Abstract
Male parental care is a vital behavior for the development as well as the physical and mental well-being of the young. However, little is known about the neurochemical regulation of male parental behavior, mainly due to the lack of appropriate animal models. In the present study, we used the socially monogamous male prairie vole (Microtus ochrogaster) to investigate the effect of pair bonding experience on paternal behavior and dopamine (DA) signaling in the nucleus accumbens (NAcc) in the brain. We compared sexually naïve males with males that were pair-bonded with a female for two weeks. Our data showed that pair-bonded males displayed enhanced paternal behavior, particularly in pup licking/grooming, associated with increased DA type-1 receptor (D1R) protein expression in the NAcc, compared to sexually naïve males. Site-specific brain microdialysis revealed a significant, but transient, increase in DA release in the NAcc associated with pup exposure in both groups of the males. Further, pharmacological blockade of D1R in the NAcc decreased pup licking/grooming in the pair-bonded males. Together, our data demonstrate that pair bonding experience with a female facilitated male parental behavior via NAcc D1R mediation in male prairie voles.
Keywords: pair bonding, paternal behavior, nucleus accumbens, dopamine, D1R
Graphical Abstract
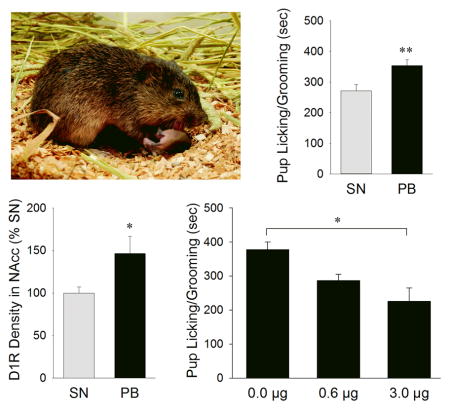
We studied paternal behavior in male prairie voles (M. ochrogaster). Compared to sexually naïve (SN) males, males that were paired-bonded with a female for 2-weeks (PB) showed enhanced pup licking/grooming associated with increased dopamine D1-type receptor (D1R) expression in the NAcc. Pup exposure induced a transient increase in dopamine release in the NAcc, and intra-NAcc administration of a D1R antagonist at 3.0μg diminished pup licking/grooming.
Introduction
In humans, strong and intimate bonds are important for individual and societal welfare and success (House et al., 1988; Lillard & Waite, 1995; Keer & Stern, 1999; Kiecolt-Glaser & Newton, 2001). In addition, pair bonding between parents and close parent-child relationships are crucial for the development as well as the physical and psychological well-being of children (Amato, 1994; Repetti et al., 2002). Therefore, understanding the neurobiological mechanisms underlying parental behavior is an important scientific inquiry that has high translational value for human health.
The vast majority of research investigating the neurobiological mechanisms of parental behavior has focused on females (i.e., maternal behavior). One important neurochemical system involved in maternal behavior is the mesolimbic dopamine (DA) system (Gaffori & Le Moal, 1979; Numan & Smith, 1984; Hansen et al., 1991a; Hansen, 1994; Keer & Stern, 1999; Lee et al., 1999). This system has been implicated in motivated and goal-directed behaviors as well as in learning and memory (Ikemoto & Panksepp, 1999; Carelli, 2004), and consists of DA producing neurons in the ventral tegmental area (VTA) that project to and release DA within various target brain regions, including the nucleus accumbens (NAcc) (Swanson, 1982). There are two families of DA receptors (DAR): D1-like (which includes D1R and D5R subtypes) and D2-like (which includes D2R, D3R, and D4R subtypes) (Missale et al., 1998). Also, D1R and D2R regulate two largely non-overlapping populations of NAcc neurons, which have different connectivity and neurochemistry (Surmeier et al., 2007). In rats, D1R signaling in the NAcc is critical for the expression of maternal behaviors (Numan et al., 2005; Stolzenberg et al., 2009). Pup licking/grooming is associated with increased VTA activity (Hernandez-Gonzalez et al., 2005) and NAcc DA release (Champagne et al., 2004; Afonso et al., 2009); it is also enhanced by D1R up-regulation in the NAcc (Champagne et al., 2004).
Relatively little is known about the neurobiology of paternal behaviors, largely because of the lack of appropriate animal models. In mammals, bi-parental care is associated with the monogamous life strategy, which occurs in only 3–5% of mammalian species (Emlen & Oring, 1977; Kleiman, 1977). Some primate species, including tamarins (Saguinus oedipus) (Ziegler & Snowdon, 1997; 2000), marmosets (Callithrix jacchus) (Almond et al., 2006; Almond et al., 2008), and titi monkeys (Callicebus cupreus) (Mendoza & Mason, 1986a; Mendoza & Mason, 1986d), have offered valuable insight into the behavioral and physiological aspects of male parental care, but few rodent models are readily available to study the underlying neurobiology. Nevertheless, testosterone (Trainor & Marler, 2002) and increased aromatase activity in the medial preoptic area (MPOA) (Trainor et al., 2003) have been found to promote paternal behavior in male California mice (Peromyscus californicus). In contrast, lesions of the MPOA disrupted male paternal behavior (Lee & Brown, 2002). Interestingly, males with NAcc lesions showed mild, but significant deficits in pup-retrieval behavior in comparison to the sham-lesioned controls (Lee & Brown, 2007) indicating a potential role of the NAcc and its neurochemicals in male parental behavior.
We used the socially monogamous male prairie vole (Microtus ochrogaster) as an animal model in the present study. Research has demonstrated that sexually naïve male prairie voles display spontaneous paternal behavior towards conspecific pups (Kirkpatrick et al., 1994; Wang et al., 1994; Nishimori et al., 1996; Winslow & Insel, 2002), and the level of paternal behavior can be influenced by the social environment/experience (Terleph et al., 2004) or by pharmacological manipulation of neurochemicals such as DA (Lonstein, 2002). Given these findings and the important role of mesolimbic DA in maternal behavior, we conducted a series of experiments to test the hypothesis that NAcc DA is involved in mediating male parental behaviors that are influenced by pair bonding experience with a female.
