Abstract
Mitochondrial cytochrome oxidase (COX) catalyzes the last step in the respiratory pathway. In the yeast Saccharomyces cerevisiae, this inner membrane complex is composed of 11 protein subunits. Expression of COX is assisted by some two dozen ancillary proteins that intercede at different stages of the assembly pathway. One such protein, Cox16p, encoded by COX16, was shown to be essential for the activity and assembly of COX. The function of Cox16p, however, has not been determined. We present evidence that Cox16p is present in Cox1p assembly intermediates and in COX. This is based on the finding that Cox16p, tagged with a dual polyhistidine and protein C tag, co-immunopurified with Cox1p assembly intermediates. The pulldown assays also indicated the presence of Cox16p in mature COX and in supercomplexes consisting of COX and the bc1 complex. From the Western signal strengths, Cox16p appears to be substoichiometric with Cox1p and Cox4p, which could indicate that Cox16p is only present in a fraction of COX. In conclusion, our results indicate that Cox16p is a constituent of several Cox1p assembly intermediates and of COX.
Keywords: cytochrome c oxidase (complex IV), electron transfer complex, membrane biogenesis, mitochondria, yeast
Introduction
Cytochrome oxidase (COX)2 is a hetero-oligomeric complex of the mitochondrial inner membrane that catalyzes the last step of the respiratory pathway by oxidizing ferrocytochrome c and reducing molecular oxygen to water. The structures and spatial organization of the 13 subunits of bovine COX have been determined from its crystal structure (1). COX of Saccharomyces cerevisiae has been determined to consist of 11 subunits that are homologous to their bovine counterparts (Table 1). Two of the bovine subunits, however, are absent in the complex of S. cerevisiae and from genomic data do not appear to have homologues in this yeast. The catalytic core of COX consists of Cox1p, Cox2p, and Cox3p that are derived from mitochondrial genes in yeast and in mammalian organisms. All the other subunits of the complex are encoded by nuclear genes. With a few exceptions, the non-catalytic subunits of yeast have been shown to be essential for COX assembly (Table 1). Recently, a new low-molecular-weight protein, Cox26p (2, 3), was reported to be associated with yeast COX in the supercomplexes. This subunit encoded by COX26 does not affect biogenesis of COX. A knock-out of the gene affects enzyme activity only when cells are exposed to high temperature (2).
Table 1.
Relationship of bovine and yeast cytochrome oxidase subunits
| Bovine subunit | Yeast subunit | Yeast gene | Subunit contacta | Activityb | Assemblyc |
|---|---|---|---|---|---|
| I | 1 | COX1 | 2, 3, 4, 5a | − | − |
| II | 2 | COX2 | 1, 6b, 7a | − | − |
| III | 3 | COX3 | 1, 4, 7, 5a, 6b | − | − |
| IV | 5a | COX5a | 1, 6 | − | − |
| Va | 6 | COX6 | 5a, 7a | − | − |
| Vb | 4 | COX4 | 1, 3 | − | − |
| VIa | 6a | COX13 | 3 | + | +/− |
| VIb | 6b | COX12 | 2, 3 | +/− | + |
| VIc | 7a | COX9 | 2, 6 | − | − |
| VIIa | 7 | COX7 | 3 | − | − |
| VIIb | |||||
| VIIc | 8 | COX8 | 1 | + | + |
| VIII |
a The subunit contacts are those reported for bovine COX (1).
b The presence (+) or absence (−) of COX activity is based on the ability of isolated yeast mitochondria to catalyze cyanide sensitive oxidation of ferrocytochrome c.
c The failure of COX subunits to assemble in the absence of a subunit is indicated as −. Normal levels of assembled COX are indicated by +, and reduced levels are indicated by +/−.
Biogenesis of COX depends on a large number of accessory proteins with functions in translation of COX-specific mitochondrial mRNAs (4–6), membrane insertion and processing of Cox2p (7, 8), regulation of Cox1p translation (9–13), insertion of heme A and copper (14, 15), and proteins such as Cox16p (16) with still uncharacterized functions. Cox16p is a low-molecular-weight protein present in all fungi, insect, and mammalian mitochondria where it is a component of the inner membrane. It is essential for COX assembly and hence activity. Mutations in the yeast COX16 gene elicit a phenotype characteristic of other COX mutants, highlighted by the absence of enzyme activity and cytochromes a and a3 and extensive proteolysis of the catalytic core subunits (16). To further probe the function of Cox16p, we have tested whether it is a component of the Cox1p module, a well studied precursor of cytochrome oxidase (10, 12, 13, 17–19). The results presented here indicate that Cox16p is a constituent of several Cox1p assembly intermediates and of COX.
