Abstract
G protein–coupled receptors (GPCRs) are essential for transferring extracellular signals into carefully choreographed intracellular responses controlling diverse aspects of cell physiology. The duration of GPCR-mediated signaling is primarily regulated via GPCR kinase (GRK)-mediated phosphorylation of activated receptors. Although many GRK structures have been reported, the mechanisms underlying GRK activation are not well-understood, in part because it is unknown how these structures map to the conformational landscape available to this enzyme family. Unlike most other AGC kinases, GRKs rely on their interaction with GPCRs for activation and not phosphorylation. Here, we used principal component analysis of available GRK and protein kinase A crystal structures to identify their dominant domain motions and to provide a framework that helps evaluate how close each GRK structure is to being a catalytically competent state. Our results indicated that disruption of an interface formed between the large lobe of the kinase domain and the regulator of G protein signaling homology domain (RHD) is highly correlated with establishment of the active conformation. By introducing point mutations in the GRK5 RHD-kinase domain interface, we show with both in silico and in vitro experiments that perturbation of this interface leads to higher phosphorylation activity. Navigation of the conformational landscape defined by this bioinformatics-based study is likely common to all GPCR-activated GRKs.
Keywords: allosteric regulation, G protein-coupled receptor (GPCR), Michaelis-Menten, molecular dynamics, serine/threonine protein kinase, structure-function
Introduction
Many important biological processes are initiated when an increase in an extracellular signaling molecule, or agonist, leads to activation of one of the >800 known human G protein–coupled receptors (GPCRs)4 located at the plasma membrane of cells (1). Upon agonist binding, these seven-transmembrane proteins undergo a structural change that exposes an intracellular binding interface for cytoplasmic binding partners (2). In canonical GPCR signaling, agonist-bound receptors bind and activate intracellular heterotrimeric G proteins that in turn modulate the activity of downstream effector enzymes (3). Thus, an extracellular chemical change, such as an increase in adrenaline, can be transduced into an organ-level physiological response, such as increased cardiac output (4). To terminate signaling, cytoplasmic GPCR kinases (GRKs) phosphorylate serines and threonines primarily in the C-terminal tails but also intracellular loops of agonist-bound GPCRs (5). These phosphorylated GPCRs are then desensitized and internalized into endosomes, via the action of arrestins, where they may either undergo arrestin-mediated signaling, be returned to the plasma membrane for reuse, or be targeted for degradation (6). GRK dysfunction has been linked to a variety of human diseases, including cancer and heart disease, and thus GRKs have emerged as an attractive target for therapeutic intervention (7).
The seven GRKs are subdivided into the GRK1 (GRKs 1 and 7), GRK2 (GRKs 2 and 3), and GRK4 (GRKs 4, 5, and 6) subfamilies based on sequence similarity but are most obviously distinguished by their C-terminal extensions and mechanisms of membrane localization (8). GRKs belong to the AGC superfamily of Ser/Thr kinases, a collection of 60 kinases related by sequence similarity to the catalytic domains of cAMP-dependent protein kinase (PKA), cGMP-dependent protein kinase (PKG), and protein kinase C (PKC) (9). Kinases in the AGC superfamily share a common catalytic core comprising small and large lobes with the ATP-binding site formed at their interface (10). AGC kinases differ from the broader superfamily of eukaryotic protein kinases by featuring a conserved C-terminal tail involved in regulation of kinase activation and localization (11). Within this C-terminal tail is the active site tether (AST), a loop that makes contact with ATP and is involved in nucleotide entry and exit from the kinase active site (12). In addition to their common catalytic subunit fold, over two-thirds of AGC kinases contain additional domains outside of the kinase domain (KD) that are also involved in kinase regulation and localization (13).
The best structurally characterized AGC kinase is PKA, currently with 190 entries in the Protein Data Bank. Molecular mechanisms of AGC kinase activation have thus been determined primarily based on the spectrum of PKA crystal structures in their various conformational states with a Mg2+–ADP–AlF3–substrate peptide complex considered to be the most reflective of an active, transition state-like configuration (Protein Data Bank code 1L3R) (14). The transition from inactive to active PKA involves full closure of the small and large lobes around a bound ATP along with phosphorylation of typically three conserved motifs: the active site loop, the turn motif, and the hydrophobic motif (13). This brings active site residues into optimal position for catalyzing phosphotransfer to proteins bound to the large lobe (15). Interestingly, some GRKs only retain the turn motif, and thus GRK activity is more dependent on its selective interactions with activated GPCRs, although the structural motifs required for this interaction are not well-understood. GRKs also possess several unique features not found in other AGC kinases that are important for their regulation, including an N-terminal helix that interacts with GPCRs, C-terminal extensions that are important for membrane localization, and an regulator of G protein signaling homology domain (RHD), which, among other roles, maintains the small lobe in a catalytically competent configuration that, in other AGC kinases, would require phosphorylation of two or more of the AGC kinase motifs (16). Because the RHD also bridges the small and large lobes of the kinase domain, another potential regulatory role for the RHD is to control the conformation of the kinase domain (17).
The mechanisms underlying receptor-mediated GRK activation are complex and not fully understood. Membrane localization and allosteric activation by GPCRs play an important role in GRK function (18). Structural analysis, kinetic experiments, and comparison with active structures of PKA suggest that GRK activation at the very least involves ordering of the N-terminal helix, ordering of the AST loop, phospholipid binding in some subfamilies, and kinase domain closure (19–24). These hypotheses have been difficult to probe structurally because most GRK crystal structures are trapped in open, inactive conformations where the small and large lobes are not fully closed and the N terminus and AST loop are disordered. The GRK6–sangivamycin complex (Protein Data Bank code 3NYN) (22) was the first to resolve a GRK in what is thought to be close to an active conformation where the full N-terminal helix and AST loop are ordered. Recent structures of GRK5 in complex with sangivamycin (Protein Data Bank code 4TNB) and AMP-PNP (Protein Data Bank code 4TND) (25) also feature well-ordered AST loops, but their kinase domains are not fully closed, and their AST loops do not follow the expected trajectory. Instead the loops are involved in extensive protein-protein interactions in the crystal lattice. With 32 structures of GRKs now deposited in the Protein Data Bank with many different classes of inhibitors and substrate analogs bound, it remains ambiguous how close these structures are to being “active” as judged by the transition-state complex of PKA and whether there are preferred principal domain motions, especially in light of the fact that GRKs contain a bridging RHD that may dictate preferred conformational pathways. Facilitating conformationally restricted pathways may be a component of GRK activation by lipids and GPCRs.
Here we use a combined approach of principal component and contact map analysis to identify the primary domain motions encompassed by the deposited GRK structures, how these various structures relate to the conformational profile of PKA, and how close each of these states is to the most active structures of PKA. This analysis also suggests that GRK5 residues at the RHD-large lobe interface, N terminus, and AST loop, as expected, play an important role in GRK allosteric regulation. Changes in the frequency of kinase domain closure observed in molecular dynamics simulations when the GRK5 RHD-large lobe interface is mutated correlated with shifts in the steady-state kinetic parameters of the mutants in vitro, supporting our overall hypothesis that the RHD-KD interface plays a role in GRK allosteric activation at least in the GRK4 subfamily.