Materials and methods
Experimental design
Experiment 1 examined the effects of pair bonding experience on paternal behavior and DA marker gene and protein expression in the brain of male prairie voles. Subjects were assigned into one of two experimental groups: sexually naïve or pair-bonded with a female for 2 weeks. This time frame was chosen based on previous data demonstrating stable pair bond formation between male and female voles after 2 weeks (Williams et al., 1992; Aragona et al., 2006). Males in both groups underwent the paternal behavior test for 10-mins and were then rapidly decapitated. Brains were subsequently harvested, and brain tissues were randomly assigned into one of the two sets that were processed for either DA marker (including D1R, D2R, and TH) mRNA levels via in-situ hybridization or protein expression via Western immunoblot, respectively. Experiment 2 measured DA release in the NAcc during pup exposure using brain microdialysis with HPLC-ECD analysis in both sexually naïve and pair-bonded males that were exposed to a conspecific pup. Novelty control groups were also created in which sexually naïve and pair-bonded males were exposed to a piece of a pup-sized plastic. In Experiment 3, pair-bonded males received intra-NAcc injections of vehicle or vehicle containing a low (0.6μg) or a high (3.0μg) dose of a D1R antagonist, SCH-23390, and were then tested for paternal behaviors.
Subjects
Subjects were adult (90–140 days of age) male prairie voles (M. ochrogaster) from a laboratory colony descended from populations in southern Illinois. Subjects were weaned at 21 days of age and then housed in same sex pairs in Plexiglas cages (29 × 18 × 13 cm) containing cedar chip bedding. Food and water were provided ad libitum. All cages were maintained at 21±1°C under a 14:10 hr light:dark photoperiod (lights on at 0700). Subjects either remained sexually naïve or were paired with a female for 2 weeks (pair-bonded). For the pair-bonded group, non-related female conspecifics were used as stimulus animals and were unilaterally ovarectomized and hysterectomized in a contralateral fashion, thereby preventing pregnancy during the 2-wk pair-bonding period. Sexually naïve and pair-bonded males were age-matched. Unrelated stimulus pups (2–4 days of age) were used in the paternal behavior tests. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Florida State University and were in accordance with the guidelines set forth by the National Institutes of Health.
Parental behavior test
The behavioral test was conducted in a Plexiglas cage (20 × 25 × 45 cm), which contained clean bedding, water, and food. Prior to behavioral testing, subjects were transferred to the testing cage for a 3-hr habituation period, at the end of which food and water were removed. A stimulus pup then was placed in the corner of the testing cage opposite the subject, and the subject was allowed to freely interact with the pup for 10-min. Behaviors were digitally recorded and subsequently quantified using the JWatch V1.0 software program (Macquarie University and UCLA: http://www.jwatcher.ucla.edu/). Paternal behaviors, including pup licking/grooming and huddling, as well as non-paternal behaviors, including locomotion (subject’s horizontal movement from one place to another) and resting away from the pup, were quantified. Huddling behavior was defined as at least 10-sec of crouching over the pup and not engaged in any other activities. An overall paternal duration was calculated as the sum of all bouts of pup licking/grooming and huddling. The latency, frequency, and duration of each behavior were measured. As reported in previous studies (Bamshad et al., 1994; Bales et al., 2004; Kenkel et al., 2017), some prairie voles showed aggressive behaviors towards the pup in the present study. At the first sign of pup-directed aggression, the pup was immediately removed, and the test was terminated. Aggressive subjects were reported below in experiments and were not included in the behavioral analyses.
In-situ hybridization labeling of DA marker mRNAs
Coronal brain sections at 14-μm thickness were thaw mounted onto microscope slides. Three sets of brain sections at 98μm intervals were processed respectively for in-situ hybridization labeling of D1R, D2R, and tyrosine hydroxylase (TH) mRNA in the NAcc, caudate putamen (CP), and ventral tegmental area (VTA), using an established method and antisense and sense riboprobes for D1R, D2R, and TH mRNA labeling which were validated in our previous studies (Liu et al., 2010; Young et al., 2011). Briefly, probes were labeled individually at 37° C for 1 hr in a transcription-optimized buffer consisting of 0.5 μg/μl of the respective DNA template, [35S]-CTP, 4mM of ATP, UTP, and GTP, 0.2M dithiothreitol (DTT), RNasin (40U/μl), and RNA polymerase (20U/μl). The DNA template was then digested with 1U/μl DNaseI. Probes were purified using Chromatography Columns (Bio-Rad, Hercules, CA) and then diluted in hybridization buffer comprised of 50% deionized formamide, 10% dextran sulfate, 3 × SSC, 10mM sodium phosphate buffer (PB, pH 7.4), 1 × Denhardt’s solution, 0.2 mg/ml yeast tRNA, and 10mM DTT to yield 5 × 106 cpm/ml. Each slide received 100μl hybridization solution containing the appropriate 35S-labeled probe, was cover-slipped and then incubated at 55°C in a humidified chamber for 14 hrs. Thereafter, cover-slips were removed. Slides were then washed, dehydrated through increasing concentrations of ETOH, and air-dried. Sections were exposed to BioMax MR film (Kodak, Rochester, NY) to generate optimal autoradiograms. Optical densities (OD) of D1R, D2R, and TH mRNAs from the autoradiograms were quantified from 3–4 matched brain sections per brain area/subject using the NIH IMAGE software program (NIH IMAGE 1.64). Data for each of the DA markers from the pair-bonded males are presented as percent changes of the mean OD of the sexually naïve males (Liu et al., 2010).