Results
Cox16p is associated with the Cox1p module
The presence of Cox16p in the Cox1p module was assessed in a strain of yeast (W303/COX16-CH) expressing Cox16p with a C-terminal double tag consisting of the protein C epitope followed by polyhistidine (CH). W303/COX16-CH grew as well as the parental wild type strain on non-fermentable carbon sources (Fig. 1A), indicating that the presence of the tag (Fig. 1B) did not significantly affect the function of Cox16p. In-gel assays of COX activity were also consistent with a full measure of active enzyme in the strain expressing tagged COX16 (Fig. 1C). Other strains used in this study expressing different subunits of COX tagged with HAC (hemagglutinin followed by the protein A tag) at their C terminus (Table 2) grew as well as the wild type on non-fermentable carbon sources.
Figure 1.
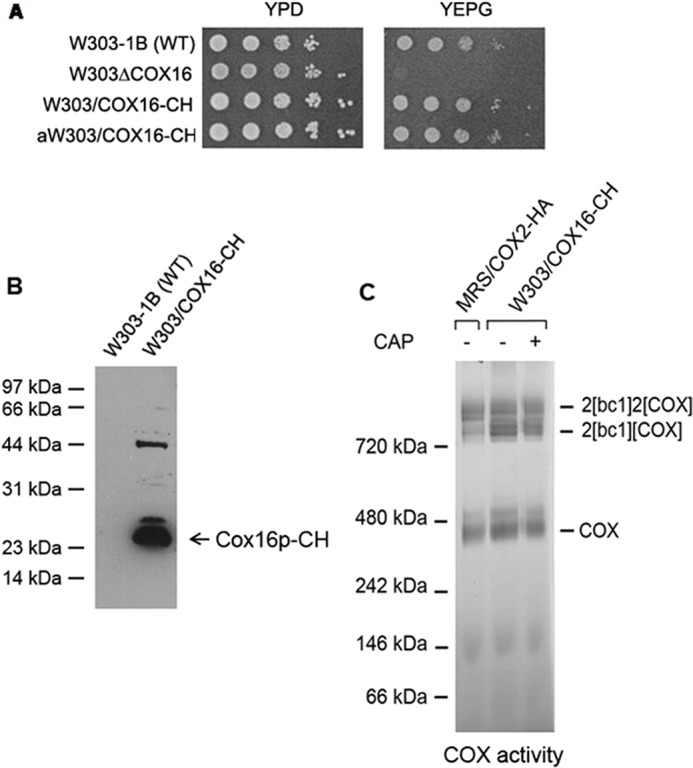
Properties of strains expressing COX16-CH. A, the indicated strains were serially diluted and spotted on YPD (rich glucose) and YEPG (rich ethanol plus glycerol) plates. The photograph was taken after 2 days of growth at 30 °C. B, mitochondria (50 μg of protein) prepared from W303/COX16-CH and the parental strain W303-1B were separated by SDS-PAGE on a 12% polyacrylamide gel. The blot was first reacted with a polyclonal antibody to the protein C tag followed by a second anti-rabbit antibody coupled to peroxidase. Proteins were detected with SuperSignal chemiluminescent substrate kit (Pierce). C, mitochondria (500 μg of protein) from the indicated strains were extracted with digitonin, purified on protein C antibody beads, and separated by BN-PAGE on a 4–13% polyacrylamide gel. The gel was stained for COX activity as described previously (13).
Table 2.
Genotypes and sources of S. cerevisiae strains
| PRIVATE strain | Genotype | mtDNA | Source |
|---|---|---|---|
| W303-1A | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 | ρ+ | a |
| W303-1B | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 | ρ+ | a |
| MRSIo/COX1-HAC | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 arg8::HIS3 | Intronless mtDNA with COX1-HAC | Ref. 13 |
| W303/COX4-HAC | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox4:: URA3 trp1::COX4-HAC | ρ + | Ref. 13 |
| W303/COX5-HAC | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox5:: URA3 trp1::COX5-HAC | ρ + | Ref. 13 |
| W303/COX6-HAC | MATα ade2-1 his3-1,15 leu2--3,112 trp1-1 ura3-1 cox6:: URA3 trp1::COX6-HAC | ρ + | Ref. 13 |
| aW303/COX16-CH | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox16:: URA3 trp1::pG22/ST13 | ρ + | This study |
| W303/COX16-CH | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox16:: URA3 trp1::pG22/ST13 | ρ + | This study |
| W303ΔCBP3/COX16-CH | MATα ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox16:: URA3 cbp3:: HIS3 trp1::pG22/ST13 | ρ + | This study |
| aW303ΔCOX16/COX1-HAC | MATa ade2-1 his3-1,15 leu2-3,112 trp1-1 ura3-1 cox16::URA3 | Intronless mtDNA with COX1-HAC | This study |
a R. Rothstein, Department of Human Genetics and Development, Columbia University.
