Abstract
The inflammatory form of epidermolysis bullosa acquisita is caused by autoantibodies against type VII collagen (C7), a component of the dermal-epidermal junction. We have previously shown that myeloid Src family kinases mediate skin inflammation triggered by anti-C7 antibodies. Here we identify the Syk tyrosine kinase as a critical component of autoantibody-induced skin inflammation downstream of Src family kinases. Immobilized C7–anti-C7 immune complexes triggered neutrophil activation and Syk phosphorylation in a Src family kinase-dependent manner. Bone marrow chimeric mice lacking Syk in their hematopoietic compartment were completely protected from skin inflammation triggered by anti-C7 antibodies despite normal circulating anti-C7 levels. Syk deficiency abrogated the accumulation of CXCL2, IL-1β, and leukotriene B4 at the site of inflammation and resulted in defective in vivo neutrophil recruitment. Syk–/– neutrophils had a normal intrinsic migratory capacity but failed to release CXCL2 or leukotriene B4 upon activation by immobilized C7–anti-C7 immune complexes, indicating a role for Syk in the amplification of the inflammation process. These results identify Syk as a critical component of skin inflammation in a mouse model of epidermolysis bullosa acquisita and as a potential therapeutic target in epidermolysis bullosa acquisita and other mechanistically related inflammatory skin diseases such as bullous pemphigoid.
Abbreviations: C7, type VII collagen; GST-C7, GST fusion protein containing a fragment of the immunodominant NC1 domain of murine C7; His-C7, His-tagged fragment of the immunodominant NC1 domain of murine C7; HSA, human serum albumin; KO, knockout; LTB4, leukotriene B4; WT, wild-type
Introduction
Typical examples of subepidermal blistering skin diseases are bullous pemphigoid and epidermolysis bullosa acquisita, the latter disease having both an inflammatory and a noninflammatory (mechanobullous) form (Kim and Kim, 2013, Ludwig and Zillikens, 2011). Both bullous pemphigoid and epidermolysis bullosa acquisita are caused by autoimmune targeting of components of the dermal-epidermal junction and are triggered by autoantibodies against the hemidesmosomal proteins BP230/BP180 or the dermal anchoring fibril protein type VII collagen (C7), respectively (Baum et al., 2014, Schmidt and Zillikens, 2013, Turcan and Jonkman, 2015). In the case of bullous pemphigoid and the inflammatory form of epidermolysis bullosa acquisita, this leads to local inflammation and disruption of the dermal-epidermal junction, mediated by complement activation and leukocyte (mainly eosinophil and neutrophil) infiltration (Schmidt and Zillikens, 2013). In contrast, autoantibodies appear to directly decrease the adhesive function of the basement membrane without initiating significant inflammation in the noninflammatory form of epidermolysis bullosa acquisita (Kim and Kim, 2013). Unfortunately, further details of the pathomechanism of bullous pemphigoid and epidermolysis bullosa are poorly understood, and therefore, the therapeutic options are rather limited (Kasperkiewicz and Schmidt, 2009).
A number of in vivo models mimic bullous pemphigoid or the inflammatory form of epidermolysis bullosa acquisita by using active or passive immunization against components of the dermal-epidermal junction in experimental animals (Iwata et al., 2015). The most widely used model relies on the passive immunization of mice with IgG antibodies against the immunodominant NC1 domain of C7 (Ludwig, 2012, Sitaru et al., 2005, Sitaru, 2007). Because this intervention triggers substantial inflammation and inflammatory pathway components are critical to disease pathogenesis (see below), this model is believed to mimic the inflammatory but not the noninflammatory form of epidermolysis bullosa acquisita.
Animal models of bullous pemphigoid and epidermolysis bullosa acquisita have shown striking similarities between the pathomechanism of the two disease models. Critical components include activating Fc-receptors (Kasperkiewicz et al., 2012, Schulze et al., 2014, Sitaru et al., 2005, Zhao et al., 2006), the alternative pathway of complement activation (Liu et al., 1995, Mihai et al., 2007, Sitaru et al., 2005) and myeloid cells such as neutrophils (Chiriac et al., 2007, Liu et al., 1997). Our recent signaling studies showed complete protection of mice lacking three Src family kinases expressed in myeloid cells (Hck, Fgr and Lyn) from skin inflammation triggered by antibodies against C7 (Kovács et al., 2014), whereas mice lacking phosphatidylinositol 3-kinase β (PI3Kβ) (Kulkarni et al., 2011) or the CARD9 adapter protein (Németh et al., 2016) were partially protected. Additional experiments pointed to a critical role for those molecules within the neutrophil compartment in all three cases.
Syk is a nonreceptor tyrosine kinase primarily expressed in cells of hematopoietic origin (Mócsai et al., 2010). Syk is involved in signal transduction of a number of tyrosine kinase-coupled receptors including β2-integrins and various Fc-receptors (Mócsai et al., 2002, Mócsai et al., 2003, Mócsai et al., 2010, Németh et al., 2016). Src family kinases are involved in Syk activation in most of those cases (Kovács et al., 2014, Mócsai et al., 2002, Mócsai et al., 2006, Mócsai et al., 2010). Hematopoietic or neutrophil-specific deletion of Syk protects mice from arthritis development in the K/B × N serum-transfer model (Elliott et al., 2011, Jakus et al., 2010), and Syk has been proposed to be a potential target in rheumatoid arthritis and other autoimmune inflammatory diseases (Geahlen, 2014, Mócsai et al., 2010, Weinblatt et al., 2010). However, the role of Syk in autoimmune inflammatory skin diseases is still unclear.
The discussed findings and the lack of information on the role of Syk in autoimmune dermatitis prompted us to test the role of Syk in an in vivo model of the inflammatory form of epidermolysis bullosa acquisita in experimental mice. Our results indicate that Syk is critically involved in skin inflammation triggered by autoantibodies against C7, identifying Syk as a potential therapeutic target in epidermolysis bullosa acquisita and, possibly, other inflammatory skin diseases of similar pathomechanism.
