Abstract
Evidence indicates that the neuropeptide substance P (SP) can act through neurokinin receptors to alter sleep and/or non-rapid eye movement (NREM) sleep slow-wave activity. Consequently, drugs acting on SP receptors could potentially be used as a novel treatment for sleep-related disorders. In the present study, we used SP conjugated with cholera toxin A subunit (SP-CTA), which enhances its duration of activity on SP receptors, to determine the effects of selectively activating SP-expressing brain cells on sleep regulation in mice. Herein, we found that intracerebroventricular administration of SP-CTA enhanced amounts of NREM sleep which was highly fragmented. This result suggests that the activation of SP receptor-expressing cells in the brain can produce not only arousal effects as shown in previous studies but also sleep-inducing effects.
Keywords: Sleep, Substance P, Cholera Toxin A Subunit, Slow-Wave Activity, Sleep Fragmentation
INTRODUCTION
Substance P (SP) is a neuropeptide which is found widely throughout the central nervous system[1]. SP is co-localized with neurotransmitters within cells and brain areas known to regulate sleep, such as serotonin within the raphe nucleus, dopamine within the midbrain and striatum, and corticotropin releasing hormone within the hypothalamus[2;3]. SP exerts its effects by binding to neurokinin receptors, particularly neurokinin-1 (NK1) and neurokinin-2 (NK2) receptors[4]. NK1 and NK2 receptors are also widely distributed throughout the brain including areas that regulate sleep[5–7].
Substance P is shown to have varying effects on sleep in humans and rodents [8–11]. For example, the systemic administration of non-nociceptive doses of SP has been reported to increase wakefulness in mice[8] and the latency to rapid eye movement (REM) sleep and time spent awake in healthy young men[9]. However, local bilateral microinjections of SP into the ventrolateral preoptic area (VLPO) increased non-rapid eye movement (NREM) sleep amounts in rats[10]. In addition, local microinjections of SP into the cerebral cortex enhanced slow-wave activity [SWA; NREM sleep electroencephalogram (EEG) power in the delta frequency range (~0.5–4 Hz)]in mice[11]. A likely explanation of these diverse results is likely that SP activates both wake- and sleep-promoting cells in the brain and that the activation of wake-active brain areas prevails to result in an increase in wakefulness. Nevertheless, this explanation is hindered by the complexity of SP actions, which include either an enhancement or reduction in the excitability of neurons[12] or desensitization of NK1 receptor function[13].
Interestingly, SP has a direct depolarizing action upon neurons[14] and a modulatory effect upon the action of other neurotransmitters, e.g. excitatory amino acids[15]. The direct activation of neurons typically takes place via the modulation of ion channels, e.g. suppression of K+ conductance[16;17] or the activation of non-selective cation conductance[18;19]. However, a decrease in excitation following SP treatment has also been demonstrated[12]. SP hyperpolarized approximately 80% of ferret vagal sensory neurons (nodose ganglion neurons), in part, through NK1 receptor-mediated activation of a potassium current (IK)[20]. In addition, the activation of a potassium channel by SP is reported to hyperpolarize and block the hyperpolarization-activated Ih current in neurons synergistically to reduce neuronal excitability[20;21]. Other examples of neuronal inhibitory mechanisms of SP include blocking calcium channels in neurons[22] and feed-forward inhibition[23]. Thus, SP appears to produce diverse responses in different types of neurons.
In the present study, we used a new tool to activate cells expressing the SP receptors NK1 and NK2—a conjugate of SP with the cholera toxin catalytic subunit A (SP-CTA). This conjugate was specifically designed to be taken up selectively by neurokinin receptor expressing neurons, resulting in long-lasting stimulation of these neurons[24]. Injection of SP-CTA into the intrathecal space induces the phosphorylation of the transcription factor cyclic adenosine mono-phosphate (AMP) response element binding protein (CREB) and also enhances the expression of the immediate early gene cFos[24], which are molecular mechanisms linked to altering sleep and/or SWA. Taking into account these specific mechanisms of action of SP-CTA, we expected that at least some of the effects of SP-CTA on sleep may be different from those elicited by SP. Thus, we determined the effects intracerebroventricular (ICV) infusions of SP-CTA on sleep and SWA in mice. Amounts of NREM sleep were greatly increased and sleep was highly fragmented after the administration of the SP-CTA conjugate.