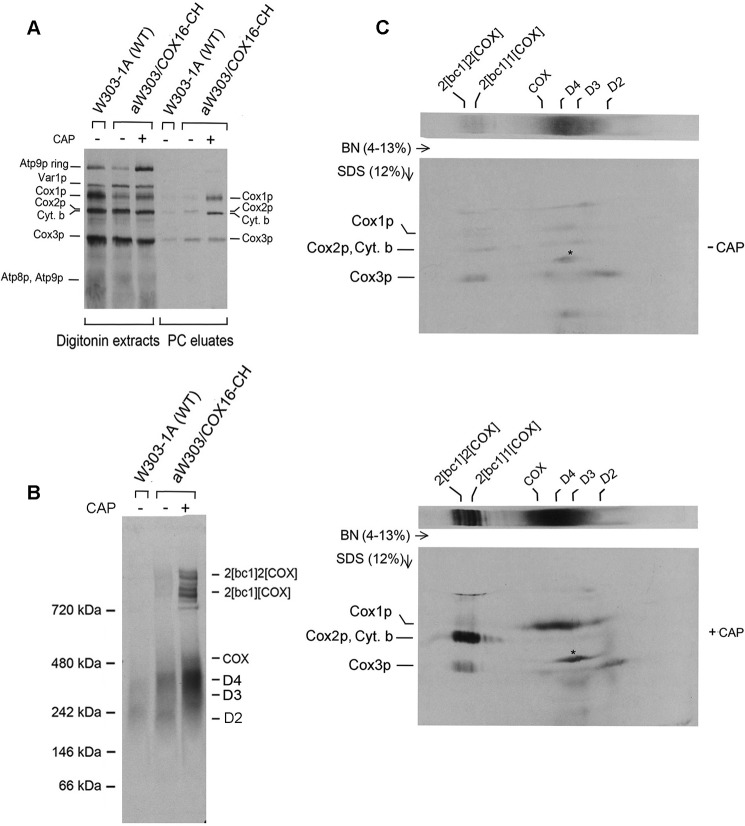
Mitochondria of W303/COX16-CH were isolated from cells grown in galactose with and without an additional 2 h of growth in medium containing chloramphenicol. The chloramphenicol treatment has been shown in other studies to enhance translation of some mitochondrial gene products (20), presumably as a result of the increased pools of nuclear gene products needed for assembly of respiratory and ATP synthase complexes. Mitochondria were pulse-labeled with [35S]methionine plus cysteine, extracted with digitonin and the CH-tagged Cox16p in the digitonin extract purified on protein C antibody beads. Analysis of the digitonin extracts by SDS-PAGE indicated a small increase in incorporation of the radioactive precursors into Cox1p of mitochondria isolated from chloramphenicol-treated cells. Consistent with prior results, the chloramphenicol incubation substantially increased translation of cytochrome b and Atp9p (Fig. 2A). The increase in translation of cytochrome b and Cox1p in mitochondria of chloramphenicol-treated cells was also evident in the SDS gel of the fractions containing affinity-purified Cox16p-CH (Fig. 2A).
Figure 2.
Cox16p is a constituent of the Cox1p module. A, mitochondria were prepared from the wild-type W303-1B and aW303/COX16-CH expressing COX16-CH. The latter strain was grown with and without a terminal 2-h growth in the presence of chloramphenicol (CAP). Mitochondria (250 μg of protein) were labeled with [35S]methionine plus [35S]cysteine for 20 min, extracted with digitonin, and purified on protein C beads as described under “Experimental procedures.” The eluate (10%) was separated by SDS-PAGE on a 12% polyacrylamide gel. The radiolabeled mitochondrial gene products were transferred to a PVDF membrane and exposed to X-ray film. B, BN-PAGE of 30% of the eluates obtained in A were separated on a 4–13% polyacrylamide gel. The positions of the COX intermediates with Cox1p (D2, D3, and D4) are marked in the margin. C, the remaining 60% of the eluate in A was separated in the first dimension by BN-PAGE on a 4–13% polyacrylamide gel, followed by SDS-PAGE on a 12% polyacrylamide gel in the second dimension. The increase in incorporation of radiolabel by mitochondria as a result of the chloramphenicol treatment is seen in increased translation of cytochrome b and Cox1 intermediates D2–D4. The identity of the radioactive band labeled with an asterisk was not studied.