Results
Principal component analysis (PCA) reveals distinct crystallographic conformers and domain configurations that are related to GRK activation
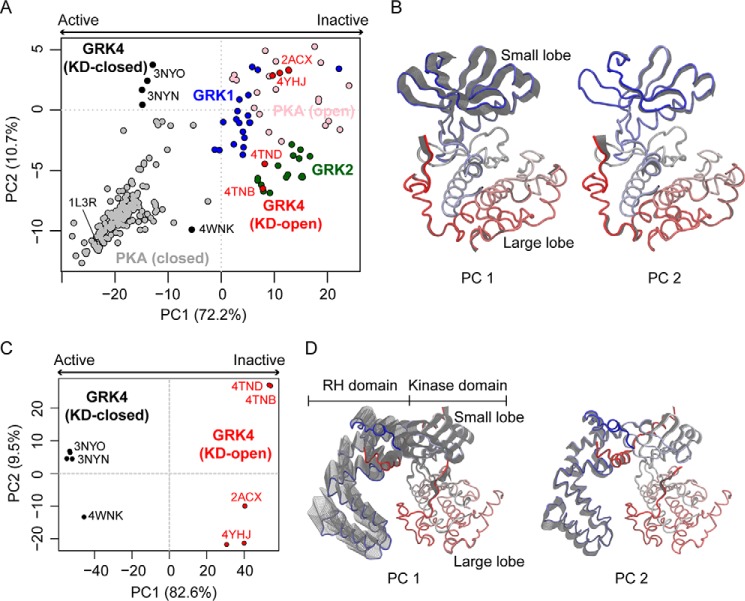
PCA focused on the kinase domain of 11 crystallographic structures of the GRK4 subfamily (see the full list of structures in supplemental Table S1) reveals two distinct groups along the dominant principal component, PC1 (Fig. 1A, black and red circles). The major variation in accumulated structures as revealed by PC1 represents opening/closing of the kinase domain as mediated by the hinge linking its small and large lobes (Fig. 1B and supplemental Movie S1). This lobe motion accounts for over 72% of the total mean square displacements (i.e. variance) in atomic positions among these structures. Subclustering of structures along PC2 within each major group is also observed; this reflects a smaller magnitude twisting of the small lobe relative to the large lobe that accounts for a further ∼10% of structural variance (Fig. 1, A and B, and supplemental Movie S2). The same structural variation associated with PC1 also separates 201 PKA crystal structures into analogous closed and open conformations (Fig. 1A, gray and pink, respectively). The five available GRK4 subfamily crystal structures that contain a relatively closed KD (Fig. 1A, black) likely represent a form that is the closest of any other GRK subfamily to the active kinase state based on the comparison of their clustering along PC1 with active PKA structures. In contrast, structures of GRK1 and GRK2 subfamilies all cluster along the KD-open side of PC1 (Fig. 1A, blue and green). However, the majority of PKA structures, including the structure of a transition-state mimic of PKA (Protein Data Bank code 1L3R), show a more closed form than is evident in any currently deposited GRK structure. This suggests that the relatively closed kinase domain GRK4 structures represent an intermediate state that has not reached the fully closed and active form, consistent with the observation that catalytic residues such as the catalytic base (Asp311 in GRK5; corresponding to Asp166 in PKA) are not quite in the expected active configuration when small lobes are aligned.
Figure 1.
PCA reveals distinct crystallographic conformers and collective motions connecting conformers. A, KD-closed (black circles) and -open (red circles) GRK4, GRK1 (blue circles), GRK2 (green circles), and closed (gray circles) and open (pink circles) PKA crystal structures are projected into the PC subspace of a PCA performed on the crystal structures of GRK4 subfamily kinase domain (see supplemental Table S1). KD-closed structures represent a conformational state closer to the kinase active state, whereas KD-open structures represent the kinase inactive state. The numbers in axis labels indicate the percentage of total structural variance captured by the corresponding PC. B, the collective motions associated with PC1 and PC2. Only the kinase domain is shown and is color-coded by residue index (from blue N terminus to red C terminus). The gray shadow indicates the motion along the corresponding PC. C, projection of GRK4 subfamily structures into the PC subspace of a PCA performed on the crystal structures of full-length GRK4 subfamily members. D, the corresponding collective motions revealed by the full-length structure PCA.
A second PCA performed on the structures of full-length GRK4 subfamily members reveals motions of the RHD that are coupled to the motions of the KD. In particular, KD closure is shown to be associated with movement of the RHD away from the KD large lobe, henceforth referred to as RHD “opening” (Fig. 1, C and D, and supplemental Movie S3). This correlation suggests a potential regulatory role of the RHD on kinase activity through its interaction with the large lobe. The PCA also reveals a small (<10% variance) lateral rotation of the RHD along PC2, which results in a subclustering of structures especially in the KD-open group (Fig. 1, C and D, and supplemental Movie S4).
Residue-wise contact map analysis identifies potential allosteric sites important for GRK activation
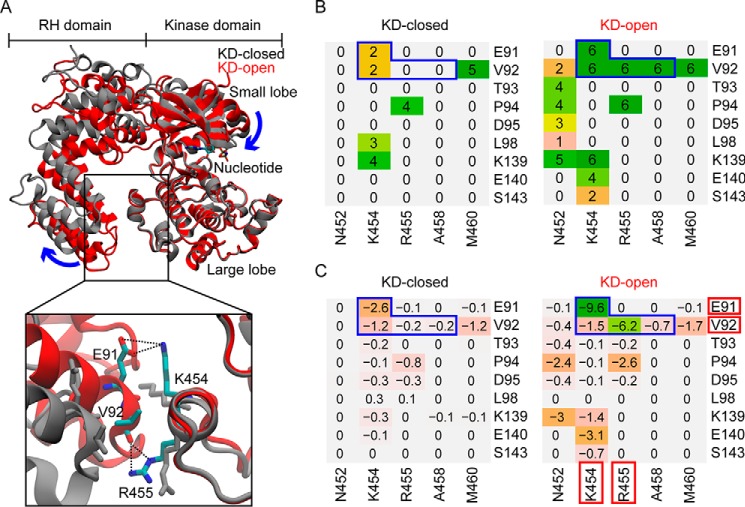
The coupled domain motions revealed by PCA suggest a potential regulatory role of the RHD on kinase activity. We hypothesized that interactions between the RHD and the KD large lobe may be important for stabilizing the RHD-closed form and thus the inactive KD form. Essentially, disruption of these interactions may promote RHD opening and induce KD closure, thereby enhancing kinase activity allosterically (Fig. 2A). To test this hypothesis, we first identified key residues that mediate the RHD-KD interactions in the GRK4 subfamily with a detailed analysis of residue-residue contacts and interaction energies. This analysis shows that overall the KD-open structures display many more residue contacts at the RHD-KD interface than the KD-closed structures (Fig. 2B). In particular, residue pairs Glu91/Lys454, Val92/Lys454, Val92/Arg455, and Val92/Ala458 (RHD/KD) form a contact in all six KD-open structures, whereas they either lose the contact completely or form the contact much less frequently in the five KD-closed structures. Further calculation of the residue-residue interaction energies indicates that, among the four residue pairs, Glu91/Lys454 and Val92/Arg455 have the strongest average interaction across the KD-open structures (Fig. 2C). Inspection of structures reveals a salt bridge formed between Glu91 and Lys454 and hydrogen bonds formed between the backbone carbonyl of Val92 and the side chain of Arg455 (Fig. 2A). In addition, because Val92 makes contacts with surrounding residues, the hydrophobic effect of burying the valine may be important for the RHD-KD interaction. In summary, our analysis of residue-residue atomic contacts and interaction energies in open and closed GRK4 subfamily structures highlighted four residues, Glu91, Val92, Lys454, and Arg455, as the major favorable determinants of the RHD-KD interaction. Next we sought to test whether mutation of these residues could modulate kinase function allosterically.
Figure 2.
Residue contact and energy analyses focused on the RHD-large lobe interface identify potential residues involved in GRK activation. A, superposed GRK4 subfamily KD-closed (gray) and KD-open (red) structures. Bottom, a closer view of the RHD-large lobe interface with key residues shown as sticks and their interactions shown as black dotted lines. B, residue contact analysis for each structural group. The value associated with each residue pair indicates the number of structures in the group in which the two residues are in contact. Regions outlined by blue lines contain residue pairs that form contact in all the KD-open structures but either lose the contact completely or form a contact much less frequently in the KD-closed structures. C, residue-residue interaction energy (kcal/mol) averaged over structures calculated for the same residue pairs as in B (see “Experimental procedures”). Residues that form the strongest polar and/or hydrophobic interactions are highlighted by red blocks.