Western immunoblot analysis of DA marker proteins
Brains were coronally sectioned at 300μm thickness and mounted onto microscope slides. Tissue punches were taken from the NAcc (containing both the core and shell sub regions) and CP (control region). D1R and D2R proteins were measured using Western immunoblot analysis as previously established (Liu et al., 2010; Young et al., 2014). Proteins from each tissue sample were extracted and transferred to a PVDF membrane at 4°C. For DA receptor immunoreactive labeling, PVDF membranes were blocked in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T) and 5% nonfat milk for 1 hr at 23°C. The membrane was then incubated overnight at 4°C in TBS-T plus milk containing a 1:1000 concentration of D2 receptor (D2R; H-50) primary antibody (catalog #sc-9113, Santa Cruz Biotechnology), or a 1:3000 concentration of D1R primary antibody (catalog #D1897, Sigma). Membranes were then washed for 1 hr at 23°C in TBS-T, incubated for 1 hr at a 1:40,000 concentration of peroxidase-labeled secondary antibody, and then washed for 1 hr in 0.1% TBS-T. Thereafter, all bands were visualized by Super Signal West Dura-enhanced chemiluminescence (catalog #34706, Pierce). D1R-ir bands were visualized at a molecular weight of 74 kDa and D2R-ir bands at 51 kDa. Protein levels were quantified based on the OD of the protein bands using the NIH IMAGE software program. The OD of each band was normalized for loading variations by using the OD of β-actin of each loaded sample. Data for each of the DA markers from the pair-bonded males are presented as percent changes of the mean OD of the sexually naïve males that were exposed to pups. All these procedures were established and validated in our previous studies in prairie voles (Liu et al., 2010; Young et al., 2014).
Brain microdialysis of DA release
Microdialysis probes were constructed as previously described (Curtis & Wang, 2007). Briefly, each probe had an active area of 1.5mm, and the membrane had a molecular weight cutoff of 18kDa. Probes were continuously perfused at a rate of 1.7μL/min with a solution isotonic for potassium (2.8mM KCl), sodium (144mM NaCl), magnesium (0.9mM MgCl2), and calcium 91.2mM CaCl2). SN or PB subjects were anesthetized with an intraperitoneal (ip) injection of an anesthesia solution (0.6mL/40g body weight of a solution consisting of 60% 10mg/mL Ketamine and 40% 10mg/mL Domitor). The probes were stereotaxically implanted into the left NAcc (coordinates from bregma: anterior 1.7mm, lateral 0.8mm, and ventral 6.2mm). All subjects were allowed to recover overnight following the surgery. At 0930 hr the next morning, baseline dialysate samples were collected every 10-min for 1-hr prior to behavioral testing. Then either a stimulus pup (PUP) or a pup-sized piece of plastic (novelty control; CON) was introduced into the subject’s cage for 2 hrs while dialysate samples were continuously collected at 10-min intervals. DA concentrations in the dialysate from the last 10-min baseline sample and the first 10-min samples from the first (T1) and second (T2) hr of the experiment were analyzed by the Neurochemistry Core Lab at Vanderbilt University, Nashville, TN, using HPLC-ECD. Brains were harvested after the test for placement verification of the probe.
Site-specific brain cannulation and D1R antagonism
As pup exposure facilitated DA release in the NAcc and pair-bonded males showed enhanced D1R expression in the NAcc compared to sexually naïve males, we focused on pair-bonded males to test whether intra-NAcc D1R blockade affected paternal behavior. Subjects were anesthetized intraperitoneally (ip) with ketamine/Domitor. Bilateral 26-gauge guide cannulae (Plastics One Inc.) were stereotaxically implanted and aimed at the NAcc shell (coordinates from bregma: anterior 1.8mm, lateral ±0.8mm and ventral 4.7mm). These males were allowed to recover for at least 7 days with their female partner. On the day of testing, subjects were transferred to the testing cage for a 15-min habituation. The drug was microinjected into the NAcc, using a 33-gauge needle that extended 1mm past the guide cannula, at a rate of 0.2μL/min for 1-min. Subjects received 200nl/side saline containing 20% dimethyl sulfoxide (DMSO, Sigma; vehicle) with 0.0, 0.6, or 3.0 μg of the D1R antagonist, SCH-23390 (Sigma). These dosages were chosen based on previous work of D1R regulation of maternal behavior in rats (Numan et al., 2005; Stolzenberg et al., 2009) and pair bonding behavior in prairie voles (Liu & Wang, 2003; Aragona et al., 2006). The injection needles were left in the guide cannulae for an additional 20-sec after injections to allow for the drug infusion. Subjects were returned to the cage for an additional 30-min to allow the drug to take effect, and were then tested with a stimulus pup for 10-min. After the behavioral test, subjects were euthanized by injections (ip) of overdose sodium pentobarbital (150mg/kg). Brains were harvested to verify cannula placement.
Data analysis
All behavioral data were statistically analyzed by SPSS Statistics (IBM Corporation) using either a t-test (Experiment 1) or one-way ANOVA followed by Student Newman-Keuls (SNK) posthoc tests (Experiment 3). Group differences in the levels of DA marker mRNA or protein expression in each brain area were analyzed by t-test. Brain microdialysate data were analyzed by a two-way ANOVA with repeated measures, followed by a SNK posthoc test, to compare DA release in the NAcc in response to pup or plastic stimulus over time.