BN-PAGE analysis of affinity-purified Cox16p-CH revealed a dramatic increase in co-immunoadsorption of newly assembled supercomplexes as a result of the chloramphenicol incubation. The chloramphenicol treatment led to an increase in radiolabeled material that migrated between the 480- and 242-kDa markers (Fig. 2B). This region was previously shown to contain the D3 and D4 assembly intermediates of Cox1p (13, 21). Two-dimensional electrophoresis of the Cox16-CH enriched fraction confirmed a large increase of labeled Cox1p in the region corresponding to the D3 and D4 assembly intermediates of COX (Fig. 2C). Thus, most of the radioactivity between the 242- and 480-kDa markers migrated as Cox1p following depolymerization and separation in the second dimension by SDS-PAGE (Fig. 2C). These results indicate that Cox16p is a component of the Cox1p module (13, 21). The two-dimensional gels also revealed a large chloramphenicol-induced increase of radiolabeled cytochrome b in the supercomplexes (Fig. 2C).
Cox16p is associated with COX and the supercomplexes
The observation that the supercomplexes are pulled down with Cox16p-CH suggested that it may be associated with COX. This was borne out by Western analysis of the fraction affinity-purified on the protein C antibody beads. The fraction eluted from the beads was separated by BN-PAGE and visualized with protein C antibody. The blot indicated the presence of Cox16p in COX and the supercomplexes (Figs. 2B and 3, A and B). The concentrations of the radiolabeled D3 and D4 intermediates are detected as radiolabeled proteins, but their concentrations are too low to be detected immunochemically, even after a long exposure to X-ray film.
Figure 3.
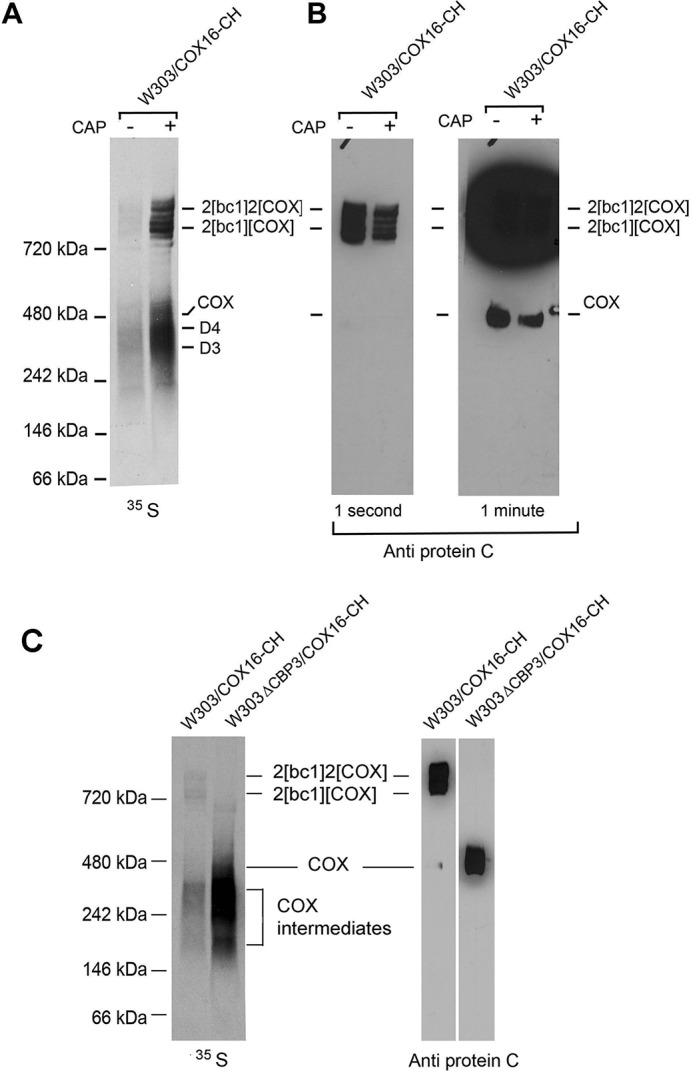
Cox16p is associated with COX. A, W303/COX16-CH mitochondria were labeled and Cox16p-CH purified on protein C antibody beads as in Fig. 2A. The eluate from the protein C antibody beads was separated by BN-PAGE on a 4–13% polyacrylamide gel and exposed to X-ray film. The positions of the supercomplexes, COX, and D2–D4 intermediates wit Cox1p are indicated in the margin. B, a duplicate of the blue native gel shown in A was transferred to nitrocellulose and reacted with antibody to protein C and further processed as in Fig. 1A. The gel on the right was exposed longer to detect the radiolabel associated with COX. The concentrations of the D2–D4 intermediates were too low to detect on the Western. C, mitochondria of W303/COX16-CH and W303ΔCBP3/COX16-CH carrying a mutation in CBP3 were first grown in rich galactose followed by 2 h in galactose medium containing 2 mg/ml chloramphenicol. Mitochondria were labeled, and the fraction was purified on protein C antibody beads separated by BN-PAGE as in A.
