Abstract
Soybean oil consumption is increasing worldwide and parallels a rise in obesity. Rich in unsaturated fats, especially linoleic acid, soybean oil is assumed to be healthy, and yet it induces obesity, diabetes, insulin resistance, and fatty liver in mice. Here, we show that the genetically modified soybean oil Plenish, which came on the U.S. market in 2014 and is low in linoleic acid, induces less obesity than conventional soybean oil in C57BL/6 male mice. Proteomic analysis of the liver reveals global differences in hepatic proteins when comparing diets rich in the two soybean oils, coconut oil, and a low-fat diet. Metabolomic analysis of the liver and plasma shows a positive correlation between obesity and hepatic C18 oxylipin metabolites of omega-6 (ω6) and omega-3 (ω3) fatty acids (linoleic and α-linolenic acid, respectively) in the cytochrome P450/soluble epoxide hydrolase pathway. While Plenish induced less insulin resistance than conventional soybean oil, it resulted in hepatomegaly and liver dysfunction as did olive oil, which has a similar fatty acid composition. These results implicate a new class of compounds in diet-induced obesity–C18 epoxide and diol oxylipins.
Introduction
While humans have been cultivating soybeans for ~5000 years1, soybean oil has become a substantial part of our diet only in the last few decades2. This increase in soybean oil consumption is due in part to a reaction to large-scale population studies in the 1950s and 60s, which showed that a typical American diet rich in saturated fats from animal products was linked to an increased risk of cardiovascular disease3,4. It was subsequently assumed that most if not all saturated fats are unhealthy and conversely that all unsaturated fats are healthy, this despite the ambiguity of evidence of cardio-protective effects of vegetable oils, which are rich in unsaturated fats5,6. Similarly, it was assumed that whatever is healthy for the heart is also healthy for the rest of the body although this assumption was never rigorously tested7,8. Nonetheless, vegetable oil, and, in particular, soybean oil, began to replace animal fat in the American diet starting in the 1970s, resulting in an exponential rise in soybean oil consumption that parallels the increase in obesity in the U.S. and worldwide2,9,10. Indeed, soybean oil is the component in the American diet that has increased the most in the last 100 years2. It constitutes >60% of all edible vegetable oil consumption in the U.S11. and is ubiquitous in the American diet, especially in cooking oil and processed foods.
Soybean oil is comprised of primarily polyunsaturated fatty acids (PUFAs), particularly linoleic acid (LA, C18:2), an omega-6 (ω6) fatty acid that makes up ~55% of soybean oil. Omega-3 (ω3) fatty acids, especially those found in fish oil, and their ratio to ω6 fatty acids have also received considerable attention. Numerous studies have shown that high ω3:ω6 (and hence low ω6:ω3) ratios are generally healthful12. However, like saturated and unsaturated fats, a distinction between different types of ω3 and ω6 fatty acids is often not made, even though this could be relevant to their metabolic effects.
While most experimental diet-induced obesity studies use high fat diets composed of lard or milk fat (rich in saturated fats), a few recent studies (including one from our group) have examined the effects of a diet rich in soybean oil and found that this vegetable oil does in fact increase adiposity, diabetes, insulin resistance and fatty liver9,13–15. Furthermore, soybean oil induces more metabolic effects than an isocaloric diet made from coconut oil13, which is nearly all saturated fats, albeit of shorter chain length than those in animal fat.
One study proposed, but did not formally prove, that linoleic acid (LA) drives the metabolic effects of soybean, and other vegetable oils16. To investigate the role of LA in soybean oil-induced metabolic disease, we compared conventional soybean oil to a new genetically modified (GM) soybean oil (Plenish) which was engineered to generate fewer trans-fats by blocking the desaturase gene FAD2-1 which converts oleic acid (C18:1) to LA17 (Supplementary Fig. S1a). The net result is an oil low in LA and high in oleic acid, similar to that of olive oil (Supplementary Fig. S1b), which, as a component of the Mediterranean diet, is considered to be healthful18,19. Our results show that the GM oil Plenish does indeed induce less obesity and insulin resistance than conventional soybean oil, although not less diabetes or fatty liver. Plenish also induced hepatomegaly and liver dysfunction, as does olive oil. Importantly, extensive metabolomic and proteomic analyses indicate that oxylipin metabolites of LA and α-linolenic acid (ALA, C18:3ω3) correlate positively with obesity.
Results
Genetic modification of soybeans reduces the obesogenic effects of soybean oil
We designed a series of isocaloric, high fat diets with a total fat content similar to that of the American diet (40 kcal%)20 (Supplementary Table S1). The control high fat diet is comprised of coconut oil (CO), which is primarily saturated fat and naturally low in LA. The conventional soybean oil diet contains 50% CO and 50% SO (SO + CO) to yield ~10% LA, comparable to that in the current American diet2,20 while the PL + CO diet has only 1.4% LA. Normal lab chow (referred to as vivarium chow, Viv) was used as a low fat control and has 1.2% LA. For comparison, the American diet had ~2% LA in the early 1900s2.
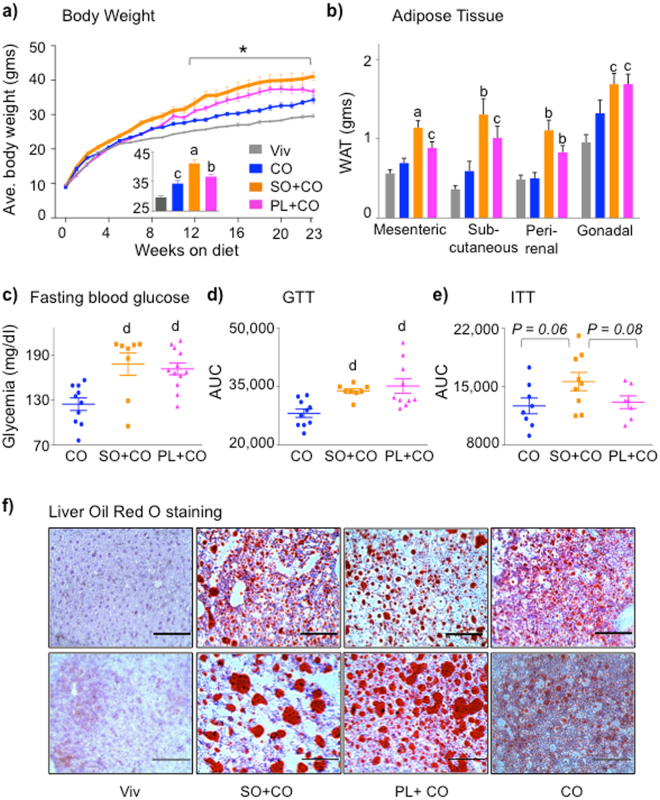
As we observed previously13, starting at ~8 weeks on the diet, SO + CO induced significantly greater weight gain than CO in C57BL/6 N male mice, primarily due to increased adipose tissue (Fig. 1a,b) and despite the fact that the two groups of mice had a similar caloric intake (Supplementary Fig. S1c). Importantly, PL + CO caused significantly less weight gain and less adiposity than did SO + CO, although still more than CO (Fig. 1a,b). Both SO + CO and PL + CO induced elevated fasting blood glucose levels and glucose intolerance (Fig. 1c,d and Supplementary Fig. S1d) but only SO + CO increased insulin resistance to near significant levels (P = 0.06) (Fig. 1e Supplementary Fig. S1e). An SO only diet also yielded significantly higher insulin resistance (see Fig. 6d).