Results
Pair bonding experience enhanced the display of paternal behavior
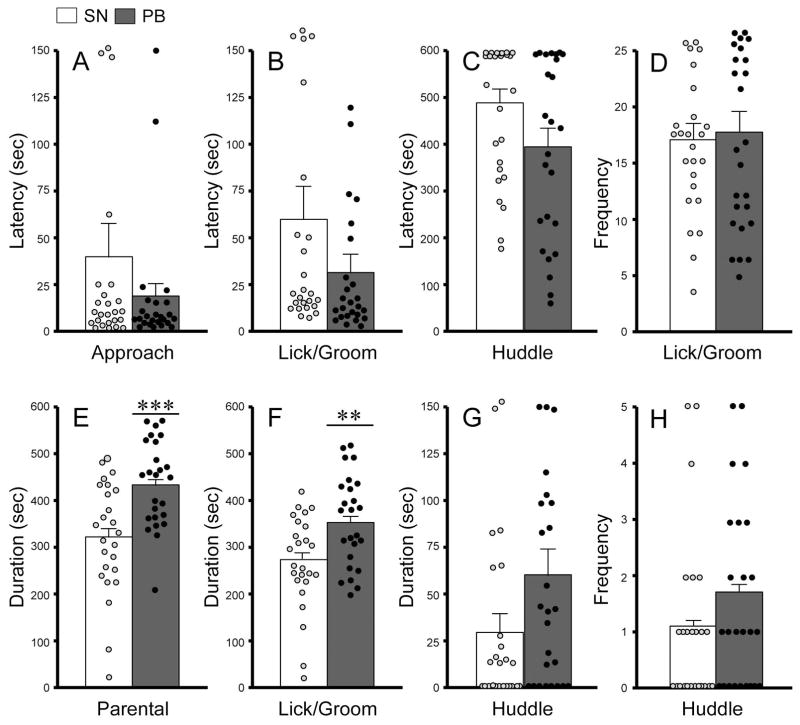
We first examined whether pair bonding experience with a female would alter paternal behaviors by comparing sexually naïve (n=34) and pair-bonded (n=33) male voles. Nine sexually naïve and 8 pair-bonded subjects showed aggressive behavior and were excluded from the data analysis. Both groups of males displayed several patterns of paternal behaviors, such as licking/grooming and huddling over the pup. No group differences were found in any of the latency and frequency measurements (Figure 1). However, pair-bonded males spent significantly more time engaged in overall paternal behavior (combination of pup licking/grooming and huddling) (t48 = 3.692, p = 0.001; Figure 1E), and particularly in pup licking/grooming (t48 = 2.906, p = 0.006; Figure 1F), compared to sexually naïve males. No group differences were found in pup huddling (Figure 1G–H) or in resting away from the pup (Table 1). Compared to pair-bonded males, sexually naïve males spent significantly more time engaged in locomotion (Table 1).
Figure 1.
Paternal behaviors displayed by male prairie voles that were sexually naïve (SN) or pair-bonded with a female for two weeks (PB). No group differences were found in the latencies of approaching (A), licking/grooming (B), or huddling the pup (C) or in the frequencies of pup licking/grooming (D) and huddling (H). PB males spent significantly more time displaying overall paternal behavior (E) and particularly pup licking/grooming (F) but not pup huddling (G), compared to SN males. Bars indicate mean ± SEM. **p<0.01 and ***p<0.001.
Table 1.
Non-social behaviors during pup-interaction test in male prairie voles.
| Behavior | Sexually naive | Pair-bonded | t | p | |
|---|---|---|---|---|---|
| locomotion | Duration (sec) | 224.19±23.40 | 127.50±18.29 | 3.256 | 0.002 |
| Frequency (#) | 14.24±1.07 | 13.60±1.97 | 0.286 | 0.776 | |
| Rest | Duration (sec) | 25.21±13.51 | 9.40±5.12 | 1.095 | 0.279 |
| Frequency (#) | 1.52±0.22 | 1.2±0.10 | 1.339 | 0.187 |
Data are presented as mean ± SEM.
Pair bonding experience altered DAR expression in the NAcc
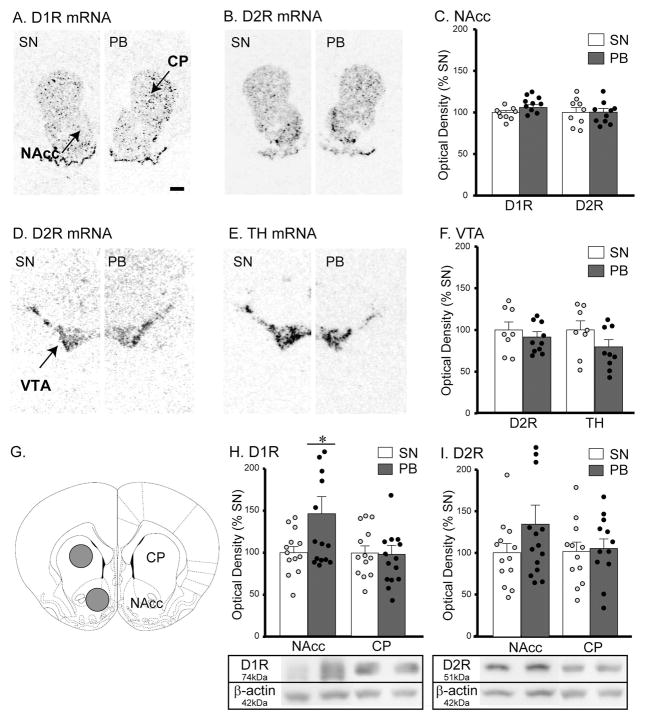
Tissues from the selected brain areas of sexually naïve (n=9) and pair-bonded (n=10) males were processed for DA marker mRNA labeling using in-situ hybridization. In the NAcc, no group differences were found in the levels of D1R and D2R mRNA expression (Figure 2A–C). Sexually naive and pair-bonded males also did not differ in the levels of D2R or TH mRNA expression in the VTA (Figure 2E–F), or in D1R or D2R mRNA expression in the CP (data not shown).
Figure 2.
DA markers in the brains of male prairie voles that were sexually naïve (SN) or pair-bonded with a female for two weeks (PB). D1R, D2R, and TH mRNAs were measured in the nucleus accumbens (NAcc), caudate putamen (CP), and ventral tegmental area (VTA) (A–F). Scale bar = 1mm. No group differences were found in any of the mRNA measurements. D1R and D2R protein levels were measured by Western immunoblotting in the NAcc and CP (G–I). PB males showed a significantly higher level of D1R in the NAcc but not CP (H). No group differences were found in the levels of D2R protein expression in either the NAcc or CP (I). Bars indicate mean ± SEM. *p<0.05.
DAR protein expression in the NAcc and CP was measured by Western immunoblot. Pair-bonded males (n=14) had a significantly higher level of D1R protein expression in the NAcc compared to sexually naïve males (n=13) (t25 = 2.086, p = 0.047; Figure 2G–H). A similar, but not statistically significant, trend was found for D2R protein expression (t25 = 1.319, p = 0.199; Figure 2I). No group differences were found in the levels of D1R and D2R protein expression in the CP (Figure 2H–I).