Molecular simulations of select mutants display enhanced RHD opening and KD closure
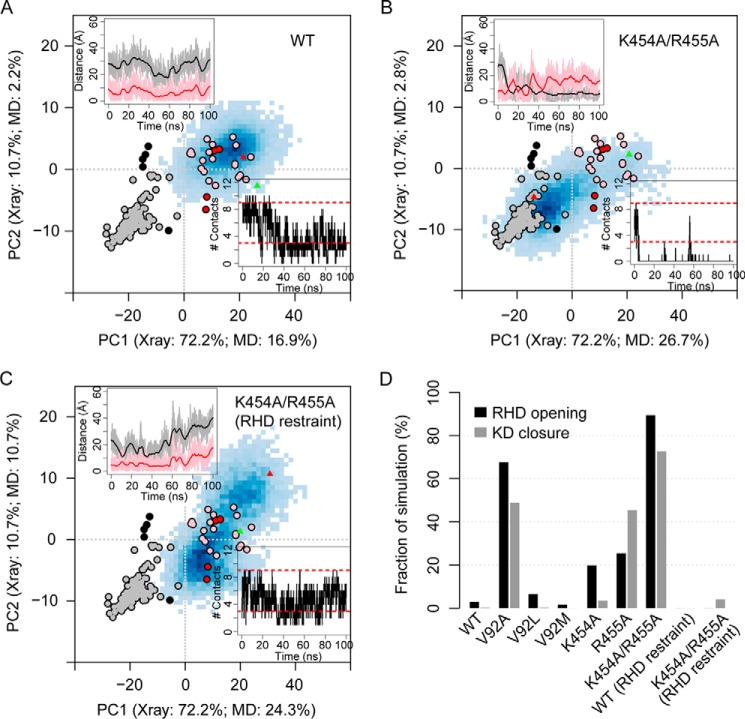
Molecular dynamics (MD) simulations confirm that the conformation of the KD can indeed be modulated by mutating the identified residues located between the RHD and the KD large lobe. Based on our contact map and energy analysis, we constructed six GRK5 mutants to test the effect of two distinct types of interdomain perturbation: 1) disruption of the RHD-KD ionic interactions (K454A, R455A, and the double mutant K454A/R455A) and 2) modulation of the RHD-KD hydrophobic interactions (V92A, V92L, and V92M). Multiple 100-ns MD simulations were performed for wild-type GRK5 and the six GRK5 mutants (supplemental Table S2, simulation numbers 9–15, 20, and 21), all starting from the GRK5–AMP-PNP crystal structure (Protein Data Bank code 4TND), which adopts an open KD conformation. The AST loop (residues 468–491) was deleted in these simulations because the unusual position of the AST loop in the GRK5–AMP-PNP structure is mostly like due to crystal packing contacts that will not be present in solution. In addition, 14 residues from the N terminus (NT) are excluded from our models because they are disordered in most GRK4 subfamily crystal structures (and in GRK1 and GRK2 structures). We added a distance restraint between the truncated NT and the RHD to keep the NT in its initial conformation and thereby reduce the potential artifact that a free truncated NT may introduce. The effects of the presence of the AST loop and/or the free truncated NT in our MD simulations are discussed in a later subsection. Although in the wild-type simulation neither RHD opening nor KD closure occurs significantly (Fig. 3, A and D, and supplemental Table S2), in the simulation for the double mutant K454A/R455A both events are largely enhanced with RHD-open and KD-closed conformations occurring in 89.4 and 72.7% of the simulation time, respectively (Fig. 3, B and D, and supplemental Table S2). Additional simulations with an artificial distance restraint imposed between the RHD and the KD large lobe to prevent RHD opening were performed in which the KD remains in the open conformation (Fig. 3, C and D, and supplemental Table S2). This indicates that KD closure is indeed promoted by RHD opening. The enhanced domain motions are in large part due to the disruption of ionic interactions between the RHD and the KD large lobe by the double mutation. Similarly, the single mutation that disrupts the most important hydrophobic interactions between domains (V92A) significantly enhances RHD opening and KD closure (Fig. 3D and supplemental Table S2 and Fig. S1). Intriguingly, neutral mutations that do not dramatically change hydrophobicity (V92L and V92M) show a pattern of conformational distribution similar to the wild type (Fig. 3D and supplemental Table S2 and Fig. S1). Furthermore, single mutations K454A and R455A only display mild enhancement of RHD opening (Fig. 3D and supplemental Table S2 and Fig. S1), indicating that neither mutation alone can completely disrupt the ionic interactions. Note that although the occurrence of RHD opening in R455A is only slightly higher than that in K454A (25.4 versus 19.8%), KD closure is much more frequently observed in R455A than in K454A (45.5 versus 3.4%). This suggests that a certain degree of RHD-opening enhancement may be necessary to gain a significant stabilization of the KD-closed conformation. However, we cannot exclude the possibility that this difference is merely a result of insufficient sampling due to the currently limited simulation time.
Figure 3.
Select mutations disrupting domain interactions promote RHD opening and KD closure as revealed by MD simulations. A–C, protein conformations are projected into the PC subspace of a PCA performed on the crystal structures of the GRK4 subfamily kinase domain. Blue shaded areas in each panel represent the conformations sampled by MD with darkness of color indicating sample density. The KD-closed (black circles) and -open (red circles) GRK crystal structures as well as the closed (gray circles) and open (pink circles) PKA crystal structures are shown as references. The start (green triangles) and end (red triangles) of simulation are indicated in the map. The numbers in axis labels indicate the percentage of total variance of the GRK crystal structures and the simulation snapshots, respectively, captured by the corresponding PC. Top left inset, time series of the minimal distance between simulation snapshots and the KD-closed (black; dc) or KD-open (red; do) GRK structures. Bottom right inset, time series of the number of contacts between the RHD and the KD large lobe that are not observed in the KD-closed (RHD-open) crystal structures (Nc). Red dashed lines indicate the lower and upper bounds of Nc in the KD-open (RHD-closed) GRK structures. D, summary of the conformational sampling by the wild-type (WT) and mutant simulations. RHD opening and KD closure are defined by Nc = 0 and dc < do, respectively. All simulations are under the NT restraint and AST removal. Some simulations also contain an additional distance restraint between the RHD and the large lobe. (see supplemental Table S2, simulation numbers 9–15, 20, and 21).
In vitro kinase assays confirm predictions from molecular dynamics simulations
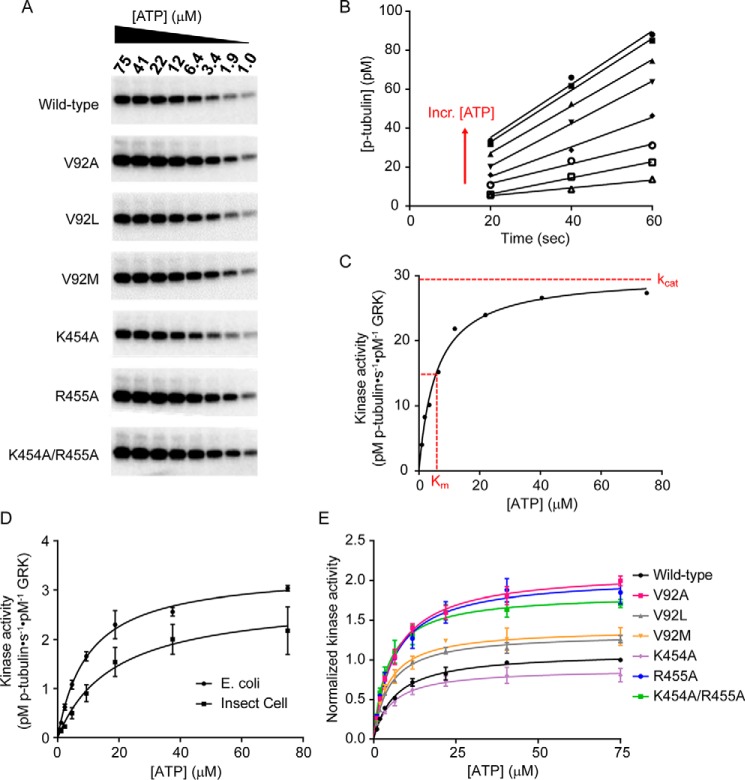
To validate our molecular dynamics results, we expressed wild-type and RHD-KD interface mutant GRK5 variants in Escherichia coli and determined their steady-state parameters using an in vitro kinase assay (see Fig. 4, A–C, for a representative analysis procedure). Because this is the only report to our knowledge of GRK5 expressed from E. coli, we first compared the steady-state parameters of human wild-type GRK5 purified from either insect cells, as has been done in the past, or E. coli. Steady-state tubulin phosphorylation assays were performed to assess changes in their activity in vitro. The full Michaelis-Menten curves and comparison of the steady-state parameters are shown in Table 1 and Fig. 4E. Because we observed no major differences in kinase activity between the E. coli and insect cell-purified wild-type GRK5 for tubulin phosphorylation (in fact, the E. coli GRK5 seems to have higher activity; Fig. 4D), all of our GRK5 mutants were purified from E. coli.
