Abstract
Interactions of soluble proteins with the cell membrane are critical within the blood coagulation cascade. Of particular interest are the interactions of γ-carboxyglutamic acid-rich domain-containing clotting proteins with lipids. Variability among conventional analytical methods presents challenges for comparing clotting protein–lipid interactions. Most previous studies have investigated only a single clotting protein and lipid composition and have yielded widely different binding constants. Herein, we demonstrate that a combination of lipid bilayer nanodiscs and a multiplexed silicon photonic analysis technology enables high-throughput probing of many protein–lipid interactions among blood-clotting proteins. This approach allowed direct comparison of the binding constants of prothrombin, factor X, activated factor VII, and activated protein C to seven different binary lipid compositions. In a single experiment, the binding constants of one protein interacting with all lipid compositions were simultaneously determined. A simple surface regeneration then facilitated similar binding measurements for three other coagulation proteins. As expected, our results indicated that all proteins exhibit tighter binding (lower Kd) as the proportion of anionic lipid increases. Interestingly, at high proportions of phosphatidylserine, the Kd values of all four proteins began to converge. We also found that although koff values for all four proteins followed trends similar to those observed for the Kd values, the variation among the proteins was much lower, indicating that much of the variation came from the kinetic binding (kon) of the proteins. These findings indicate that the combination of silicon photonic microring resonator arrays and nanodiscs enables rapid interrogation of biomolecular binding interactions at model cell membrane interfaces.
Keywords: factor VIII (FVIII), lipid, membrane bilayer, phosphatidic acid, phosphatidylserine, prothrombin, nanodisc, coagulation factors, protein–lipid interaction, silicon photonic microring resonators
Introduction
The study of complex, multifactorial biomolecular binding interactions using direct physical methods, such as surface plasmon resonance, can be a time-consuming and complicated process that often requires large quantities of expensive reagents. This holds especially true for monitoring interactions at the cell membrane surface, which plays a critical role in the regulation of important biological processes such as cell signaling and blood coagulation, the latter of which involves protein–protein and protein–lipid interactions, and is of particular relevance to this study.
Protein–membrane interactions governing processes such as blood coagulation are difficult to study because of their multimodal nature, but also because of the complicated environment of the cell membrane. The many varieties of lipids, including phospholipids, sphingolipids, and glycolipids, as well as small molecules such as sterols and polyketides (1), can influence direct binding of proteins to the cell membrane or membrane proteins. Therefore, the use of a model membrane system is an attractive approach to probing the biophysics of interactions in a more well defined and simplified environment. Among several different model lipid bilayer systems (2–4), nanodiscs offer many advantages for probing biomolecular interactions at membrane surfaces. Nanodiscs are discoidal lipid bilayers ∼10 nm in diameter, held together by two membrane scaffold proteins (MSPs)3 (3). Their small size and ease of assembly give a high degree of control over lipid composition (5–7). Previous work has also shown that MSP offers a handle-like structure to which tags can be attached (8), thereby not interfering with the lipid bilayer. Unlike liposomes, the small size of nanodiscs allows for a wider range of lipid compositions to be probed without complications from aggregation (9). The unique versatility of nanodiscs has been proven through a variety of studies, including determination of membrane protein orientation (10), dimer versus monomer activity of rhodopsin (6), and solid-state NMR experiments (11). A recent review describes the versatility and stability of nanodiscs across an array of biochemical and biophysical characterization applications (12).
Silicon photonic microring resonators have emerged as a promising, array-based detection technology that has been applied to a number of bioanalytical applications, including the quantitation of protein (13–16) and nucleic acid (17, 18) biomarkers. Notably, the technology has also been applied to the determination of kinetic and thermodynamic binding constants (19–21). Microring resonators are chip-integrated waveguide structures that support optical resonances, and the wavelength of these resonances is sensitive to the local refractive index environment (22). Previous proof-of-concept experiments demonstrated that binding interactions between solution phase proteins and nanodiscs immobilized onto a microring array could be probed; however, the nature of these interactions was extremely simple (23).
For this study, we combined the versatility of nanodiscs with highly multiplexible silicon photonic microring resonators to study protein–lipid interactions involved in the blood coagulation cascade. Many of the key regulatory processes of both thrombosis and hemostasis are initiated through the successive action of a series of serine proteases that ultimately leads to the formation of a blood clot. Most of these enzymatic cleavage events occur at the membrane surface and are strongly influenced by the underlying lipid composition (9, 24, 25). Of particular interest are the seven γ-carboxyglutamate (GLA) domain-containing coagulation proteins, which include prothrombin (PT), factor VII (fVII), factor X (fX), and activated protein C (APC). These proteins reversibly bind membrane surfaces through their GLA domains, each rich in post-translationally modified GLA residues and containing 7–9 divalent metal binding sites (26–30). Ca2+ is absolutely required for proper folding and binding of the GLA domain to membrane surfaces (27). Furthermore, it is well established that GLA domains bind anionic phosphatidylserine (PS) lipids, but despite structural similarities, the GLA domains of coagulation proteins have affinities for PS spanning 3 orders of magnitude (31, 32). Both APC and fVIIa bind poorly to PS-containing membranes; however, these proteins have been shown to bind the lipid phosphatidic acid (PA) with much higher affinity (33). This higher affinity also affects enzyme activity, with APC and fVIIa having greater activity when interacting with membranes containing specific mixtures of PA and PS compared with membranes with PS alone (33). It has also been demonstrated that other lipids, including phosphatidylethanolamine, phosphatidylglycerol, and phosphatidylinositol are able to effectively reduce the concentration of PS required to achieve maximal activity. This “anything but choline” hypothesis postulates that any lipids not containing the bulky choline headgroup found on PC will synergize with PS and dramatically reduce the PS content required for maximal enzymatic activity (24).