Results
Neutrophil activation by C7-containing immune complexes
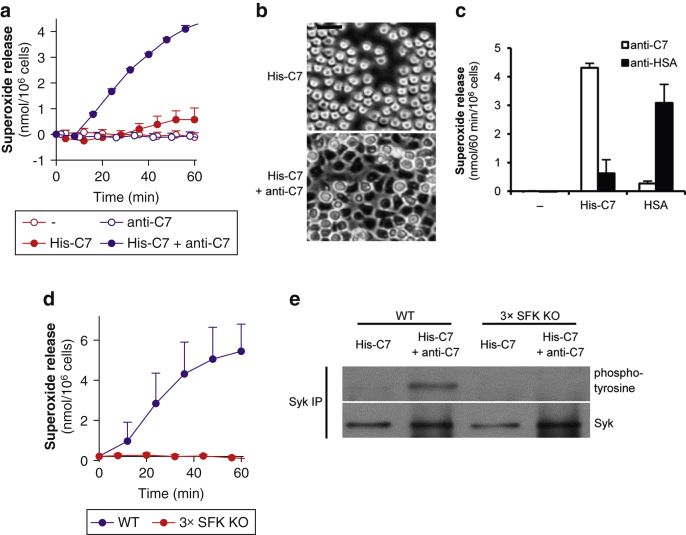
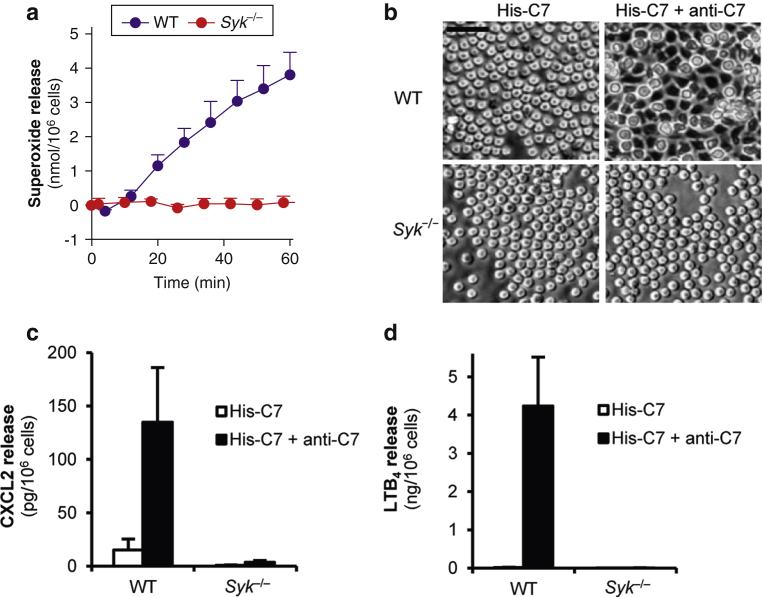
We have previously shown that immobilized human serum albumin (HSA)–anti-HSA immune complexes trigger robust activation of neutrophils (Jakus et al., 2008). To determine whether immune complexes containing dermal-epidermal junctional proteins can also activate neutrophils, we generated immobilized immune complexes using a His-tagged fragment of the immunodominant NC1 domain of murine C7 (His-C7) and rabbit polyclonal IgG (anti-C7) against the GST fusion protein of the same fragment (Csorba et al., 2010, Sitaru et al., 2002). Plating wild-type (WT) mouse neutrophils on such immobilized immune complexes triggered robust activation of the cells as measured by the release of superoxide anions (Figure 1a; P = 4 × 10–6). Neutrophil activation required both the antigen and the antibody components (Figure 1a). His-C7–anti-C7 immune complexes also triggered spreading of the cells over the immune complex-coated surface (Figure 1b).
Figure 1.
Type VII collagen-containing immune complexes trigger neutrophil activation and Syk phosphorylation through Src family kinases. (a) Respiratory burst of WT mouse neutrophils plated on surfaces treated with His-C7 and/or anti-C7. (b) His-C7–anti-C7 immune complexes trigger spreading of WT neutrophils. (c) Specificity of the His-C7‒anti-C7- and HSA–anti-HSA-induced responses. (d–e) Src family kinases are essential for His-C7‒anti-C7 immune complex-triggered superoxide release and Syk-phosphorylation. Kinetic curves and graphs in a, c, and d show mean and standard error of the mean of 2 to 3 independent experiments. In panels a and d, control data points were subtracted. Panels b and e are representative of 2 to 3 independent experiments. See the text for actual P-values. Scale bar = 20 μm. 3 × SFK KO, Hck–/–Fgr–/–Lyn–/–; C7, collagen type VII; HSA, human serum albumin; IP, immunoprecipitation; KO, knockout; min, minute; WT, wild-type.
To test the antigen specificity of neutrophil activation, ELISA plates were coated with His-C7 or HSA, blocked, and then incubated with anti-C7 or anti-HSA antibodies. As shown in Figure 1c, neutrophils mounted a robust respiratory burst in the presence of matching antigen-antibody pairs (His-C7 with anti-C7 or HSA with anti-HSA; P = 8 × 10–6 and P = 1.9 × 10–3, respectively) but not when nonmatching antigen-antibody pairs were used.
Taken together, C7-containing immune complexes are able to specifically activate neutrophil functions.
Src family kinases mediate neutrophil respiratory burst and Syk activation by C7-containing immune complexes
We have recently shown that the Src family kinases Hck, Fgr, and Lyn are critically involved in neutrophil activation triggered by HSA–anti-HSA immune complexes (Kovács et al., 2014). To test whether this also applies to C7-containing immune complexes, we tested the responses of Hck–/–Fgr–/–Lyn–/– (3 × SFK knockout [KO]) neutrophils. As shown in Figure 1d, 3 × SFK KO neutrophils failed to release superoxide under such conditions.
Syk is a nonreceptor tyrosine kinase activated downstream of Src family kinases in several immunoreceptor-induced signaling pathways (Futosi et al., 2013, Mócsai et al., 2010). We next tested whether this is also true for neutrophil activation by C7-containing immune complexes. WT neutrophils plated on immobilized His-C7–anti-C7 immune complexes showed Syk phosphorylation (a measure of Syk activation), whereas no such response could be observed in 3 × SFK KO neutrophils (Figure 1e, and see the entire blots in Supplementary Figure S1 online).
Taken together, neutrophil activation by C7-containing immune complexes triggers Syk activation downstream of the Src family kinases Hck, Fgr, and Lyn.