METHODS
All studies were conducted in accordance with the principles and procedures described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committees at the West Roxbury, MA VA Medical Center.
Surgical procedures
Under anesthesia, twelve 6-week-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were implanted with a guide cannula into the left ventricle and electrodes to record EEG and electromyogram (EMG). Two contralateral cortical screw electrodes (±2 mm from midline, +3 mm and −6 mm from bregma) recorded the EEG and two flexible multistrand wires placed into the nuchal muscles recorded the muscle activity. Immediately after the surgery, animals were housed singly in plastic cages in a room with controlled temperatures and light-dark cycles (12h light/12h dark). Food and water were available ad libitum.
Materials
SP-CTA (Catalog # IT-39) was purchased from Advanced Targeting Systems, San Diego, CA. It was stored frozen at −80°C and diluted in saline to the concentration of 1 μg/μl within 1 hour prior to its use.
Experimental protocol
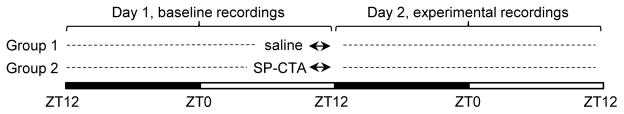
Seven to ten days after surgical recovery, the animals were connected to light weight recording cables via commutators where they adapted to the tether for at least one week. The cables permitted complete mobility and normal behavior including rearing, turning, and assuming a curled sleep posture. The mice remained attached to the cables throughout the experiment. EEG and EMG signals were continuously recorded for 48-h period on a Grass amplifier polysomnograph. One mouse from the saline group had a noisy EEG signal and was excluded from the analysis. After baseline recordings, the mice were slowly infused through implanted ICV cannula with either saline (4 μl; n=6) or SP-CTA (1 μg/μl in saline; 4 μl; n=6) between 11.5 h and 12 h of the light period [Zeitgeber time (ZT) 11.5–12] (Figure 1). Twenty-four hours later, the mice were anesthetized with pentobarbital, perfused with saline followed by 10% formalin, and the brains were used to verify proper cannula placement (data not shown).
Figure 1.
Schematic drawing of the experimental design. Two groups of mice were used in the experiment. EEG and EMG recordings were performed throughout the experiment. Mice were infused with either saline (Group 1) or SP-CTA diluted in saline (Group 2) within a half an hour preceding the light-off period (marked with double-headed arrows in the figure).
Analysis of sleep data
EEG and EMG data were scored manually in 12-s epochs for sleep states (wake, NREM and REM sleep) by an investigator who was blinded to the experimental treatment groups. As previously described,[25] wakefulness was identified by the presence of desynchronized EEG and high EMG activity. NREM sleep consisted of high-amplitude slow waves together with a low EMG tone relative to waking. REM sleep was identified by the presence of regular theta activity coupled with low EMG. The amount of time spent in wakefulness, NREM sleep, and REM sleep was determined for each hour. EEG data were filtered at 70 Hz (low-pass filter) and 0.3 Hz (high-pass filter) using a Grass electroencephalograph and continuously sampled at 128 Hz by a computer with an A/D board (National Instruments). A fast Fourier analysis was performed on the EEG data using the ICELUS program (Mark Opp, Ann Arbor, MI). The average SWA (delta power, 1–4 Hz) was summed for each NREM sleep epoch and averaged over all NREM sleep epochs. To reduce within-group variability, the SWA measures for each mouse were calculated as the change from the baseline level for that mouse. A two-way analysis of variance (ANOVA) and the Student–Newman–Keuls post-hoc test were used to compare changes in sleep parameters across days (day 1 vs day 2) and groups (group 1 vs group 2). Data are presented as means ± SEM. Differences were considered significant at P < 0.05.