Past studies of coagulation protein–lipid binding using techniques like surface plasmon resonance (SPR) (9, 24, 33) have yielded important insights into the mechanisms of interaction; however, the relatively low throughput nature of the most common configuration of this technology (Biacore) presents practical limitations in terms of the number of both proteins and lipid compositions that can be evaluated in a reasonable amount of time. Additionally, to assess numerous conditions using SPR, multiple experimental runs—each consisting of the adsorption of lipids, experimentation, and regeneration of the surface—are required, increasing experimental variability and reagents used.
Herein, we demonstrate the utility of nanodiscs and silicon photonic microring resonator technologies as a platform for the facile and thorough interrogation of protein–membrane interactions through the investigation of 28 different clotting protein–lipid interactions: 4 clotting proteins, each at 7 different binary lipid combinations. Both the absolute values and relative trends of equilibrium binding constants were determined. We also find that the coagulation proteins investigated—fX, PT, fVIIa, and APC—not only exhibit varying binding affinities for PS and PA, but also require differing proportions of these lipids to achieve maximal membrane binding. In addition to determining the dissociation equilibrium constant, Kd, we report the off-rate constants (koff) for each of the 28 interactions probed. These studies demonstrate the powerful biochemical analysis combination of the silicon photonic microring detector platform coupled with nanodiscs to rapidly interrogate binding interactions at model cell membrane interfaces.
Results
In previous work (23), a four-channel microfluidic chamber was used to direct solutions of four nanodiscs, each having a different lipid composition, across different regions of a microring resonator substrate to create a four-component sensing array. That method demonstrated that unique binding to each of the four distinct types of nanodiscs could be monitored without cross-reactivity, but fluidic immobilization did not facilitate higher throughput studies. In this study we used a microarrayer to create higher density arrays and used these arrays to probe biophysical interactions between blood coagulation proteins and nanodiscs of variable lipid composition. Using physisorption as an immobilization method, seven different compositions of nanodiscs were deposited onto clusters of four microrings using a Bioforce Nano eNabler. The lipid compositions investigated included 100% PC, as well as binary combinations of PC and PS (10, 30, 50, and 70% PS, with the balance being PC) or PC and PA (30 and 50% PA, with the balance being PC). It is important to note that anionic lipid percentages higher than those naturally occurring are required because of the discrete size of nanodiscs, which limit the spatial area over which lipids can be recruited into clusters by divalent cations and/or GLA domains. Because the nanodiscs naturally physisorb to the silicon oxide surface, neither the surface nor the nanodiscs were modified for attachment.
Arrays of these nanodiscs with seven different lipid compositions were used to determine both the equilibrium dissociation constant, Kd, and kinetic dissociation rate, koff, for interactions with four different proteins involved in the blood coagulation pathway: PT, fX, fVIIa, and APC. Fig. 1A shows a representative Kd determination titration for PT. PT was flowed across the nanodisc array at increasing concentrations, and the shift in microring resonance wavelength was monitored in real time. At each step, the binding response was allowed to equilibrate before introducing the next concentration. Because PT does not specifically bind to PC lipids, the small response measured from the 100% PC nanodisc-functionalized rings was subtracted to correct for nonspecific protein adsorption. The real time resonance shift data, replotted as a function of PT concentration, is presented in Fig. 1B. Because all of the coagulation proteins studied bind in a Ca2+-dependent manner, the nanodisc array could be completely regenerated by simply flowing a Ca2+-free buffer, followed by interrogation of subsequent coagulation protein–lipid interactions. In this way, identical titrations for fX, fVIIa, and APC were performed and can be found in supplemental Figs. S2–S5. To verify the ability to regenerate nanodisc arrays for subsequent titrations and also to investigate the role of Ca2+ in the spotting buffer, an additional set of PT titrations was performed, as described under “Experimental procedures.” The determined Kd values are shown in supplemental Table S2. The regeneration studies verified consistent Kd values across multiple surface regenerations; however, the determined values from these measurements are slightly different from those measured for the multiplexed array. This discrepancy is attributed to completely different lots of all reagents. The studies of Ca2+-containing spotting buffers did reveal that PT had higher Kd values to nanodiscs spotted with Ca2+. The reason for this difference is unknown and will be the subject of future studies.
Figure 1.
