Abstract
Endophyte is a factor that affects the physiology and metabolism of plant. However, limited information is available on the mechanism of interaction between endophyte and plant. To investigate the effects of endophytic fungus ZPRs-R11, that is, Trimmatostroma sp., on salidroside and tyrosol accumulations in Rhodiola crenulata, signal transduction, enzyme gene expression, and metabolic pathway were investigated. Results showed that hydrogen peroxide (H2O2), nitric oxide (NO), and salicylic acid (SA) involved in fungus-induced salidroside and tyrosol accumulations. NO acted as an upstream signal of H2O2 and SA. No up- or down-stream relationship was observed, but mutual coordination existed between H2O2 and SA. Rate-limiting enzyme genes with the maximum expression activities were UDP-glucosyltransferase, tyrosine decarboxylase (TYDC), monoamine oxidase, phenylalanine ammonialyase (PAL), and cinnamic-4-hydroxylase sequentially. Nevertheless, the genes of tyrosine transaminase and pyruvate decarboxylase only indicated slightly higher activities than those in control. Thus, TYDC and PAL branches were the preferential pathways in ZPRs-R11-induced salidroside and tyrosol accumulation. Trimmatostroma sp. was a potential fungus for promoting salidroside and tyrosol accumulations. The present data also provided scientific basis for understanding complex interaction between endophytic fungus and R. crenulata.
Introduction
Rhodiola crenulata (HK. f. et. Thoms) H. Ohba is an alpine plant and a functional food and medicine with adaptogenic bioactivity, which is mostly attributed by its main ingredients, namely, salidroside (2-(4-hydroxyphenyl)ethyl-β-D-glucopyranoside) and tyrosol (4-(2-hydroxyethyl)phenol) (Supplementary Fig. S1)1. This plant is commonly eaten by people working in deep sea or high mountain and those sportsmen and astronauts2. The high cold regions at Qinghai–Tibet Plateau with altitude of more than 3,500 m are the main distribution areas of geo-authentic R. crenulata (Supplementary Fig. S1), which is known as “plateau ginseng” due to its pharmacological efficacy, such as antifatigue, antianoxia, antimicrowave radiation, and prolongation of life1,3. However, in recent years, the largely declined wild resource of R. crenulata is not easily addressed due to the slow growth and overexploitation of this plant4. Although people tried to introduce this plant artificially to areas at low altitude, the salidroside and tyrosol contents are relatively low in R. crenulata after growing 5–7 years4,5. Currently, guaranteeing salidroside and tyrosol contents in R. crenulata and how to improve their cultivation away from their native environment are both important topics. As an important influencing factor in plant growth, development, and secondary metabolite accumulation, endophytic fungi received considerable attention6.
Endophytes can establish symbiotic relationship with host plant, but they will not cause evident symptoms and strong hypersensitive reaction6,7; as an important environmental constituent of healthy plant, endophytes can largely influence the secondary metabolite profile of the host plant7. With the invasion of endophytic fungus, a series of events will occur; these events include recognition to fungus; release of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2); flow of ion, such as K+/H+ and Cl−/Ca2+; cross-talk of signal molecules, such as nitric oxide (NO); signal message integration by transcription factors (TFs); and up- or down-regulation of gene expression, thereby resulting in closing or opening of some special secondary metabolic pathways in the accumulation of desired defense secondary metabolites8.
Signal molecules mediate the interaction between endophyte and plant8,9. In a specific fungus-induced reaction, not all signals (pathways) are involved; they do not work alone, but they are in a cross-talk10. Knowledge about which and how signal molecules are involved in a special endophyte-induced secondary metabolite accumulation is largely limited. When the oxidation–reduction is not in equilibrium with the invasion of endophyte, oxidative stress would lead to ROS generation, which depends on NADPH or peroxidase11. H2O2 is a key signaling molecule in ROS that can activate and regulate the expression of defense genes, such as lipoxygenase pathway12. Nevertheless, H2O2 release is not always related with the accumulation of secondary metabolites. H2O2 is not involved in endophytic fungus-induced isoeuphpekinensin accumulation in Euphorbia pekinensis 13. NO is a common and key node of complicated transduction network in fungus-induced secondary metabolite accumulation14; NO is also the upstream signal molecule of other signals, such as salicylic acid (SA), H2O2, and methyl jasmonate15. Except for Ca2+, NO, H2O2, and SA are the first signals detected sequentially in endophytic Gilmaniella sp.; they regulate sesquiterpenoid production in Atractylodes lancea 16. SA is also a high frequency signaling molecule in response to biotic stress; notably, this molecule is involved in fungus-induced products17. However, SA is not present in all fungus-induced secondary metabolite accumulation in plant. SA treatment exerts no influence on betacyanin synthesis in Amaranthus seedlings under light or dark conditions18. Thus, whether and how the signals work in a special endophyte and plant need further scientific basis.
Signal transductions caused by endophyte invasion will be integrated by TF, and some gene expressions would be regulated to produce the desired secondary metabolites in host plant in response to defense reaction8,9. When A. lancea is infected with endophytic Acinetobacter sp., 3-hydroxy-3-methylglutaryl-CoA reductase and 1-deoxy-D-xylulose 5-phosphate reductoisomerase genes are up-regulated; these genes are related with mevalonate pathway in bacterium-induced sesquiterpenoid production17. In Rhodiola plant, the synthesis of salidroside and tyrosol originated from the cinnamic acid pathway. Nevertheless, the branch pathway that is preferential among phenylalanine ammonialyase (PAL), tyrosine aminotransferase (TAT), and tyrosine decarboxylase (TYDC) branch pathways is still unknown19,20. The enzyme gene expression and metabolism of salidroside and tyrosol in R. crenulata affected with endophytic fungus are also unclear. Further investigation should be performed to understand the complex synthesis mechanism of fungus-induced salidroside and tyrosol in R. crenulata 19.