Pup exposure increased DA release in the NAcc
Brain microdialysis with HPLC-ECD analysis was conducted to examine intra-NAcc DA release by pup exposure in sexually naïve (n=6) and pair-bonded (n=7) males, as well as in males that were exposed to a piece of a pup-sized plastic to control for novelty (n=7 for sexually naïve and n=4 for pair-bonded groups) (Figure 3A). There was no group difference in basal DA concentration in the NAcc (Figure 3B). Pup exposure induced an increase in DA release in the NAcc in a time-dependent manner (F(6,40) = 3.370, p = 0.008). Ten-min following pup exposure (T1), a significant increase in the extracellular concentration of DA was found in the NAcc of both groups (F(2,40) = 14.134, p = 0.000), but this increase was not sustained 1-hr later (T2; Figure 3C).
Figure 3.
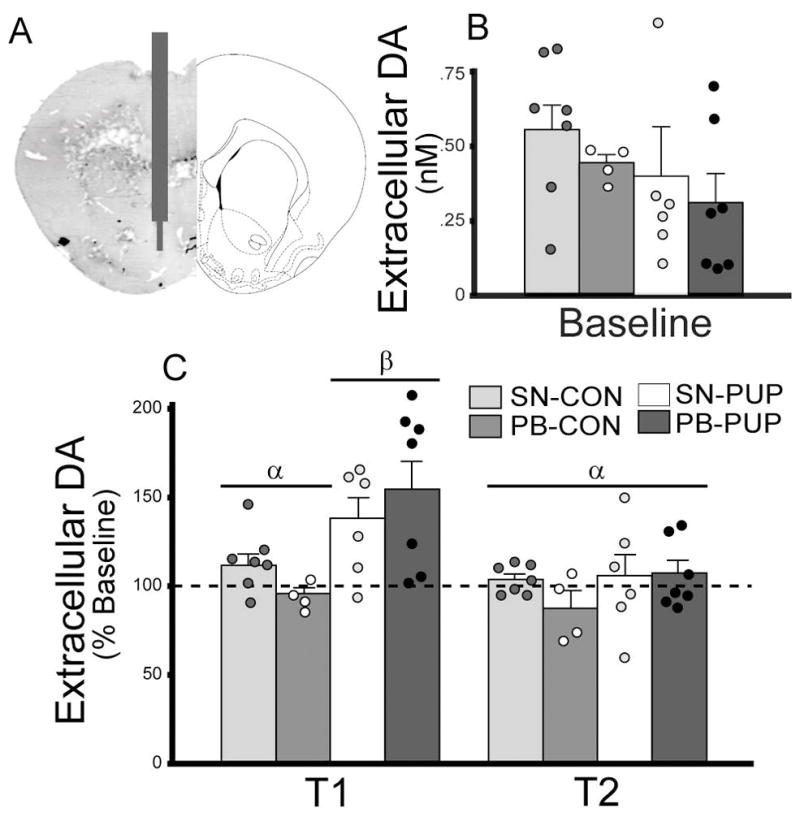
DA release in the nucleus accumbens (NAcc) in male prairie voles that were sexually naïve (SN) or pair-bonded with a female for two weeks (PB). Schematic drawing illustrates the placement of microdialysis probes (A). Microdialysis samples were collected every 10-min for 1-hr during the baseline period and for an additional 2 hr after a stimulus pup (PUP) or a pup-sized piece of plastic (novelty control; CON) was introduced into the cage. The last baseline sample and the first samples during the first (T1) and second (T2) hrs of the experiment were analyzed for DA concentrations. No group differences were found for baseline DA concentrations (B). Pup exposure significantly increased DA release in the NAcc in both SN and PB males but only during T1 (C). Bars indicate mean ± SEM. Bars with different Greek letters differ significantly from each other.
D1R antagonism in the NAcc affected paternal behavior
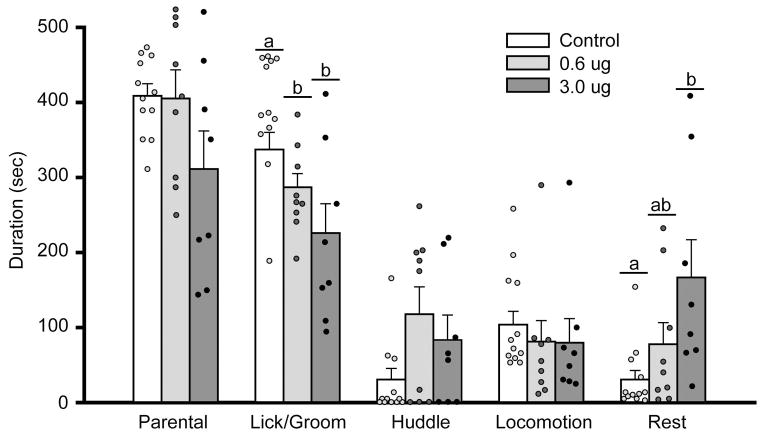
Male voles received intra-NAcc injections of the vehicle (control; n=17) or vehicle containing a low (0.6μg; n=11) or a high (3.0μg; n=10) dose of a D1R antagonist, SCH-23390, and were then tested for paternal behaviors. Nine males (5 for vehicle-only, 2 for 0.6μg, and 2 for 3.0μg groups) showed aggressive behavior and were excluded from the data analysis. Intra-NAcc injections of SCH-23390 at both doses significantly decreased the duration of pup licking/grooming (F(2,28) = 8.449, p = 0.001), and tended to have an opposite effect on pup huddling but this effect was not statistically significant (F(2,28) = 2.812, p = 0.078) (Figure 4). The high dose of SCH-23390 also tended to decrease the overall paternal duration (F(2,28) = 2.461, p = 0.105), significantly increased the duration and frequency of the subject resting-away from the pup, and had no effects on locomotor activities (Table 2).
Figure 4.
Administration of a D1R antagonist, SCH-23390, in the nucleus accumbens (NAcc) altered behaviors of male prairie voles that were pair-bonded with a female for two weeks. SCH-23390 at both low and high doses significantly decreased the duration of pup licking/grooming. Further, SCH-23390 at the high dose increased the duration of the male vole resting-away from the pup. Bars indicate mean ± SEM. Bars with different Greek letters differ significantly from each other.
Table 2.