Generation and characterization of Syk–/– bone marrow chimeras
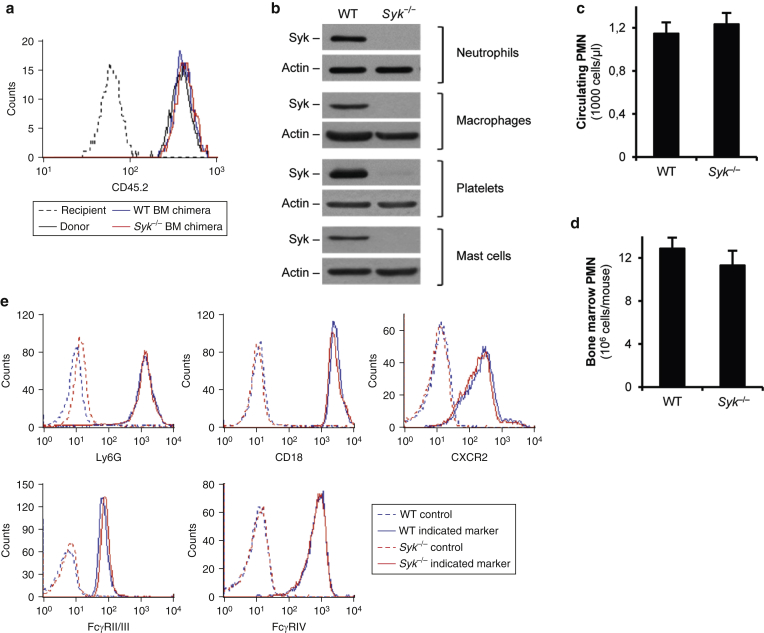
These findings raised the possibility that Syk may also be involved in anti-C7 antibody-induced skin inflammation. Unfortunately, the perinatal lethality of genetically Syk-deficient (Syk–/–) animals (Abtahian et al., 2003, Mócsai et al., 2010, Turner et al., 1995) did not allow us to test this in intact animals. To overcome that problem, we generated bone marrow chimeras lacking Syk only in their hematopoietic compartment (termed Syk–/– bone marrow chimeras) by transplanting fetal liver cells of Syk–/– fetuses into lethally irradiated WT recipients (Mócsai et al., 2002). Control chimeras (termed WT bone marrow chimeras) were generated by parallel transplantation of Syk+/+ or Syk+/– fetal liver cells. Differential expression of CD45 alleles in the donor (CD45.2) and the recipient (CD45.1) cells allowed identification of donor- and recipient-derived cells. As shown in Figure 2a, practically all peripheral blood neutrophils of WT or Syk–/– bone marrow chimeras expressed the donor-derived CD45.2 allele, indicating complete repopulation of the hematopoietic compartment by donor-derived cells. In addition, Syk was present in lysates of freshly isolated neutrophils and platelets, as well as of bone marrow-derived macrophages and mast cells, from WT but not Syk–/– bone marrow chimeras (Figure 2b). Therefore, we were able to replace the WT hematopoietic compartment of the recipient mice with donor-derived (e.g., Syk–/–) cells.
Figure 2.
Characterization of WT and Syk–/– bone marrow chimeras and their neutrophils. (a) Flow cytometric analysis of the donor-specific CD45.2 epitope in peripheral blood neutrophils tested 4 weeks after BM transplantation. (b) Immunoblot analysis of Syk expression in various cell types from WT and Syk‒/‒ BM chimeras. (c) Circulating neutrophil (PMN) counts in WT and Syk‒/‒ chimeras. (d) Average neutrophil number isolated from the BM of the different chimeras. (e) Cell surface molecule expression of WT and Syk‒/‒ BM-isolated neutrophils. Panels a, b, and e are representative of 3 to 10 independent experiments. Graphs in panel c and d show mean and standard error of the mean from 9 to 11 mice. See the text for actual P-values. BM, bone marrow; PMN, polymorphonuclear cell; WT, wild-type.
We did not observe any difference in circulating neutrophils numbers between WT and Syk–/– bone marrow chimeras (Figure 2c) (P = 0.69), and similar numbers of bone marrow neutrophils could be isolated from chimeras of the two genotypes (Figure 2d) (P = 0.37). In addition, Syk–/– neutrophils expressed normal levels of the neutrophil maturation marker Ly6G, the β2 integrin component CD18, the major neutrophil chemokine receptor CXCR2, and the Fc-receptors FcγRII/III and FcγRIV (Figure 2e). Therefore, the Syk–/– mutation did not interfere with the generation or maturation of neutrophils or with the expression of critical cell surface receptors.
Syk–/– bone marrow chimeras are completely protected from skin inflammation triggered by anti-C7 antibodies
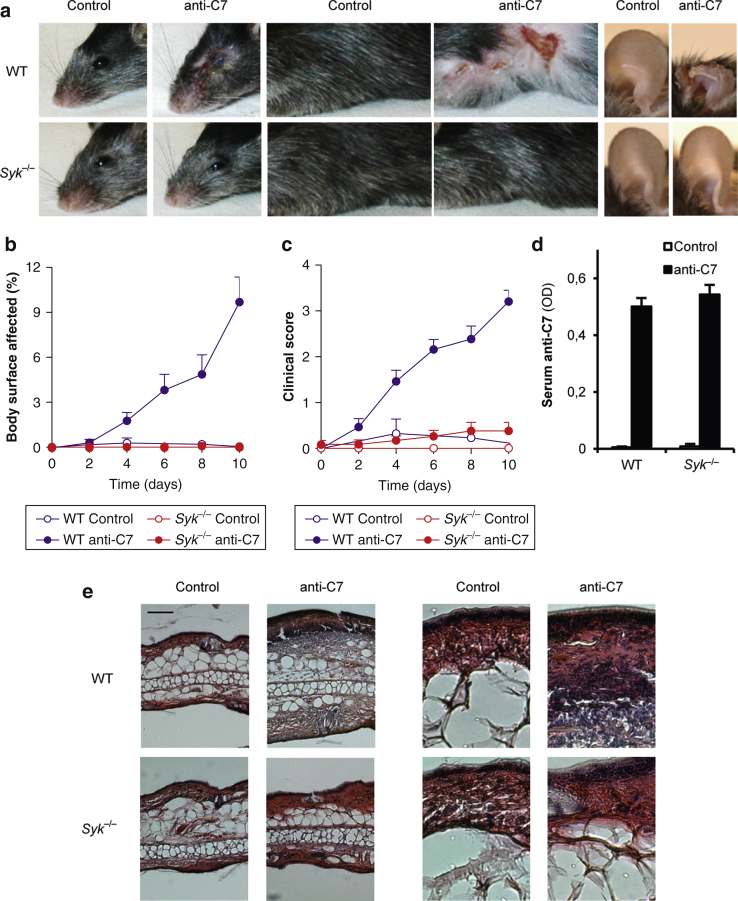
We next subjected WT and Syk–/– bone marrow chimeras to repeated subcutaneous injection of anti-C7 or normal rabbit IgG/phosphate buffered saline as a model of inflammatory autoimmune blistering skin diseases (Sitaru et al., 2005). Our previous studies have indicated a critical role for the Fc portion of the pathogenic antibodies in this model (Sitaru et al., 2005). As shown in Figure 3a, WT chimeras treated with anti-C7 antibodies developed significant signs of skin inflammation at several body areas, ranging from loss of the superficial layer of the skin (most prominent in the buccal/periorbital area) to severe inflammation and scarring at the most severely affected places (most prominent on the ears). Syk–/– bone marrow chimeras were completely protected from development of all those skin inflammatory changes (Figure 3a).
Figure 3.
Syk is required for experimental epidermolysis bullosa acquisita. Skin disease was triggered in WT or Syk–/– bone marrow chimeras by anti-C7 antibodies and was followed by (a) photographing the heads, the trunks, and the ears and clinical assessment of (b) the body surface affected and (c) the overall disease severity. (d) Serum anti-C7 levels were tested by ELISA. (e) Hematoxylin and eosin stained ear histological sections are presented from WT and Syk–/– chimeras. The right images were magnified from the pictures seen on the left. Original magnification ×10. (a, e) Representative images or (b, c) mean and standard error of the mean from 3 control and 12 to 13 anti-C7–treated mice per genotype from 3 independent experiments are shown. Panel d shows mean and standard error of the mean from 2 to 3 control and 9 to 11 anti-C7–treated mice from 2 independent experiments. See the text for actual P-values. Scale bar = 100 μm. C7, collagen type VII; OD, optical density; WT, wild-type.