Although many events are involved in endophytic fungus-induced secondary metabolite accumulation in plant8, the molecular mechanism mediating secondary metabolite production, especially in rare functional plants, such as R. crenulata, has not been investigated widely. In the present study, several bioactive endophytic fungi, such as Lachnum sp. (Rac12)21, Phialocephala fortinii (ZPRa-R-1)22, and Trimmatostroma sp. (ZPRs-R11), were obtained from Rhodiola spp21., which could grow healthily with R. crenulata plantlet and promote salidroside and tyrosol accumulations. In this paper, we report the function and interaction of NO, SA, and H2O2 and the key enzyme gene expression activity in synthesis pathway of salidroside and tyrosol in R. crenulata plantlet, as induced by endophytic Trimmatostroma sp. Furthermore, we revealed the mechanism of signal transduction and biosynthesis branch pathway of salidroside and tyrosol.
Results
Endophytid fungus-induced salidroside and tyrosol accumulations
According to the quantitative results of 1H-NMR, UPLC-Q/TOF/-MS, and HPLC, the endophyte ZPRs-R11 could promote salidroside and tyrosol accumulations. Figure 1 shows the time course of salidroside and tyrosol accumulations in R. crenulata inoculated with ZPRs-R11. The maximum contents of salidroside and tyrosol reached 2.424 and 10.759 mg g−1 on the 10th day of fungus-inoculated plantlet, and they were 13.7- and 9.7-fold of those of the controls, respectively. The symbiosis relationship was verified through microtome investigation (Supplementary Fig. S1). Endophytic fungus was re-isolated and re-identified through comparison of its r-DNA ITS sequence.
Figure 1.
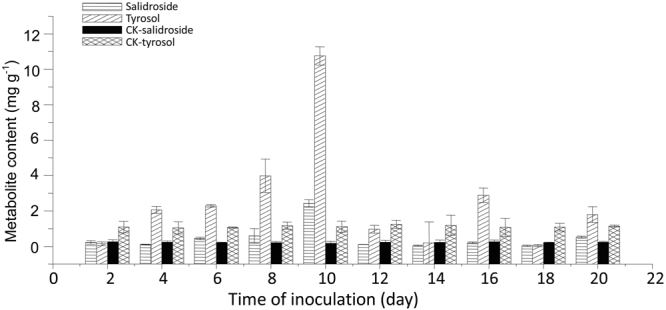
Effects of endophytic fungus ZPRs-R11 (Trimmatostroma sp.) on salidroside and tyrosol accumulations in R. crenulata plantlets in a test cycle of 20 days. The endophytic fungus-induced salidroside and tyrosol contents reached 2.424 and 10.759 mg g−1 on the 10th day, which were13.7- and 9.7-fold those of the control, respectively. Data are represented with means ± standard deviation (SD) to three replicates by Microsoft.
Involvement of H2O2, NO, and SA signaling pathways
Detection results showed that H2O2, NO, and SA were involved the accumulation of ZPRs-R11-induced salidroside and tyrosol. As indicated in Fig. 2, higher levels of H2O2, NO, and SA were observed in ZPRs-R11 treatment than those in control, which exhibited that the endophytic fungus might trigger the biosynthesis of H2O2, NO, and SA in R. crenulata. The maximum contents of H2O2, NO, and SA could reach 0.082 μmol g−1, 0.156 μmol g−1, and 0.183 μg g−1, and they were 6.56-, 1.77-, and 3.52-fold of those in controls on the 10th day, respectively.
Figure 2.
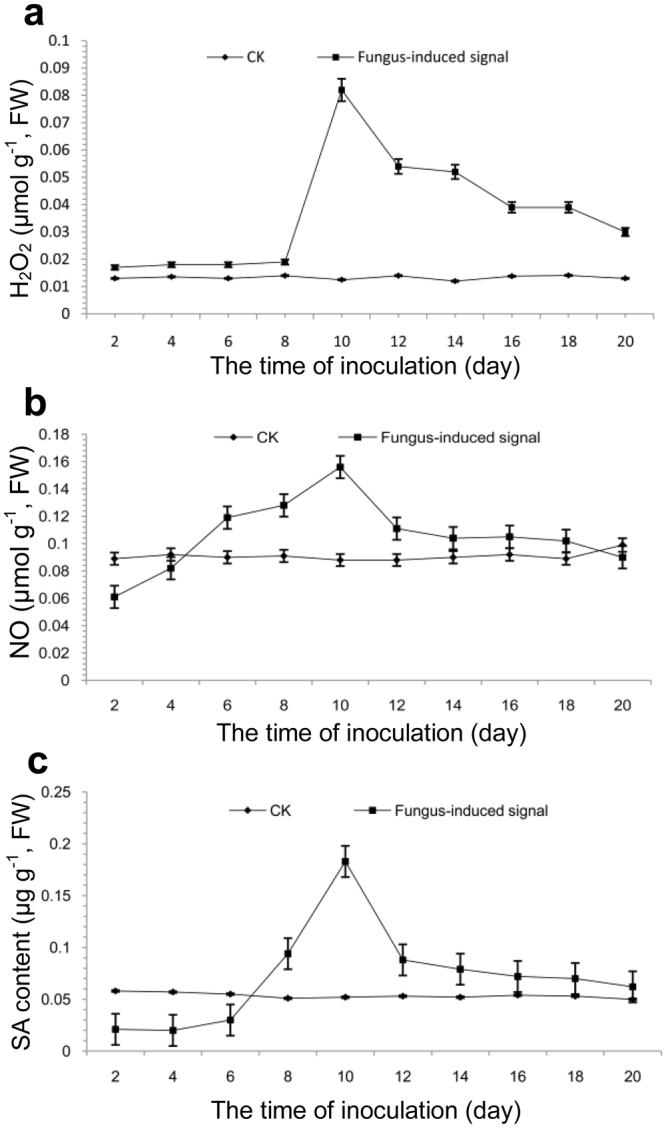
Time course of signals, including hydrogen peroxide (H2O2) (a), nitric oxide (NO) (b), and salicylic acid (SA) (c), produced in R. crenulata plantlets inoculated with endophytic fungus ZPRs-R11 (Trimmatostroma sp.) in a test cycle of 20 days. The maximum contents of H2O2, NO, and SA could reach 0.082 μmol g−1, 0.156 μmol g−1, and 0.183 μg g−1, which were 6.56-, 1.77-, and 3.52-fold those of controls on the 10th day, respectively. Results are presented as the means ± SD of three replicates. *P < 0.05, **P < 0.01, statistically significant in comparison with the control. FW: fresh weight.