RESULTS
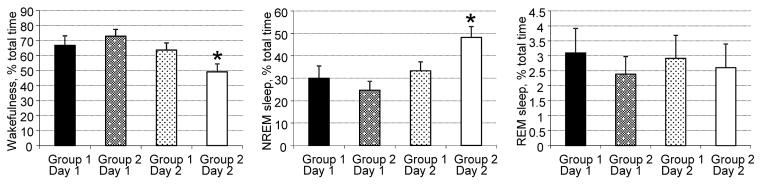
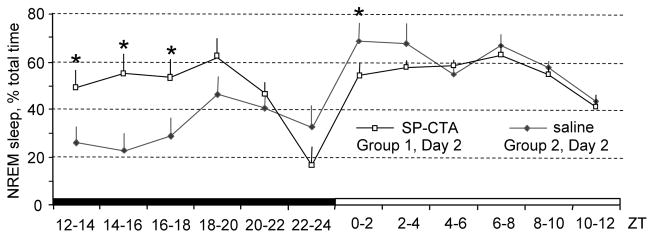
Compared to responses after the vehicle, SP-CTA greatly increased NREM sleep amounts during the 12-hour light-off period after the infusion (45% increase; Figure 2). However, the amounts of REM sleep and wakefulness did not change significantly (Figure 2). NREM sleep amounts differed between the groups (F(12,112)=4.34, p<0.0001; Figure 3). They increased during the first hour following the SP-CTA infusion and remained elevated for 8 hours (ZT12-ZT20; Figure 3). Amounts of NREM sleep were lower at the beginning of the light-on period of the Day 2 (ZT0-ZT2) in the SP-CTA treated mice compared to the saline treated mice, which could be a compensatory reaction to the increased NREM sleep amounts during the preceding light-off period (Figure 3).
Figure 2.
Amounts of wakefulness, NREM sleep and REM sleep during light-off periods in mice during baseline and experimental recordings. NREM sleep amounts increased in mice infused with SP-CTA (Group 2, Day 2). *P < 0.05 compared to other groups/days.
Figure 3.
Profile of NREM sleep changes in mice infused with SP-CTA or saline. SP-CTA highly induced NREM sleep amounts, which remained increased for up to 8 hours. *P < 0.05 compared to corresponding saline-infused mice.
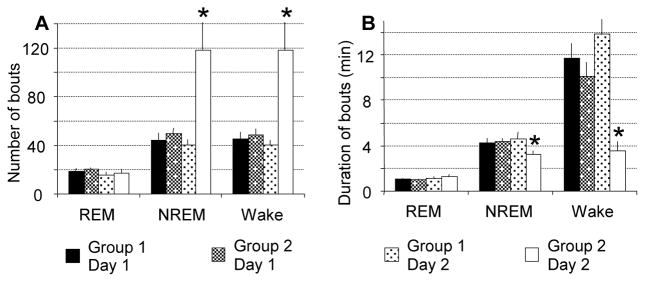
The number of bouts of both NREM sleep (F(1,18)=8.68, p=0.0086 between days; F(1,18)=9.91, p=0.0056 between groups) and wakefulness (F(1,18)=8.51, p=0.0092 between days; F(1,18)=9.64, p=0.0061 between groups) increased almost 3 times after SP-CTA infusion, whereas duration of the bouts was reduced (30.3% reduction for NREM sleep, F(1,18)=4.74, p=0.043 and 74.5% reduction for wakefulness, F(1,18)=10.3, p=0.0049; Figure 4). This result indicates that sleep was highly fragmented after SP-CTA infusion.
Figure 4.
Number and duration of bouts of wakefulness, and NREM and REM sleep. Number of NREM sleep and wakefulness bouts was increased (A), and duration of NREM sleep bouts and wakefulness bouts was decreased (B) in SP-CTA treated mice. Number or duration of bouts was averaged per hour during the 12-h light off period. *P < 0.05 compared to other groups/days.
SWA was reduced soon after the beginning of SP-CTA infusion (ZT14) and remained diminished during the light-off period (ZT18–ZT22) on Day 2 (Figure 5). There were no significant differences in SWA between SP-CTA and saline-treated mice on Day 1.