Effects of NAcc D1R antagonism on non-social behaviors in pair-bonded male prairie voles.
| Behavior | 0μg | 0.6μg | 3.0μg | F(2,28) | p | |
|---|---|---|---|---|---|---|
| locomotion | Duration (sec) | 103.81±17.62 | 81.20±28.01 | 79.75±32.15 | 0.320 | 0.729 |
| Frequency (#) | 16.42±2.68 | 11.56±2.38 | 14.00±2.63 | 0.909 | 0.415 | |
| Rest | Duration (sec) | 30.60±12.13α | 77.72±28.84αβ | 166.68±50.35β | 5.246 | 0.012 |
| Frequency (#) | 4.75±1.02α | 4.11± 1.11α | 11.50±1.89β | 8.803 | 0.001 |
Data are presented as mean ± SEM. Greek letters indicate group differences illustrated by the SNK tests.
Discussion
Although studies have examined the neurochemical regulation of maternal behavior, particularly the role of NAcc DA (Hansen et al., 1991c; a; Hansen, 1994; Keer & Stern, 1999; Champagne et al., 2004; Hernandez-Gonzalez et al., 2005; Afonso et al., 2009), we know very little about the neurobiology of paternal behavior. In the present study using socially monogamous male prairie voles, we characterized the effects of pair-bonding experience with a female on changes in paternal behaviors and NAcc DA activities. We then examined the effects of pharmacological blockade of D1R in the NAcc on paternal behavior of pair-bonded males. Together, our data demonstrate that NAcc DA is involved in regulating male parental behavior in a behavior-specific manner via D1R mediation in prairie voles.
Our data show that 2 weeks of pair bonding experience significantly increased the duration of overall paternal behavior, particularly pup licking/grooming, in male prairie voles. These data are consistent with the previous finding that sociosexual experience with a female increased the display of paternal behavior in prairie voles (Bamshad et al., 1994; Terleph et al., 2004). These data are also consistent with data from other monogamous, bi-parental rodent species. For example, in California mice (Peromyscus californicus), pair-bonded males, expectant fathers, and new fathers were more paternal towards young pups than virgin males (Gubernick & Nelson, 1989; de Jong et al., 2009). Pair-bonded male mandarin voles (Lasiopodomys mandarinus) displayed enhanced pup licking/grooming and huddling behaviors, compared to their sexually naïve counterparts (Song et al., 2010). Similar increases in the display of paternal behavior by social experience has also been reported in a number of monogamous primate species, including marmoset and titi monkeys (Mendoza & Mason, 1986a; Mendoza & Mason, 1986d). Together, these data support the notion that sociosexual experience with a female can enhance various forms of paternal behavior in species with a monogamous life strategy (Mendoza & Mason, 1986a; Mendoza & Mason, 1986d; Gubernick & Nelson, 1989; Bamshad et al., 1994; Terleph et al., 2004; de Jong et al., 2009). In our present study, this effect is behavior-specific, as it had more robust effects on pup licking/grooming than on pup huddling in male prairie voles.
In female rats, interaction with pups is associated with increased DA release in the NAcc (Hansen et al., 1993), and released DA and increased DAR signaling are associated with the enhanced display of pup licking/grooming (Champagne et al., 2004; Afonso et al., 2009). Our data indicate a similar phenomenon in male prairie voles. Pup exposure induced a significant, but transient, increase in DA release in the NAcc in both sexually naïve and pair-bonded males. This transient increase in DA release may be important during the initial onset/display of paternal behavior, and sustained DA release may not be necessary to maintain this behavior. It should also be noted that in response to pup exposure, pair-bonded males showed a 54.7% increase in DA release in the NAcc compared to 38.3% of sexually naïve males. Although that difference was not statistically significant, its potential role on enhancing pup licking/grooming in pair-bonded males is worth investigating in future studies.
A significant increase in D1R protein expression in the NAcc was found in pair-bonded, compared to sexually naive, males. As voles were sacrificed immediately after the 10-min paternal behavioral test, this increase in D1R expression in the NAcc is better explained by the pair bonding experience rather than pup stimulation. This is in agreement with the previous finding that pair bonding with a female elevated NAcc D1R which, in turn, plays a role in pair bond maintenance in male prairie voles (Aragona et al., 2006). We found no group differences in all measured DA marker expression in the CP and VTA as well as in D2R expression in the NAcc, indicating brain region- and receptor-specific effects of pair bonding on the NAcc DAR. One drawback of the present study is that the DAR mRNA and protein expressions were only measured at a single time point, which might attribute to the observed mismatches between altered gene and protein expression.
The functional role of NAcc D1R in paternal behavior is demonstrated by our pharmacological data showing that D1R blockade in the NAcc decreased pup licking/grooming in pair-bonded males. Blockade of D1R in the NAcc disrupted maternal behavior in postpartum rats (Byrnes et al., 2002; Silva et al., 2003; Numan et al., 2005; Stolzenberg et al., 2007; Parada et al., 2008) and abolished mate guarding behavior in male prairie voles (Aragona et al., 2006). Further, in a previous study in prairie voles, systemic administration of a non-selective DAR antagonist, haloperidol, decreased pup licking/grooming in both mothers and fathers (Lonstein, 2002). Global inhibition of DARs could also act on the D2R autoreceptors in the VTA to decrease DA release (Missale et al., 1998), which in turn could decrease pup/licking/grooming behavior. Our data suggests that such effects could be due to decreased D1R signaling activities in the NAcc. In mandarin voles, the NAcc D2R protein levels are decreased in fathers that developed pup-induced place preference (Fang & Wang, 2017), suggesting that the rewarding effects of pups could be due to a shift in the NAcc D1R/D2R ratio that favors D1R signaling. Thus, NAcc D1R blockade could lower saliency of the stimulus pup, leading to lower paternal behavior, in our case, decreased licking/grooming. Furthermore, as D1Rs are coupled with stimulatory G-proteins and their activation increases cAMP production (Missale et al., 1998), decreased pup licking/grooming by D1R blockade could be due to decreased cAMP signaling activities in the NAcc. This has been demonstrated in DAR regulation of pair bonding behavior in prairie voles (Aragona & Wang, 2007) and cocaine seeking behavior in rats (Self et al., 1996). Finally, our data also indicate that treatment with the high dose of the D1R antagonist increased subject’s resting behavior away from the pup. These data should be interpreted with caution as the D1R antagonism may induce pup avoidance and/or affect other motion activities (e.g., climbing, leaping, upright posturing, and olfactory sniffing without location movement) that were not measured in the present study, yet may have secondary effects on paternal behavior.