Figure 1.
Plenish induces less obesity and insulin resistance than conventional soybean oil, but similar levels of diabetes and hepatic steatosis. (a) Average weekly body weight of male C57BL/6 N mice on Vivarium chow (Viv) and 40 kcal% high fat diets: CO, coconut oil; SO + CO, conventional soybean oil-enriched; PL + CO, Plenish oil-enriched. Inset, average weight after 23 weeks on diet. N = 12 per group except for Viv (N = 23). *All diets are significantly different from each other, asignificantly greater than all others, bthan Viv and CO, cthan Viv. (b) Average weight of white adipose tissues. N = 11–12. aSignificantly greater than all others, bthan Viv and CO, cthan Viv. (c) Fasting blood glucose. (d) GTT area under the curve (AUC) of mice on diets for 22 weeks. N = 7–12. dSignificantly greater than CO. (e) ITT AUC of mice on diets for 20 weeks. N = 8-9. (c–e) See Fig. 6d for Viv values: CO is not significantly different from Viv. ITT AUC: SO + CO vs. Viv (P < 0.05). (f) Representative Oil Red O staining of livers from mice on the diets for 24 weeks. Scale bar is 100 microns. Each section is from one of 4-6 mice per group. (See Supplementary Fig. S2 for additional stains.) Data are presented as ± SEM (a–e).
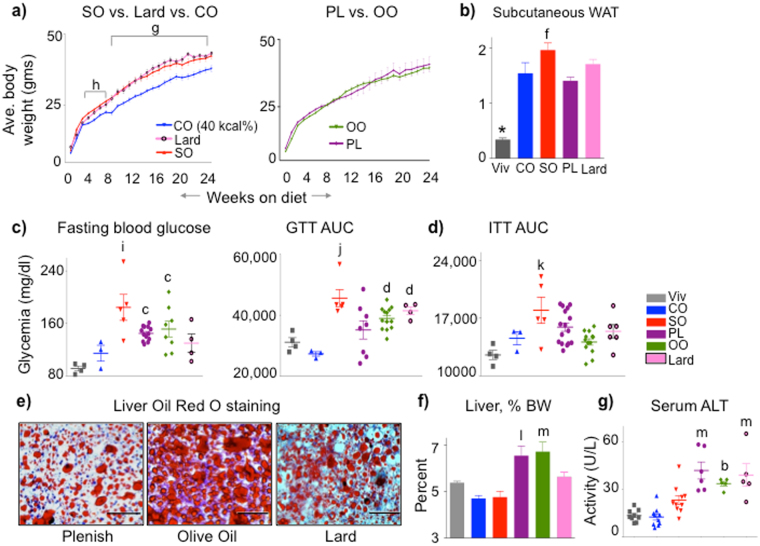
Figure 6.
Plenish induces similar metabolic effects as olive oil; conventional soybean oil is similar to lard. (a) Average weekly body weights of C57BL/6 N male mice started on the indicated diets at weaning. High fat diets (35 kcal%) with a single fat source: SO, soybean oil only; PL, Plenish oil only; OO, olive oil only. CO (40 kcal%) is as in Fig. 1. N = 7–16. gCO significantly different from SO and lard, or hfrom SO. (b) Average mass of subcutaneous (flank) fat pads from mice on diets for 24 weeks. *Significantly different from all others, ffrom PL. (c) Fasting blood glucose (18–20 weeks). N = 10–13. iSignificantly greater than Viv, CO and lard, cthan Viv. AUC, area under the curve of a GTT assay (18-20 weeks). N = 4–13. jSignificantly greater than CO, PL and Viv or dthan CO. (d) AUC of ITT (18 weeks). N = 5-12 except CO (N = 3) and Viv (N = 4). kSignificantly greater than Viv and OO. (e) Representative Oil Red O staining of livers. Scale bar is 100 microns. N = 4–6 per group. (See Supplementary Fig. S6e for SO and Supplementary Fig. S2 for additional sections) (f) Liver weight as percent body weight. N = 10–13. (See Supplementary Fig. S6g for absolute liver body weight) (g) Serum ALT activity. N = 5–10. For (f) and (g), lsignificantly greater than all others except OO, mthan SO, CO and Viv or bthan Viv and CO. All data are presented as ± SEM.
Since between 30 and 40% of adult Americans have non alcoholic fatty liver disease (NAFLD)21, and since fatty liver is a common co-morbidity with obesity, diabetes and insulin resistance, we stained the livers with Oil Red O. PL + CO generated the same striking phenotype of large lipid droplets and hepatocyte ballooning observed previously with SO + CO (Fig. 1f)13. In contrast, coconut oil resulted in a less severe fatty liver phenotype (Fig. 1f)13: the size and number of lipid droplets were less than in the SO + CO and PL + CO livers.
Metabolomic analysis reveals a potential role for oxylipins in obesity
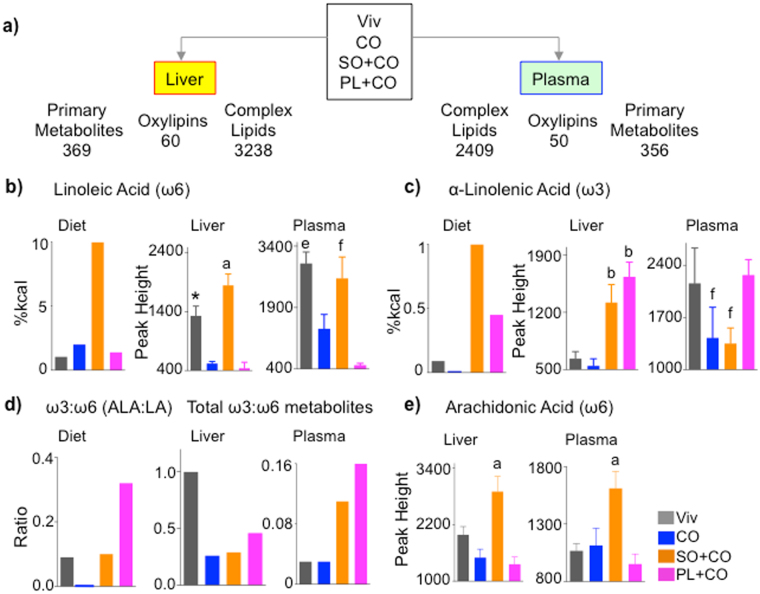
To investigate the mechanism by which soybean oil induces its metabolic effects, we performed metabolomics on the liver and plasma of mice fed Viv, CO, SO + CO and PL + CO diets for 24 weeks using three different platforms –primary metabolites, complex lipids and oxylipins (oxidative metabolites of PUFAs22,23) (Fig. 2a). We identified 369 primary metabolites in the liver, of which 55 to 75 differed between any two of the high fat diets; 60 oxylipins of which 35 to 49 differed; and 3,238 complex lipids of which ~1,000 to ~1,800 differed. Similar global differences were found in the plasma (Supplementary Fig. S1f and Table S2).
Figure 2.
Metabolomic analysis reveals variations in fatty acid accumulation in liver and plasma between conventional soybean oil and Plenish. (a) Schematic of total number of metabolites identified by the various platforms in liver and plasma of mice fed the indicated diets. (b,c,e) Levels of the indicated fatty acids in the diets and liver and plasma of mice fed the respective diets. N = 7–8. (d) Ratio of ω3:ω6 fatty acids in diet (ALA:LA) and total ω3:ω6 oxylipins in liver and plasma. *Significantly different from all others. aSignificantly different than all others, bthan CO and Viv, ethan CO and PL + CO, fthan PL + CO. Data are presented as ± SEM, except for graphs showing levels or ratios in diets.