Figure 4.
Disrupting the RHD-KD interface causes in vitro changes in steady-state parameters. Excess tubulin was incubated with 50 nm kinase, and the [ATP] was varied from 1 to 75 μm to determine kinase activity for wild-type GRK5 expressed in either E. coli or insect cells and for the RHD-KD interface mutants from E. coli. A, tubulin phosphorylation reactions at varying ATP concentrations were quenched at 20, 40, and 60 s; separated by SDS-PAGE; and exposed to a phosphor storage screen overnight. Band intensities were quantified using ImageQuant. A representative gel of the 40-s time points is shown. B, after converting band intensities to pm p-tubulin using an ATP standard curve, [p-tubulin] was plotted as a function of time for each ATP concentration, and lines were fit in Prism to determine initial velocities (i.e. slopes in pm·s−1). Each line represents the three time points at a single ATP concentration, and the direction of the lines corresponding to increasing (Incr.) [ATP] is indicated with a red arrow. Only the wild-type data are shown for clarity. C, the slopes calculated from B were divided by the GRK concentration to calculate kinase activity (pm p-tubulin·s−1·pm−1 GRK), plotted as a function of [ATP], and fit to the Michaelis-Menten curve in Prism to determine kcat and Km. Approximate kcat and Km values are indicated with red dotted lines. D, comparison of wild-type human GRK5 purified from either insect cells or E. coli shows no major differences in kinase activity, kcat, or Km. E, for each independent experiment, kinase activity of the RHD-KD mutants was normalized to wild type and fit to the Michaelis-Menten curve to determine Km values and kcat values relative to wild type. Each curve is the average of n = 3 experiments, and error bars represent S.E.
Table 1.
Comparison of steady-state parameters
Each value is calculated from n = 3 experiments.
| GRK5 variant | Km ± S.E. | kcat (relative to WT) | kcat/Km (relative to WT) |
|---|---|---|---|
| μm | |||
| Wild type | 6.7 ± 0.6 | 1 | 1 |
| V92A | 6.4 ± 0.6 | 1.9 | 1.9 |
| V92L | 4.8 ± 0.4a | 1.2 | 1.6 |
| V92M | 4.4 ± 0.5a | 1.2 | 1.9 |
| K454A | 5.5 ± 0.9 | 0.8 | 0.9 |
| R455A | 6.1 ± 0.2 | 1.9 | 1.9 |
| K454A/R455A | 4.5 ± 0.6 | 1.7 | 2.5 |
a p < 0.05 for Km values by one-sample t test.
To test the effect of disrupting the size and hydrophobicity of Val92, we generated V92A, V92L, and V92M mutants. In agreement with the MD simulations, increasing the size of the side chain at this position (V92L and V92M) had no effect on kcat relative to wild type, although there was a slight yet significant decrease by one-sample t test in the Km for ATP of these mutants (4.8 μm, p = 0.04, and 4.4 μm, p = 0.05, respectively) relative to wild type (6.7 μm), resulting in 1.6- and 1.9-fold increases in catalytic efficiency (kcat/Km) relative to wild type. This modest decrease in Km may be reflective of a decreased propensity to release ATP from the active site once it is bound due to potential steric clashes with the larger side chains at the RHD-KD interface in these mutants. V92A had the largest effect on kcat out of any of the mutants tested with a 1.9-fold increase in kcat relative to wild type, consistent with the enhanced RHD opening and KD closure in the MD experiments. This result suggests that a smaller residue at this position does not make as many stabilizing hydrophobic interactions bridging the RHD and KD, thus shifting the equilibrium to an open RHD and favoring the closed KD state.
Next, we sought to test the effects of perturbing the ionic interactions at the RHD-KD interface by generating K454A, R455A, and K454A/R455A mutants. Although Lys454 is positioned to form a salt bridge with Glu91 when the RHD and KD are engaged, we saw no significant changes in either the Km or kcat relative to wild type in the single K454A mutant. This result agrees with the K454A MD simulation where the frequency of KD closure (3.4%) is close to that observed in the wild-type simulation (0.3%). With both R455A and the K454A/R455A double mutant, we observed a significant increase in kcat (1.9- and 1.7-fold relative to wild type, respectively) and a decrease in the K454A/R455A Km. These mutants had 1.9- and 2.5-fold increases in catalytic efficiency, again in agreement with a large increase in KD closure (45.5 and 72.7%) from the MD simulations for these mutants. The increase in activity dependent on the R455A mutation may be reflective of a change in the ionic environment and/or a loss of stabilizing polar interactions between the Val92 backbone carbonyl and the guanidinium group of Arg455. Overall our in vitro results, in particular the increases in kcat values for V92A, R455A, and K454A/R455A, mirror the MD simulations, supporting our hypothesis that the equilibrium toward a more closed and active GRK kinase conformation can be influenced by interactions at the RHD-KD interface.
The AST and NT regions may play essential roles in GRK activation
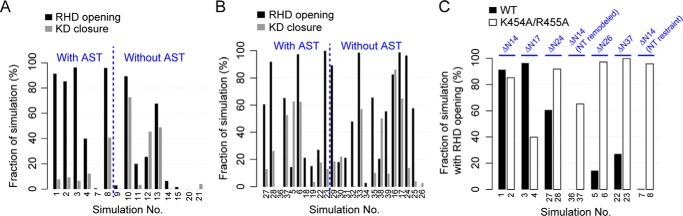
The deletion of the AST loop (including part of the extended C-tail) was assumed to be critical for the observed KD closure in the MD simulations. To test this assumption, we performed additional simulations with the presence of the AST loop (supplemental Table S2, simulation numbers 1–4, 7, and 8). The initial conformation of the AST was taken from the KD-open crystallographic structure (Protein Data Bank code 4TND). It shows that, in the presence of the AST, KD closure is largely restricted even in the presence of a largely opened RHD (Fig. 5A). In contrast, deletion of the AST allows substantial KD closure that is coupled to RHD opening (Fig. 5A). Although the detailed conformational dynamics of the AST region is still unclear due to the structural ambiguity of this region in crystal structures, our simulations suggest that interactions between the AST and the two lobes can have large effects on KD closure and subsequent GRK activation. In this sense, our models with the AST removed may represent a “simplified” version of GRK in which the KD closure might in part be accelerated due to the lack of AST interactions.
Figure 5.
Distinct models of the AST and NT substantially affect the dynamics of RHD and KD in MD simulations. A, fraction of simulation time of observing RHD opening and KD closure under the conditions with or without the AST loop. In the simulations shown, the NT either has the same length as that present in the initial crystallographic structure (Protein Data Bank code 4TND), i.e. is truncated by 14 residues or ΔN14, or is only slightly shorter (i.e. ΔN17). B, the same as A except that in all the displayed simulations the NT is either largely truncated (ΔN24, ΔN26, and ΔN37) or remodeled as a random loop for residues 15–24. C, RHD opening observed in the simulations for the wild-type (WT) and the double mutant K454A/R455A under various NT truncation, remodeling, and restraint conditions.