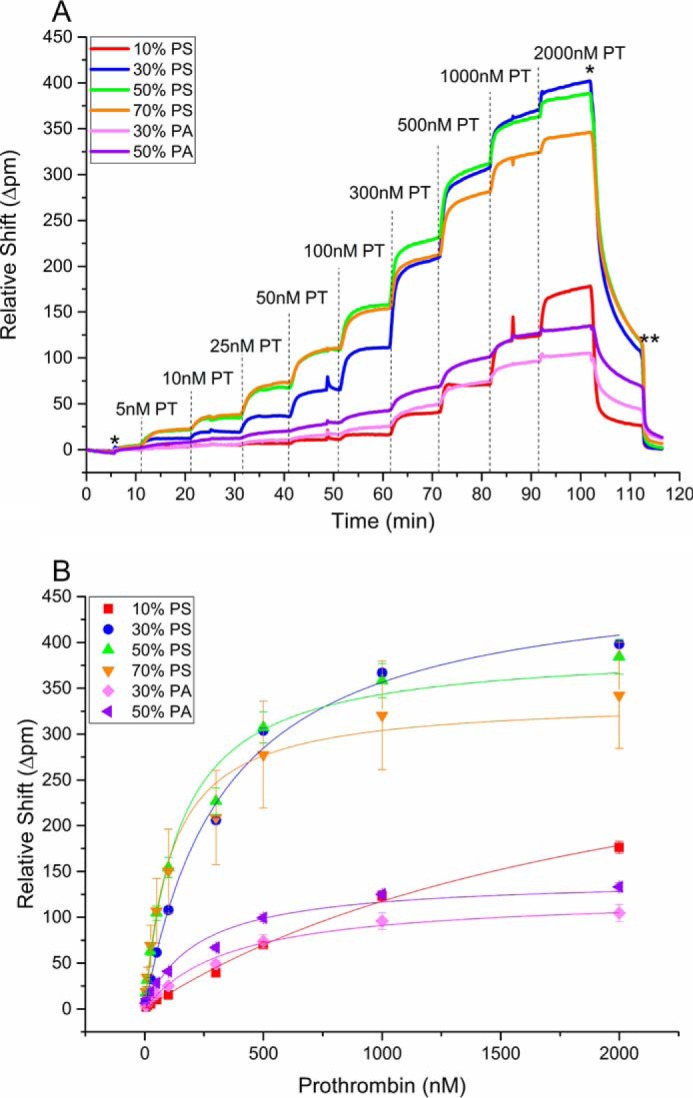
A, real-time shifts in resonance wavelength during PT Kd titration at different nanodisc compositions. The dashed lines indicate the addition of a new concentration of protein of interest; *marks the transition to HEPES buffer, and ** marks the transition to HEPES(−). Minimal binding to the 100% PC microrings were subtracted from the other responses to account for nonspecific binding. The different concentrations of PT (ranging from 50 to 2000 nm) flowed across the sensor array at different times are indicated. B, plots of relative wavelength shift as a function of PT concentration. In each panel, the error bars represent the standard deviation from at least n = 8 microrings in a single detection experiment.
To determine Kd values for each coagulation protein at each lipid composition, the concentration-dependent shifts in resonance wavelength were fit according to a single-site binding model using the following equation,
| (Eq. 1) |
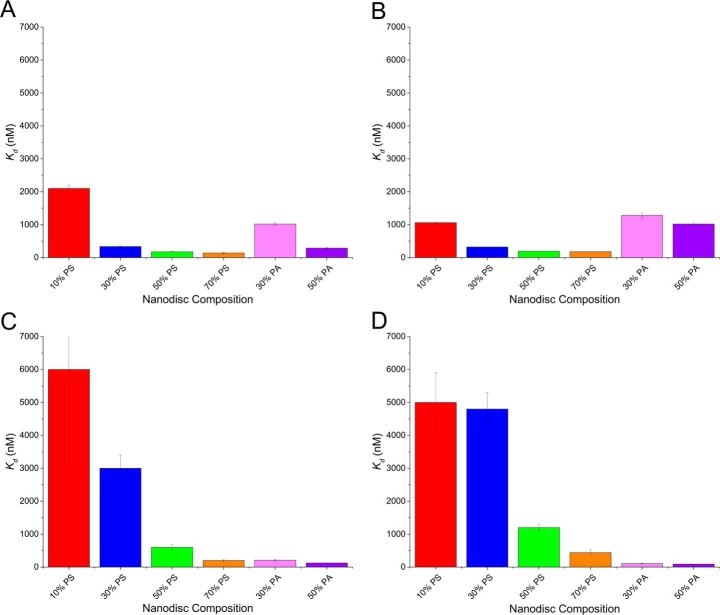
where Bmax is the maximum shift the protein is approaching, and Kd is the equilibrium dissociation constant. Fig. 2 shows Kd values determined for the binding of PT, fX, fVIIa, and APC to the different nanodisc compositions. These values are also tabulated in and supplemental Table S1. Overall, binding increased (Kd decreased) as the percentage of PS composition of the nanodisc increased. At 10% PS, fX exhibited the lowest Kd, followed by PT, APC, and fVIIa; these relative binding affinities are consistent with those observed previously (24). At 30% PS, PT and fX (Fig. 2, A and B) bound to the membrane surface with Kd values approximately an order of magnitude smaller than fVIIa or APC (Fig. 2, C and D); however, when the PS content was increased to 70%, all four proteins bound with similar Kd values of 140–440 nm (supplemental Table S1). To investigate how the PS content affected the Kd, we evaluated the percentage change in Kd as a function of %PS. For both PT and fX, the largest percentage of change in Kd occurred between 10 and 30% PS (84% decrease). Conversely, fVIIa and APC binding to nanodiscs increased most significantly when the PS content was increased from 30 to 50% (80 and 75% decrease, respectively). fVIIa and APC were previously demonstrated to be PA-binding proteins (24). To confirm, we next evaluated the binding of PT, fX, fVIIa, and APC to 30 and 50% PA-containing nanodiscs (Fig. 2, A–D); as expected, fVIIa and APC bound more tightly to PA than PS nanodiscs exhibiting Kd values for binding to 50% PA nanodiscs of 125 nm for fVIIa and 90 nm for APC, compared with 600 and 1200 nm, respectively, to 50% PS.