Figure 5.
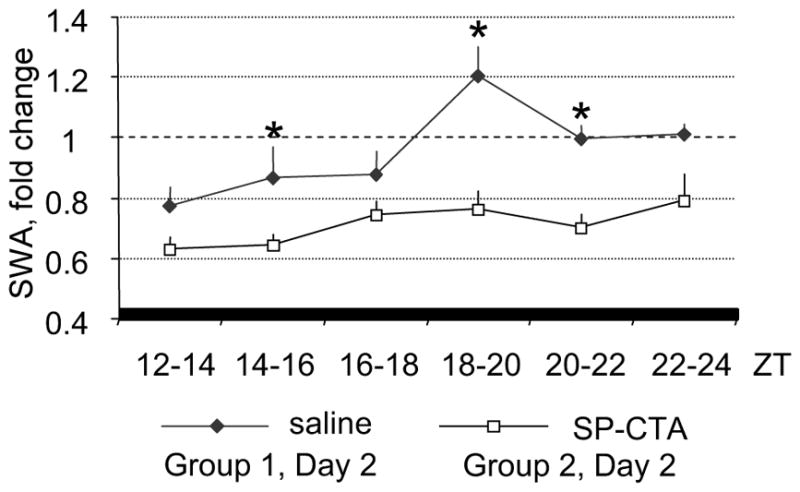
Profile of changes of NREM sleep SWA in SP-CTA and saline-treated mice. SWA was calculated as change from the average value of SWA recorded during the corresponding 12-h baseline period. *P < 0.05 between groups.
DISCUSSION
As discussed, systemic administration of SP has been reported to increase wakefulness in both mice and humans[8;9]. Since SP tends to be excitatory within the central nervous system,[26] our present findings indicating SP-CTA inducing sleep fragmentation suggest that global activation of cells expressing SP-receptor has an arousal effect. However, that we did not observe increased wakefulness after treating mice centrally with SP-CTA suggests that the conjugate designed to strongly activate cells expressing SP receptors are not wake promoting [24]. Herein, we found that amounts of NREM seep more than doubled soon after the beginning of the SP-CTA infusion and remained highly elevated for up to 8 hours (Figure 3). Collectively, our findings suggest that SP and its receptors have a complex arousing effect and sleep promoting effects in the central nervous system.
A plausible explanation of our current findings are that SP-CTA strongly activates neurons in sleep-active areas, such as the VLPO, which overpowers arousing effect of SP-CTA in other brain areas. Indeed, a previous study showed that local injections of SP into the VLPO induced NREM sleep[10]. It is possible that the activation of sleep-active neurons in the VLPO is stronger and more sustained after SP-CTA administration than SP administration. Differences in neuronal activation could relate to the variability in responses to internalized CTA between different cell types, so that sleep-active neurons would be more activated than wake-active neurons. To our knowledge, such a possibility has not been tested and requires additional studies.
In previous studies, the simultaneous activation of sleep and arousal systems has been observed with the cage exchange model of insomnia in rats[27]. The psychological stressor (cage exchange) initially induced an acute stress response, but several hours later generated a pattern of sleep disturbances characterized by increased sleep latency, decreased NREM and REM sleep, and increased fragmentation[27]. In the present study, the administration of SP-CTA produced a highly fragmented pattern of sleep, during which the number of NREM bouts increased almost 3 times compared to baseline values in the same animal or saline-treatment (Figure 4). Although speculative, it seems that the simultaneous activation of sleep- and wake-active brain areas may destabilize sleep and cause an increase in sleep fragmentation. This explanation is plausible because the cells expressing NK1 receptor are widely distributed in both wake-active and sleep-active brain regions (e.g., the locus coeruleus, substantia nigra, dorsal raphe, preoptic area, etc.)[1–3]
Another possible explanation for the increased sleep amounts that we observed after SP-CTA administration could be related to the pro-inflammatory effects of SP. SP is thought to be a potent initiator of neurogenic inflammation due, in part, to its association with increased vascular permeability and subsequent plasma protein extravasation[28]. It also potentiates inflammation by stimulating the production of inflammatory mediators such as histamine, nitric oxide, cytokines and kinins, in addition to interacting with adhesion molecules causing leukocyte migration[29]. Histamine and SP are able to stimulate microglial activation and the subsequent production of reactive oxygen species and pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interluekin-6 (IL-6)[30;31]. Because pro-inflammatory cytokines, such as TNF-α and IL-6 are somnogenic,[32–34] increased sleep amounts observed in SP-CTA treated mice could be explained by their increased activity.