The NT region has long been suggested to play important roles in the GRK-receptor interaction and GRK activation (19, 20, 22–24). To test how different models of the NT may affect results, we performed additional simulations with various truncations of the NT. In addition, we ran simulations in which the NT was remodeled up to the 24th residue as a random loop protruding into solvent. In all the models, the first 14 residues are absent; these residues are disordered in the initial structure (Protein Data Bank code 4TND) as well as in all other GRK structures except for GRK6–sangivamycin (Protein Data Bank code 3NYN). Moreover, to compare with models having an NT restraint, additional simulations were performed with the NT region unrestrained and free to move. Results indicate that, with a long (≥24-residue) NT truncation or NT remodeling, KD closure can be restricted even in the absence of the AST (Fig. 5B, simulation numbers 24, 25, 29, 35, and 39). In particular, in simulation numbers 24 and 29 (with 37 and 24 NT residues truncated, respectively) the percentage of KD-closed conformations remains low (<20%) even though the RHD opens in >90% simulation time. Moreover, significant KD closure (>50%) is observed in the presence of the AST regardless of the state of RHD interface (Fig. 5B, simulation numbers 5, 6, and 37). This weakened domain coupling is possibly caused by the loss of interactions between the NT and the small lobe due to the truncation. Furthermore, our simulations indicate that a free NT can perturb the dynamics of RHD and enhance RHD opening in the wild type to a level similar to that observed in the double mutant (Fig. 5C). With an NT restraint or the NT region largely truncated, this enhancement is substantially reduced (Fig. 5C). Note that we cannot exclude the possibility that in the cell the NT region indeed interacts with the RHD and/or the KD large lobe and enhances domain opening; this enhancement should apply to both the wild type and the mutant, the latter of which is not detected with current short-time MD simulations. In summary, although the conformational dynamics of the NT region is still unclear, our results indicate that the NT likely plays a critical role in GRK activation by modulating GRK dynamics through its interactions with the KD large lobe, KD small lobe, and potentially the RHD.
Discussion
GRKs are essential regulators of GPCR-mediated signaling. Here, using a combined approach of computational and in vitro experiments, we explore the structural dynamics and function of GRKs and propose an activation mechanism of the kinases that involves cooperative subdomain motions. Specifically, the small and large lobes close via an “asymmetric bite” (via two major conformational changes) to achieve a configuration required for catalyzing phosphotransfer. This closure is coupled with the opening of the RHD relative to the large lobe of the KD. Based on this model, potential allosteric residues, which are over 20 Å away from the active site, are predicted to be located at the RHD-KD interface. Tests show that perturbation introduced by site-directed mutations of these residues can indeed affect the dynamics of the kinase domain and the catalytic activity, thereby supporting our hypothesis that the RHD can allosterically impact kinase activation. In particular, mutants V92A, R455A, and K454A/R455A are shown to have increased frequency of KD closure in silico and enhanced efficiency for phosphorylation in vitro.
Accumulating evidence shows that both the AST loop and the N-terminal regions are involved in GRK activation (19–24). In a previous hypothetical model, an activated GPCR is proposed to dock to the ∼20-amino acid N-terminal α-helix (αN) of GRKs (26). This is similar to how receptors bind the C-terminal α5 helix of the Gα subunit of GDP-bound heterotrimeric G proteins, leading to G protein activation. Intriguingly, the αN region also contributes to the ordering of the AST loop (22), a requisite for the kinase domain closure. The αN to AST thereby forms a bridge between the closed large and small lobes by which activated receptors can regulate kinase activation allosterically. Here, our MD simulation results further suggest that residues right after the αN helix observed in Protein Data Bank code 3NYN that dock near the interface between the regulator of G protein signaling homology domain and the small lobe of the kinase domain play an important role in the coupling of these domain motions. It is also possible that, via αN, the receptor promotes the RHD opening and thereby the kinase domain closure. Integrating these results, we conclude that GRK activation is associated with a set of collaborative events, including N terminus ordering and binding to the receptor and anionic phospholipids, AST ordering, RHD opening, and kinase domain closure.
As many AGC kinases contain extra domains involved in cellular localization or regulation of kinase activity, a regulatory role for the RHD of GRKs has always been speculated, especially given that the GRK RHD has little or no GTPase-activating protein activity (27). Structural clues about the role of the RHD were first provided by the crystal structure of the GRK2–Gβγ complex (Protein Data Bank code 1OMW) (17). In this structure, the GRK RHD was found to adopt the canonical α-helical bilobal fold (28) with the bundle subdomain adjacent to the small lobe of the kinase domain and the terminal subdomain adjacent to the kinase large lobe. In addition to the contacts made between the RHD terminal subdomain and the KD large lobe that are explored in the present study, the α10 helix from the bundle subdomain of the RHD was identified as a potential site of regulation because it makes extensive contacts with two loops in the kinase small lobe that are adjacent to the active site. Thus, the α10 helix can be thought of as a bridge between the KD and RHD and could be involved either in the allosteric activation of GRKs or in the maintenance of an active small lobe conformation. When superimposed on the structure of c-Src, a distantly related protein kinase, it was found that the terminal and bundle subdomains of the RHD roughly overlay with the c-Src SH3 and SH2 domains, respectively, which are well-known to play a regulatory role in c-Src kinase activation (29), suggesting an analogous role for the RHD in GRKs.
While our study was being conducted, evidence for a regulatory role of the RHD was similarly provided by functional analysis of GRK5 activity toward the β2-adrenergic receptor (30). In this study, neutralizing the charged residues at the RHD-KD large lobe interface by making multiple alanine mutations caused a 60% increase in catalytic efficiency for β2-adrenergic receptor phosphorylation by GRK5. In addition, MD simulations showed more frequent and prolonged separation between the KD and RHD upon neutralizing charges at this interface, and covalently linking the RHD bundle domain to the KD large lobe with an engineered disulfide bond dramatically reduced GRK activity toward bleached rhodopsin, indicating, as expected, that a separation of the RHD and KD is required for formation of a catalytically competent kinase domain. Interestingly, the hinge for the domain motion from the authors' MD simulations was near the α10 helix in agreement with the hypothesis that perturbations in the RHD can be translated into the changes in kinase domain activity via the bridging α10 helix.
Our MD and functional analyses of the RHD contact in GRK5 are largely consistent with this parallel study, but in either case mutations at the interface only result in mild activation of kinase activity versus receptor or soluble substrates. Activated receptors, in contrast, have been reported to activate GRKs 100–1000-fold. Thus, this interface makes at best a mild contribution to the overall activation of GRK5 by GPCRs. Based on our prior crystallographic results, the majority of the activation likely involves modulation of the αN and AST regions, stabilizing the active form of the kinase domain. Although disulfide trapping of the RHD to the KD large lobe dramatically squelches activity, published GRK crystal structures and our MD analysis show that this interface is already quite labile. It is unclear whether other GRK subfamilies are subject to the same allosteric control at this interface. Mutating Arg458 in bovine GRK1 (analogous to Arg455 in human GRK5) to alanine has no effect on arrestin recruitment as measured by a Tango assay (31) and suggests that the level of allosteric control through the RHD-KD interface varies from GRK to GRK. Our unique contribution to the story of GRK5 activation is that we empirically determined the coupling of kinase domain closure with separation of the RHD and KD by performing a bioinformatics analysis of all currently available GRK crystal structures and then supported our findings with in silico and in vitro experiments. In addition to neutralizing charged residues at the RHD-KD interface (i.e. R455A and K454A/R455A, the so-called GRK “ionic lock” from Komolov et al. (30)), we also show that the size of a hydrophobic residue (i.e. V92A) is equally if not more important for this interaction network. Moreover, by the nature of our experimental design, which used the soluble substrate tubulin to assess catalytic efficiency, we remove confounding effects of anionic detergents or lipids and show that perturbing the RHD-KD interface with single or double amino acid mutations is sufficient to cause enhancement in GRK5 activity. Importantly, our analysis describes the known conformational landscape for all reported GRK structures, allowing in the future a straightforward assessment of how active a new GRK structure may be as measured by the yardstick of PKA. We have shown that there are two dominant conformational transitions of the GRK kinase domain. Regardless of the precise molecular mechanism of GPCR-mediated activation, we hypothesize that it must lower energetic barriers for these transitions. Our results thus make a significant contribution to our understanding of GRK allosteric activation, which, not inconsistent with other AGC kinases, likely involves structural perturbations of regions outside the kinase domain.
Experimental procedures
Software
Molecular dynamics and energy calculations were performed with Amber12 and AmberTools12 (32), respectively. Structural modeling was performed with Modeller 9.12 (33). All structure and trajectory analyses were performed with Bio3D 2.0 (34, 35). Molecular graphics were generated with VMD 1.9 (36).