Cox16-CH also pulls down COX when it is not part of the supercomplexes. This was shown by introducing a null cbp3 allele into the strain containing Cox16p with the CH tag. (W303ΔCBP3/COX16-CH). The cbp3 mutant lacks the bc1 complex because Cbp3p is required for expression of cytochrome b (22, 23). The absence of the bc1 complex resulted in a higher accumulation of radiolabeled COX and Cox1 intermediates (Fig. 3C). As expected, in the absence of the bc1 complex, all the COX migrated as a dimer. The antibody signal of COX in the bc1 mutant was comparable with that of COX in the supercomplexes of the strain expressing the bc1 complex (Fig. 3C).
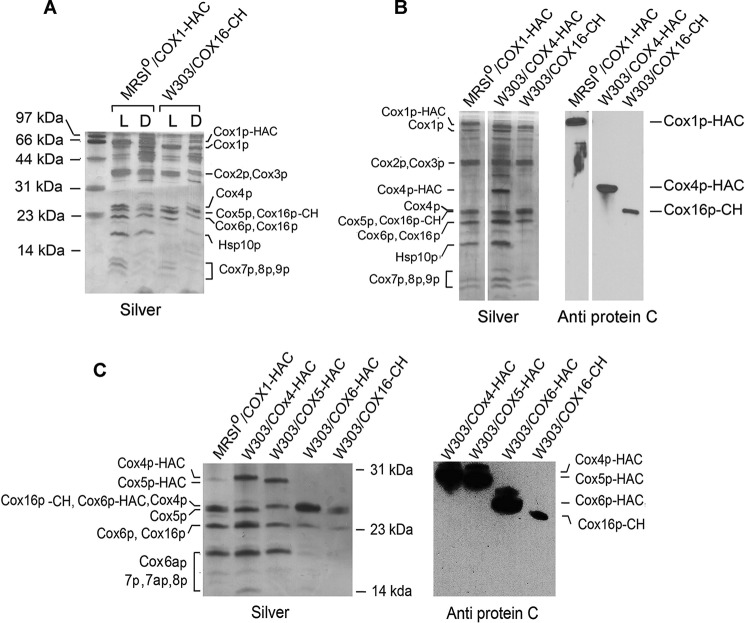
The presence of Cox16p in COX was also was confirmed by purifying the enzyme from strains that express Cox16p with a CH tag. As a control, COX was also purified from a strain expressing Cox1p with a double HA and protein C tag (Cox1p-HAC). COX was extracted from mitochondria with lauryl maltoside or with digitonin and purified on protein C antibody beads. Proteins were separated by SDS-PAGE under conditions that resolve low-molecular-weight proteins (24). In contrast to digitonin, lauryl maltoside dissociates the supercomplexes. The silver-stained gel indicated that the major proteins purified from the lauryl maltoside extract corresponded to known subunits of COX, although there were some contaminating proteins in the high molecular weight region of the gel (Fig. 4A).
Figure 4.
Purification of COX with Cox16. A, mitochondria were prepared from MRSIo/COX1-HAC and W303/COX16-CH expressing Cox1p-HAC and Cox16-CH, respectively. The mitochondria (2 mg) were extracted with either 2% lauryl maltoside (L) or 2% digitonin (D). The extracts were purified on protein C antibody beads, and of the eluates were separated by SDS-PAGE on a 15% gel (22). The silver-stained proteins are identified in the margin. Some of the extra bands of the bc1 complex seen in the digitonin extract are absent in Cox16p-CH purified from lauryl maltoside extracted mitochondria because this detergent dissociates the supercomplexes, and only the Cox16p-CH associated with COX is recovered from the protein C beads. B, to confirm identity of the bands migrating in the region between Cox6p and smaller subunits in A, COX was purified on protein C antibody beads from strains expressing Cox1p-HAC, Cox4p-HAC, and Cox16p-CH. Proteins were separated and silver-stained as in A. A duplicate gel was transferred to a PVDF membrane and probed with an antibody to the protein C epitope as in Fig. 1A. C, Western analysis of COX affinity-purified from strains expressing Cox1p-HAC with and without a cox16 null mutation and from strains expressing either Cox4p-HAC Cox5p-HAC, Cox6p-HAC, and Cox16p-CH.
The purified fraction obtained from the digitonin extract contained additional proteins, which are probably subunits of the bc1 complex because this detergent does not dissociated the yeast supercomplexes. No attempt was made to identify the extra bands in the digitonin extract that co-purify with either Cox1p-HAC or Cox16p-CH. The only significant difference between the enzyme pulled down with the tagged Cox1p and Cox16p was the presence in the former of a protein of ∼18 kDa (Fig. 4A). This protein was absent in COX purified recovered in the Cox16p-CH pulldown (Fig. 4A). This protein was identified as Hsp10p by mass spectrometry. The failure of tagged Cox16p to pull down Hsp10p suggests that that different assembly intermediates may exist, some of which contain Cox1p and Hsp10p but not Cox16p.