It is plausible that SP-CTA could also change sleep patterns, in part, by acting on the nNOS-containing cells in the cerebral cortex. We recently performed infusions of an NK1 receptor agonist (SP fragment 1–7) and antagonist (CP96345) into the cerebral cortex and found that the SWA was locally enhanced by the NK1 receptor agonist and reduced by the NK1 receptor antagonist[11]. Because NK1 receptors are expressed exclusively in nNOS cells in the cerebral cortex,[35] this result suggested that changes in the activity of cortical sleep-active nNOS cells are involved in the local modulation of the SWA production. Surprisingly, SWA was reduced by the SP-CTA treatment in our current study (Figure 5). This result could be explained by the reduced duration of NREM sleep bouts in SP-CTA-treated mice, which were too short for the mice to enter into a deeper sleep stage associated with a high SWA. However, the activation of cells in subcortical regions might have also served to interfere with SWA production in SP-CTA-treated mice.
SP-CTA binds to both NK1 and NK2 receptors[24]. While the role of NK1 receptor in the regulation of sleep has been demonstrated,[8;11;35] the role of NK2 receptor is not well understood. NK2 binding sites are present in several limbic structures in rats, including the hippocampus, thalamus, septum and prefrontal cortex, suggesting involvement in the modulation of emotional processes[36]. Thus, it is possible that some of the sleep effects produced by SP-CTA in the present study were mediated by NK2 receptor-expressing cells.
Conclusions
The main findings of the present study are that ICV administration of SP-CTA produces increased amounts of NREM sleep but induces sleep fragmentation. These results suggests that the activation of SP-expressing cells in the brain can produce not only arousal effects as shown in previous studies,[8;9] but also sleep-inducing effects. These effects should be taken into accounts when novel drugs acting on SP receptor are developed for treatment of sleep-related disorders. For example, SP receptor antagonist vestipitant has been recently shown to improve sleep maintenance without causing next-day cognitive impairment in patients with primary insomnia[37]. Long-term consequences of the simultaneous activation or inhibition of the sleep and arousal systems are not known and require further investigations.
HIGHLIGHTS.
The of substance P (SP) and cholera toxin A subunit conjugate (SP-CTA) induces sleep
Sleep induced by SP-CTA is highly fragmented
SP-CTA applied intracerebroventricularly reduces slow-wave activity
Acknowledgments
This study was supported by the National Institutes of Health grants NS064193 (DG), NS092926 (DG) and the Department of Veterans Affairs grant IBX002823 (MRZ)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Ribeiro-da-Silva A, Hokfelt T. Neuroanatomical localisation of Substance P in the CNS and sensory neurons. Neuropeptides. 2000;34:256–271. doi: 10.1054/npep.2000.0834. [DOI] [PubMed] [Google Scholar]
- 2.Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- 3.Sergeyev V, Hokfelt T, Hurd Y. Serotonin and substance P co-exist in dorsal raphe neurons of the human brain. Neuroreport. 1999;10:3967–3970. doi: 10.1097/00001756-199912160-00044. [DOI] [PubMed] [Google Scholar]
- 4.Yip J, Chahl LA. Localization of tachykinin receptors and Fos-like immunoreactivity induced by substance P in guinea-pig brain. Clin Exp Pharmacol Physiol. 2000;27:943–946. doi: 10.1046/j.1440-1681.2000.03366.x. [DOI] [PubMed] [Google Scholar]
- 5.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides. 1998;32:1–49. doi: 10.1016/s0143-4179(98)90015-4. [DOI] [PubMed] [Google Scholar]