Crystallographic structure preparation
Atomic coordinates for GRK and PKA were obtained from the RCSB Protein Data Bank (37) via sequence search utilities in the Bio3D package. Structures with missing active site residues in the kinase domain were excluded from analysis, leading to a data set containing 49 GRK and 201 PKA structures (see supplemental Table S1 for full listing). Within the GRK and PKA families, sequences were aligned with MUSCLE (38), whereas the alignment between the GRK and PKA families was made with the structure-based alignment program MUSTANG (39) and confirmed by visual inspection.
Principal component analysis
PCA was performed to characterize interconformer relationships. The application of PCA to both experimental structures and MD trajectories along with its ability to provide considerable insight into the nature of conformational differences in a range of protein families has been discussed previously (40–43). Prior to PCA, iterated rounds of structural superposition were performed to identify the most structurally invariant region. During this procedure, residues with the largest positional variance (measured as the volume of an ellipsoid determined from the Cartesian coordinates of Cα atoms) were removed, before each round of superposition, until only invariant core residues remained (44). The identified “core” residues were used as the reference frame for the superposition of crystal structures and MD trajectories prior to further analysis. For GRK structures, the core residues are mostly found in the large lobe of the kinase domain, including Lys244, Asp282–Gly312, Tyr323-Arg324, Asn330, Leu332–Asp335, Pro351, Gly353-Asp354, Val364–Gly379, Val413, Arg415–His426, Glu431–Lys433, and Lys445–Leu448 (human GRK5 sequence). Briefly, PCA involves diagonalization of the variance–covariance matrix, ∑, with elements ∑ij calculated from the Cartesian coordinates of Cα atoms, r, after structure superposition.
| (Eq. 1) |
where i and j enumerate all 3N (with N being the number of aligned residues) Cartesian coordinates. The eigenvectors, or principal components (PCs), of ∑ form a new basis set in which structure coordinates are linearly uncorrelated. Structural variance along each PC is given by the corresponding eigenvalue. The projection of structures into the subspace spanned by PCs with the largest eigenvalues provides a low-dimensional representation of structures facilitating the analysis of interconformer relationships. PCA was performed for the 11 crystallographic structures of the GRK4 subfamily, and then all GRK and PKA structures as well as MD trajectories were projected into the PC1-PC2 subspace for further analysis.
Molecular dynamics simulation
MD simulations were performed with Amber12 (32) using the ff99SB force field (45). Additional parameters for ATP were taken from Meagher et al. (46). The Mg2+–AMP-PNP-bound human GRK5 crystal structure (Protein Data Bank code 4TND) (25) was used as the starting model for all simulations. The AMP-PNP was replaced with an ATP by simply changing the nitrogen of the γ-imidophosphate to oxygen. Both N and C termini were capped with acetyl and methylamide groups, respectively. In addition, in simulations with AST removed, intrachain termini of the broken chain were capped. In all systems, Arg and Lys were protonated, whereas Asp and Glu were deprotonated. The protonation states for His residues were determined based on their pKa values at pH 7.0 calculated by PROPKA (47). Simulation structures were solvated in a truncated cubic box of pre-equilibrated TIP3P water molecules that extended 12 Å in each dimension from the surface of the solute. Na+ or Cl− counter-ions were added to neutralize the systems. Energy minimization was performed in four stages with each stage using 500 steps of steepest decent followed by 1500 steps of conjugate gradient. First, minimization for solvent only was performed with fixed positions of protein and ligand atoms. Second, side chain and ligand were relaxed with backbone still fixed. Third, all protein and ligand atoms were relaxed with fixed solvent. Fourth, all atoms were free to move without any restraint. Following minimization, a 10-ps MD was performed to heat the system from 0 to 300 K under constant-volume periodic boundary conditions. A further 1-ns equilibration simulation was performed at constant temperature (300 K) and constant pressure (1 bar). Subsequent 100-ns (or 200-ns) production-phase MD was then performed under the same conditions as equilibration. For both energy minimization and MD simulations, the particle mesh Ewald summation method was adopted to treat long-range electrostatic interactions. In addition, an 8-Å cutoff was used to truncate the short-range nonbonded van der Waals interactions. Additional operational parameters included a 2-fs time step, removal of the center-of-mass motion every 1000 steps, and an update of the nonbonded neighbor list every 25 steps. All hydrogen atoms were constrained using the SHAKE algorithm. In simulations with restraint, an additional linear-parabolic-flat well potential was applied to restrain the distance between specified atoms to around its initial value. The potential changes linearly when R < r1 or R ≥ r4 where R is the atomic distance, r1 = 1.3 Å, r4 = R0 + 0.5 Å, and R0 is the target distance value. When r1 ≤ R < r2 or R0 ≤ R < r4, the potential is parabolic where r2 = 1.8 Å. The force constants for the left-hand and right-hand parabola are 20 and 80 (kcal·mol−1·Å−2), respectively. The potential is zero when r2 ≤ R < R0.
Structural modeling
The structure of AST loop (residues 468–491) or a fraction of the N terminus (residues 15–24) was remodeled in some simulations using Modeller 9.12 (33). For the AST loop, one initial model was generated that was then subjected to 100 runs of loop refinement. The model with lowest Molecular Probability Density Function (MOLPDF) score was picked up as the new AST structure. For the N terminus, 20 initial models were generated without further refinement. The models were visually inspected, and the one in which the N-terminal region does not form any contact with other protein regions was selected for simulations.
Residue contact and energy analysis
Residues were considered in contact if the minimal distance between any heavy atoms from each residue is less than 4.5 Å and the residues are separated by three amino acids in sequence. Residue-residue interactions were calculated with AmberTools12 using the ff99SB force field and the Generalized-Born/Surface-Area (GB/SA) implicit solvent model. All GRK4 subfamily structures were analyzed with ligands excluded from calculations. Treatment for ionizable amino acids similar to that in MD simulations was applied. Energy minimization was performed in two stages with each stage using 500 steps of steepest descent followed by 1500 steps of conjugate gradient. In the first stage, the positions of backbone atoms were restrained. Then, in the second stage, energy minimization was performed without any restraint. The single point total energy was decomposed into pairwise residue interaction energies. A 0.1 m salt concentration was used in the calculations.
GRK5 plasmid preparation and mutagenesis
Human GRK5 with a tobacco etch virus-cleavable C-terminal hexahistidine tag was cloned into pFastBac HTB for insect expression. For human GRK5 expressed in E. coli, the pMalEL17 plasmid was generated as follows. Bacterial vector pMalC2H10T was digested with NdeI and EcoRI, and the human GRK5 gene containing a non-cleavable C-terminal hexahistidine tag separated by a Val-Asp linker was inserted into the modified vector using the same restriction sites and transformed into E. coli BL21 DE3 pLysS for expression. Inverse PCR was used to generate all of the GRK5 point mutations in the context of the full-length E. coli construct. All mutants were confirmed by DNA sequencing prior to expression and purification.
Protein expression and purification
GRK5 from insect cells was prepared by first generating high-titer recombinant baculovirus, which was used to infect Sf9 or Hi5 insect cells at a density of 2 × 106 cells/ml for 48–55 h. Cells were pelleted and flash frozen in liquid nitrogen. GRK5 was purified as described previously (48). Briefly, GRK5 was obtained via Ni-NTA resin and further purified through cation exchange and size exclusion chromatography before concentrating to ∼5 mg/ml. Protein concentrations were determined by absorbance at 280 nm.
For GRK5 expressed in E. coli, suspension cultures were grown at 37 °C to an optical density at 600 nm of ∼0.6 before protein expression was induced with 0.5 mm isopropyl β-d-1-thiogalactopyranoside at 20 °C. Cells were harvested 16 h postinduction by centrifugation at 3000 relative centrifugal force at 4 °C. Cell pellets were immediately frozen at −80 °C and stored until future use.