While LA, not surprisingly, was highest in the SO + CO livers, the Viv-fed livers unexpectedly had LA levels that were nearly as high as SO + CO; a similar profile was found in the plasma (Fig. 2b). This could be due to the fact that LA, as an essential fatty acid, is preferentially retained in the body. In contrast, both the CO and PL + CO diets resulted in much lower levels of LA compared to Viv, suggesting that coconut oil may actively impede the accumulation of LA (Fig. 2b).
The other essential fatty acid, ALA, was also highest in the SO + CO diet but its profile differed from that of LA in the liver and plasma. The PL + CO liver accumulated as much ALA as SO + CO livers and the PL + CO plasma had significantly more ALA than SO + CO (Fig. 2c). The PL + CO diet had the highest ω3:ω6 ratio (ALA:LA), a ratio that was maintained in the plasma for total ω3 and ω6 metabolites but reduced in the liver in which Viv chow had the highest ratio (Fig. 2d). Arachidonic acid (AA, C20:4ω6), which is derived from LA and associated with inflammation that often accompanies obesity24, was also highest in SO + CO liver and plasma (Fig. 2e). As anticipated, oleic acid was highest in PL + CO liver and plasma and saturated fatty acids abundant in coconut oil–myristic (C14:0) and lauric (C12:0) – were highest in the CO-fed plasma and liver (Supplementary Fig. S3a,c,d). The saturated fat palmitic acid (C16:0) did not vary significantly among any of the diets nor in the plasma, although it was significantly elevated in the SO + CO liver (Supplementary Fig. S3b).
Spearman’s rank correlation coefficient for all annotated complex lipids, primary metabolites, and oxylipins in the livers of mice fed CO, SO + CO or PL + CO revealed 45 primary metabolites (including 14 lipids), 12 complex lipid classes and 16 oxylipins that correlated significantly (P < 0.05) with individual values for body weight and total adipose tissue from each mouse (Supplementary Fig. S4a). In contrast, plasma had only half the number of significant correlations compared to the liver (Supplementary Fig. S4b). While the primary metabolites LA and AA correlated positively with body weight and adipose tissue in both liver and plasma, the saturated fats lauric and myristic acid negatively correlated only in the liver (Supplementary Fig. S4a,b). Various complex lipids (e.g., tri- and di-acylglycerides, phosphatidylcholines, and acylcarnitines) correlated either positively or negatively with body/adipose weight in liver and/or plasma. In contrast, oxylipins were the only class of metabolites to show exclusively positive correlations with body and adipose weight in both liver and plasma, with only one exception in the plasma (Supplementary Fig. S4a,b and Table S2).
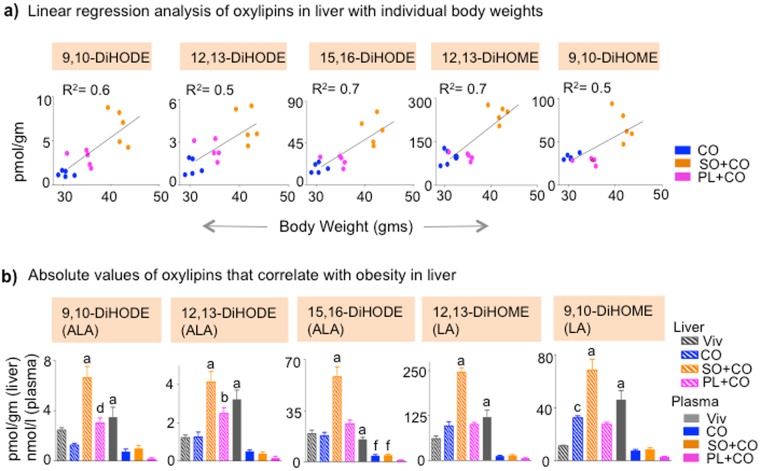
Interestingly, nearly all of these oxylipins are generated by the cytochrome P450 (CYP)/soluble epoxide hydrolase (sEH) pathway. A linear regression analysis showed that among all the oxylipins that significantly correlated in CO, SO + CO and PL + CO in Supplementary Fig. S4a,b, there were only four in the liver that had a significant R2 (R2 ≥ 0.5) (9,10-DiHODE, 12,13-DiHODE, 15,16-DiHODE and 12,13-DiHOME); all four also had a significant Spearman’s coefficient (r ≥ 0.6) (Fig. 3a). A fifth oxylipin (9,10-DiHOME) that missed the P-value cut-off in Supplementary Fig. S4a,b also showed a significant R2 and Spearman’s coefficient (Fig. 3a) The significance of the linear regression and correlation was maintained or increased when the Viv diet was included (Supplementary Fig. S4c). Interestingly, these five oxylipins are all derived from LA (DiHOMEs) or ALA (DiHODEs) (Supplementary Fig. S5) and were highest in the SO + CO livers (Fig. 3b). Furthermore, their absolute levels were lower in plasma, where they did not correlate significantly with obesity (Fig. 3b).
Figure 3.
Liver oxylipins correlate with soybean oil-induced obesity. (a) Correlation between body weight and concentration of liver oxylipins of individual mice. Spearman correlation coefficient (r) is 0.8 for 9,10-DiHODE (P = 0.0007), 0.8 for 12,13-DiHODE (P = 0.0009), 15,16-DiHODE (P = 0.0009), 0.6 for 12,13-DiHOME (P = 0.02) and 0.5 for 9,10-DiHOME (P = 0.06). Goodness of fit or R2 values for linear regression are indicated on the graphs. (The Viv group was not included in the correlation analyses in Supplementary Fig. S4a,b,c). (b) Absolute levels of oxylipins that correlate only in liver (hatched bars). Values in plasma (solid bars) are shown as a comparison. N = 4–5 mice per group. aSignificantly different (within same tissue) from all others, bfrom CO and Viv, cfrom Viv, dfrom CO, ffrom PL + CO. The fatty acid from which the oxylipin was derived is shown in parentheses. Data are presented as ± SEM.
Another 14 oxylipins had a P < 0.05 in the liver or plasma but they did not have a significant R2 (Supplementary Fig. S4c–e). They were all higher in liver than plasma and are a mix of metabolites from AA, ALA, eicosapentaenoic acid (EPA, C20:5ω3) and docosahexaenoic acid (DHA, C22:6ω3): EPA and DHA are both derived from ALA. Among these metabolites was 8,9 EpETrE (from AA), the only oxylipin with a negative correlation with body weight (in plasma), consistent with a previous study25.
Since the oxylipins of LA and ALA showed a significant, positive correlation with body weight in the liver, we also calculated the linear regression for LA, ALA, and other fatty acids (AA, DHA, oleic acid, palmitic acid, myritstic acid, lauric acid). LA, AA and DHA, which were all elevated in SO + CO livers (Fig. 2b,e and Supplementary Fig. S3e), were the only fatty acids that showed significant R2 values (Supplementary Fig. S4f). However, unlike the C18 diols for which the R2 values remained (or became more) significant when the Viv chow values were included, the LA, AA and DHA R2 values lost their significance (R2 ≤ 0.3) when the low-fat diet values were included (Supplementary Fig. S4g).