Alignment of a silver and antibody-stained gel revealed that the antibody detected Cox16p-CH at the position of Cox5p (Fig. 4B). In the absence of an antibody to Cox16p, it was not possible to determine the position of the untagged protein. However, assuming that the tag on Cox16p elicits a shift in its migration similar to that observed for Cox4p, Cox5p, and Cox6p, untagged Cox16p would be expected to migrate in the region of Cox 6p. This is supported by the silver-stained gel of COX purified from a strain expressing Cox16-CH. The upward shift in the migration of Cox16p-CH elicits an increase in staining at the position of Cox4p and Cox5p with a concomitant decrease but not complete disappearance of stain at the position of Cox6p (third lane in Fig. 4C). The remaining stain at the position of Cox6p in this strain is probably Cox16p. The strain expressing Cox16p-CH also showed a decrease of silver staining at the position of Cox6 (last lane in Fig. 4C). The silver-stained band remaining in this strain is probably Cox6p.
Discussion
COX is a hetero-oligomeric member of bacterial and mitochondrial respiratory chains. X-ray crystallography of bovine COX indicates that it is composed of 13 unique subunit polypeptides (1). Three core subunits that are in encoded in mtDNA are homologous to the three subunits of the bacterial complex. Whereas bacterial COX is composed of three or four subunits (25), mitochondrial COX has additional subunits that appear to play a structure role but do not contribute to the mechanism of electron transfer and proton translocation across the inner membrane. These nuclear encoded subunits surround the three catalytic core subunits. The eight structural subunits of yeast COX are all homologous to the bovine subunits. Two of the bovine subunits (VIIb and VIII), however, have no homologues in yeast (Table 1). Although the composition of bovine COX has been determined from crystallographic studies, that of yeast has been inferred from a combination of SDS-PAGE separations of the subunits in purified COX (26), purification of isolated polypeptides from the complex (27, 28), and genetic and genomic data (29, 30).
Recent studies indicate the presence in the yeast supercomplexes of COX with a 12th low-molecular-weight subunit protein (Cox26p) (2, 3). In the present study, we present evidence that Cox16p, a protein previously proposed to be a COX-specific assembly factor (16), is associated with yeast with either COX itself or with the supercomplexes. This is based on the following evidence. 1) Pull down assays with tagged Cox16p co-immunopurify the D2, D3 and D4 but not the smaller D1 (13, 21) assembly intermediates of Cox1p. 2) Cox16p is also associated with mature COX and with COX present in the supercomplexes. This was shown both by immune adsorption of Cox16p-CH and by Western analysis of affinity-purified COX separated by BN-PAGE. Mass spectrometry analysis also indicated the presence of Cox16p in the yeast supercomplexes (31).
Cox16p-CH migrates in the region of Cox4p and Cox5p when the purified complex is separated by SDS-PAGE. Native Cox16p, however, probably migrates together with Cox6p. Based on the antibody-antigen signal strength, Cox16p appears to be substoichiometric with Cox1p and Cox4p. This could be a consequence of a difference in the accessibility to the protein C antibody of the tags on the two proteins. Alternatively, Cox16p may be associated with only a fraction of COX.
Based on protein sequence alignments and hydrophilicity profiles, Cox16p does not appear to be related to either subunit VIIB or VIII of bovine COX. The yeast protein, however, has homologues in a broad range of organisms including fungi and mammals. An alignment of the yeast, mushroom, and human proteins are shown in Fig. 5A. The three proteins also share a similar hydrophilicity profile, although yeast Cox16p has a longer hydrophilic N-terminal sequence. The presence in the bovine genome of a Cox16p homologue and its absence in the purified COX complex are perplexing. One possible explanation is that the interaction of the mammalian Cox16p with mammalian COX is weaker than it is in yeast and as a consequence is shed during purification, which involves use of strong detergents such as lauryl maltoside or bile acids.
Figure 5.