Quantification of the overall skin changes showed that the percentage of the affected body surface area gradually increased over the assay period in WT chimeras, whereas no signs of the disease could be observed in Syk–/– chimeras (Figure 3b) (P = 0.022). Similarly, the overall clinical score also gradually increased during the assay period in WT chimeras without any signs of the disease in Syk–/– chimeras (Figure 3c) (P = 6.0 × 10–3).
A possible explanation for these findings could be that the Syk–/– mutation affects the metabolism of the injected pathogenic antibodies. However, no differences in circulating anti-C7 antibody titers could be observed between the WT and Syk–/– genotypes (Figure 3d) (P = 0.69).
We next tested skin inflammation by histological analysis of the ear tissue. As shown in Figure 3e, anti-C7 antibody treatment triggered substantial thickening of the ear of WT chimeras with prominent leukocyte infiltration. However, no such inflammatory changes could be observed in Syk–/– bone marrow chimeras.
Taken together, the Syk–/– mutation blocks the capability of anti-C7 antibodies to trigger skin inflammation both at the macroscopic and microscopic levels, without affecting their removal from the circulation.
The Syk–/– mutation blocks neutrophil recruitment without affecting the intrinsic migratory capacity of neutrophils
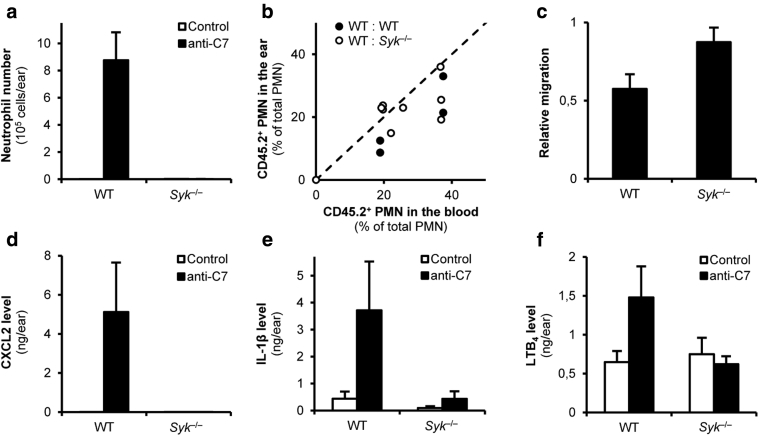
The inflammatory form of epidermolysis bullosa acquisita is characterized by a significant influx of granulocytes (eosinophils and neutrophils), which are likely involved in the inflammation process (Schmidt and Zillikens, 2013). As shown in Figure 4a, anti-C7 antibodies triggered dramatic influx of neutrophils to the ear of WT bone marrow chimeras, which appears to be a peculiarity of this mouse model (compared with the dominant infiltration by eosinophils in the inflammatory form of human epidermolysis bullosa acquisita). In agreement with the histological pictures (Figure 3e), no such accumulation of neutrophils could be observed in Syk–/– chimeras (Figure 4a) (P = 7.7 × 10–3).
Figure 4.
Syk is essential for forming the inflammatory environment. (a) The accumulation of neutrophils in the ear upon anti-C7 injection was determined by flow cytometry. (b, c) Mixed bone marrow chimeras with CD45.1-expressing WT and CD45.2-expressing WT or Syk–/– hematopoietic cells were injected with anti-C7 antibodies and neutrophil accumulation was determined by flow cytometric analysis. In panel b, each dot represents an individual ear, and panel c shows the relative migration of WT and Syk–/– neutrophils. (d–f) Decreased in vivo CXCL2, IL-1β, and LTB4 levels in the ears of Syk-deficient chimeras. Graphs represent mean and standard error of the mean from (a) 3 to 5 or (c) 2 to 6 mice and (d–f) 3 to 4 control or 5 to 7 anti-C7–treated mice per group from 2 to 3 independent experiments. See the text for actual P-values. C7, collagen type VII; LTB4, leukotriene B4; PMN, polymorphonuclear cell; WT, wild-type.
The simplest explanation for these findings would be an intrinsic migration defect of Syk–/– neutrophils. However, we previously reported normal migration of Syk–/– neutrophils toward various proinflammatory agonists both under in vitro and in vivo conditions (Mócsai et al., 2002, Mócsai et al., 2003). To test this question in anti-C7–induced skin inflammation, we generated mixed bone marrow chimeras carrying leukocytes (including neutrophils) from both CD45.1-expressing WT and CD45.2-expressing WT or Syk–/– bone marrow cells. Such chimeras developed signs of inflammation because of the presence of WT cells. Comparative flow cytometric analysis of CD45.1/2 expression in the inflammatory infiltrate and the peripheral blood then allowed us to compare the migration of WT and Syk–/– neutrophils within the same animal. The percentage values for the peripheral blood and ear samples are presented in Figure 4b, whereas the relative migratory capacity of the CD45.2 (WT or Syk–/–) cells relative to the CD45.1 (WT) reference cells (calculated based on the data in Figure 4b) is shown in Figure 4c. As expected, the percentage of WT CD45.2 cells was comparable in the peripheral blood and the inflamed ear in the chimeras transplanted with cells from two WT donors (WT:WT chimeras) (Figure 4b and c). The percentage of Syk–/– CD45.2 cells in the inflamed ear tissue was also similar to that in the peripheral blood in WT:Syk–/– chimeras (Figure 4b), and the calculated relative migration of Syk–/– cells was similar to, or even higher than, that of WT cells (Figure 4c) (P = 4.4 × 10–6). Those results indicate that Syk–/– neutrophils are intrinsically capable of migrating to the site of inflammation, arguing against an intrinsic migration defect in Syk–/– neutrophils.
Taken together, although neutrophils failed to accumulate in the ear of anti-C7 antibody-treated Syk–/– bone marrow chimeras, this was not due to an intrinsic migration defect of Syk–/– neutrophils, because those cells were able to accumulate at the site of inflammation when WT cells were also present in mixed bone marrow chimeras.
The Syk–/– mutation blocks the accumulation of proinflammatory mediators in vivo
An alternative explanation for the abrogation of neutrophil accumulation in Syk–/– chimeras (Figure 4a) is that Syk deficiency may interfere with the development of a proper inflammatory (chemoattractant) microenvironment. As shown in Figure 4d, systemic administration of anti-C7 antibodies dramatically increased tissue levels of CXCL2 (MIP-2) in the ear of WT but not Syk–/– bone marrow chimeras (P < 10–17). Similarly, hematopoietic deficiency of Syk abrogated the accumulation of the proinflammatory cytokine IL-1β (Figure 4e) (P = 1.6 × 10–7). Moreover, anti-C7 antibodies also moderately increased the level of the lipid mediator leukotriene B4 (LTB4) (a major neutrophil chemoattractant in various inflammatory conditions) in WT but not Syk–/– bone marrow chimeras (Figure 4f) (P = 0.038).