Cell pellets were thawed and resuspended in Lysis Buffer (25 mm HEPES, pH 7.5, 300 mm NaCl, 0.1 mm EDTA, pH 8.0, 10 mm BME, 10 μm leupeptin, 100 μm PMSF) by 10–20 passes with a 40-ml Dounce homogenizer. Resuspended cells were lysed by three passes through a high-pressure homogenizer (Avestin Emulsi-Flex-C3). GRK5 is initially insoluble, but the addition of 2 μg/ml DNase I and 5 mm CaCl2 to the resuspended cell pellet during lysis yielded soluble protein, suggesting that it is associated with genomic DNA. The soluble fraction was separated from the insoluble fraction by ultracentrifugation at 40,000 rpm at 4 °C for 40 min. Clarified lysate was filtered through a 0.45-μm PVDF filter (Millipore Sigma) and passed over 3 ml of Ni-NTA-agarose resin (Qiagen) per 1-liter expression volume by gravity at 4 °C. Ni-NTA resin was washed with 10 column volumes of High-salt Wash Buffer (25 mm HEPES, pH 7.5, 300 mm NaCl, 20 mm imidazole, 10 mm BME, 10 μm leupeptin, 100 μm PMSF) and 10 column volumes of Low-salt Wash Buffer (25 mm HEPES, pH 7.5, 25 mm NaCl, 20 mm imidazole, 10 mm BME, 10 μm leupeptin, 100 μm PMSF) prior to elution with 5 column volumes of Elution Buffer (25 mm HEPES, pH 7.5, 25 mm NaCl, 300 mm imidazole, 10 mm BME). The Ni-NTA elution fraction was concentrated to ∼5 ml using a 30-kDa -cutoff Amicon Ultra-15 centrifugal filter unit (Millipore Sigma) and diluted to 50 ml in Anion Exchange Buffer A (20 mm HEPES, pH 7.5, 10 mm NaCl, 2 mm DTT) to reduce the NaCl and imidazole concentrations prior to anion exchange chromatography using a 5-ml HiTrap Q HP column (GE Healthcare). Following a 10-column volume Anion Exchange Buffer A wash, GRK5 was eluted by a 20-column-volume linear gradient prepared from Anion Exchange Buffers A and B (20 mm HEPES, pH 7.5, 250 mm NaCl, 2 mm DTT). GRK5 was eluted between 100 AND 150 mm NaCl, and purity was assessed by SDS-PAGE. Fractions with >90% pure GRK5 were pooled and concentrated to 3.5 mg/ml, flash frozen in liquid nitrogen, and stored at −80 °C until use in steady-state kinetics assays. Protein yields of all GRK5 mutants were 1–2 mg/liter.
Comparison of steady-state parameters
Steady-state parameters (kcat and Km) for tubulin phosphorylation by GRK5 were determined at room temperature. 10-μl reactions were prepared whereby GRK5 (50 nm) was incubated with 500 nm soluble tubulin dimer (PurSolutions; bovine brain lyophilized tubulin) in Reaction Buffer (20 mm HEPES, pH 7.5, 10 mm NaCl, 10 mm MgCl2, 2 mm DTT). Kinase reactions were initiated by addition of 1–75 μm ATP supplemented with radioactive [γ-32P]ATP (PerkinElmer Life Sciences) and stopped in the linear initial velocity range at 20, 40, and 60 s by addition of 1× SDS running buffer. Samples were separated on a 4–15% Criterion TGX precast gel (Bio-Rad), gels were exposed to a storage phosphor screen overnight and scanned using a Typhoon scanner, and bands corresponding to phosphorylated tubulin were quantified using ImageQuant software and converted to phosphorylated tubulin concentration ([p-tubulin]) (pm). [p-tubulin] was plotted as a function of time for each ATP concentration, and initial velocity values (pm p-tubulin·s−1) were calculated as the slope of the line. These initial velocity values were then converted to kinase activity by dividing by the enzyme concentration (pm p-tubulin·s−1·pm−1 GRK), plotted as a function of ATP concentration, and fit to the Michaelis-Menten equation to determine kcat (s−1) and Km (μm) for each GRK5 variant. For the steady-state parameter comparisons shown in Table 1, the kinase activity of each mutant was normalized to the wild-type kinase activity by dividing all data points by the maximal wild-type kinase activity value for each independent experiment and plotted as shown in Fig. 4E. Each experiment was performed with n = 3. A one-sample t test was performed to determine which mutants have significantly different (p < 0.05) kcat and Km values relative to wild type. All calculations, curve fitting, and statistical analyses were performed using GraphPad Prism 7.03.
Kinetic comparisons
Kinetic assays were carried out as described above using GRK5 purified from E. coli or insect cells. No significant difference was observed for the Km or kcat.
Author contributions
B. J. G., J. J. G. T., X.-Q. Y., and M. C. C. designed the project and oversaw the work. X.-Q. Y. performed all computational analysis. M. C. C. purified GRK5 variants and performed all associated in vitro experiments. E. L. and T. S. B. optimized GRK5 cloning and purification from E.coli. X.-Q. Y., M. C. C., T. S. B., J. J. G. T., and B. J. G. wrote the paper. All authors contributed reagents and analysis tools and critically reviewed the manuscript.
Supplementary Material
This work was supported by National Institutes of Health Grant 2R01GM070862-10A1 (to B. J. G.), the University of Michigan Chemistry-Biology Interface training program through National Institutes of Health Grant 5T32GM008597 (to M. C. C.), National Institutes of Health Pharmacological Sciences Training Program Fellowship T32-GM007767 and a United States Department of Education Graduate Assistance in Areas of National Need Fellowship P200A150164 (to T. S. B.), and National Institutes of Health Grants R01DK100319, R01HL122416, and R01HL071818 (to J. J. G. T.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Movies S1–S4, Tables S1 and S2, and Fig. S1.
- GPCR
- G protein–coupled receptor
- GRK
- GPCR kinase
- AST
- active site tether
- RHD
- regulator of G protein signaling homology domain
- KD
- kinase domain
- PCA
- principal component analysis
- NT
- N terminus
- MD
- molecular dynamics
- AMP-PNP
- adenosine 5′-(β,γ-iminotriphosphate)
- αN
- N-terminal α-helix
- SH
- Src homology
- PC
- principal component
- Ni-NTA
- nickel-nitrilotriacetic acid
- BME
- β-mercaptoethanol
- p-tubulin
- phosphorylated tubulin.