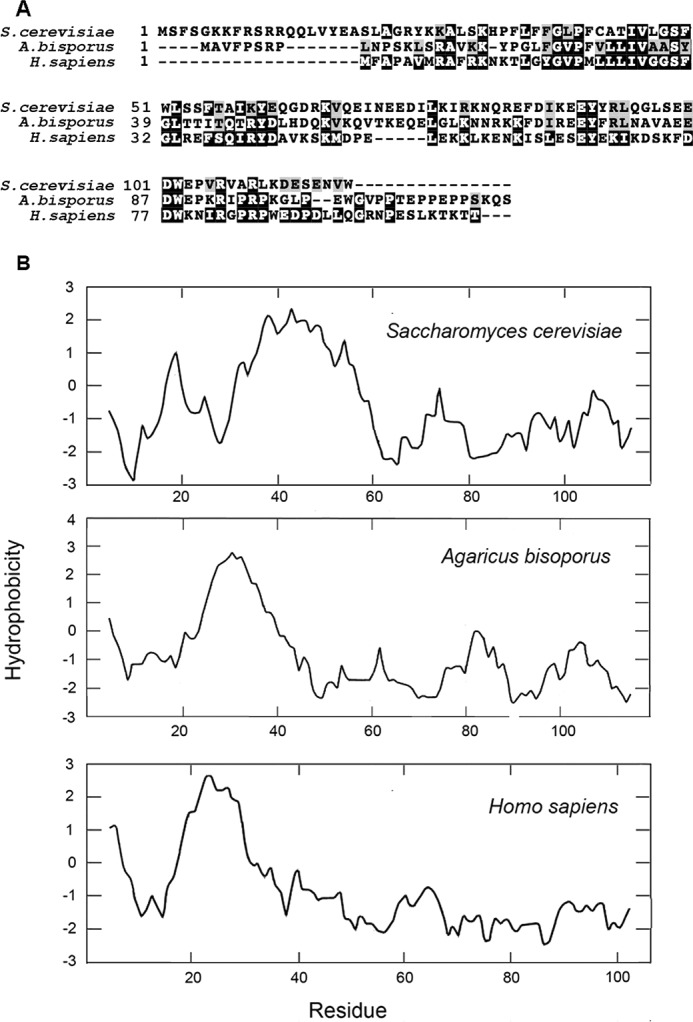
Homology and Kyte–Doolittle hydrophobicity plots of yeast, mushroom and human Cox16p. A, proteins were aligned by the Clustal Omega and formatted with BlockShade. B, the Kyte–Doolittle parameters with window size of 9 was used to plot the hydrophobicity of yeast Cox16p and of the homologous mushroom and human proteins.
Experimental procedures
Strains and growth media
The genotypes and sources of the S. cerevisiae strains used in this study are described in Table 2. The compositions of solid and liquid YPD, YPGal, and YEPG have been described previously (32).
Construction of COX16-CH
COX16-CH, expressing Cox16p with a C-terminal tandem protein C followed by a polyhistidine tag, was constructed by PCR amplification of the gene with 500 bp of 5′-UTRs plus the coding sequences (minus the termination codon) from purified nuclear DNA of W303-1B with primers 5′-ggcgagctctctgggaaggtttcaactcat and 5′-ggcctgcagccagacattctcagattcatc. The PCR product was digested with a combination of SacI plus PstI and was ligated to YIp349-CH, an integrative plasmid with a TRP1 selectable marker and the CH cassette (gaagatcaggtagatccacggttaatcgatggtaagggaggaggacaccatcaccatcatcactaa) inserted between PstI and HindIII sites of the multiple cloning sequence of YIp349. YIp349 is similar to YIp351 (33), except that LEU2 is replaced with TRP1. The resultant plasmid (pG22/ST13) was linearized by digestion with BstX1 internal to TRP1 and was integrated at the trp1 locus of W303-1B (34).
Growth of yeast, isolation of mitochondria and labeling of mitochondria, and affinity purification of CH-tagged proteins
Yeast was grown in YPGal and mitochondria isolated by the method of Herrmann et al. (35). Small aliquots of mitochondria were frozen in liquid nitrogen and stored at −80 °C. Unless otherwise indicated, the mitochondria (250 μg of protein) were labeled for 20 min at 25 °C with [35S]methionine/cysteine (3,000 Ci/mmol; MP Biochemicals, Solon, OH) (13). The labeled mitochondria were extracted with digitonin and purified on protein C antibody beads as described previously.
Miscellaneous procedures
Purification, ligation and transformation of DNA in Escherichia coli were done under standards conditions (36). Yeast was transformed by the lithium acetate method of Schiestl and Gietz (37). Unless otherwise indicated, the proteins were separated by either by SDS-PAGE on 12% in standard Laemmli buffer (38) or in 17.5% polyacrylamide with a ratio of acrylamide to bis-acrylamide of 30:0.2 and run in Laemmli buffer (36) adjusted to pH 8.3. Proteins were separated by BN-PAGE on 4–13% polyacrylamide gels (39). Western blots were treated with a polyclonal antibody to the protein C epitope followed by a second reaction with anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase (Sigma), and proteins were detected with SuperSignal chemiluminescent substrate kit (Pierce). The method of Lowry (40) was used to estimate protein concentration.
Author contributions
A. T. and C.-H. S. contributed to conception and design, acquisition of data, and analysis and interpretation of data. A. T. and C.-H. S. contributed toward drafting the article. Both authors approved the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This work was supported by NIGMS National Institutes of Health Grant 5R01GM111864. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- COX
- cytochrome oxidase
- BN-PAGE
- blue native PAGE.
