholar]
- 23.Bazett HC. An Analysis of the Time-Relations of Electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2:177–194. [Google Scholar]
- 24.Goldberger AL, Amaral LAN, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng C-K, Stanley HE. PhysioBank, PhysioToolkit, and PhysioNet Components of a New Research Resource for Complex Physiologic Signals. Circulation. 2000;101:e215–e220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 25.Martínez JP, Almeida R, Olmos S, Rocha AP, Laguna P. A wavelet-based ECG delineator: evaluation on standard databases. IEEE Trans Biomed Eng. 2004;51:570–581. doi: 10.1109/TBME.2003.821031. [DOI] [PubMed] [Google Scholar]
- 26.Metelka R. Heart rate variability--current diagnosis of the cardiac autonomic neuropathy. A review. Biomed Pap Med Fac Univ Palacký Olomouc Czechoslov. 2014;158:327–338. doi: 10.5507/bp.2014.025. [DOI] [PubMed] [Google Scholar]
- 27.Peng C-K, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos Interdiscip J Nonlinear Sci. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 28.Goldberger JJ, Challapalli S, Tung R, Parker MA, Kadish AH. Relationship of Heart Rate Variability to Parasympathetic Effect. Circulation. 2001;103:1977–1983. doi: 10.1161/01.cir.103.15.1977. [DOI] [PubMed] [Google Scholar]
- 29.Balcıoğlu AS, Müderrisoğlu H. Diabetes and cardiac autonomic neuropathy: Clinical manifestations, cardiovascular consequences, diagnosis and treatment. World J Diabetes. 2015;6:80–91. doi: 10.4239/wjd.v6.i1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 31.Rathmann W, Ziegler D, Jahnke M, Haastert B, Cries Fa. Mortality in Diabetic Patients with Cardiovascular Autonomic Neuropathy. Diabet Med. 1993;10:820–824. doi: 10.1111/j.1464-5491.1993.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 32.Suarez GA, Clark VM, Norell JE, Kottke TE, Callahan MJ, O’Brien PC, Low PA, Dyck PJ. Sudden cardiac death in diabetes mellitus: risk factors in the Rochester diabetic neuropathy study. J Neurol Neurosurg Psychiatry. 2005;76:240–245. doi: 10.1136/jnnp.2004.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckers F, Verheyden B, Ramaekers D, Swynghedauw B, Aubert AE. Effects of autonomic blockade on non-linear cardiovascular variability indices in rats. Clin Exp Pharmacol Physiol. 2006;33:431–439. doi: 10.1111/j.1440-1681.2006.04384.x. [DOI] [PubMed] [Google Scholar]
- 34.Billman GE. The effect of heart rate on the heart rate variability response to autonomic interventions. Front Physiol. 2013;4:222. doi: 10.3389/fphys.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, Chakravarti A, Folsom AR, Siscovick DS, Rosamond WD. Diabetes and the risk of sudden cardiac death, the Atherosclerosis Risk in Communities (ARIC) study. Acta Diabetol. 2010;47:161–168. doi: 10.1007/s00592-009-0157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]