Taken together, hematopoietic deficiency of Syk somehow prevented the accumulation of various proinflammatory agonists at the site of inflammation, likely leading to the defective accumulation of Syk–/– neutrophils and subsequent protection from clinical disease.
Syk–/– neutrophils fail to respond to C7-containing immune complexes in vitro
Because neutrophils are capable of releasing various chemokines, cytokines, and lipid mediators (Kovács et al., 2014, Németh et al., 2016, Tecchio et al., 2014), we hypothesized that the discussed in vivo findings may be due to defective responses of Syk–/– neutrophils to C7-containing immune complexes. Indeed, Syk–/– neutrophils failed to release superoxide (Figure 5a) (P = 8.6 × 10–4) or spread over the adhesive surface (Figure 5b) when plated on immobilized His-C7–anti-C7 immune complexes. In addition, WT neutrophils released large amounts of both CXCL2 and LTB4, whereas no such responses could be observed in Syk–/– samples (Figure 5c and d) (P = 3.8 × 10–10 and 1.1 × 10–16, respectively). Those results suggest that Syk is indispensable for neutrophil activation by C7-containing immune complexes, explaining the defective development of an in vivo inflammatory environment in Syk–/– bone marrow chimeras.
Figure 5.
Syk is required for neutrophil responses on immobilized His-C7‒anti-C7 immune complexes. (a) Respiratory burst, (b) cell spreading, (c) CXCL2, and (d) LTB4 release of WT and Syk‒/‒neutrophils. Curves in panel a show mean and standard error of the mean of 3 independent experiments; control data points were subtracted. Panel b is representative of 3 independent experiments. Graphs in c and d show mean and standard error of the mean from 3 independent experiments. See the text for actual P-values. Scale bar = 40 μm. C7, collagen type VII; His-C7, His-tagged fragment of the immunodominant NC1 domain of murine C7; LTB4, leukotriene B4; min, minute; WT, wild-type.
Discussion
Here we report that the Syk tyrosine kinase is indispensable for an in vivo model of the inflammatory form of epidermolysis bullosa acquisita in experimental mice. This study raises the possibility that Syk may be a suitable therapeutic target in that disease and, possibly, in other diseases with a similar pathogenesis.
Syk has diverse biological functions (Mócsai et al., 2010), primarily by mediating signaling from immunoreceptors such as Fc-receptors. Fc-receptors play a critical role in mouse models of bullous pemphigoid and the inflammatory form of epidermolysis bullosa acquisita (Kasperkiewicz et al., 2012, Schulze et al., 2014, Sitaru et al., 2005, Zhao et al., 2006). Together with our studies on the role of Src family kinases (Kovács et al., 2014) and CARD9 (Németh et al., 2016), our experiments suggest that anti-C7–induced skin inflammation is mediated by Syk linking Fc-receptor–mediated activation of Src family kinases to downstream signaling through the CARD9 adapter protein. This is reminiscent of signal transduction in K/B × N serum transfer arthritis and recognition of fungal pathogens (Gross et al., 2009).
In agreement with other studies on neutrophil function (Németh and Mócsai, 2012, Mócsai, 2013), our results suggest an important role for neutrophils in certain inflammatory autoimmune subepidermal blistering skin disease models. Our results of defective in vivo recruitment of Syk–/– neutrophils despite apparently normal intrinsic migratory capacity (Figure 4a–c) and the defective accumulation of neutrophil-attracting proinflammatory mediators (CXCL2 and LTB4) in vivo (Figure 4d and f) and in in vitro neutrophil assays (Figure 5c and d) suggest that the principal role of Syk is to promote feedback amplification loops through proinflammatory mediator release by neutrophils (Németh and Mócsai, 2016). Although we cannot exclude the role of Syk expression in another cell type, neutrophil-specific deletion of Syk completely protected mice from anti-C7–induced skin pathology (Németh and Mócsai, unpublished observation), indicating the central role for neutrophil-derived Syk in the disease process.
Besides identifying Syk as a critical player in autoantibody-induced skin inflammation, our results also raise a number of questions. What is the role of Syk in the initial immunization phase of autoimmune bullous skin diseases or the very first steps of anti-C7–induced skin inflammation before the initiation of the neutrophil-mediated feedback amplification process? Would it be possible to document differential chemokine gene expression of WT and Syk–/– neutrophils in mixed bone marrow chimeras? Do our conclusions also apply to other autoimmune subepidermal blistering skin diseases where the autoantigen is other than C7 or where the inflammatory component is less pronounced? How much can we extrapolate our findings on mouse models to human autoimmune and other inflammatory skin diseases? Those and other questions need to be addressed by future follow-up studies.
Parallel to our own study initiated by the relationship between Src family kinases and Syk (Berton et al., 2005, Futosi and Mócsai, 2016, Kovács et al., 2014), another study also identified Syk as a core signaling hub in the inflammatory form of epidermolysis bullosa acquisita (Ralf Ludwig, unpublished observations). Although both studies conclude that Syk is critical for disease development, they also complement each other by addressing different additional aspects, such as additional signaling and mechanistic experiments in this paper and bioinformatics and pharmacological aspects in the other study.
The pharmacological therapy of bullous pemphigoid and epidermolysis bullosa acquisita still relies on rather nonspecific immunosuppressive and anti-inflammatory agents. We have identified Syk as a critical component of autoantibody-induced skin inflammation in experimental mice. Although there are clear differences between our mouse model and the corresponding human disease(s) (passive vs. active immunization and neutrophils vs. eosinophils dominating the inflammatory infiltrate, respectively), we believe that our mouse studies relatively closely mimic important aspects of human pathology. Syk has emerged as a potential therapeutic target in other autoimmune inflammatory diseases such as rheumatoid arthritis, as well as in Syk-dependent hematologic malignancies (Geahlen, 2014). Although fostamatinib, the first Syk inhibitor reaching clinical development, raised safety concerns likely related to its poor specificity (Anastassiadis et al., 2011, Genovese et al., 2014, Rolf et al., 2015, Weinblatt et al., 2014), there is a large effort from the pharmaceutical industry to develop much more specific Syk inhibitors (Norman, 2014, Walker and Croasdell, 2016). Our results suggest that such Syk-specific inhibitors may be suitable for the pharmacological control of certain inflammatory autoimmune subepidermal blistering skin diseases such as the inflammatory form of epidermolysis bullosa acquisita or, possibly, the mechanistically related bullous pemphigoid.