There were other primary metabolites that were statistically different between SO + CO and PL + CO but none correlated with obesity in the mega analysis (Supplementary Fig. S3e–g, Fig. S4a,b). The level of α-tocopherol, which is enriched in soybean oil, was not significantly different in the Plenish mice (Supplementary Fig. S3h). Taken together, the metabolomic data indicate that CYP/sEH oxylipin metabolites of LA and ALA in the liver (but not the plasma) were the only metabolites to consistently and significantly show a positive correlation with SO-induced obesity.
Integration of proteomic and metabolomics analysis converges on the CYP/sEH pathway
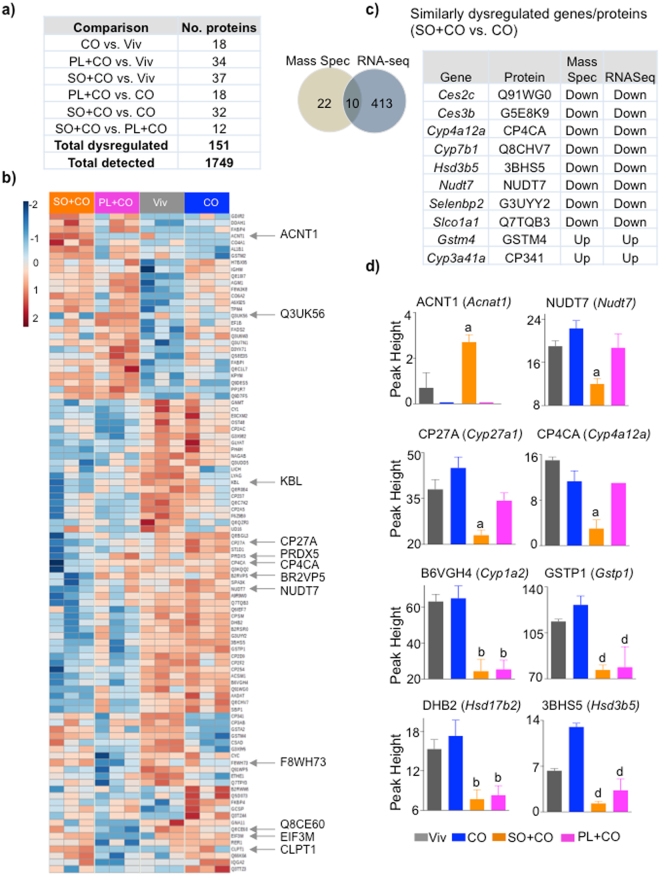
To elucidate the mechanism responsible for the changes in the liver metabolites, we performed proteomics on the livers of mice fed Viv, CO, SO + CO or PL + CO for 24 weeks. Out of 1,749 proteins detected, there were 151 proteins (8.6%) that were significantly dysregulated between any two of the diets (Fig. 4a,b). SO + CO had the greatest number of differences: 37 versus Viv and 32 versus CO as well as 12 proteins that differed between SO + CO and PL + CO, underscoring the effect that dietary oils, especially soybean oil, and even a single modification in a dietary oil (LA to oleic acid), can have on the liver proteome.
Figure 4.
Proteomic analysis of liver reveals changes induced by both conventional and GM soybean oil. (a) Number of significantly dysregulated proteins in the livers of C57BL/6 N male mice fed the indicated diets (P ≤ 0.05, Tukey’s post hoc test). (b) Heatmap showing liver proteins that are significantly different between any two diets. Arrows, significantly different proteins between SO + CO and PL + CO. N = 3 livers per group. (c) Left: Venn diagram showing overlap of dysregulated proteins between CO and SO + CO fed livers identified by mass spectrometry and RNA-Seq analysis13. Right: List of similarly dysregulated proteins in SO + CO versus CO livers. (d) Proteins that are different in SO + CO or PL + CO compared to any other diet. aSignificantly different from all others, bfrom CO and Viv, dfrom CO. P ≤ 0.05 by One-way ANOVA, Tukey’s post-hoc analysis. Data are presented as ± SEM.
Comparison of the proteomic data to our previous RNAseq data from the livers of mice fed SO + CO and CO for 35 weeks13 revealed 10 proteins with altered levels in SO + CO versus CO that also had altered mRNA levels. Additional proteins (22 total) that were altered in the proteomic but not the transcriptomic data suggest that non-transcriptional mechanisms may also be implicated (Fig. 4c). Notably, several CYP (1A2, 4A12A, 27 A) and lipid metabolizing enzymes (ACNT1, NUDT7, HSD3B5/17B2) were altered in the SO + CO and PL + CO diets (Fig. 4d). These alterations, as well as that of the Phase II enzyme GSTP1, indicate that different dietary oils lead not only to different fatty acid metabolites, but also to differences in metabolic and detoxification enzymes, which in turn could impact both the metabolomic and xenobiotic profile.
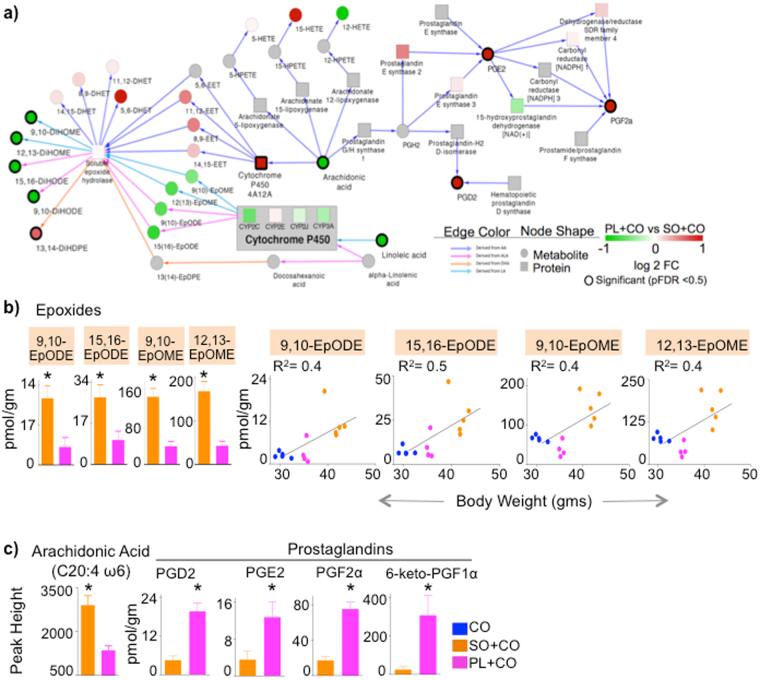
A network analysis generated by cross-referencing the liver SO + CO versus PL + CO proteome with their respective metabolomes revealed a modest, albeit insignificant, down regulation in Plenish of CYP2C and CYP3A families, which metabolize LA and ALA to EpOME and EpODE epoxides, respectively (Fig. 5a). Although the change in individual CYP enzymes did not reach significance, the combined effect was sufficient to decrease the C18 epoxide levels in PL + CO livers; this decrease reached significance once an outlier was removed (Fig. 5b left). Importantly, linear regression analysis showed a modest positive correlation of the C18 epoxides with body weight (Fig. 5b right, R2 > 0.4). The epoxides in turn are converted by sEH to C18 diols (Supplementary Fig. S5a,c), which strongly correlated with body weight (Fig. 3) and were significant in the network analysis (Fig. 5a). Although the C18 epoxides and diols were impacted by the diets, the sEH activity was largely unaffected as shown by the similar diol:epoxide ratios between PL + CO and SO + CO. Only the 9,10-DiHODE/EpODE ratio was statistically different in the liver (Supplementary Fig. S5b).