References
- 1. Katritch V., Cherezov V., and Stevens R. C. (2012) Diversity and modularity of G protein-coupled receptor structures. Trends Pharmacol. Sci. 33, 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ritter S. L., and Hall R. A. (2009) Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 10, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shukla A. K., Singh G., and Ghosh E. (2014) Emerging structural insights into biased GPCR signaling. Trends Biochem. Sci. 39, 594–602 [DOI] [PubMed] [Google Scholar]
- 4. Rockman H. A., Koch W. J., and Lefkowitz R. J. (2002) Seven-transmembrane-spanning receptors and heart function. Nature 415, 206–212 [DOI] [PubMed] [Google Scholar]
- 5. Ferguson S. S. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 53, 1–24 [PubMed] [Google Scholar]
- 6. Hanyaloglu A. C., and von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 7. Gurevich E. V., Tesmer J. J., Mushegian A., and Gurevich V. V. (2012) G protein-coupled receptor kinases: More than just kinases and not only for GPCRs. Pharmacol. Ther. 133, 40–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pitcher J. A., Freedman N. J., and Lefkowitz R. J. (1998) G protein-coupled receptor kinases. Annu. Rev. Biochem. 67, 653–692 [DOI] [PubMed] [Google Scholar]
- 9. Hanks S. K., and Hunter T. (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9, 576–596 [PubMed] [Google Scholar]
- 10. Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N.-H., Taylor S. S., and Sowadski J. M. (1991) Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253, 407–414 [DOI] [PubMed] [Google Scholar]
- 11. Kannan N., Haste N., Taylor S. S., and Neuwald A. F. (2007) The hallmark of AGC kinase functional divergence is its C-terminal tail, a cis-acting regulatory module. Proc. Natl. Acad. Sci. U.S.A. 104, 1272–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Narayana N., Cox S., Nguyen-huu X., Ten Eyck L. F., and Taylor S. S. (1997) A binary complex of the catalytic subunit of cAMP-dependent protein kinase and adenosine further defines conformational flexibility. Structure 5, 921–935 [DOI] [PubMed] [Google Scholar]
- 13. Pearce L. R., Komander D., and Alessi D. R. (2010) The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22 [DOI] [PubMed] [Google Scholar]
- 14. Madhusudan Akamine P., Xuong N.-H., and Taylor S. S. (2002) Crystal structure of a transition state mimic of the catalytic subunit of cAMP-dependent protein kinase. Nat. Struct. Biol. 9, 273–277 [DOI] [PubMed] [Google Scholar]
- 15. Arencibia J. M., Pastor-Flores D., Bauer A. F., Schulze J. O., and Biondi R. M. (2013) AGC protein kinases: from structural mechanism of regulation to allosteric drug development for the treatment of human diseases. Biochim. Biophys. Acta 1834, 1302–1321 [DOI] [PubMed] [Google Scholar]
- 16. Homan K. T., and Tesmer J. J. (2014) Structural insights into G protein-coupled receptor kinase function. Curr. Opin. Cell Biol. 27, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lodowski D. T., Pitcher J. A., Capel W. D., Lefkowitz R. J., and Tesmer J. J. (2003) Keeping G proteins at bay: a complex between G protein-coupled receptor kinase 2 and Gβγ. Science 300, 1256–1262 [DOI] [PubMed] [Google Scholar]
- 18. Kohout T. A., and Lefkowitz R. J. (2003) Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 63, 9–18 [DOI] [PubMed] [Google Scholar]
- 19. Huang C. C., Yoshino-Koh K., and Tesmer J. J. (2009) A surface of the kinase domain critical for the allosteric activation of G protein-coupled receptor kinases. J. Biol. Chem. 284, 17206–17215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pao C. S., Barker B. L., and Benovic J. L. (2009) Role of the amino terminus of G protein-coupled receptor kinase 2 in receptor phosphorylation. Biochemistry 48, 7325–7333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterne-Marr R., Leahey P. A., Bresee J. E., Dickson H. M., Ho W., Ragusa M. J., Donnelly R. M., Amie S. M., Krywy J. A., Brookins-Danz E. D., Orakwue S. C., Carr M. J., Yoshino-Koh K., Li Q., and Tesmer J. J. (2009) GRK2 activation by receptors: role of the kinase large lobe and carboxyl-terminal tail. Biochemistry 48, 4285–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boguth C. A., Singh P., Huang C. C., and Tesmer J. J. (2010) Molecular basis for activation of G protein-coupled receptor kinases. EMBO J. 29, 3249–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang C.-C., Orban T., Jastrzebska B., Palczewski K., and Tesmer J. J. (2011) Activation of G protein-coupled receptor kinase 1 involves interactions between its N-terminal region and its kinase domain. Biochemistry 50, 1940–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orban T., Huang C. C., Homan K. T., Jastrzebska B., Tesmer J. J., and Palczewski K. (2012) Substrate-induced changes in the dynamics of rhodopsin kinase (G protein-coupled receptor kinase 1). Biochemistry 51, 3404–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Komolov K. E., Bhardwaj A., and Benovic J. L. (2015) Atomic structure of GRK5 reveals distinct structural features novel for G protein-coupled receptor kinases. J. Biol. Chem. 290, 20629–20647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C. C., and Tesmer J. J. (2011) Recognition in the face of diversity: Interactions of heterotrimeric G proteins and G protein-coupled receptor (GPCR) kinases with activated GPCRs. J. Biol. Chem. 286, 7715–7721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carman C. V., Parent J. L., Day P. W., Pronin A. N., Sternweis P. M., Wedegaertner P. B., Gilman A. G., Benovic J. L., and Kozasa T. (1999) Selective regulation of Gq/11 by an RGS domain in the G protein-coupled receptor kinase, GRK2. J. Biol. Chem. 274, 34483–34492 [DOI] [PubMed] [Google Scholar]
- 28. Tesmer J. J. (2009) Structure and function of regulator of G protein signaling homology domains. Prog. Mol. Biol. Transl. Sci. 86, 75–113 [DOI] [PubMed] [Google Scholar]
- 29. Parsons S. J., and Parsons J. T. (2004) Src family kinases, key regulators of signal transduction. Oncogene 23, 7906–7909 [DOI] [PubMed] [Google Scholar]
- 30. Komolov K. E., Du Y., Duc N. M., Betz R. M., Rodrigues J. P., Leib R. D., Patra D., Skiniotis G., Adams C. M., Dror R. O., Chung K. Y., Kobilka B. K., and Benovic J. L. (2017) Structural and functional analysis of a β2-adrenergic receptor complex with GRK5. Cell 169, 407.e16–421.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He Y., Gao X., Goswami D., Hou L., Pal K., Yin Y., Zhao G., Ernst O. P., Griffin P., Melcher K., and Xu H. E. (2017) Molecular assembly of rhodopsin with G protein-coupled receptor kinases. Cell Res. 27, 728–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Case D. A., Darden T. A., Cheatham T. E. 3rd, Simmerling C. L., Wang J., Duke R. E., Luo R., Walker R. C., Zhang W., Merz K. M., Roberts B., Hayik S., Roitberg A., Seabra G., Wails J., et al. (2012) AMBER 12, University of California, San Francisco [Google Scholar]
- 33. Eswar N., Webb B., Marti-Renom M. A., Madhusudhan M. S., Eramian D., Shen M. Y., Pieper U., and Sali A. (2007) Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci. Chapter 2, Unit 2.9 [DOI] [PubMed] [Google Scholar]
- 34. Grant B. J., Rodrigues A. P., ElSawy K. M., McCammon J. A., and Caves L. S. (2006) Bio3d: an R package for the comparative analysis of protein structures. Bioinformatics 22, 2695–2696 [DOI] [PubMed] [Google Scholar]
- 35. Skjærven L., Yao X.-Q., Scarabelli G., and Grant B. J. (2014) Integrating protein structural dynamics and evolutionary analysis with Bio3D. BMC Bioinformatics 15, 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Humphrey W., Dalke A., and Schulten K. (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 [DOI] [PubMed] [Google Scholar]
- 37. Berman H. M., Westbrook J., Feng Z., Gilliland G., Bhat T. N., Weissig H., Shindyalov I. N., and Bourne P. E. (2000) The Protein Data Bank. Nucleic Acids Res. 28, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edgar R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Konagurthu A. S., Whisstock J. C., Stuckey P. J., and Lesk A. M. (2006) MUSTANG: a multiple structural alignment algorithm. Proteins 64, 559–574 [DOI] [PubMed] [Google Scholar]
- 40. van Aalten D. M., Conn D. A., de Groot B. L., Berendsen H. J., Findlay J. B., and Amadei A. (1997) Protein dynamics derived from clusters of crystal structures. Biophys. J. 73, 2891–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caves L. S., Evanseck J. D., and Karplus M. (1998) Locally accessible conformations of proteins: multiple molecular dynamics simulations of crambin. Protein Sci. 7, 649–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grant B. J., McCammon J. A., Caves L. S., and Cross R. A. (2007) Multivariate analysis of conserved sequence-structure relationships in kinesins: coupling of the active site and a tubulin-binding sub-domain. J. Mol. Biol. 368, 1231–1248 [DOI] [PubMed] [Google Scholar]
- 43. Gorfe A. A., Grant B. J., and McCammon J. A. (2008) Mapping the nucleotide and isoform-dependent structural and dynamical features of Ras proteins. Structure 16, 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gerstein M., and Altman R. B. (1995) Average core structures and variability measures for protein families: application to the immunoglobulins. J. Mol. Biol. 251, 161–175 [DOI] [PubMed] [Google Scholar]
- 45. Hornak V., Abel R., Okur A., Strockbine B., Roitberg A., and Simmerling C. (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65, 712–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meagher K. L., Redman L. T., and Carlson H. A. (2003) Development of polyphosphate parameters for use with the AMBER force field. J. Comput. Chem. 24, 1016–1025 [DOI] [PubMed] [Google Scholar]
- 47. Li H., Robertson A. D., and Jensen J. H. (2005) Very fast empirical prediction and rationalization of protein pKa values. Proteins 61, 704–721 [DOI] [PubMed] [Google Scholar]
- 48. Homan K. T., Waldschmidt H. V., Glukhova A., Cannavo A., Song J., Cheung J. Y., Koch W. J., Larsen S. D., and Tesmer J. J. (2015) Crystal Structure of G protein-coupled receptor kinase 5 in complex with a rationally designed inhibitor. J. Biol. Chem. 290, 20649–20659 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.