Dependence of fungus-induced metabolite accumulation on H2O2
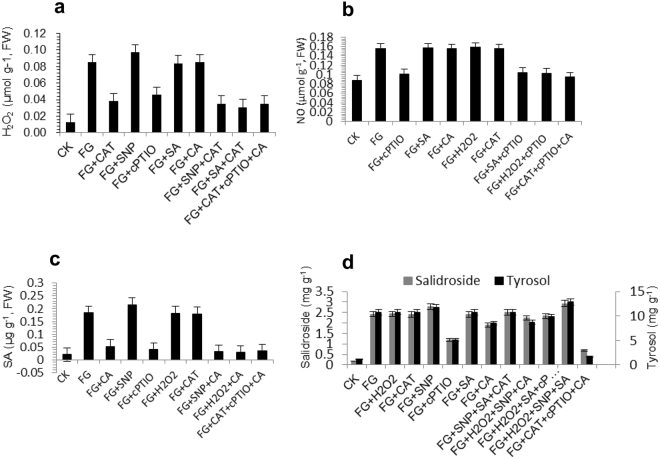
As shown in Fig. 3a, ZPRs-R11 could significantly induce H2O2 accumulation (0.085 μmol g−1) compared with that of control (0.013 μmol g−1). Nevertheless, H2O2 accumulation was significantly suppressed to 0.038 μmol g−1 by CAT (2 mkat L−1) on the 10th day after inoculation. SNP could enhance H2O2 accumulation (0.097 μmol g−1), and its scavenger cPTIO could significantly inhibit at 0.046 μmol g−1 of H2O2 triggered with ZPRs-R11. The donor (SA) and inhibitor (CA) of SA exerted no remarkable effects on H2O2 accumulation. CAT could inhibit the promotion effects of exogenous SNP and SA in R. crenulata infected with ZPRs-R11. However, CAT, cPTIO, and CA were not the only factors suppressing H2O2 production because combined CAT, cPTIO, and CA could not completely inhibit the H2O2 content to the control level in plantlets inoculated with ZPRs-R11. The contents of ZPRs-R11-induced salidroside and tyrosol could slightly increase to 10.802 and 2.435 mg g−1 by adding exogenous H2O2 (1 mM) (Fig. 3d). Nonetheless, the chemical contents of the two metabolites were not evidently affected by inhibitor CAT (Fig. 3d). All these results indicated that H2O2 was an essential signal, but it played limited role in fungus-induced salidroside and tyrosol accumulations.
Figure 3.
Interaction relationships among H2O2, NO, and SA (a,b, and c) and the effects of these signals on fungus-induced salidroside and tyrosol accumulations (d) in R. crenulata plantlets inoculated with endophytic fungus ZPRs-R11. NO was a key node signal molecule. NO and SA exhibited remarkable activities in accumulation of the two metabolites. The working concentration of SNP, SA, H2O2, and CA was 1 mM, and the cPTIO and CAT concentrations were 30 μM and 2 mkat L−1, respectively. SNP: sodium nitroprusside; SA: salicylic acid, H2O2: hydrogen peroxide; CA: trans-cinnamic acid; cPTIO: 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt; CAT: catalase; FW: fresh weight. Plantlets were treated on the 10th day. Values were the means of three repeats ± standard error.
NO as a necessary signal in fungus-induced product accumulation
NO content increased from 0.088 μmol g−1 to 0.156 μmol g−1 in R. crenulata inoculated with ZPRs-R11 on the 10th day. Figure 3b shows that the scavenger cPTIO (30 μM) could considerably inhibit the NO accumulation triggered by endophytic fungus ZPRs-R11. However, both CAT (2 mkat L−1) and CA (1 mM) could not suppress NO accumulation, and their H2O2 (1 mM) and SA (1 mM) donors could not also promote NO to obtain higher yield than that of plantlet inoculated with ZPRs-R11. These results indicated that NO was the upstream signal of H2O2 and SA. Figure 3d illustrates that exogenous NO (1 mΜ SNP) exhibited its advantage in promoting fungus-induced salidroside (2.784 mg g−1) and tyrosol (11.768 mg g−1) accumulations. In addition, cPTIO prevented these metabolites from accumulation induced by ZPRs-R11. These results showed that NO played a vital role in fungus-induced salidroside and tyrosol accumulations, and it was a necessary signaling pathway.
Involvement of fungus-induced metabolite accumulation on SA
Figure 3c shows that CA, the inhibitor of SA, can decrease SA content from 0.184 μg g−1 to 0.053 μg g−1 in R. crenulata triggered with ZPRs-R11 on the 10th day. As the NO donor, SNP (1 mM) could promote SA content to increase slightly to 0.216 μg g−1. cPTIO (30 μM) largely suppressed fungus-induced SA accumulation, which showed that SA was a downstream signal molecule of NO. The other signal molecule H2O2 and its inhibitor could slightly affect SA accumulation. Figure 3d illustrates that the contents of fungus-induced salidroside and tyrosol increased slightly, which was related with SA (1 mM). On one hand, CA could decrease the fungus-induced salidroside and tyrosol from 2.424 mg g−1 to 1.895 mg g−1 and 10.759 mg g−1 to 8.524 mg g−1, respectively. On the other hand, cPTIO and CAT could not suppress the function of SA-induced salidroside and tyrosol accumulations in R. crenulata inoculated with ZPRs-R11. These results showed that SA was an important signaling pathway in fungus-induced salidroside and tyrosol accumulations.
Cross-talk of H2O2, NO, and SA in synthesis pathways
Figure 2 shows that the contents of three signals, namely, H2O2, NO, and SA, could be increased by inducing endophytic fungus ZPRs-R11 in R. crenulata. However, combined CAT, cPTIO, and CA only could partly suppress H2O2, NO, and SA production. This observation showed that these three signals were only a small part in the signal network, which was also affected with other factors (Fig. 3). cPTIO could suppress the increase in all of these three signals. Nevertheless, CAT could decrease H2O2 but partly decrease SA. CA could decrease SA but partly decrease H2O2. Both them could not suppress NO increase, which showed that NO was an upstream signal of H2O2 and SA. A mutual interaction occurred between H2O2 and SA, but no up- or down-stream relationship existed between them. As shown in Fig. 3, combined H2O2, SNP, and SA could promote fungus-induced salidroside and tyrosol accumulations, but their inhibitor or scavenger could not totally decrease the metabolite contents to the level of the control; this result showed that H2O2, NO, and SA were only parts of the overall signal network (Fig. 4a).