Figure 2.
Kd values in nm of PT (A), fX (B), fVIIa (C), and APC (D) binding to variable lipid content nanodiscs, as indicated. Each was determined by plotting relative shift of binding versus concentration of protein and fitting to equation 1. The error bars represent the standard deviation from at least n = 8 microrings in a single detection experiment.
In addition to determining equilibrium binding constants, the real-time analysis capabilities also permit interrogation of binding kinetics. Interactions between anionic lipids and the GLA domain are multivalent, making extraction of binding rate (kon) difficult; however, an apparent unbinding rate (koff) can be determined through a kinetic titration. To achieve this, a given concentration of a coagulation protein was flowed across the surface, and after reaching steady stage, a Ca2+-containing buffer is flowed to watch the dissociation of the protein from the immobilized nanodiscs. An entire representative titration for PT binding to variable lipid content nanodiscs is shown in supplemental Figs. S6 and S7. For clarity, the overlaid binding and unbinding curves for PT interacting with 50% PS-containing nanodiscs is shown in Fig. 3. The real-time dissociation was fit for each titration according to the following equation,
| (Eq. 2) |
where Δpm is the resonance shift as a function of time, A is a constant, t is time, and koff is the dissociation off rate. This process was repeated for the other coagulation proteins again using a multiplexed nanodisc composition array to simultaneously allow determination of multiple koff values for a particular coagulation protein. Full titrations for fX, fVIIa, and APC, as well as overlaid binding and unbinding curves for each, are presented in supplemental Figs. S8–S13.
Figure 3.
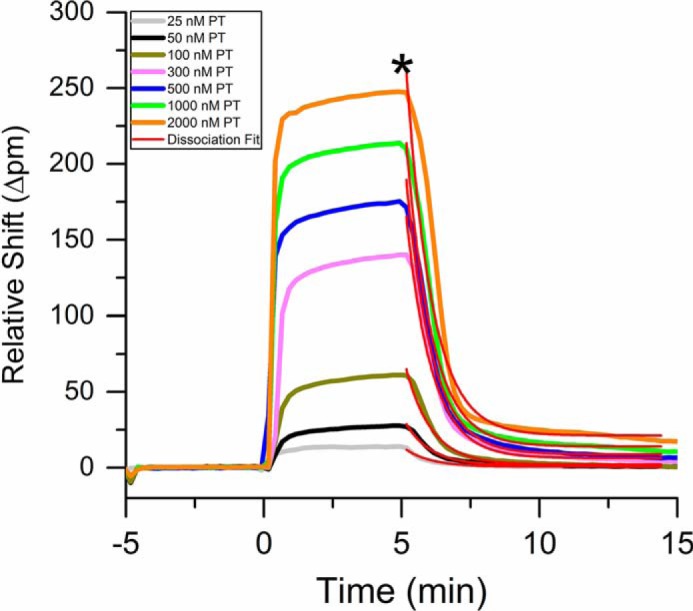
Real-time resonance shifts measured during the binding and unbinding of PT to nanodiscs with a lipid composition of 50% PS 50% PC. After achieving a stable baseline in buffer, PT was introduced at varying conditions at t = 0. After 5 min, the solution was changed to HEPES buffer (*), and dissociation was observed. The red traces are fits to the dissociation phase obtained by globally fitting all concentrations using Equation 2.
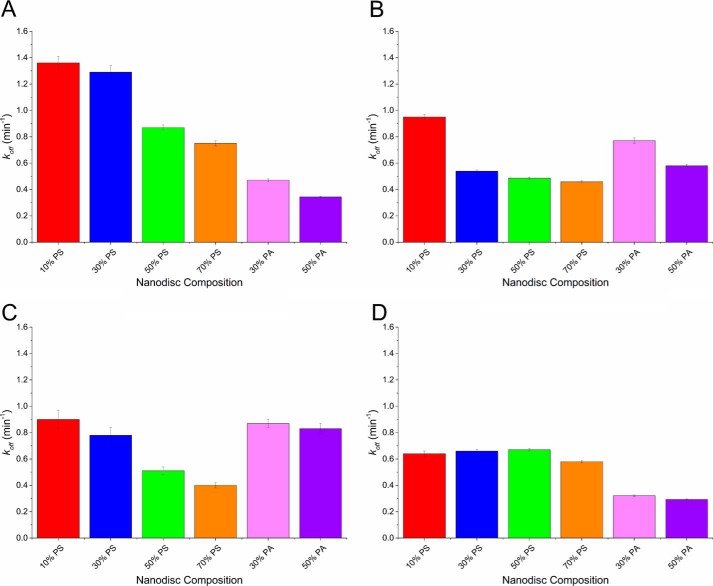
The resulting koff values for each coagulation protein and lipid composition are shown graphically in Fig. 4 and numerically in supplemental Table S3. Like Kd, the overall trend showed a decrease in koff as the percentage of PS increased, but the overall changes were much smaller (Fig. 4, red bars). As the percentage of PS was increased from 10 to 70%, fVIIa saw the largest decrease (55%), and APC saw the lowest (10%). PT and fX decreased 45 and 52%, respectively. The koff values of each protein were much closer than was seen for Kd values. At 50% PS, the range of koff values was 0.66–0.87 min−1 (supplemental Table S3). Another interesting difference was that APC had the lowest koff at 10% PS, whereas PT had the highest (0.64 and 1.36 min−1, respectively). When analyzing the koff values for PA containing nanodiscs, there was a decrease in koff as the percentage of PA increased. Both fVIIa and APC had the lower koff values when comparing PA to PS containing nanodiscs with koff values of 50% PA nanodiscs of 0.344 and 0.293 min−1, compared with 0.51 and 0.67 min−1 for 50% PS, respectively (supplemental Table S3).