In summary, our data demonstrate that pair-bonding experience with a female enhances paternal behavior and induces neuroadaptations in NAcc DA signaling in male prairie voles. We also show that enhanced D1R expression in the NAcc in pair-bonded males is responsible for their increased pup licking/grooming behavior. While pregnancy and its associated physiological changes can prime females for motherhood (Rosenblatt, 1975), pair-bonding experience with a female may facilitate male parental behavior in monogamous species (Bamshad et al., 1994). It is speculated that repeated exposure to female-associated stimuli (e.g. sexual and social interactions) may increase NAcc D1R responsiveness to released DA, enhancing the retention of paternal pup licking/grooming as suggested for the role of NAcc D1R in maternal behavior (Byrnes et al., 2002; Silva et al., 2003; Numan et al., 2005; Stolzenberg et al., 2007; Parada et al., 2008). Finally, it is worth mentioning that our data also showed a 34.5% increase in D2R protein expression in the NAcc of pair-bonded, compared to sexually naïve, males. Although this increase was not statistically significant, the potential role of D2R in paternal behavior should not be ignored. NAcc D2R mediates the onset of maternal behavior in rats (Silva et al., 2003). In prairie voles, NAcc D2R signaling plays a critical role in the formation of pair bonds (Aragona et al., 2003; Aragona & Wang, 2007), and haloperidol, a D2R bias antagonist, might also act on D2R to mediate dose-dependent decreases in pup licking/grooming (Lonstein, 2002). Further, increased D2R activation may facilitate D1R-mediated behavior via an inhibition of the pathways that reduce the probability/efficacy of D1R regulation of the behavior (Numan & Insel, 2003; de Jong et al., 2009; D’Aquila, 2010; Galistu & Paolo, 2013). Perhaps epigenetic events induced by pair bonding experience are involved in modulating paternal behaviors, as has been demonstrated in maternal behavior in rats (Champagne & Curley, 2008; Stolzenberg et al., 2014) and pair bonding behavior in prairie voles (Wang et al., 2013).
Acknowledgments
This work was supported by National Institutes of Health grant NIMHR01-058616 and MHR01-108527 to ZW. We thank J. Chalcraft for his help with the graphics.
Abbreviations
- CP
caudate putamen
- DA
dopamine
- DAR
dopamine receptors
- D1R
dopamine D1-type receptors
- D2R
dopamine D2-type receptors
- DMSO
dimethyl sulfoxide
- ip
intraperitoneal
- MPOA
medial preoptic area
- NAcc
nucleus accumbens
- OD
optical density
- PB
pair-bonded
- SN
sexually naïve
- SNK
Student Newman-Keuls
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
Footnotes
Conflict of interest
The authors have no financial disclosures and have no potential conflicts of interest.
Author contributions
KL, YL, and ASS performed experiments. KL analyzed data and drafted the manuscript. JSL participated in experimental design, data interpretation and manuscript writing. ZW designed experiments and wrote the final version of the paper.
Data accessibility
All primary data generated in this study will be available upon request.
References
- Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Horm Behav. 2009;56:11–23. doi: 10.1016/j.yhbeh.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Almond RE, Brown GR, Keverne EB. Suppression of prolactin does not reduce infant care by parentally experienced male common marmosets (Callithrix jacchus) Horm Behav. 2006;49:673–680. doi: 10.1016/j.yhbeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Almond RE, Ziegler TE, Snowdon CT. Changes in prolactin and glucocorticoid levels in cotton-top tamarin fathers during their mate’s pregnancy: the effect of infants and paternal experience. Am J Primatol. 2008;70:560–565. doi: 10.1002/ajp.20529. [DOI] [PubMed] [Google Scholar]
- Amato PR. Father-child relations, mother-child relations, and offspring psychological well-being in early adulthood. Journal of Marriage and the Family. 1994:1031–1042. [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Opposing regulation of pair bond formation by cAMP signaling within the nucleus accumbens shell. J Neurosci. 2007;27:13352–13356. doi: 10.1523/JNEUROSCI.3216-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, Sue Carter C. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, de Vries GJ. Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster) Physiol Behav. 1994;56:751–758. doi: 10.1016/0031-9384(94)90238-0. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacol Biochem Behav. 2002;73:869–875. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Nucleus accumbens cell firing and rapid dopamine signaling during goal-directed behaviors in rats. Neuropharmacology. 2004;47(Suppl 1):180–189. doi: 10.1016/j.neuropharm.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Maternal regulation of estrogen receptor α methylation. Current opinion in pharmacology. 2008;8:735–739. doi: 10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Amphetamine effects in microtine rodents: a comparative study sing monogamous and promiscuous vole species. Neuroscience. 2007;148:857–866. doi: 10.1016/j.neuroscience.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aquila PS. Dopamine on D2-like receptors “reboosts” dopamine D1-like receptor-mediated behavioural activation in rats licking for sucrose. Neuropharmacology. 2010;58:1085–1096. doi: 10.1016/j.neuropharm.2010.01.017. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Fang Q, Wang J. Place preferences associated with pups or cocaine change the expression of D2R, V1aR and OTR in the NAcc and MeA and the levels of plasma AVP, OT, T and E2 in mandarin vole fathers. Psychoneuroendocrinology. 2017;80:147–154. doi: 10.1016/j.psyneuen.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Gaffori O, Le Moal M. Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiol Behav. 1979;23:317–323. doi: 10.1016/0031-9384(79)90373-1. [DOI] [PubMed] [Google Scholar]