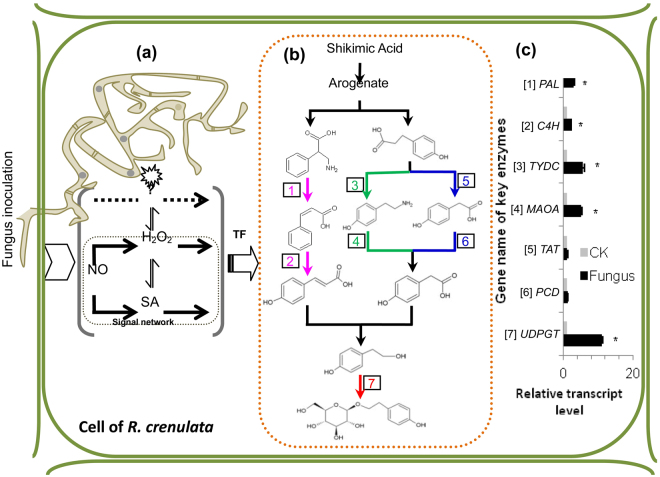
Figure 4.
Proposed signal network, synthetic metabolic pathway, and the key enzyme activities of fungus-induced salidroside and tyrosol in R. crenulata plantlets inoculated with ZPRs-R11 on the 10th day. With the invasion of fungus ZPRs-R11, NO, H2O2, and SA were involved in transmitting signals, which were integrated by transcription factors (a), which could activate the synergic expression of multiple genes and activate the expression of genes regulating similar secondary metabolites, such as salidroside and tyrosol (b,c)8. Results showed that [3] tyrosine decarboxylase gene (TYDC) and [4] monoamine oxidase gene (MAOA) exhibited the highest relative transcript level, followed by that of [1] phenylalanine ammonialyase gene (PAL), [2] cinnamic-4-hydroxylase gene (C4H), [5] tyrosine transaminase gene (TAT), and [6] pyruvate decarboxylase gene (PCD). Such result indicated that TYDC and PAL branch pathways played more vital role than that of TAT branch pathway in R. crenulata plantlets inoculated with ZPRs-R11. The relative expression levels were evaluated using 2−ΔΔCT method40. Data were represented with means ± SD (n = 3) and normalized against the double-reference gene phytochelatin synthase (PCS) and ubiquitin (UBQ). Bars with asterisks denote significant difference from the control (*P < 0.05).
Transcriptional responses of enzyme genes and synthesis branch pathway
As shown in Fig. 4, the UDPGT (⑦) gene expression level and salidroside content were considerably significantly correlated, and the Pearson correlation coefficient (PCC) was 0.996 (P < 0.01). The PCCs of TYDC (③) and MAOA (④) genes were 0.850 and 0.840, and those of PAL (①) and C4H (②) genes were 0.806 and 0.869, which showed significant correlation (P < 0.05). However, the PCCs of TAT (⑤) and PCD (⑥) were only 0.524 and 0.687. The relative expression levels of UDPGT, TYDC, MAOA, PAL, C4H, TAT, and PCD were 11.06-, 5.71-, 5.13-, 2.90-, 2.49-, 1.20-, and 1.26-fold of those of control levels, respectively. The salidroside and tyrosol contents were 13.69- and 9.67-fold of those of control in R. crenulata inoculated with ZPRs-R11 on the 10th day (Fig. 3). The presented results showed that ZPRs-R11 could promote tyrosol and salidroside accumulations, and TYDC and PAL branch pathways, followed by TAT branch pathway, showed the most contribution to the syntheses these metabolites (Fig. 4).
Discussion
The opinion that fungal endophyte plants, as a fate community, live and respond together to the environmental stress in natural conditions is supported by many biologists6. Recently, scientists found that elicitor, such as oligosaccharide, polyunsaturated fatty acid, and chitosan from fungal endophyte cell, can also regulate secondary metabolite accumulation in host plant9,23. In our study, protein and amino acid, oligosaccharide, polysaccharide, lipid, and glycoprotein were isolated from the mycelium of ZPRs-R11 and its fermentation broth. Subsequently, their effects on salidroside and tyrosol accumulations in R. crenulata were investigated. Results indicated that only polysaccharide (1 mg/mL) slightly promoted salidroside and tyrosol accumulations by 1.27- and 1.62-fold of those in controls after six days of treatment. No results better than that of living fungus application could be obtained from any single elicitor component. Similar finding also indicated that endogenous fungal component as elicitor didn’t cause the oxidative increase and reactive ROS release in E. pekinensis cells24. Presumably, exceptions exist in complicated interaction between special endophytic fungus and plant. Living fungi exhibit several advantages over an elicitor: (i) fungi can grow continually with host growth and release metabolites or constantly interact with host tissue or cell, which can constantly trigger host defense response. (ii) Fungi can establish permanent symbiosis relationship with host without causing evident symptoms of infection. People need not to maintain the fungus after inoculating them. Several whole endophytic fungi can already be applied in agricultural production in large-scale commercialization. For example, modified Neotyphodium lolii (EAR1 and EAR37) is inoculated on Lolium perenne to improve the quality of feeding livestock25, and Trichoderma harziamum, Paecilomyces lilicinus, Beauveria bassiana, and Fusarium oxysporum are used as commercial biological control agents26.
Compared with millions of plant, only about dozens of species were investigated regarding the signal transduction interaction between endophytic fungus and host to date8. Among them, Cupressus lusitanica, Catharanthus roseus, and Panax ginseng are the most commonly researched plants about signal transduction27,28. In the signal network, many signal molecules, such as NO, IP3, O2 −, ROS, H2O2, JA, SA, ABA, ethylene, Ca2+, cAMP, and G protein, are related with the secondary metabolite accumulation. NO, H2O2, JA, SA, and ROS are regarded as essential signaling molecules in the process of fungus-induced metabolite production9. NO can improve the whole lines of signal transduction8, and it is a key signal that can trigger many signals, such as H2O2 and ethylene increase8, and induce taxol29, ginseng saponin30, and catharanthine accumulation31. Considerable amount of evidence proved that mutual coordination is a common phenomenon among signals, such as Ca2+ and brassinolide32, and coordination among JA, ethylene, SA, H2O2, and NO results in sesquiterpenoid accumulation in A. lancea induced with endophytic fungus Gilmaniella sp16. However, further basis is needed to support this argument because of exception13,18. Thus, our results provided further understanding of signal transduction in fungus-induced secondary metabolites production.