Figure 4.
The koff values in min−1 of PT (A), fX (B), fVIIa (C), and APC (D) binding to variable lipid content nanodiscs, as indicated. The koff of each protein nanodisc was determined by stacking the association and dissociation curves of different protein concentrations to a single lipid composition and fitting protein dissociation curves to Equation 2. The error bars represent the standard deviation from at least n = 8 microrings in a single detection experiment.
Discussion
PT, fX, fVIIa, and APC bind to the membrane surface through their GLA domains (24, 25). Each of the proteins' GLA domains are homologous and have multiple, specific binding sites for PS head groups; however, PS is not the only lipid that can affect clotting protein binding and activation. Other lipids have been shown to synergize with PS to reduce the amount of PS lipids needed for full activation of fVIIa and fX (24). One such lipid is PA, and its effects on fVIIa and APC binding are especially clear from the presented microring resonator array results. Both of these coagulation proteins bind with lower Kd to PA lipid-presenting nanodiscs, compared with those with equivalent amounts of PS. They also show tighter binding as the amount of PA is increased.
Examining Kd values reveals several interesting trends in protein binding as a function of lipid composition. As expected from previous studies (9, 33), the Kd had an inverse relationship with PS and PA concentration. That is, coagulation protein binding was generally tighter (smaller Kd) with greater amounts of anionic lipid in the nanodiscs. The fact that all of the proteins bound more tightly as PS content increased is consistent with the literature (9).
A considerable advantage to using microring resonator technology is the direct comparison of different proteins binding to the same nanodisc-modified sensor array. This unprecedented continuity across multiple experiments allows for a more confident analysis of results. Although our results largely support those found previously, the ability to directly compare across proteins allows for a deeper interpretation. One such insight comes from the finding that at a high percentage of PS, binding affinities for the coagulation proteins tested converge, with fVIIa and APC binding significantly better than at high percent PS. Interestingly, PS has been shown to undergo calcium-induced clustering in vitro (11), and thus the presence of PS-rich nanodomains within the lipid bilayer may act to recruit fVIIa and APC to PA-deficient membrane surfaces under certain conditions.
PT, fX, fVIIa, and APC bind to the membrane surface through their GLA domains (24, 25). Each of the proteins' GLA domains are homologous and have multiple, specific binding sites for PS head groups, but PS is not the only lipid that can affect clotting protein binding and activation. Other lipids have been shown to synergize with PS to reduce the amount of PS lipids needed for full activation of fVIIa and fX (24). One such lipid is PA, and its effects on fVIIa and APC binding are especially clear from the presented microring resonator array results. Both of these coagulation proteins bind with lower Kd values to PA lipid-presenting nanodiscs, compared with those with equivalent amounts of PS. They also show tighter binding as the amount of PA is increased.
It is worth pointing out that the increased binding affinity does not strictly lead to a larger magnitude of observed resonance shift caused by variable amounts of specific nanodisc loaded onto the surface. As we showed previously (23), the charge of lipids within nanodiscs plays a large role in determining the relative amount of physisorption, with nanodiscs with higher percentages of negatively charged lipids (PS and PA) having reduced loading because of electrostatic repulsion with the natively negatively charges SiOx surface of the microring. This can cause a reduced magnitude of resonance shift. However, Kd values mathematically determined from fitting of the data to the single-site binding model do reflect the expected trends as a function of lipid composition. In general, Kd values obtained using the silicon photonic microring resonator platform are lower than those generally obtained using SPR (33); however, the trends as a function of lipid composition are identical. Furthermore, the internal consistency of these simultaneously performed measurements lends further confidence in the resulting trends.
Importantly, by using the nanodisc-functionalized microring sensor array, a single experiment yielded internally consistent Kd values for six distinct interactions per coagulation protein. This is in contrast to lower throughput methods that would require many more measurements across multiple sensor substrates and multiple days of experimentation to obtain this amount of binding interaction data, a laborious process that introduces experimental uncertainties and is prone to run-to-run variation.
Similar to Kd values, koff values also show trends related to the anionic lipid content in nanodiscs. Specifically, the rate of unbinding is lower as the amount of PS or PA is increased, which is consistent with the measured lower Kd values (tighter binding). For fVIIa and APC, the koff values are also proportionally lower for PA-containing discs, compared with equivalent amounts of PC. Notably, the variation within koff alone is not enough to fully explain differences in Kd, suggesting that there must also be corresponding differences in the kinetic rate of binding, kon (Kd = kon/koff) for different protein–lipid combinations. However, as mentioned earlier, direct measurement of kon is difficult because of the multivalent nature of the GLA domain interaction (6–8 lipids bound/GLA domain (24)) and the likely need for lipid rearrangement during binding of GLA domains (leading to complicated binding kinetics). However, the coupled Kd and koff values obtained using this technology platform provide confidence in inferring variations in kon.