- 6.Yang CY, Chen YF, Lee CW, Huang A, Shen Y, Wei C, Liu HM. Multiphase CT angiography versus single-phase CT angiography: comparison of image quality and radiation dose. AJNR Am J Neuroradiol. 2008;29:1288–1295. doi: 10.3174/ajnr.A1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caudle RM, King C, Nolan TA, Suckow SK, Vierck CJ, Jr, Neubert JK. Central sensitization in the trigeminal nucleus caudalis produced by a conjugate of substance P and the A subunit of cholera toxin. J Pain. 2010;11:838–846. doi: 10.1016/j.jpain.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen ML, Nascimento DC, Machado RB, Roizenblatt S, Moldofsky H, Tufik S. Sleep disturbance induced by substance P in mice. Behav Brain Res. 2006;167:212–218. doi: 10.1016/j.bbr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Lieb K, Ahlvers K, Dancker K, Strohbusch S, Reincke M, Feige B, Berger M, Riemann D, Voderholzer U. Effects of the neuropeptide substance p on sleep, mood, and neuroendocrine measures in healthy young men. Neuropsychopharmacology. 2002;27:1041–1049. doi: 10.1016/S0893-133X(02)00369-X. [DOI] [PubMed] [Google Scholar]
- 10.Zhang G, Wang L, Liu H, Zhang J. Substance P promotes sleep in the ventrolateral preoptic area of rats. Brain Res. 2004;1028:225–232. doi: 10.1016/j.brainres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Zielinski MR, Karpova SA, Yang X, Gerashchenko D. Substance P and the neurokinin-1 receptor regulate electroencephalogram non-rapid eye movement sleep slow-wave activity locally. Neuroscience. 2015;284:260–272. doi: 10.1016/j.neuroscience.2014.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moraes ER, Kushmerick C, Naves LA. Characteristics of dorsal root ganglia neurons sensitive to Substance P. Mol Pain. 2014;10:73. doi: 10.1186/1744-8069-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McConalogue K, Corvera CU, Gamp PD, Grady EF, Bunnett NW. Desensitization of the neurokinin-1 receptor (NK1-R) in neurons: effects of substance P on the distribution of NK1-R, Galphaq/11, G-protein receptor kinase-2/3, and beta-arrestin-1/2. Mol Biol Cell. 1998;9:2305–2324. doi: 10.1091/mbc.9.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi S, Otsuka M. Excitatory action of hypothalamic substance P on spinal motoneurones of newborn rats. Nature. 1974;252:734–735. doi: 10.1038/252734a0. [DOI] [PubMed] [Google Scholar]
- 15.Rusin KI, Jiang MC, Cerne R, Randic M. Interactions between excitatory amino acids and tachykinins in the rat spinal dorsal horn. Brain Res Bull. 1993;30:329–338. doi: 10.1016/0361-9230(93)90261-9. [DOI] [PubMed] [Google Scholar]
- 16.Stanfield PR, Nakajima Y, Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985;315:498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- 17.Tokimasa T, Tsurusaki M, Akasu T. Chemosensitivity of C-cells in bullfrog dorsal root ganglia to substance P and adenosine 5′-triphosphate. Neurosci Lett. 1993;163:169–172. doi: 10.1016/0304-3940(93)90374-t. [DOI] [PubMed] [Google Scholar]
- 18.Spigelman I, Puil E. Substance P actions on sensory neurons. Ann NY Acad Sci. 1991;632:220–228. doi: 10.1111/j.1749-6632.1991.tb33110.x. [DOI] [PubMed] [Google Scholar]
- 19.Shen KZ, North RA. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience. 1992;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- 20.Jafri MS, Weinreich D. Substance P regulates Ih via a NK-1 receptor in vagal sensory neurons of the ferret. J Neurophysiol. 1998;79:769–777. doi: 10.1152/jn.1998.79.2.769. [DOI] [PubMed] [Google Scholar]