Figure 5.
Alterations in LA-, ALA- and AA-metabolizing enzymes in SO + CO versus PL + CO livers correlate with oxylipin and prostaglandin levels. (a) Integrated proteomic-metabolomic network analysis comparing PL + CO and SO + CO livers based on significant metabolites/proteins (black borders) (pFDR < 0.05) and their connecting nodes. Node colors: red, up in PL + CO; green, up in SO + CO; white, no change; gray, no data mapped to nodes. (b) Left, absolute levels of EPOMEs and EPODEs shown in (a) (one outlier mouse in the PL + CO group was removed). Right, Correlation between body weight and concentration of liver epoxides of individual mice. Spearman correlation coefficient (r) is 0.6 for 9,10-EpODE (P = 0.02) and 15,16-EpODE (P = 0.03), 0.5 for 12,13-EpOME (P = 0.07) and 9,10-EpOME (P = 0.08). Goodness of fit or R2 values for linear regression are indicated on the graphs. c) Levels of arachidonic acid (AA) and prostaglandins in liver. N = 4–5 mice per group. *Significantly different (P < 0.05). Data are presented as ± SEM (b and c).
CYP4A12A was significantly up regulated in PL + CO versus SO + CO livers (Figs 4d and 5a). It hydroxylates AA to epoxyeicosatrienoic acids (EpETrEs or EETs), which in turn are converted by EPHX2 to dihydroxytrienoic acids (DiHETrEs or DHETs)26. Both EETs and DHETs were elevated in PL + CO relative to SO + CO (Fig. 5a), albeit not significantly, and have been reported to have anti-obesogenic properties25.
Finally, several prostaglandins (PGD2, PGE2, PGF2α and 6-keto-PGF1α) were significantly elevated in PL + CO livers, whereas AA, from which they are derived, was higher in SO + CO livers (Fig. 5a,c). The increases could be explained by the modest (but not significant) increase in prostaglandin E synthase 2 (Ptges2) and dehydrogenase/reductase SDR family member 4 (Dhrs4) (Fig. 5a), demonstrating again that small changes in enzyme levels can have significant effects on metabolites.
Taken together, these results implicate both ω3 and ω6 hepatic C18 oxylipins (epoxides and diols) derived from the essential fatty acids ALA and LA in obesity induced by conventional soybean oil. In contrast, AA-derived prostaglandins do not correlate positively with obesity, but were elevated in Plenish livers.
Plenish induces similar effects to olive oil, including hepatomegaly and liver dysfunction
To rule out potential confounding effects of the coconut oil in the diets, we reformulated the diets to include only a single source of fat (just soybean oil or Plenish) and compared them to isocaloric diets made with olive oil or animal fat (lard) (35% kcal total fat) (Supplementary Table S1). The conventional soybean oil-only diet (SO) induced an identical weight gain and adiposity to lard while the Plenish-only diet (PL) was identical to olive oil (OO), despite comparable food intake (Fig. 6a,b and Supplementary Fig. S6a,b,c). The SO, PL and OO diets, but not lard or CO, all produced elevated fasting glucose levels with SO inducing the highest level (Fig. 6c left). SO, OO and lard induced glucose intolerance, with SO again having the largest effect (Fig. 6c right and Supplementary Fig. S6d). Interestingly, the conventional soybean oil diet was still the only one to induce insulin resistance (Fig. 6d and Supplementary Fig. S6e).
There were unanticipated effects on liver morphology and function: while all four diets induced fatty livers with large lipid droplets and hepatocyte ballooning (Fig. 6e, Supplementary Figs S2 and S6f), mice fed PL or OO but not CO, SO or lard, had excessive liver weights (Fig. 6f and Supplementary Fig. S6g). These mice, along with those on the lard diet, also had significantly reduced liver function, as determined by elevated levels of circulating alanine transaminase (ALT) (Fig. 6g). Taken together, these results indicate that the fatty liver phenotype does not always track with obesity, diabetes or insulin resistance. They also show that the genetic modification of soybean oil may induce detrimental health effects in terms of liver function even though it induces less obesity and insulin resistance than conventional soybean oil.
Discussion
This is the first report to compare the metabolic effects of conventional soybean oil to those of GM oil (Plenish) with low LA but high oleic acid. It is also the first study to compare the metabolomic and proteomic profiles induced by these oils high in unsaturated fats to those generated by an oil rich in saturated fatty acids (coconut oil). Out of >3,000 known compounds, the only class of metabolites that consistently correlated positively with obesity across all three high fat diets (CO, SO + CO, and PL + CO) were the oxylipins of both ω-6 LA and ω-3 ALA generated by the CYP/sEH pathway. The correlation was primarily in the liver, not the plasma, which could have clinical implications.
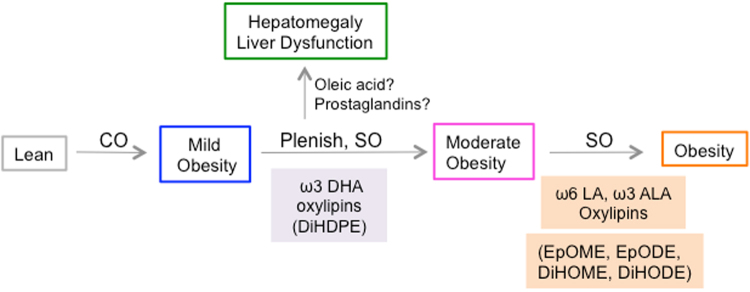
Based on these results, we propose a model for diet-induced obesity that is divided into three steps or stages that are modulated by the availability of different types of fatty acids and their metabolites (Fig. 7). In the first stage, coconut oil (CO) high in medium chain saturated fats induces mild obesity. This could be due simply to the greater number of calories in the CO diet compared to the Viv chow, since the saturated fats in CO did not correlate with body weight (Supplementary Fig. S3b–d and Fig. S4f). Importantly, mice on the high fat diet consisting of coconut oil alone do not progress beyond this first stage of metabolic disease even after long-term feeding (up to 35 weeks): they do not develop diabetes or insulin resistance, only moderately fatty liver13.
Figure 7.
Proposed model for the role of hepatic oxylipin metabolites in diet-induced obesity. CO, coconut oil; PL, Plenish (high oleic acid), low linoleic acid (LA); SO, conventional soybean oil (high LA). Prostaglandins, PGD2, PGE2, PGF2α, 6-keto-PGF1α. Oxylipin boxes are color-coded with other figures (see Supplementary Fig. S5c for overview); box outlines of phenotypes are color-coded with diets (Figs 1 and 6). See text for details.
In the second stage, mice fed the high soybean oil diets (either conventional or Plenish) developed more obesity. DiHDPE metabolites of ω3 DHA generated by the CYP/sEH pathway, which are significantly elevated in both SO + CO and PL + CO livers, may play a role in this second phase (Fig. 7, Supplementary Fig. S4c,d). However, they did not correlate with obesity across all the diets and if anything tended to be higher in Plenish than conventional soybean oil, raising the possibility that the DiHDPEs could also have a positive effect. Indeed, certain DiHDPEs are referred to as resolvins for their anti-inflammatory effects27.