The interactions of clotting proteins with cell membrane lipids is critical to the blood coagulation cascade, as well as many other biological processes. Previous work studying protein–lipid interactions has been relatively low throughput, so only a limited number of proteins or lipid compositions are examined. This paper introduces a multiplexed technique where interactions with many different lipid compositions can be monitored simultaneously in real time. Because nanodiscs were spotted and allowed to physisorb to the surface of the chip, no tags were required to monitor binding. Here, the binding constants of four coagulation proteins (PT, fX, fVIIa, and APC) binding to nanodiscs of six different lipid compositions were determined. Although absolute Kd values were lower than those reported by SPR, qualitative trends in terms of binding as a function of anionic lipid content was consistent. The benefits of utilizing both nanodiscs and microring resonator technologies for probing protein–membrane interactions are numerous. Unlike liposomes, the local (nanoscale) lipid composition of nanodiscs can be precisely controlled because of their small size, making them an ideal substrate on which to study these interactions. The simultaneous interrogation of numerous binding interactions from a single measurement using the photonic microring resonators increases intra-assay precision and eliminates experimental variability. Additionally, because of its multiplexed nature, utilization of microring resonators decreases the consumption of precious reagents and reduces instrument time compared with traditional techniques such as Biacore. Finally, the ability to evaluate the binding of numerous proteins to the same lipid surface eliminates inherent differences in substrate (i.e. nanodisc) loading and the associated run-to-run variability.
In the future, this technique can be applied to further examine the effect of different lipids on the binding of clotting proteins to membranes, as well as ternary and quaternary lipid mixtures. Previous work has shown that combinations of lipids with PS have led to synergistic binding and activation for fVIIa and APC (9, 33). Similar binding studies of other clotting proteins containing GLA domains could be obtained in a high throughput manner using the silicon photonic microring resonator platform in combination with multiplex nanodiscs.
Experimental procedures
Materials
POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine), POPS (1-palmitoyl-2-oleoyl-sn-glycero-2-phosphoserine), and POPA (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidic acid) were purchased from Avanti Polar Lipids (Alabaster, AL). MSP1D1 was expressed in Escherichia coli and purified as described previously (3). fX, PT, and APC were purchased from Enzyme Research Laboratories (South Bend, IN). A non-clinical, recombinant human factor VIIa produced in mammalian milk was generously provided by rEVO Biologics (Framingham, MA). Amberlite XAD-2 hydrophobic beads, and all other chemicals were purchased from Sigma-Aldrich and used as received, unless otherwise noted. Buffers were prepared with 18.2 mΩ deionized water and sterile filtered prior to use.
Solution preparation
Nanodisc solutions were prepared in a TBS buffer (20 mm Tris-HCl, 100 mm NaCl, and 0.01% (w/v) NaN3, pH 7.4). Clotting protein solutions were made in TBS buffer with 2.5 mm CaCl2 added. All clotting protein solutions were prepared in a HEPES buffer (10 mm HEPES, 150 mm NaCl, 50 μm EDTA, 2.5 mm CaCl2, 0.1% (w/v) PEG 8000). Solutions of fVIIa and APC also contained 0.2% (w/v) BSA, pH 7.4. The HEPES rinse buffer (HEPES(−)) for surface regeneration was made without CaCl2.
Nanodisc preparation and purification
Nanodisc preparation and purification has been described in detail previously (3–5). Briefly, lipids solubilized in chloroform were measured into test tubes and dried under nitrogen. For nanodiscs containing mixtures of POPS and POPC or POPA and POPC, the lipids were mixed at appropriate ratios prior to drying. After drying, lipids were placed in a lyophilizer under vacuum for 60–90 min. Once completely dry, lipids were dissolved in TBS buffer with 100 mm deoxycholate to give a final ratio of 2:1 dexoycholate:phospholipids. Dissolved lipids were then combined with MSP1D1 in TBS to give a final ratio of 70:1 phospholipid:MSP. The solution of MSP and lipids was actively mixed at room temperature for ∼1 h. Half the volume of the MSP/lipid solution of Amberlite XAD-2 hydrophobic beads was added and then left to mix at room temperature for ∼1.5 h. Bio-beads were then removed by filtering through a 0.22-μm syringe filter. Nanodiscs were then purified using size exclusion chromatography. For studies of regeneration stability, nanodiscs were made in an identical fashion but using the larger MSP1E3D1 construct.
Silicon photonic microring resonators
The Maverick M1 optical scanning instrumentation and microring resonator sensor chips were purchased from Genalyte, Inc. (San Diego, CA). The operation of the instrumentation has been previously described (22). The sensor chips were each 4 × 6-mm sensor chips and contained 30-μm diameter active sensor microrings, 128 in total grouped into sets of four, plus four temperature control microrings and two dedicated to detecting leaks from the microfluidic gasket positioned atop the sensor chip during microring detection experiments.
Sensor chip array functionalization
Prior to use, sensor chips were cleaned with a freshly made piranha solution (3:1 H2SO4:30% HOOH) for 30 s and then rinsed with water and dried with N2 (Caution! Piranha solution must be handled with extreme care and will react explosively with organics). The Bioforce Nano eNablerTM was then used to spot nanodisc solutions onto individual clusters of four microrings. A spotting map showing the arrangement of the nanodisc solutions on the sensor substrate is shown in supplemental Fig. S1. Nanodisc solutions containing unique lipid compositions and ranging in concentration from 0.5 to 10 μm. Nanodiscs containing greater percentages of negative charge were spotted at higher concentrations because their physisorption was less efficient because of electrostatic repulsion (23), because the bare silicon microring surface also bears a negative charge. After spotting, chips were stored in a humidity chamber at 4 °C for at least 4 h before use. For array studies, nanodiscs were spotted in buffer lacking Ca2+; however, titrations were also performed using 50% PS and 50% PA nanodiscs spotted in the presence of 2.5 mm Ca2+.