Selection of suitable reference gene is important to reduce error on yield, quality, and efficiency of reverse transcription of desired gene mRNAs using qRT-PCR. Most often, glyceraldehydes-3-phosphate dehydrogenase (GAPDH), beta-actin (ACT), and elongation factor 1 alpha (EF-1α) are used as reference genes33. Nevertheless, the result would be different from the true value because no gene remains stable under different conditions34. Gutierrez et al.34 found that the expression levels of target gene showed 100-fold difference between validated and nonvalidated reference genes. Therefore, the reference genes should be carefully selected in a particular study, and the numbers should not be only one, as shown in the latest consensus33. For example, UBI1 is selected as reference gene in Rosa hybrid treated with drought stress, and ACT4 is suitable for R. hybrid treated with gibberellin or abscisic acid35. GAPDH combined with EF-1α is a double-reference-gene used in the study of the reproductive stage of Jatropha curcas 36. In the present study, the expression levels of PCS and UBQ were stable, as evaluated by geNorm, Normfinder, and BestKeeper software. PCS combined with UBQ as a double-reference-gene could obtain precise results on the gene expression in R. crenulata plantlet inoculated with Trimmatostroma sp., which is a novel discovery.
The synthesis pathway of salidroside and tyrosol was mainly based on TYDC and PAL branch pathways sequentially in R. crenulata induced with Trimmatostroma sp. (Fig. 4). In Phialocephala sp., PAL and TYDC branch pathways sequentially are the most prioritized branch in the synthesis of fungus-induced salidroside and tyrosol22. Nonetheless, TAT branch pathway is an important pathway to synthesize salidroside and tyrosol in natural R. crenulata uninfected with fungus37. This result indicated that R. crenulata would select optimum metabolic pathway or its combination according to special spatiotemporal conditions, and change varied with different fungal species. Similar examples supported this natural phenomenon. The ergot alkaloid synthesis pathway in Lolium arundinaceum induced with endophytic fungus Epichloë sp. has been studied extensively for the past two decades38. Currently, the content and type of ergot alkaloid in L. arundinaceum could be mediated through infection with genetically modified endophytic fungus Epichloë sp.; therefore, host plant would change the metabolic pathway in response to endophytic fungus infection39. Furthermore, a RNAi-based approach of reducing or increasing the concentration of mRNA from single gene could change alkaloid flux, in which the gene is related with endophytic fungus Epichloë typhina 40.
Conclusion
Endophytic fungus plays important role in secondary metabolite accumulation in plant. Special endophytic fungus exhibits distinct promotion of particular secondary metabolite accumulation in a particular plant. In this study, endophytic fungus ZPRs-R11, that is, Trimmatostroma sp., was first proven to induce NO, SA, and H2O2 increase. This fungus subsequently induced gene to change the expression activities of some key enzyme genes, which resulted in secondary metabolite accumulation in Rhodiola plant. The use of exogenous donor of NO, SA, and H2O2 and their scavenging or inhibiting agents indicated the absence of interaction among NO, SA, and H2O2 and their involvement in fungus induced-salidroside and tyrosol production. Analysis results of key enzyme gene expression activity stated that R. crenulata can select particular synthesis pathway to respond to Trimmatostroma sp. and produce the bioactive product salidroside and tyrosol. This study will help further understand the interaction mechanism between endophyte and plant and provide scientific basis to improve the quality of R. crenulata and industrialization of salidroside and tyrosol.
Materials and Methods
Plant materials and treatments
Meristem cultures were established from R. crenulata explants (5,007 m, E92°22′31″–92°24′88″, N29°44′33″–29°46′56″) (Supplementary Fig. S1) according to the method of György et al.41. The explants were surface sterilized (75% ethanol, 30 s; washing with sterile water, three times; treatment with 0.1% HgCl2, 3–5 min) and grown in modified Murshige and Skoog (MS) medium supplemented with 0.5 mg L−1 thidiazuron, 0.5 mg L−1 naphthaleneacetic acid, 30 g L−1 sucrose, and 8 g L−1 agar in 300 mL tissue culture vessels. Rooting medium contained 0.5 mg L−1 indole butyric acid, 30 g L−1, sucrose and 8 g L−1 agar. All media pH were adjusted to 5.7–5.8 before autoclaving at 121 °C for 20 min. Plants were cultured in a climatron at 28 °C/22 °C day/night with a 14 h/10 h cycle, 30 µmol·m−2·s−1 light intensity, and 70–75% humidity and subcultured every 30 days (Supplementary Fig. S1).
Endophytic fungus culture, inoculation, and reisolation
The endophytic fungus ZPRs-R11 (Trimmatostroma sp., GenBank No. KJ542345), isolated from R. sachalinensis, was subcultured with potato dextrose agar medium at 25 °C in the dark. ZPRs-R11 mycelian disks (5 mm) were obtained and placed near the rooting plantlet caudexes of seven-day-old R. crenulata. Equal-sized PDA disks were set as control. The tested plantlets were collected every two days. The test cycle was 20 days, and each experiment was performed in triplicate. The fungus ZPRs-R11 was re-identified to verify authenticity through morphological characteristics and 18S-rDNA-ITS sequence (Supplementary Fig. S1).
H2O2 measurement
H2O2 was measured by modified titanium sulfate method42. Leaf samples (1 g) were ground with 5 mL of cold acetone in ice bath. The homogenate was centrifuged at 10,000 g for 20 min, and 2 mL of supernatant and 0.5 mL of 5% titanium sulfate were completely mixed fully. Afterward, 2 mL of strong ammonia water was added in the mixture, which was subsequently homogenized. The mixture was centrifuged at 10,000 g for 15 min. The supernatant was removed, and the sediment was dissolved with 5 mL of concentrated sulfuric acid (2 M) The H2O2 value was calculated through the H2O2 external standard at 415 nm with spectrophotometer (UV2700, Shimadzu, Japan).