Materials and Methods
Animals
Mice carrying a deleted Syk allele (Syktm1Tyb, referred to as Syk–) were from Victor Tybulewicz (Turner et al., 1995) and kept in heterozygous form. Recipients carrying the CD45.1 allele on the C57BL/6 genetic background (B6.SJL-Ptprca mice from the Jackson Laboratory, Bar Harbor, ME) were lethally irradiated by 11 Gy from a 137Cs source using a Gamma-Service Medical D1 irradiator (Gamma-Service Medical GmbH, Leipzig, Germany) and then injected intravenously with fetal liver cells from Syk–/– and control fetuses obtained from timed mating of Syk+/– carriers (Jakus et al., 2010, Mócsai et al., 2002). Hcktm1Hev/tm1HevFgrtm1Hev/tm1HevLyntm1Sor/tm1Sor triple KO mice (referred to as Hck–/–Fgr–/–Lyn–/– or 3 × SFK KO mice) were described previously (Kovács et al., 2014, Meng and Lowell, 1998). Mice were kept in individually sterile ventilated cages (Tecniplast, Buguggiate, Italy) in a conventional facility. All animal experiments were approved by the Animal Experimentation Review Board of the Semmelweis University.
Autoantibody-induced skin blistering model
The murine model of the inflammatory form of human epidermolysis bullosa acquisita was triggered by systemic administration of antibodies against C7, which triggers skin inflammation through the antibody Fc portions (Sitaru et al., 2005). Rabbits were immunized with a GST fusion protein containing a fragment of the immunodominant NC1 domain of murine C7 (GST-C7), followed by total IgG preparation (anti-C7) (Csorba et al., 2010, Sitaru et al., 2005). The reactivity of the antibody preparation was tested with a His-tagged murine type VII collagen fragment (His-C7) (Csorba et al., 2010). Normal rabbit IgG (Sigma-Aldrich, St. Louis, MO) or phosphate buffered saline (Thermo Fisher, Waltham, MA) was used as control.
Twelve milligrams of pathogenic or control IgG was injected subcutaneously under isoflurane anesthesia on days 0, 2, 4, 6, and 8 (60 mg total IgG/mouse). Disease onset and progression were followed by clinical assessment, and quantitative scoring was based on the specific dermatological abnormalities and the size of the affected skin area (Kovács et al., 2014, Németh et al., 2016, Sitaru et al., 2005). Serum anti-C7 levels were determined by ELISA using His-C7. For histological analysis, mice were killed on day 8 (when no open wounding had occurred), and their ears were fixed in paraformaldehyde, dehydrated, embedded in paraffin, sectioned at 9 μm thickness, and stained with hematoxylin and eosin.
In vivo analysis of neutrophil accumulation
WT and Syk-deficient bone marrow chimeras were injected with control or pathogenic IgG. On days 8 through 10, mice were killed, and their ears were removed. The samples were digested with Liberase II (Roche, Basel, Switzerland) (Weber et al., 2015). Single-cell suspensions were obtained, and neutrophil numbers were determined by flow cytometry on the basis of their forward and side scatter characteristics and Ly6G-positivity (clone 1A8, BD Biosciences, San Jose, CA).
In vivo neutrophil migration
A competitive migration assay in mixed bone marrow chimeras was used to assess in vivo migration of neutrophils (Jakus et al., 2009, Kovács et al., 2014, Mócsai et al., 2002). WT (C57BL/6) or Syk–/– bone marrow cells (carrying the CD45.2 allele) were mixed at varying ratios with bone marrow cells from congenic mice expressing CD45.1 on the C57BL/6 genetic background. The cell suspension was injected intravenously into lethally irradiated CD45.1-expressing recipient mice. After bone marrow repopulation, the chimeras were subjected to experimental epidermolysis bullosa acquisita, and their ears were digested (before any open wounds occurred). The percentage of CD45.2-expressing neutrophils was analyzed by flow cytometry with staining for Ly6G and CD45.2 (clone 104, BD Biosciences).
Relative migration of the CD45.2-positive neutrophils (relative to the CD45.1-expressing WT cells) was calculated as described (Jakus et al., 2009).
Isolation and activation of neutrophils
Mouse neutrophils were isolated from the bone marrow by Percoll (GE Healthcare, Chicago, IL) gradient centrifugation (Mócsai et al., 2003). Neutrophil assays were performed at 37 °C in HBSS (GE Healthcare, Little Chalfont, UK) supplemented with 20 mmol/L HEPES, pH 7.4 or in DMEM (Sigma-Aldrich). Cell surface molecule expression was detected by anti-Ly6G-phycoerythrin (clone 1A8), anti-CD18-biotin (clone C71/16), anti-CXCR2-phycoerythrin (clone #242216, R&D Systems, Minneapolis, MN), anti-FcγRII/III (clone 2.4G2) and anti-FcγRIV-phycoerythrin (clone 9E9) antibodies. Where required, secondary staining with Streptavidin-phycoerythrin or mouse anti-rat–FITC (clone MRK-1) was performed. If not otherwise stated, the antibodies were from BD Biosciences.
Immobilized immune complex-coated surfaces were obtained by binding His-C7 at 20 μg/ml to Nunc MaxiSorp F96 plates (Thermo Fisher) or tissue culture dishes, blocked, and then treated with anti-C7 at a 1:10 dilution, otherwise as described (Jakus et al., 2008). HSA and anti-HSA immune complexes were generated as described (Jakus et al., 2008).
For the analysis of mediator release, neutrophils were stimulated for 1 (LTB4) or 6 (cytokines) hours, and supernatants were collected for further analysis (Kovács et al., 2014, Németh et al., 2016). Superoxide release was followed by a cytochrome c reduction test (Németh et al., 2010).
Isolation of other cell types
Platelets were isolated from peripheral blood by mild centrifugation in the presence of heparin. Mast cells were cultured from the bone marrow by murine IL-3 and stem cell factor (both from PeproTech, Rocky Hill, NJ). Macrophages were obtained by culturing bone marrow cells in the presence of recombinant murine macrophage colony-stimulating factor from a conditioned medium (Kertész et al., 2012).
Analysis of inflammatory mediators
The removed ears were either crushed under liquid nitrogen and lysed by a Triton-based buffer or were cut into small pieces and were digested. The release of mediators was tested from the ear lysates, the cell-free supernatants of digested samples, or of in vitro stimulated neutrophils. The levels of CXCL2, IL-1β, and LTB4 were tested by commercial ELISA kits (R&D Systems).
Biochemical studies
For analysis of Syk phosphorylation, neutrophils were plated on immune complex-covered 6-cm tissue culture dishes, incubated for 10 minutes at 37 °C, and lysed on ice (adherent and nonadherent cells combined) (Kovács et al., 2014). Immunoprecipitation was performed by an anti-Syk antibody (5F5, BioLegend, San Diego, CA) followed by capturing with Protein G-Agarose (Invitrogen, Waltham, MA) (Mócsai et al., 2000, Mócsai et al., 2004, Mócsai et al., 2006, Németh et al., 2016). Whole-cell lysates from the same experiments were used as controls.