Protein binding titrations
Laser cut Mylar gaskets that directed fluid flow across the chip were aligned onto the functionalized sensor chips, assembled into a Teflon cartridge, and loaded into the sensor scanner instrument. A 2% BSA in TBS buffer was first flowed across the chip surface at 10 μl/min to prevent nonspecific binding of proteins. For the PT and fX titrations in the Kd determination titrations, the proteins were flowed across the chip in increasing concentrations at 10 μl/min, and the response was allowed to equilibrate before the next solution injection. The same was done for fVIIa and APC, but at a 5 μl/min flow rate. Following the series of increasing concentrations, all of the proteins were released from the surface by flowing HEPES(−) buffer solution. The titration was then repeated with the next protein. For the PT and fX titrations used to determine koff rates, a given concentration of protein was flowed across the chip for 5 min at 10 μl/min followed by a 10-min rinse with HEPES buffer to observe dissociation and then a 5-min rinse in HEPES(−) to regenerate the surface. The next concentration of protein was then introduced, and the process was repeated throughout the concentration series. All protein solutions were made in HEPES buffer. This protocol was subsequently repeated for the other three proteins. The same procedure was used for fVIIa and APC koff titrations, except with a flow rate of 5 μl/min, and each rinse step was 15 min.
Data analysis
Data analysis was performed using software provided by Genalyte, Inc., as well as custom scripts written in Origin 9.1. Sensor traces were corrected for temperature fluctuations and any residual nonspecific binding by subtraction of response of 100% POPC nanodiscs. Data fits for determining Kd were performed in Prism and fitting for koff in Origin 9.1.
Author contributions
E. M. M. conducted the majority of the experiments, analyzed the results, and helped in the writing of the paper. J. M. G. prepared nanodiscs, helped with experimental design, and helped write the paper. S. M. M. and Z. S. B. W. helped perform some of the experiments. J. H. M. and R. C. B. conceived the project, advised on experiments and data analysis, and helped write the paper. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
This work was supported by National Institutes of Health Grant GM110432. R. C. B. has a financial interest in Genalyte, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Tables S1–S3 and Figs. S1–S13.
- MSP
- membrane scaffold protein
- PT
- prothrombin
- fX
- factor X
- fVIIa
- activated factor VII
- APC
- activated protein C
- PS
- phosphatidylserine
- PA
- phosphatidic acid
- PC
- phosphatidylcholine
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPS
- 1-palmitoyl-2-oleoyl-sn-glycero-2-phosphoserine
- POPA
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidic acid
- GLA
- γ-carboxyglutamate
- SPR
- surface plasmon resonance.
References
- 1. Fahy E., Subramaniam S., Murphy R. C., Nishijima M., Raetz C. R., Shimizu T., Spener F., van Meer G., Wakelam M. J., and Dennis E. A. (2009) Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 50, S9–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seddon A. M., Curnow P., and Booth P. J. (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim. Biophys. Acta 1666, 105–117 [DOI] [PubMed] [Google Scholar]
- 3. Bayburt T. H., Grinkova Y. V., and Sligar S. G. (2002) Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2, 853–856 [Google Scholar]
- 4. Bayburt T. H., and Sligar S. G. (2010) Membrane protein assembly into nanodiscs. FEBS Lett. 584, 1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denisov I. G., Grinkova Y. V., Lazarides A. A., and Sligar S. G. (2004) Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. Am. Chem. Soc. 126, 3477–3487 [DOI] [PubMed] [Google Scholar]
- 6. Bayburt T. H., Leitz A. J., Xie G., Oprian D. D., and Sligar S. G. (2007) Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 282, 14875–14881 [DOI] [PubMed] [Google Scholar]
- 7. Marty M. T., Zhang H., Cui W., Blankenship R. E., Gross M. L., and Sligar S. G. (2012) Native mass spectrometry characterization of intact nanodisc lipoprotein complexes. Anal. Chem. 84, 8957–8960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schuler M. A., Denisov I. G., and Sligar S. G. (2013) Nanodiscs as a new tool to examine lipid-protein interactions. Methods Mol. Biol. 974, 415–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shaw A. W., Pureza V. S., Sligar S. G., and Morrissey J. H. (2007) The local phospholipid environment modulates the activation of blood clotting. J. Biol. Chem. 282, 6556–6563 [DOI] [PubMed] [Google Scholar]