The third stage correlates with different CYP/sEH metabolites. Oxylipins of ω3 ALA (9,10-, and 15,16-EpODE; 9,10-, 12,13- and 15,16-DiHODE) and ω6 LA (9,10- and 12,13-EpOME; 9,10- and 12,13-DiHOME) were all significantly increased in the livers of mice fed conventional soybean oil compared to Plenish and correlated positively with body weight across all three high fat diets (Figs 3, 5b and 7). Finally, Plenish and olive oil, both rich in oleic acid, caused liver dysfunction and hepatomegaly (Figs 6f,g and 7). We could find no relevant literature about olive oil affecting liver size or ALT levels and studies on olive oil and hepatic steatosis do not reach a consensus28. It is also possible that the elevated prostaglandin levels in the PL + CO liver play a role since prostaglandins have been shown to regulate hepatic growth either directly29 or indirectly via their interaction with peroxisome proliferator-activated receptors (PPARs)30 Fig. 5c and 7).
Another category of oxylipins generated by the 12/15 LOX pathway–metabolites of AA (LXA4 and 9-HETE) and ALA (9-HOTrE)–may also play a role, although they did not reach statistical significance between conventional and Plenish soybean oil, only between SO + CO and CO or Viv chow (Supplementary Fig. S4c). LXA4 and 9-HETE are both elevated in the plasma of humans with metabolic syndrome: 9-HETE was suggested as a causal factor in oxidative stress, while LXA4 is thought to be involved in the down regulation of inflammation31. Although we could find no reports of 9-HoTRE in metabolic syndrome, 13-HoTRE has been cited as an anti-inflammatory oxylipin32. The only non-enzymatic oxylipin detected was EKODE (12,13-epoxy-9-keto-10(trans)-octadecenoic acid), which was slightly higher in SO + CO versus PL + CO in the liver but not significantly different from CO (Supplementary Table S2).
Oxylipins in general, as bioactive signaling lipids, are increasingly being associated with inflammation, vascular permeability, and cardiovascular disease as well as diabetes, obesity-induced hypertriglyceridemia, and insulin signaling23,33–36. However, we found only two published reports on C18 diols and obesity. One report found a negative correlation between obesity and esterified LA/ALA-derived oxylipins37 while the other observed a positive correlation with non esterified (free) oxylipins38, which are the ones we analyzed since they are considered to be bioactive23,39. Many of the negative effects of epoxy derivatives of LA, such as cytotoxicity and inflammation, are actually attributed to their sEH metabolites such as 12,13-DiHOME40–45. EpODEs were also recently linked to the obese phenotype in humans31. In contrast, not much is known about the biological action of the ALA-derived DiHODEs although it has been reported that 9,10-DiHODE and 12,13-DiHODE concentrations are lower in serum of hyperlipidemic men compared to normolipidemic men46. Additional investigation of the role of the C18 oxylipins in obesity and other aspects of the metabolic syndrome – diabetes, insulin resistance, hepatocyte ballooning and large lipid droplets–is clearly warranted.
Our results also show that, just as not all saturated and unsaturated fats have the same effects, not all ω3 fatty acids may be healthful, since we found a positive correlation between ω3 (ALA) oxylipins and obesity. Furthermore, even though the proper balance of ω6:ω3 fatty acids in the diet is often emphasized12,47, we found that Plenish and olive oil have identical metabolic effects even though they have very different ω6:ω3 ratios (3.4 and 10.0, respectively) (Supplementary Table S1). Interestingly, all the oxylipins that correlated well with obesity are derived from fatty acids that must be obtained from the diet (LA and ALA), suggesting that a dietary overload of even essential fatty acids can have significant implications for health.
The vast majority of diet-induced obesity studies use lard as the source of fat and assume that they are looking at the effects of saturated fat, as well as cholesterol. In the U.S., lard comes from animals that are typically fed soybean meal48 and consequently the levels of LA in lard can be quite high (11% or higher)12,49. Hence, it is possible that some of the metabolic effects in the literature attributed to saturated fats in these lard-based studies could actually be due to high LA from soybean oil, as others have found with farmed salmon14. It will be of interest to determine whether the C18 oxylipins identified in this study are also elevated in the livers of animals fed conventional high fat diets based on animal fat. In terms of cholesterol, contrary to the widely held belief that PUFAs such as those in soybean oil lower plasma cholesterol levels, in our experiments neither the conventional nor the GM soybean oil ameliorated the increase in plasma cholesterol induced by coconut oil. (The increase in cholesterol with coconut oil has been reported previously50–52). Additionally, both soybean oils increased cholesteryl esters in the liver (Supplementary Fig. S3i,j), consistent with a number of recent reports debunking the putative cholesterol lowering effects of vegetable oils8,53.
In summary, we show that while the GM soybean oil Plenish induces less obesity and insulin resistance than conventional soybean oil in mice, it also produces negative effects on liver function, as does olive oil. Our results also implicate various ω6 as well as ω3 oxylipin metabolites of ALA and LA in obesity, although it remains to be determined if they act in a causal fashion.
Methods
Diets
Three isocaloric diets with 40 kcal% fat and four isocaloric diets with 35%kcal fat were formulated in conjunction with Research Diets, Inc. (New Brunswick, NJ) (Supplementary Table S1). Normal lab chow referred to as Vivarium diet (Viv) was included as a low-fat control. See Supplemental Experimental Procedures for more details on diet formulation.
Animals
Male C57BL/6 N mice (Charles River Laboratories) were weaned at three weeks of age and assigned randomly to one of the diets. The animals were maintained on a 12:12 h light-dark cycle in a specific pathogen free vivarium (SPF) for the 40 kcal% diet feeding experiment. The mice in the 35 kcal% experiment (Fig. 6 and Supplementary Fig. S6) and the mice used for the 24-week vivarium (Viv) chow liver oxylipin analysis were housed in a non-SPF facility. At least 12 mice were put on each diet with three to four animals per cage. Food intake was recorded twice a week on a per cage basis; individual mouse weights were recorded once a week (Supplementary Figs S1c and S6c).
Ethics Statement
Care and treatment of animals was in accord with guidelines from and approval by the University of California Riverside Institutional Animal Care and Use Committee (AUP#20140014). All mice had ad libitum access to food and water (other than the indicated fasting times). At the end of the study, mice were euthanized by carbon dioxide inhalation (before noon), in accordance with stated NIH guidelines.
Glucose and Insulin Tolerance Tests
Glucose tolerance (GTT) and insulin tolerance tests (ITT) were performed as described previously13.
Alanine Transaminase (ALT) Activity Test
Blood for the Alanine Transaminase (ALT) colorimetric assay to measure liver disease or injury54 was collected by cardiac puncture (without anti-coagulant) and allowed to clot at room temperature for 30 min, followed by centrifugation at 9,300 × g for 15 min at 4 °C. Serum was stored immediately at −80 °C. The assay and data analysis were done according to manufacturer’s instructions (Catalog#: 700260 Cayman Chemicals, Ann Arbor USA).
Tissue Samples and Staining
Liver was collected, stored and analyzed by Oil Red O staining as described previously13.