- 21.Jafri MS, Weinreich D. Substance P hyperpolarizes vagal sensory neurones of the ferret. J Physiol. 1996;493(Pt 1):157–166. doi: 10.1113/jphysiol.1996.sp021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sculptoreanu A, de Groat WC. Protein kinase C is involved in neurokinin receptor modulation of N- and L-type Ca2+ channels in DRG neurons of the adult rat. J Neurophysiol. 2003;90:21–31. [Google Scholar]
- 23.Wu LJ, Xu H, Ko SW, Yoshimura M, Zhuo M. Feed-forward inhibition: a novel cellular mechanism for the analgesic effect of substance P. Mol Pain. 2005;1:34. doi: 10.1186/1744-8069-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caudle RM, Mannes AJ, Keller J, Perez FM, Suckow SK, Neubert JK. Sensitization of spinal cord nociceptive neurons with a conjugate of substance P and cholera toxin. BMC Neurosci. 2007;8:30. doi: 10.1186/1471-2202-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zielinski MR, Gerashchenko D, Karpova SA, Konanki V, McCarley RW, Sutterwala FS, Strecker RE, Basheer R. The NLRP3 inflammasome modulates sleep and NREM sleep delta power induced by spontaneous wakefulness, sleep deprivation and lipopolysaccharide. Brain Behav Immun. 2017;62:137–150. doi: 10.1016/j.bbi.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otsuka M, Konishi S, Takahashi T, Saito K. Substance P and primary afferent transmission. Adv Biochem Psychopharmacol. 1976;15:187–191. [PubMed] [Google Scholar]
- 27.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 29.Averbeck B, Reeh PW. Interactions of inflammatory mediators stimulating release of calcitonin gene-related peptide, substance P and prostaglandin E(2) from isolated rat skin. Neuropharmacology. 2001;40:416–423. doi: 10.1016/s0028-3908(00)00171-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Qu C, Lu X, Zhang S. Activation of microglia by histamine and substance P. Cell Physiol Biochem. 2014;34:768–780. doi: 10.1159/000363041. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi K, Kumakura S, Murakami T, Someya A, Inada E, Nagaoka I. Ketamine suppresses the substance P-induced production of IL-6 and IL-8 by human U373MG glioblastoma/astrocytoma cells. Int J Mol Med. 2017;39:687–692. doi: 10.3892/ijmm.2017.2875. [DOI] [PubMed] [Google Scholar]
- 32.Zielinski MR, McKenna JT, McCarley RW. Functions and mechanisms of sleep. AIMS Neuroscience. 2016;31:67–104. doi: 10.3934/Neuroscience.2016.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zielinski MR, Gerashchenko D. Role of sleep in cognition, immunity, and disease and its interaction with exercise. In: Farooqui AA, Farooqui T, editors. Diet and exercise in cognitive function and neurological diseases. Wiley; Hoboken, NJ, USA: 2015. pp. 225–240. [Google Scholar]
- 34.Krueger JM, Clinton JM, Winters BD, Zielinski MR, Taishi P, Jewett KA, Davis CJ. Involvement of cytokines in slow wave sleep. Prog Brain Res. 2011;193:39–47. doi: 10.1016/B978-0-444-53839-0.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dittrich L, Heiss JE, Warrier DR, Perez XA, Quik M, Kilduff TS. Cortical nNOS neurons co-express the NK1 receptor and are depolarized by Substance P in multiple mammalian species. Front Neural Circuits. 2012;6:31. doi: 10.3389/fncir.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saffroy M, Torrens Y, Glowinski J, Beaujouan JC. Autoradiographic distribution of tachykinin NK2 binding sites in the rat brain: comparison with NK1 and NK3 binding sites. Neuroscience. 2003;116:761–773. doi: 10.1016/s0306-4522(02)00748-0. [DOI] [PubMed] [Google Scholar]
- 37.Ratti E, Carpenter DJ, Zamuner S, Fernandes S, Squassante L, Danker-Hopfe H, Archer G, Robertson J, Alexander R, Trist DG, Merlo-Pich E. Efficacy of vestipitant, a neurokinin-1 receptor antagonist, in primary insomnia. Sleep. 2013;36:1823–1830. doi: 10.5665/sleep.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]