- 10. Baylon J. L., Lenov I. L., Sligar S. G., and Tajkhorshid E. (2013) Characterizing the membrane-bound state of cytochrome P450 3A4: structure, depth of insertion, and orientation. J. Am. Chem. Soc. 135, 8542–8551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boettcher J. M., Davis-Harrison R. L., Clay M. C., Nieuwkoop A. J., Ohkubo Y. Z., Tajkhorshid E., Morrissey J. H., and Rienstra C. M. (2011) Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry 50, 2264–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denisov I. G., and Sligar S. G. (2017) Nanodiscs in membrane biochemistry and biophysics. Chem. Rev. 117, 4669–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Washburn A. L., Gunn L. C., and Bailey R. C. (2009) Label-free quantitation of a cancer biomarker in complex media using silicon photonic microring resonators. Anal. Chem. 81, 9499–9506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Qavi A. J., Washburn A. L., Byeon J. Y., and Bailey R. C. (2009) Label-free technologies for quantitative multiparameter biological analysis. Anal. Bioanal. Chem. 394, 121–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kindt J. T., Luchansky M. S., Qavi A. J., Lee S. H., and Bailey R. C. (2013) Subpicogram per milliliter detection of interleukins using silicon photonic microring resonators and an enzymatic signal enhancement strategy. Anal. Chem. 85, 10653–10657 [DOI] [PubMed] [Google Scholar]
- 16. Wade J. H., Alsop A. T., Vertin N. R., Yang H., Johnson M. D., and Bailey R. C. (2015) Rapid, multiplexed phosphoprotein profiling using silicon photonic sensor arrays. ACS Cent. Sci. 1, 374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kindt J. T., and Bailey R. C. (2012) Chaperone probes and bead-based enhancement to improve the direct detection of mRNA using silicon photonic sensor arrays. Anal. Chem. 84, 8067–8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qavi A. J., Kindt J. T., Gleeson M. A., and Bailey R. C. (2011) Anti-DNA:RNA antibodies and silicon photonic microring resonators: increased sensitivity for multiplexed microRNA detection. Anal. Chem. 83, 5949–5956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Byeon J.-Y., and Bailey R. C. (2011) Multiplexed evaluation of capture agent binding kinetics using arrays of silicon photonic microring resonators. Analyst 136, 3430–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marty M. T., Sloan C. D., Bailey R. C., and Sligar S. G. (2012) Nonlinear analyte concentration gradients for one-step kinetic analysis employing optical microring resonators. Anal. Chem. 84, 5556–5564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Washburn A. L., Gomez J., and Bailey R. C. (2011) DNA-encoding to improve performance and allow parallel evaluation of the binding characteristics of multiple antibodies in a surface-bound immunoassay format. Anal. Chem. 83, 3572–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iqbal M., Gleeson M. A., Spaugh B., Tybor F., Gunn W. G., Hochberg M., Baehr-Jones T., Bailey R. C., and Gunn L. C. (2010) Label-free biosensor arrays based on silicon ring resonators and high-speed optical scanning Instrumentation. IEEE J. Selected Topics Quantum Electronics 16, 654–661 [Google Scholar]
- 23. Sloan C. D., Marty M. T., Sligar S. G., and Bailey R. C. (2013) Interfacing lipid bilayer nanodiscs and silicon photonic sensor arrays for multiplexed protein–lipid and protein–membrane protein interaction screening. Anal. Chem. 85, 2970–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tavoosi N., Davis-Harrison R. L., Pogorelov T. V., Ohkubo Y. Z., Arcario M. J., Clay M. C., Rienstra C. M., Tajkhorshid E., and Morrissey J. H. (2011) Molecular determinants of phospholipid synergy in blood clotting. J. Biol. Chem. 286, 23247–23253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zwaal R. F., Comfurius P., and Bevers E. M. (1998) Lipid-protein interactions in blood coagulation. Biochim. Biophys. Acta 1376, 433–453 [DOI] [PubMed] [Google Scholar]
- 26. Ohkubo Y. Z., and Tajkhorshid E. (2008) Distinct structural and adhesive roles of Ca2+ in membrane binding of blood coagulation factors. Structure 16, 72–81 [DOI] [PubMed] [Google Scholar]
- 27. Sunnerhagen M., Forsén S., Hoffrén A.-M., Drakenberg T., Teleman O., and Stenflo J. (1995) Structure of the Ca2+-free GLA domain sheds light on membrane binding of blood coagulation proteins. Nat. Struct. Biol. 2, 504–509 [DOI] [PubMed] [Google Scholar]
- 28. Stenflo J., and Suttie J. W. (1977) Vitamin K-dependent formation of gamma-carboxyglutamic acid. Annu. Rev. Biochem. 46, 157–172 [DOI] [PubMed] [Google Scholar]
- 29. Furie B., and Furie B. C. (1988) The molecular-basis of blood-coagulation. Cell 53, 505–518 [DOI] [PubMed] [Google Scholar]
- 30. Banner D. W., D'Arcy A., Chène C., Winkler F. K., Guha A., Konigsberg W. H., Nemerson Y., and Kirchhofer D. (1996) The crystal structure of the complex of blood coagulation factor VIIa with soluble tissue factor. Nature 380, 41–46 [DOI] [PubMed] [Google Scholar]
- 31. McDonald J. F., Shah A. M., Schwalbe R. A., Kisiel W., Dahlbäck B., and Nelsestuen G. L. (1997) Comparison of naturally occurring vitamin K-dependent proteins: correlation of amino acid sequences and membrane binding properties suggests a membrane contact site. Biochemistry 36, 5120–5127 [DOI] [PubMed] [Google Scholar]
- 32. Nelsestuen G. L., Kisiel W., and Di Scipio R. G. (1978) Interaction of vitamin K dependent proteins with membranes. Biochemistry 17, 2134–2138 [DOI] [PubMed] [Google Scholar]
- 33. Tavoosi N., Smith S. A., Davis-Harrison R. L., and Morrissey J. H. (2013) Factor VII and protein C are phosphatidic acid-binding proteins. Biochemistry 52, 5545–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.