Samples were run on SDS-PAGE and immunoblotted using antibodies against phosphotyrosine (clone 4G10, Merck Millipore, Billerica, MA), Syk (N19, Santa Cruz Biotechnology, Dallas, TX), or β-actin (clone AC-74, Sigma-Aldrich) followed by incubation with peroxidase-labeled secondary antibodies (GE Healthcare). The signal was developed using the enhanced chemiluminescence system (GE Healthcare) and exposed to X-ray films.
Presentation of the data and statistical analysis
Experiments were performed the indicated number of times. Quantitative graphs and kinetic curves show mean and standard error of the mean from all independent in vitro experiments or from all individual mice from the indicated number of experiments. Statistical analyses were carried out using one- or two-way (factorial) analysis of variance (with treatment and genotype being the two independent variables). Area under the curve was used for statistical analysis in kinetic measurements. P-values below 0.05 were considered statistically significant.
ORCIDs
Tamás Németh: http://orcid.org/0000-0001-6854-4301
Cassian Sitaru: http://orcid.org/0000-0002-0370-1270
Attila Mócsai: http://orcid.org/0000-0002-0512-1157
Conflict of Interest
The authors state no conflict of interest.
Acknowledgments
We thank Edina Simon for expert technical assistance; Kinga Csorba, Levente Kiss-Pápai, and Krisztina Futosi for help with experiments; Kitti Ajtay and Zoltán Jakus for the histological images; Gábor Bánhegyi for access to equipment; and Clifford Lowell and Victor Tybulewicz for transgenic animals. This work was supported by the European Union’s FP7 Cooperation Program (Project No. 282095 [TARKINAID] to AM and CS), the Lendület program of the Hungarian Academy of Sciences (LP2013-66/2013 to AM), the Deutsche Forschungsgemeinschaft (SI-1281/5-1 to CS), and the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (to TN). AM was a recipient of a Wellcome Trust International Senior Research Fellowship (grant no. 087782).
Author Contributions
TN and AM conceived the study, designed the experiments, analyzed and interpreted the data, and wrote the manuscript. TN performed the majority of the experiments. OV and CS purified the anti-C7 antibodies, performed some of the experiments, and provided further experimental tools and scientific advice. AM supervised the project.
accepted manuscript published online 30 May 2017
Footnotes
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.doi.org/10.1016/j.jid.2017.05.017.
Supplementary Material
References
- Abtahian F., Guerriero A., Sebzda E., Lu M.M., Zhou R., Mócsai A. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiadis T., Deacon S.W., Devarajan K., Ma H., Peterson J.R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1039–1045. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S., Sakka N., Artsi O., Trau H., Barzilai A. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482–489. doi: 10.1016/j.autrev.2014.01.047. [DOI] [PubMed] [Google Scholar]
- Berton G., Mócsai A., Lowell C.A. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Chiriac M.T., Roesler J., Sindrilaru A., Scharffetter-Kochanek K., Zillikens D., Sitaru C. NADPH oxidase is required for neutrophil-dependent autoantibody-induced tissue damage. J Pathol. 2007;212:56–65. doi: 10.1002/path.2157. [DOI] [PubMed] [Google Scholar]
- Csorba K., Sesarman A., Oswald E., Feldrihan V., Fritsch A., Hashimoto T. Cross-reactivity of autoantibodies from patients with epidermolysis bullosa acquisita with murine collagen VII. Cell Mol Life Sci. 2010;67:1343–1351. doi: 10.1007/s00018-009-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott E.R., Van Ziffle J.A., Scapini P., Sullivan B.M., Locksley R.M., Lowell C.A. Deletion of Syk in neutrophils prevents immune complex arthritis. J Immunol. 2011;187:4319–4330. doi: 10.4049/jimmunol.1100341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futosi K., Fodor S., Mócsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17:638–650. doi: 10.1016/j.intimp.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futosi K., Mócsai A. Tyrosine kinase signaling pathways in neutrophils. Immunol Rev. 2016;273:121–139. doi: 10.1111/imr.12455. [DOI] [PubMed] [Google Scholar]
- Geahlen R.L. Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends Pharmacol Sci. 2014;35:414–422. doi: 10.1016/j.tips.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese M.C., van der Heijde D.M., Keystone E.C., Spindler A.J., Benhamou C., Kavanaugh A. A phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study of 2 dosing regimens of fostamatinib in patients with rheumatoid arthritis with an inadequate response to a tumor necrosis factor-α antagonist. J Rheumatol. 2014;41:2120–2128. doi: 10.3899/jrheum.140238. [DOI] [PubMed] [Google Scholar]
- Gross O., Poeck H., Bscheider M., Dostert C., Hannesschlager N., Endres S. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- Iwata H., Bieber K., Hirose M., Ludwig R.J. Animal models to investigate pathomechanisms and evaluate novel treatments for autoimmune bullous dermatoses. Curr Pharm Des. 2015;21:2422–2439. doi: 10.2174/1381612821666150316122502. [DOI] [PubMed] [Google Scholar]
- Jakus Z., Németh T., Verbeek J.S., Mócsai A. Critical but overlapping role of FcγRIII and FcγRIV in activation of murine neutrophils by immobilized immune complexes. J Immunol. 2008;180:618–629. doi: 10.4049/jimmunol.180.1.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus Z., Simon E., Balázs B., Mócsai A. Genetic deficiency of Syk protects mice from autoantibody-induced arthritis. Arthritis Rheum. 2010;62:1899–1910. doi: 10.1002/art.27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus Z., Simon E., Frommhold D., Sperandio M., Mócsai A. Critical role of phospholipase Cγ2 in integrin and Fc receptor-mediated neutrophil functions and the effector phase of autoimmune arthritis. J Exp Med. 2009;206:577–593. doi: 10.1084/jem.20081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkiewicz M., Nimmerjahn F., Wende S., Hirose M., Iwata H., Jonkman M.F. Genetic identification and functional validation of FcγRIV as key molecule in autoantibody-induced tissue injury. J Pathol. 2012;228:8–19. doi: 10.1002/path.4023. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz M., Schmidt E. Current treatment of autoimmune blistering diseases. Curr Drug Discov Technol. 2009;6:270–280. doi: 10.2174/157016309789869065. [DOI] [PubMed] [Google Scholar]
- Kertész Z., Győri D., Körmendi S., Fekete T., Kis-Tóth K., Jakus Z. Phospholipase Cγ2 is required for basal but not oestrogen deficiency-induced bone resorption. Eur J Clin Invest. 2012;42:49–60. doi: 10.1111/j.1365-2362.2011.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Kim S.C. Epidermolysis bullosa acquisita. J Eur Acad Dermatol Venereol. 2013;27:1204–1213. doi: 10.1111/jdv.12096. [DOI] [PubMed] [Google Scholar]