Metabolomic, Lipidomic, Oxylipin and Proteomic Analysis
For analysis of primary metabolites, 30 µL of plasma or 5 mg of liver tissue homogenate were extracted and derivatized; metabolite levels were quantified by chromatography time-of-flight (GC-TOF) mass spectrometry as previously described55. The precipitated protein from the primary metabolite analysis was used for the proteomic analysis. For analysis of complex lipids, plasma aliquots (20 µL) or liver tissue homogenates (5 mg) were extracted using a modified liquid-liquid phase extraction approach proposed by Matyash et al.56. For analysis of non-esterified oxylipins, plasma aliquots (250 µL) or liver tissue homogenates (100 mg) were extracted and analyzed according to previously described protocols56,57. Epoxyeicosatrienoic acids are referred to as EpETrEs or EETs and dihydroxytrienoic acids are referred to as DiHETrEs or DHETs. See Supplementary Information for details on the sample collection and metabolomic and proteomic analysis and Supplementary Table S2 for all the datasets, including separate datasheets for primary metabolites, complex lipids, oxylipins and proteomics results for the plasma and liver as well as information sheets for each platform. Raw metabolomics and proteomics data is available on Metabolomics Workbench and Massive/Proteome Exchange, respectively. See Supplementary Information for accession numbers.
Network-based analysis
An integrated network of metabolites and proteins was computed by Grinn software tool58, an R-based tool that integrates biochemical and genomic relationships from several databases, including KEGG59, Reactome60 and ENSEMBL61. We used significant metabolites and proteins (pFDR < 0.05 comparing PL + CO vs SO + CO for liver at 24 weeks) to infer the metabolite-protein networks. The resulting networks were visualized in Cytoscape62.
Statistical Analysis
Data are presented as mean +/− standard error of mean (SEM). Statistical significance, using GraphPad Prism version 6 for Mac, is defined as P ≤ 0.05 using the following tests: Two-way ANOVA with Holm-Sidak post hoc analysis for differences in weight gain over time among the different diets. One-way ANOVA with Holm-Sidak post hoc analysis was performed for tissue weights at harvest and GTT, ITT and ALT assays.
For metabolomics data, values were log2 transformed and statistical significance was determined using a One-Way ANOVA. Specific group differences were determined using Tukey HSD post hoc test. ANOVA P-values were adjusted using Benjamini and Hochberg false-discovery rate adjustment. Statistical analyses were conducted using R statistical software. For major structural lipids, the summed intensities of all lipids belonging to that specific lipid class (e.g., triacylglycerides) were used. Lipids were delineated by degree of saturation. Saturated: <2 or <4 double bonds present in lipid species that contain one or more acyl chains, respectively. Unsaturated: ≥2 or ≥4 double bonds present in lipid species that contain one or more acyl chains, respectively. For correlations between metabolites and metabolic phenotypes, Spearman’s rank correlations on log10-transformed values of known compounds were performed; only significant correlations are included (P ≤ 0.05) (Supplementary Fig. S4). Linear regression analysis was performed between body weight and concentration of oxylipins or fatty acids in the liver. The following cut-offs were used to determine significance: Spearman’s coefficient r > 0.5 with P ≤ 0.05 and R2 > 0.5 (Figs 3, 5b and Supplementary Fig. S4c–f). For proteomics data, One-way ANOVA values were log10-transformed and statistical significance was determined using One-way ANOVA. Hierarchical clustering was calculated on Euclidean Distance with Ward’s agglomeration (Fig. 4). For the integrated network analysis, significance was based on one-way ANOVA on log10-transformed data. Benjamini and Hochberg tests were used for FDR adjustment. Tukey’s HSD was used to determine specific group differences (Fig. 5).
Electronic supplementary material
Acknowledgements
We thank DuPont for Plenish oil; M. Pellizon at Research Diets for advice on diets; J. Newman for critical reading of the manuscript; and C. Perea for help with histology. The work was supported by NIEHS T32 Training Grant (5T32ES018827-03) and Crohn’s and Colitis Foundation of America Career Development Award (#454808) to PD; NIH R01DK053892, USDA National Institute of Food and Agriculture (Hatch project CA-R-NEU-5680), UCR Seed grant and United Soybean Board Soy Health Research Program incentive award to FMS; NIEHS R01 ES002710 to BDH; and a WCMC Pilot Project from NIH U24 DK097154 to FMS, in collaboration with BP, OF and BDH.
Author Contributions
Conceived and designed the experiments: P.D. and F.M.S. Supervised the study: F.M.S., O.F., B.P., B.D.H. Performed the experiments: P.D., J.R.E., J.F., J.Y., A.R., M.S. Analyzed the data: P.D., J.F., D.G., K.W., F.M.S. Wrote the paper: P.D. and F.M.S. with input from the other authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Poonamjot Deol and Johannes Fahrmann contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12624-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hymowitz T. On the domestication of the soybean. Economic Botany. 1970;24:408–421. doi: 10.1007/BF02860745. [DOI] [Google Scholar]
- 2.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keys A, Grande F. Role of dietary fat in human nutrition. III. Diet and the epidemiology of coronary heart disease. Am J Public Health Nations Health. 1957;47:1520–1530. doi: 10.2105/AJPH.47.12.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., 3rd Factors of risk in the development of coronary heart disease–six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 5.Heady JA, et al. Controlled trial of soya-bean oil in myocardial infarction. Lancet. 1968;2:693–699. [PubMed] [Google Scholar]
- 6.Lawrence GD. Dietary fats and health: dietary recommendations in the context of scientific evidence. Adv Nutr. 2013;4:294–302. doi: 10.3945/an.113.003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harcombe Z, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Heart. 2015;2:e000196. doi: 10.1136/openhrt-2014-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramsden CE, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73) BMJ. 2016;353:i1246. doi: 10.1136/bmj.i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikemoto S, et al. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism. 1996;45:1539–1546. doi: 10.1016/S0026-0495(96)90185-7. [DOI] [PubMed] [Google Scholar]
- 10.CDC. Data, Trends and Maps, https://www.cdc.gov/obesity/data/databases.html (2016).
- 11.Ash, M. In USDA Economic Research Service-Related Data and Statistics. https://www.ers.usda.gov/topics/crops/soybeans-oil-crops/related-data-statistics/ (2012).