NO measurement
R. crenulata leaves (1 g) were uniformly ground with 3 mL of distilled water, aided with little silica sand. The mixture was centrifuged at 4,000 g for 20 min. Approximately 1 mL of supernatant was uniformly mixed with 1 mL of Greiss reagent (Sigma-Aldrich, USA) and incubated at 25 °C for 30 min. The NO content was measured on the basis of a standard NaNO2 curve at 550 nm according to modified Greiss reagent method41.
SA measurement
SA quantification was performed using RP-HPLC (Agilent 1200, Agilent technology, USA) equipped with Thermo-C18 column (250 mm × 4.6 mm, 5 µm) and fluorescence detector under 294 nm of excitation wavelength and 426 nm of emission wavelength. The injection volume was 10 µL, and flow rate was 0.5 mL min−1 at 25 °C. The mobile phase was sodium acetate buffer (0.2 M):methanol (9:1 v/v).
The SA extraction method was modified according to that of Yuan et al.16. Leaf samples (1 g) were ground in liquid nitrogen and extracted ultrasonically in 1 mL of methanol. After centrifugation at 10,000 g for 15 min, the residue was re-extracted two times as above. The supernatants were combined and freeze dried. The concentrate was dissolved in 0.5 mL of trichloroacetic acid. After oscillation for 2 min, the mixture was extracted twice with 0.8 mL of acetic acid ester:cyclohexane (1:1 v/v). Organic phases were combined and freeze dried. The concentrate was dissolved with 0.6 mL of HPLC mobile phase and filtered with 0.22 µm Millipore membrane for detection.
Treatment of plantlets with inhibitors and exogenous H2O2, NO, and SA
Sodium nitroprusside (SNP) was used as a NO donor. H2O2, SNP, and SA were obtained from Sigma–Aldrich (St. Louis, MO, USA). The specific scavenging or inhibiting agents of H2O2, NO, and SA were catalase (CAT), 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt (cPTIO), and trans-cinnamic acid (CA), respectively. All of these agents were purchased from Sigma–Aldrich (St. Louis, MO, USA). All the exogenous donors and inhibitors were dissolved with ultrapure water. According to preliminary test results, the most suitable working concentration of SNP, SA, H2O2, and CA was 1 mM. Moreover, the most suitable concentrations of cPTIO and CAT to evaluate the interaction mechanism of three signaling pathways in this experiment were 30 μM and 2 mkat L−1, respectively. All the above solutions were filtered through 0.22 μm Millipore filter and sprayed directly on plant explant tissues. Scavenger or inhibitor was applied on plant at one day before ZPRS-R11 inoculation or spraying of exogenous donors. Ultrapure water was set as control.
Expression activities of key enzyme genes
To examine the influence of endophytic fungi on the expression of key enzyme genes in R. crenulata, two constitutively expressed genes, namely, phytochelatin synthase (PCS) and ubiquitin (UBQ), were used as double-reference-genes. The 2−ΔΔCT method43 was performed to relatively quantify the seven key enzyme genes in the biosynthesis pathway of tyrosol and salidroside; these genes included PAL, cinnamic-4-hydroxylase (C4H), TYDC, TAT, monoamine oxidase (MAOA), pyruvate decarboxylase (PCD), and UDP-glucosyltransferase (UDPGT). The primers of these genes are listed in Supplementary Table S1. Total RNA from the tissue-cultured plantlets of R. crenulata was isolated using Plant RNA Extraction Kit (Sangon Biotech., Shanghai, China). Subsequently, the first-strand cDNA was synthesized from 500 ng of total RNA in a volume of 20 µL using the TransScrip® One-Step gDNA remover and cDNA Synthsis SuperMix (TransGen. Biotech., Beijing, China) according to manufacturer’s protocol. All final cDNA samples were diluted 20-fold for subsequent quantitative real-time PCR (RT-PCR) reactions with quantitative RT-PCR reactions (CFX96TM Real-Time Detection System, Bio-Rad Laboratories Corp., USA) with SYBR Green QPCR Master Mix (TransGenBiotech). Each reaction (final volume of 15 µL) contained 2 µL of diluted cDNA, 7.5 µ of 2 × TransStart® Tip Green qPCR SuperMix, and 0.3 µL of each primer. The PCR conditions were as follows: 94 °C for 30 s, followed by 45 cycles of 94 °C for 5 s, 56 °C for 15 s, and 72 °C for 10 s.
Extraction, identification, and qualification of salidroside and tyrosol
Dried plant powder (0.5 g) was sonicated for 30 min in 10 mL of methanol and re-extracted for another two times from the residue. After centrifugation at 5,000 g for 10 min, the combined supernatants were rotary evaporated at 50 °C. Methanol was added to a constant volume of 1 mL. The mixture was filtered with 0.22 µm Millipore membrane for qualitation and quantification.
Identification of salidroside and tyrosol was based on 1H-NMR and UPLC-Q/TOF/-MS according to our previous methods44. In brief, dried plant extracts were dissolved in methanol-d 4 (CD3OD) to elucidate the structure through comparison of parameters, such as the chemical shift of standard salidroside and tyrosol (bought from National Institute for Food and Drug Control, Beijing), using an AVANCE 300 MHz spectrometer (Bruker, Switzerland). Molecular masses of salidroside and tyrosol were confirmed through comparison of parameters with the standard based on Waters Xevo G2Q-TOF (Micromass MS Tech., UK). The detection of salidroside and tyrosol content was conducted with HPLC (Agilent 1200, Agilent Tech., USA) equipped with a diode array detector and a Thermo-C18 column (250 mm × 4.6 mm, 5 µm) at 30 °C. The mobile phase was methanol: H2O2 (32:68 v/v) with a flow rate of 0.8 µL min−1. The monitoring wavelength was 277 nm.
Statistical analyses
Data were edited with Microsoft Office Excel 2007 and Origin 8.0 (OriginLab Corp. US). The values are expressed as mean ± standard deviation. Statistical significance was evaluated using one-way ANOVA. Duncan’s multiple range tests were performed using SPSS 16.0 software (SPSS version 16.0, SPSS, Chicago, IL, USA). Correlation analysis was based on Pearson’s coefficient. Differences among means were identified significant at P < 0.05. Statistical evaluation on two means was performed by student’s t-test.