- Galistu A, Paolo S. Dopamine on D2-like receptors “reboosts” dopamine D1-like receptor-mediated behavioural activation in rats licking for a isotonic NaCl solution. Psychopharmacology. 2013;229:357–366. doi: 10.1007/s00213-013-3110-0. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Nelson RJ. Prolactin and paternal behavior in the biparental California mouse, Peromyscus californicus. Horm Behav. 1989;23:203–210. doi: 10.1016/0018-506x(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Hansen S. Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav. 1994;55:615–620. doi: 10.1016/0031-9384(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav. 1991a;39:71–77. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991c;105:588–598. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez M, Navarro-Meza M, Prieto-Beracoechea CA, Guevara MA. Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Processes. 2005;70:132–143. doi: 10.1016/j.beproc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Keer S, Stern J. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67:659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Kenkel WM, Perkeybile AM, Carter CS. The neurobiological causes and effects of alloparenting. Dev Neurobiol. 2017;77:214–232. doi: 10.1002/dneu.22465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658:112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in Mammals. Quarterly Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Lee A, Li M, Watchus J, Fleming AS. Neuroanatomical basis of maternal memory in postpartum rats: selective role for the nucleus accumbens. Behav Neurosci. 1999;113:523–538. doi: 10.1037//0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- Lee AW, Brown RE. Medial preoptic lesions disrupt parental behavior in both male and female California mice (Peromyscus californicus) Behav Neurosci. 2002;116:968–975. doi: 10.1037//0735-7044.116.6.968. [DOI] [PubMed] [Google Scholar]
- Lee AW, Brown RE. Comparison of medial preoptic, amygdala, and nucleus accumbens lesions on parental behavior in California mice (Peromyscus californicus) Physiol Behav. 2007;92:617–628. doi: 10.1016/j.physbeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Lillard LA, Waite LJ. Til death do us part: Marital disruption and mortality. American Journal of Sociology. 1995:1131–1156. [Google Scholar]
- Liu Y, Aragona BJ, Young KA, Dietz DM, Kabbaj M, Mazei-Robison M, Nestler EJ, Wang Z. Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci U S A. 2010;107:1217–1222. doi: 10.1073/pnas.0911998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lonstein JS. Effects of dopamine receptor antagonism with haloperidol on nurturing behavior in the biparental prairie vole. Pharmacol Biochem Behav. 2002;74:11–19. doi: 10.1016/s0091-3057(02)00952-8. [DOI] [PubMed] [Google Scholar]
- Mendoza S, Mason W. Primate Ontogeny, Cognition and Social Behaviour. Cambridge University Press; Cambridge: 1986a. Parenting within a monogamous society; pp. 255–266. [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (< i> Callicebus moloch</i>) Animal Behaviour. 1986d;34:1336–1347. [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A. 1996;93:11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. Springer Science & Business Media; 2003. [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Parada M, King S, Li M, Fleming AS. The roles of accumbal dopamine D1 and D2 receptors in maternal memory in rats. Behav Neurosci. 2008;122:368–376. doi: 10.1037/0735-7044.122.2.368. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychological bulletin. 2002;128:330. [PubMed] [Google Scholar]
- Rosenblatt JS. Prepartum and postpartum regulation of maternal behaviour in the rat. Ciba Found Symp. 1975:17–37. doi: 10.1002/9780470720158.ch3. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1-and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Silva MR, Bernardi MM, Cruz-Casallas PE, Felicio LF. Pimozide injections into the Nucleus accumbens disrupt maternal behaviour in lactating rats. Pharmacol Toxicol. 2003;93:42–47. doi: 10.1034/j.1600-0773.2003.930106.x. [DOI] [PubMed] [Google Scholar]
- Song Z, Tai F, Yu C, Wu R, Zhang X, Broders H, He F, Guo R. Sexual or paternal experiences alter alloparental behavior and the central expression of ERalpha and OT in male mandarin voles (Microtus mandarinus) Behav Brain Res. 2010;214:290–300. doi: 10.1016/j.bbr.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav Neurosci. 2007;121:907–919. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Stevens JS, Rissman EF. Histone deacetylase inhibition induces long-lasting changes in maternal behavior and gene expression in female mice. Endocrinology. 2014;155:3674–3683. doi: 10.1210/en.2013-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Zhang KY, Luskin K, Ranker L, Bress J, Numan M. Dopamine D(1) receptor activation of adenylyl cyclase, not phospholipase C, in the nucleus accumbens promotes maternal behavior onset in rats. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends in neurosciences. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Terleph TA, Jean-Baptiste N, Bamshad M. Mechanisms and time course for induction of paternal behavior in prairie voles (Microtus ochrogaster) Journal of Mammalogy. 2004;85:1124–1129. [Google Scholar]
- Trainor BC, Bird IM, Alday NA, Schlinger BA, Marler CA. Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. Neuroendocrinology. 2003;78:36–44. doi: 10.1159/000071704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Duclot F, Liu Y, Wang Z, Kabbaj M. Histone deacetylase inhibitors facilitate partner preference formation in female prairie voles. Nature neuroscience. 2013;16:919–924. doi: 10.1038/nn.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. The social deficits of the oxytocin knockout mouse. Neuropeptides. 2002;36:221–229. doi: 10.1054/npep.2002.0909. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Dietz DM, Wang H, Kabbaj M, Wang Z. Amphetamine alters behavior and mesocorticolimbic dopamine receptor expression in the monogamous female prairie vole. Brain Res. 2011;1367:213–222. doi: 10.1016/j.brainres.2010.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, Wang H, Wang Z. Oxytocin reverses amphetamine-induced deficits in social bonding: evidence for an interaction with nucleus accumbens dopamine. J Neurosci. 2014;34:8499–8506. doi: 10.1523/JNEUROSCI.4275-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler TE, Snowdon CT. Role of prolactin in paternal care in a monogamous New World primate, Saguinus oedipus. Ann N Y Acad Sci. 1997;807:599–601. doi: 10.1111/j.1749-6632.1997.tb51979.x. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Snowdon CT. Preparental hormone levels and parenting experience in male cotton-top tamarins, Saguinus oedipus. Horm Behav. 2000;38:159–167. doi: 10.1006/hbeh.2000.1617. [DOI] [PubMed] [Google Scholar]