- 12.Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk forObesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deol P, et al. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver. PLoS One. 2015;10:e0132672. doi: 10.1371/journal.pone.0132672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Midtbo LK, et al. Intake of farmed Atlantic salmon fed soybean oil increases insulin resistance and hepatic lipid accumulation in mice. PLoS One. 2013;8:e53094. doi: 10.1371/journal.pone.0053094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa CA, et al. Abdominal adiposity, insulin and bone quality in young male rats fed a high-fat diet containing soybean or canola oil. Clinics (Sao Paulo) 2011;66:1811–1816. doi: 10.1590/S1807-59322011001000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvheim AR, et al. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity. 2012;20:1984–1994. doi: 10.1038/oby.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaney B, et al. Subchronic feeding study of high oleic acid soybeans (Event DP-3O5423-1) in Sprague-Dawley rats. Food Chem Toxicol. 2008;46:3808–3817. doi: 10.1016/j.fct.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Salas-Salvado, J. et al. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J Nutr, doi:10.3945/jn.115.218487 (2016). [DOI] [PMC free article] [PubMed]
- 19.Perez-Martinez P, et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75:307–326. doi: 10.1093/nutrit/nux014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen E, et al. Statistical review of US macronutrient consumption data, 1965-2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition. 2015;31:727–732. doi: 10.1016/j.nut.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Spengler E, et al. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin Proc. 2015;90:1233–46. doi: 10.1016/j.mayocp.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moghaddam M, Motoba K, Borhan B, Pinot F, Hammock BD. Novel metabolic pathways for linoleic and arachidonic acid metabolism. Biochim Biophys Acta. 1996;1290:327–339. doi: 10.1016/0304-4165(96)00037-2. [DOI] [PubMed] [Google Scholar]
- 23.Gabbs, M., Leng, S., Devassy, J. G., Monirujjaman, M. & Aukema, H. M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Advances in Nutrition: An International Review Journal6(5), 513–540 (2015). [DOI] [PMC free article] [PubMed]
- 24.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 25.Zha W, et al. Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity. J Lipid Res. 2014;55:2124–2136. doi: 10.1194/jlr.M053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Vicario C, et al. Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur J Pharmacol. 2016;785:133–143. doi: 10.1016/j.ejphar.2015.03.092. [DOI] [PubMed] [Google Scholar]
- 28.Priore P, et al. Modulation of hepatic lipid metabolism by olive oil and its phenols in nonalcoholic fatty liver disease. IUBMB Life. 2015;67:9–17. doi: 10.1002/iub.1340. [DOI] [PubMed] [Google Scholar]
- 29.Nissim S, et al. Prostaglandin E2 regulates liver versus pancreas cell-fate decisions and endodermal outgrowth. Dev Cell. 2014;28:423–437. doi: 10.1016/j.devcel.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu K, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 31.Pickens CA, et al. Plasma phospholipids, non-esterified plasma polyunsaturated fatty acids and oxylipids are associated with BMI. Prostaglandins Leukot Essent Fatty Acids. 2015;95:31–40. doi: 10.1016/j.plefa.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulze-Tanzil G, et al. Effects of the antirheumatic remedy hox alpha–a new stinging nettle leaf extract–on matrix metalloproteinases in human chondrocytes in vitro. Histol Histopathol. 2002;17:477–485. doi: 10.14670/HH-17.477. [DOI] [PubMed] [Google Scholar]
- 33.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Adv Nutr. 2011;2:304–316. doi: 10.3945/an.111.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tourdot BE, Ahmed I, Holinstat M. The emerging role of oxylipins in thrombosis and diabetes. Front Pharmacol. 2014;4:176. doi: 10.3389/fphar.2013.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grapov D, Adams SH, Pedersen TL, Garvey WT, Newman JW. Type 2 diabetes associated changes in the plasma non-esterified fatty acids, oxylipins and endocannabinoids. PLoS One. 2012;7:e48852. doi: 10.1371/journal.pone.0048852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shearer GC, Newman JW. Lipoprotein lipase releases esterified oxylipins from very low-density lipoproteins. Prostaglandins Leukot Essent Fatty Acids. 2008;79:215–222. doi: 10.1016/j.plefa.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picklo MJ, Sr., Newman JW. Antioxidant supplementation and obesity have independent effects on hepatic oxylipin profiles in insulin-resistant, obesity-prone rats. Free Radic Biol Med. 2015;89:182–191. doi: 10.1016/j.freeradbiomed.2015.07.152. [DOI] [PubMed] [Google Scholar]
- 38.Midtbo LK, et al. Intake of farmed Atlantic salmon fed soybean oil increases hepatic levels of arachidonic acid-derived oxylipins and ceramides in mice. J Nutr Biochem. 2015;26:585–595. doi: 10.1016/j.jnutbio.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Schuchardt JP, et al. Comparison of free serum oxylipin concentrations in hyper- vs. normolipidemic men. Prostaglandins Leukot Essent Fatty Acids. 2013;89:19–29. doi: 10.1016/j.plefa.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moghaddam MF, et al. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edin ML, et al. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011;25:3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene JF, Newman JW, Williamson KC, Hammock BD. Toxicity of epoxy fatty acids and related compounds to cells expressing human soluble epoxide hydrolase. Chem Res Toxicol. 2000;13:217–226. doi: 10.1021/tx990162c. [DOI] [PubMed] [Google Scholar]
- 43.Greene JF, Williamson KC, Newman JW, Morisseau C, Hammock BD. Metabolism of monoepoxides of methyl linoleate: bioactivation and detoxification. Arch Biochem Biophys. 2000;376:420–432. doi: 10.1006/abbi.2000.1753. [DOI] [PubMed] [Google Scholar]
- 44.Hayakawa M, et al. Neutrophils biosynthesize leukotoxin, 9, 10-epoxy-12-octadecenoate. Biochem Biophys Res Commun. 1986;137:424–430. doi: 10.1016/0006-291X(86)91227-1. [DOI] [PubMed] [Google Scholar]
- 45.Viswanathan S, et al. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J Am Coll Nutr. 2003;22:502–510. doi: 10.1080/07315724.2003.10719328. [DOI] [PubMed] [Google Scholar]
- 46.Caligiuri SP, et al. Dietary linoleic acid and alpha-linolenic acid differentially affect renal oxylipins and phospholipid fatty acids in diet-induced obese rats. J Nutr. 2013;143:1421–1431. doi: 10.3945/jn.113.177360. [DOI] [PubMed] [Google Scholar]
- 47.Lands B. Consequences of essential fatty acids. Nutrients. 2012;4:1338–1357. doi: 10.3390/nu4091338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry, T. W. C., Lowrey, A. E. R. S. Feeds & Feeding. 6th edn, (Prentice Hall, 2002).
- 49.Kubant R, et al. A comparison of effects of lard and hydrogenated vegetable shortening on the development of high-fat diet-induced obesity in rats. Nutr Diabetes. 2015;5:e188. doi: 10.1038/nutd.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dauqan E, Sani HA, Abdullah A, Kasim ZM. Effect of four different vegetable oils (red palm olein, palm olein, corn oil, coconut oil) on antioxidant enzymes activity of rat liver. Pak J Biol Sci. 2011;14:399–403. doi: 10.3923/pjbs.2011.399.403. [DOI] [PubMed] [Google Scholar]
- 51.Eyres L, Eyres MF, Chisholm A, Brown RC. Coconut oil consumption and cardiovascular risk factors in humans. Nutr Rev. 2016;74:267–280. doi: 10.1093/nutrit/nuw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wall-Medrano, A. et al. Lipidomic and Antioxidant Response to Grape Seed, Corn and Coconut Oils in Healthy Wistar Rats. Nutrients9, 10.3390/nu9010082 (2017). [DOI] [PMC free article] [PubMed]
- 53.Veerman JL. Dietary fats: a new look at old data challenges established wisdom. BMJ. 2016;353:i1512. doi: 10.1136/bmj.i1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HC, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fahrmann J, et al. Systemic alterations in the metabolome of diabetic NOD mice delineate increased oxidative stress accompanied by reduced inflammation and hypertriglyceremia. Am J Physiol Endocrinol Metab. 2015;308:E978–989. doi: 10.1152/ajpendo.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wanichthanarak K, Fahrmann JF, Grapov D. Genomic, Proteomic, and Metabolomic Data Integration Strategies. Biomark Insights. 2015;10:1–6. doi: 10.4137/BMI.S29511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanehisa M, et al. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42:D199–205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Croft D, et al. The Reactome pathway knowledgebase. Nucleic Acids Res. 2014;42:D472–477. doi: 10.1093/nar/gkt1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cunningham F, et al. Ensembl 2015. Nucleic Acids Res. 2015;43:D662–669. doi: 10.1093/nar/gku1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.