Electronic supplementary material
Supplementary Table S1; Supplementary Figure S1; Supplementary Figure S2
Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (No. 31270383, 31670328), and Forestry Public Welfare Industry Research Special-Study on the integrated technology of vegetation restoration in the severe saline alkali soil in the north of Shanxi Province (201304326). We also express our great thanks to Dr. Jason Slot (Department of Plant Pathologh, Ohio State University) for the language polishing.
Author Contributions
J.L.C. conceived, designed and wrote the manuscript. M.L.W., Y.N.W. and J.J. performed the experiments. J.L.C, Y.G and J.H.W. prepared Figures 1–4 and supplementary material.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-12895-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jin-Long Cui, Email: cjl717@sxu.edu.cn.
Meng-Liang Wang, Email: mlwang@sxu.edu.cn.
References
- 1.Khanum F, Singh F, Bawa A, Singh B. Rhodiola rosea: a versatile adaptogen. Compr. Rev. Food Sci. F. 2005;4:55–62. doi: 10.1111/j.1541-4337.2005.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 2.Loset KN, et al. Metabolic profiling of Rhodiola rosea rhizomes by H-1 NMR spectroscopy. Phytochem. Analysis. 2011;22:158–165. doi: 10.1002/pca.1262. [DOI] [PubMed] [Google Scholar]
- 3.Li L, Wang H, Zhao X. Effects of Rhodiola on production, health and gut development of broilers reared at high altitude in Tibet. Sci. Rep. 2014;4:7166. doi: 10.1038/srep07166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peschel W, Prieto JM, Karkour C, Williamson EM. Effect of provenance, plant part and processing on extract profiles from cultivated European Rhodiola rosea L. for medicinal use. Phytochemistry. 2013;86:92–102. doi: 10.1016/j.phytochem.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Booker A, et al. Phytomedicine. 2016. The authenticity and quality of Rhodiola rosea products; pp. 754–762. [DOI] [PubMed] [Google Scholar]
- 6.Jia M, et al. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front. Microbiol. 2016;7:906–920. doi: 10.3389/fmicb.2016.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estrada C, William WT, Van Bael SA. Symbiotic fungi alter plant chemistry that discourages leaf-cutting ants. New Phytol. 2013;198:241–251. doi: 10.1111/nph.12140. [DOI] [PubMed] [Google Scholar]
- 8.Zhai X, et al. The regulatory mechanism of fungal elicitorinduced secondary metabolite biosynthesis in medical plants. Crit. Rev. Microbial. 2017;43:238–261. doi: 10.1080/1040841X.2016.1201041. [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Fan GZ, Liu YT, Wang XD, Zhan YG. Cross-talk of polyamines and nitric oxide in endophytic fungus-induced betulin production in Betula platyphylla plantlets. Trees. 2014;28:635–641. doi: 10.1007/s00468-014-0978-1. [DOI] [Google Scholar]
- 11.Hamilton CE, Gundel PE, Helander M, Saikkonen K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: a review. Fungal. Diversity. 2012;54:1–10. doi: 10.1007/s13225-012-0158-9. [DOI] [Google Scholar]
- 12.Hao W, et al. Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. Sci. World J. 2014;2014:843764–843771. doi: 10.1155/2014/843764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan WZ, Qin WM, Yu LJ, Yang X. Hydrogen peroxide from the oxidative burst is not involved in the induction of taxol biosynthesis in Taxus chinensis cells. Z. Nat. C. 2003;58:605–608. doi: 10.1515/znc-2003-7-827. [DOI] [PubMed] [Google Scholar]
- 14.Arasimowicz-Jelonek M, Floryszak-Wieczorek J. Nitric oxide: an effective weapon of the plant or the pathogen? Mol. Plant Pathol. 2014;15:406–416. doi: 10.1111/mpp.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren CG, Chen Y, Dai CC. Cross-Talk between calcium–calmodulin and brassinolide for fungal endophyte-induced volatile oil accumulation of Atractylodes lancea plantlets. J. Plant Growth. Regul. 2014;33:285–294. doi: 10.1007/s00344-013-9370-4. [DOI] [Google Scholar]
- 16.Yuan J, et al. The mechanism of ethylene signaling induced by endophytic fungus Gilmaniella sp. AL12 mediating sesquiterpenoids biosynthesis in Atractylodes lancea. Front. Plant Sci. 2016;7:361–372. doi: 10.3389/fpls.2016.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang XM, et al. Involvement of abscisic acid and salicylic acid in signal cascade regulating bacterial endophyte-induced volatile oil biosynthesis in plantlets of Atractylodes lancea. Physiol. Plantarum. 2015;153:30–42. doi: 10.1111/ppl.12236. [DOI] [PubMed] [Google Scholar]
- 18.Cao SF, et al. The effects of host defence elicitors on betacyanin accumulationin Amaranthus mangostanus seedlings. Food Chem. 2012;134:1715–1718. doi: 10.1016/j.foodchem.2012.03.129. [DOI] [PubMed] [Google Scholar]
- 19.Zhang JX, et al. A tyrosine decarboxylase catalyzes the initial reaction of the salidroside biosynthesis pathway in Rhodiola sachalinensis. Plant Cell Rep. 2011;30:1443–1453. doi: 10.1007/s00299-011-1053-7. [DOI] [PubMed] [Google Scholar]
- 20.Hu GS, et al. Effects of 2- aminoindan- 2- phosphonic acid reatment on the accumulation of salidroside and four pheny-ethanoid glycosides in suspension cell culture of Cistanche deserticola. Plant Cell Rep. 2011;30:665–674. doi: 10.1007/s00299-010-0997-3. [DOI] [PubMed] [Google Scholar]
- 21.Cui JL, et al. Diversity and antioxidant activity of culturable endophytic fungi from alpine plants of Rhodiola crenulata, R. angusta, and R. sachalinensis. Plos One. 2015;10:1–16. doi: 10.1371/journal.pone.0118204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ML, et al. Effects of endophytic fungi ZPRa-R-1 on key signal molecules and the main secondary metabolites in Rhodiola crenulata. Bull. Bot. Res. 2016;36:416–420. [Google Scholar]