- Kovács M., Németh T., Jakus Z., Sitaru C., Simon E., Futosi K. The Src family kinases Hck, Fgr, and Lyn are critical for the generation of the in vivo inflammatory environment without a direct role in leukocyte recruitment. J Exp Med. 2014;211:1993–2011. doi: 10.1084/jem.20132496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S., Sitaru C., Jakus Z., Anderson K.E., Damoulakis G., Davidson K. PI3Kβ plays a critical role in neutrophil activation by immune complexes. Sci Signal. 2011;4:ra23. doi: 10.1126/scisignal.2001617. [DOI] [PubMed] [Google Scholar]
- Liu Z., Giudice G.J., Swartz S.J., Fairley J.A., Till G.O., Troy J.L. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95:1539–1544. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Giudice G.J., Zhou X., Swartz S.J., Troy J.L., Fairley J.A. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100:1256–1263. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R.J. Model systems duplicating epidermolysis bullosa acquisita: a methodological review. Autoimmunity. 2012;45:102–110. doi: 10.3109/08916934.2011.606451. [DOI] [PubMed] [Google Scholar]
- Ludwig R.J., Zillikens D. Pathogenesis of epidermolysis bullosa acquisita. Dermatol Clin. 2011;29:493–501. doi: 10.1016/j.det.2011.03.003. xi. [DOI] [PubMed] [Google Scholar]
- Meng F., Lowell C.A. A β1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 1998;17:4391–4403. doi: 10.1093/emboj/17.15.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihai S., Chiriac M.T., Takahashi K., Thurman J.M., Holers V.M., Zillikens D. The alternative pathway of complement activation is critical for blister induction in experimental epidermolysis bullosa acquisita. J Immunol. 2007;178:6514–6521. doi: 10.4049/jimmunol.178.10.6514. [DOI] [PubMed] [Google Scholar]
- Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A., Abram C.L., Jakus Z., Hu Y., Lanier L.L., Lowell C.A. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A., Humphrey M.B., Van Ziffle J.A., Hu Y., Burghardt A., Spusta S.C. The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A., Jakus Z., Vántus T., Berton G., Lowell C.A., Ligeti E. Kinase pathways in chemoattractant-induced degranulation of neutrophils: the role of p38 mitogen-activated protein kinase activated by Src family kinases. J Immunol. 2000;164 doi: 10.4049/jimmunol.164.8.4321. 432–31. [DOI] [PubMed] [Google Scholar]
- Mócsai A., Ruland J., Tybulewicz V.L. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mócsai A., Zhang H., Jakus Z., Kitaura J., Kawakami T., Lowell C.A. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells. Blood. 2003;101:4155–4163. doi: 10.1182/blood-2002-07-2346. [DOI] [PubMed] [Google Scholar]
- Mócsai A., Zhou M., Meng F., Tybulewicz V.L., Lowell C.A. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- Németh T., Futosi K., Hably C., Brouns M.R., Jakob S.M., Kovács M. Neutrophil functions and autoimmune arthritis in the absence of p190RhoGAP: Generation and analysis of a novel null mutation in mice. J Immunol. 2010;185:3064–3075. doi: 10.4049/jimmunol.0904163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh T., Futosi K., Sitaru C., Ruland J., Mócsai A. Neutrophil-specific deletion of the CARD9 gene expression regulator suppresses autoantibody-induced inflammation in vivo. Nat Commun. 2016;7:11004. doi: 10.1038/ncomms11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh T., Mócsai A. The role of neutrophils in autoimmune diseases. Immunol Lett. 2012;143:9–19. doi: 10.1016/j.imlet.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Németh T., Mócsai A. Feedback amplification of neutrophil function. Trends Immunol. 2016;37:412–424. doi: 10.1016/j.it.2016.04.002. [DOI] [PubMed] [Google Scholar]
- Norman P. Spleen tyrosine kinase inhibitors: a review of the patent literature 2010–2013. Expert Opin Ther Pat. 2014;24:573–595. doi: 10.1517/13543776.2014.890184. [DOI] [PubMed] [Google Scholar]
- Rolf M.G., Curwen J.O., Veldman-Jones M., Eberlein C., Wang J., Harmer A. In vitro pharmacological profiling of R406 identifies molecular targets underlying the clinical effects of fostamatinib. Pharmacol Res Perspect. 2015;3:e00175. doi: 10.1002/prp2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- Schulze F.S., Beckmann T., Nimmerjahn F., Ishiko A., Collin M., Kohl J. Fcγ receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid. Am J Pathol. 2014;184:2185–2196. doi: 10.1016/j.ajpath.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Sitaru C. Experimental models of epidermolysis bullosa acquisita. Exp Dermatol. 2007;16:520–531. doi: 10.1111/j.1600-0625.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- Sitaru C., Kromminga A., Hashimoto T., Brocker E.B., Zillikens D. Autoantibodies to type VII collagen mediate Fcγ-dependent neutrophil activation and induce dermal-epidermal separation in cryosections of human skin. Am J Pathol. 2002;161:301–311. doi: 10.1016/s0002-9440(10)64182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaru C., Mihai S., Otto C., Chiriac M.T., Hausser I., Dotterweich B. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest. 2005;115:870–878. doi: 10.1172/JCI21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio C., Micheletti A., Cassatella M.A. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcan I., Jonkman M.F. Blistering disease: insight from the hemidesmosome and other components of the dermal-epidermal junction. Cell Tissue Res. 2015;360:545–569. doi: 10.1007/s00441-014-2021-7. [DOI] [PubMed] [Google Scholar]
- Turner M., Mee P.J., Costello P.S., Williams O., Price A.A., Duddy L.P. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- Walker G., Croasdell G. The European League Against Rheumatism (EULAR)—17th Annual European Congress of Rheumatology (June 8-11, 2016–London, UK) Drugs Today (Barc) 2016;52:355–360. doi: 10.1358/dot.2016.52.6.2516435. [DOI] [PubMed] [Google Scholar]
- Weber F.C., Németh T., Csepregi J.Z., Dudeck A., Roers A., Ozsvári B. Neutrophils are required for both the sensitization and elicitation phase of contact hypersensitivity. J Exp Med. 2015;212:15–22. doi: 10.1084/jem.20130062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt M.E., Genovese M.C., Ho M., Hollis S., Rosiak-Jedrychowicz K., Kavanaugh A. Effects of fostamatinib, an oral spleen tyrosine kinase inhibitor, in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheumatol. 2014;66:3255–3264. doi: 10.1002/art.38851. [DOI] [PubMed] [Google Scholar]
- Weinblatt M.E., Kavanaugh A., Genovese M.C., Musser T.K., Grossbard E.B., Magilavy D.B. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N Engl J Med. 2010;363:1303–1312. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- Zhao M., Trimbeger M.E., Li N., Diaz L.A., Shapiro S.D., Liu Z. Role of FcRs in animal model of autoimmune bullous pemphigoid. J Immunol. 2006;177:3398–3405. doi: 10.4049/jimmunol.177.5.3398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.