- 23.Erwig J, et al. Chitin-induced and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) phosphorylation-dependent endocytosis of Arabidopsis thaliana LYSIN MOTIF-CONTAINING RECEPTOR-LIKE KINASE5 (LYK5) New Phytol. 2017;215:382–396. doi: 10.1111/nph.14592. [DOI] [PubMed] [Google Scholar]
- 24.Gao FK, Ren CG, Dai CC. Signaling effects of nitric oxide, salicylic acid, and reactive oxygen species on isoeuphpekinens in accumulation in Euphorbia pekinensis suspension cells induced by an endophytic fungal elicitor. J. Plant Growth Regul. 2012;31:490–497. doi: 10.1007/s00344-012-9258-8. [DOI] [Google Scholar]
- 25.Qawasmeh A, et al. Influence of fungal endophyte infection on phenolic content and antioxidant activity in grasses: interaction between Lolium perenne and different strains of Neotyphodium lolii. J. Agric. Food Chem. 2012;60:3381–3388. doi: 10.1021/jf204105k. [DOI] [PubMed] [Google Scholar]
- 26.Wani ZA, et al. Plant-endophyte symbiosis, an ecological perspective. Appl. Microbiol. Biotechnol. 2015;99:2955–2965. doi: 10.1007/s00253-015-6487-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Fujita K, Sakai K. Reactive oxygen species, nitric oxide, and their interactions play different roles in Cupressus lusitanica cell death and phytoalexin biosynthesis. New Phytol. 2007;175:215–229. doi: 10.1111/j.1469-8137.2007.02109.x. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi S, et al. Effect of salicylicacid and yeast extract on the accumulation of jasmonic acid and sesquiterpenoids in Panax ginseng adventitious roots. Russ J. Plant Physiol. 2014;61:811–17. doi: 10.1134/S1021443714060156. [DOI] [Google Scholar]
- 29.Wang JW, Zheng LP, Tan RX. Involvement of nitric oxide in cerebroside-induced defense responses and taxol production in Taxus yunnanensis suspension cells. Appl. Microbiol. Biotechnol. 2007;75:1183–1190. doi: 10.1007/s00253-007-0927-7. [DOI] [PubMed] [Google Scholar]
- 30.Hu X, Neill SJ, Cai W, Tang Z. Nitric oxide mediates elicitorinduced saponin synthesis in cell cultures of Panax ginseng. Funct. Plant Biol. 2003;30:901–907. doi: 10.1071/FP03061. [DOI] [PubMed] [Google Scholar]
- 31.Xu MJ, Dong JF. Elicitor-induced nitric oxide burst is essential for triggering catharanthine synthesis in Catharanthus roseus suspension cells. Appl. Microbiol. Biotechnol. 2005;67:40–44. doi: 10.1007/s00253-004-1737-9. [DOI] [PubMed] [Google Scholar]
- 32.Du L, Poovaiah BW. Ca2+/calmodulin is critical for for brassinosteroid biosynthesis and plant growh. Nature. 2005;437:741–745. doi: 10.1038/nature03973. [DOI] [PubMed] [Google Scholar]
- 33.Li JT, et al. Validation of suitable reference genes for RT-qPCR data in Achyranthes bidentata Blume under different experimental conditions. Front. Plant Sci. 2017;8:1–12. doi: 10.3389/fpls.2017.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez L, et al. The lack of a systematic validation of reference genes: a serious pit fall under valued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Pant Biotechnol. J. 2008;6:609–618. doi: 10.1111/j.1467-7652.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- 35.Meng YL, et al. Identification and validation of reference genes for gene expression studies in post-harvest rose flower (Rosa hybrida) Sci. Hortic. 2013;158:16–21. doi: 10.1016/j.scienta.2013.04.019. [DOI] [Google Scholar]
- 36.Zhang L, et al. Selection of reliable reference genes for gene expression studies in the biofuel plant Jatro phacurcas using real-time quantitative PCR. Int. J. Mol. Sci. 2013;14:24338–24354. doi: 10.3390/ijms141224338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma LQ, et al. Effects of overexpression of endogenous phenylalanine ammonia-lyase (PALrs1) on accumulation of salidroside in Rhodiola sachalinensis. Plant Biol. 2008;10:323–333. doi: 10.1111/j.1438-8677.2007.00024.x. [DOI] [PubMed] [Google Scholar]
- 38.Robinson SL, Panaccione DG. Diversification of ergot alkaloids in natural and modified fungi. Toxins. 2015;7:201–218. doi: 10.3390/toxins7010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan KL, Akhmedov NG, Panaccione DG. Identification and structural elucidation of ergotryptamine, a new ergot alkaloid produced by genetically modified Aspergillus nidulans and natural isolates of Epichloë species. J. Agric. Food Chem. 2015;63:61–67. doi: 10.1021/jf505718x. [DOI] [PubMed] [Google Scholar]
- 40.Mulinti P, Florea S, Schardl CL, Panaccione DG. Modulation of ergot alkaloids in a grass-endophyte symbiosis by alteration of mRNA concentrations of an ergot alkaloid synthesis gene. J. Agric. Food Chem. 2016;64:4982–4989. doi: 10.1021/acs.jafc.6b01604. [DOI] [PubMed] [Google Scholar]
- 41.György Z, et al. Enhanced biotransformation capacity of Rhodiola rosea callus cultures for glycosid production. Plant Cell Tiss. Org. 2005;83:129–135. doi: 10.1007/s11240-005-4010-8. [DOI] [Google Scholar]
- 42.Nakayama T, et al. Generation of hydrogen peroxide and superoxide anion from active metabolites of naphthylamines and aminoazo dyes: its possible role in carcinogenesis. Carcinogenesis. 1983;4:765–769. doi: 10.1093/carcin/4.6.765. [DOI] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−[Delta][Delta]CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Cui, J. L. et al. Potential of the endophytic fungus Phialocephala fortinii Rac56 found in Rhodiola plants to produce salidroside and p-Tyrosol. Molecules 21, 502 (2016). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1; Supplementary Figure S1; Supplementary Figure S2