References
- 1. Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., and Yoshikawa S. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 272, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 2. Strecker V., Kadeer Z., Heidler J., Cruciat C. M., Angerer H., Giese H., Pfeiffer K., Stuart R. A., and Wittig I. (2016) Supercomplex-associated Cox26 protein binds to cytochrome c oxidase. Biochim. Biophys. Acta 1863, 1643–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levchenko M., Wuttke J. M., Römpler K., Schmidt B., Neifer K., Juris L., Wissel M., Rehling P., and Deckers M. (2016) Cox26 is a novel stoichiometric subunit of the yeast cytochrome c oxidase. Biochim. Biophys. Acta 1863, 1624–1632 [DOI] [PubMed] [Google Scholar]
- 4. Costanzo M. C., and Fox T. D. (1988) Specific translational activation by nuclear gene products occurs in the 5′ untranslated leader of a yeast mitochondrial mRNA. Proc. Natl. Acad. Sci. U.S.A. 85, 2677–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulero J. J., and Fox T. D. (1993) PET111 acts in the 5′-leader of the Saccharomyces cerevisiae mitochondrial COX2 mRNA to promote its translation. Genetics 133, 509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manthey G. M., and McEwen J. E. (1995) The product of the nuclear gene PET309 is required for translation of mature mRNA and stability or production of intron-containing RNAs derived from the mitochondrial COX1 locus of Saccharomyces cerevisiae. EMBO J. 14, 4031–4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hell K., Tzagoloff A., Neupert W., and Stuart R. A. (2000) Identification of Cox20p, a novel protein involved in the maturation and assembly of cytochrome oxidase subunit 2. J. Biol. Chem. 275, 4571–4578 [DOI] [PubMed] [Google Scholar]
- 8. Saracco S. A., and Fox T. D. (2002) Cox18p is required for export of the mitochondrially encoded Saccharomyces cerevisiae Cox2p C-tail and interacts with Pnt1p and Mss2p in the inner membrane. Mol. Biol. Cell 13, 1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrientos A., Zambrano A., and Tzagoloff A. (2004) Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 23, 3472–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pierrel F., Bestwick M. L., Cobine P. A., Khalimonchuk O., Cricco J. A., and Winge D. R. (2007) Coa1 links the Mss51 post-translational function to Cox1 cofactor insertion in cytochrome c oxidase assembly. EMBO J. 26, 4335–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fontanesi F., Clemente P., and Barrientos A. (2011) Cox25 teams up with Mss51, Ssc1, and Cox14 to regulate mitochondrial cytochrome c oxidase subunit 1 expression and assembly in Saccharomyces cerevisiae. J. Biol. Chem. 286, 555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mick D. U., Fox T. D., and Rehling P. (2011) Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat. Rev. Mol. Cell Biol. 12, 14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McStay G. P., Su C. H., and Tzagoloff A. (2013) Modular assembly of yeast cytochrome oxidase. Mol. Biol. Cell 24, 440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khalimonchuk O., Bestwick M., Meunier B., Watts T. C., and Winge D. R. (2010) Formation of the redox cofactor centers during Cox1 maturation in yeast cytochrome oxidase. Mol. Cell. Biol. 30, 1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rigby K., Cobine P. A., Khalimonchuk O., and Winge D. R. (2008) Mapping the functional interaction of Sco1 and Cox2 in cytochrome oxidase biogenesis. J. Biol. Chem. 283, 15015–15022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carlson C. G., Barrientos A., Tzagoloff A., and Glerum D. M. (2003) COX16 encodes a novel protein required for the assembly of cytochrome oxidase in Saccharomyces cerevisiae. J. Biol. Chem. 278, 3770–3775 [DOI] [PubMed] [Google Scholar]
- 17. Perez-Martinez X., Broadley S. A., and Fox T. D. (2003) Mss51p promotes mitochondrial Cox1p synthesis and interacts with newly synthesized Cox1p. EMBO J. 22, 5951–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bareth B., Dennerlein S., Mick D. U., Nikolov M., Urlaub H., and Rehling P. (2013) The heme a synthase Cox15 associates with cytochrome c oxidase assembly intermediates during Cox1 maturation. Mol. Cell. Biol. 33, 4128–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fontanesi F., Soto I. C., Horn D., and Barrientos A. (2010) Mss51 and Ssc1 facilitate translational regulation of cytochrome c oxidase biogenesis. Mol. Cell. Biol. 30, 245–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rak M., Gokova S., and Tzagoloff A. (2011) Modular assembly of yeast mitochondrial ATP synthase. EMBO J. 30, 920–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McStay G. P., Su C. H., Thomas S. M., Xu J. T., and Tzagoloff A. (2013) Characterization of assembly intermediates containing subunit 1 of yeast cytochrome oxidase. J. Biol. Chem. 288, 26546–26556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu M., and Tzagoloff A. (1989) Identification and characterization of a new gene (CBP3) required for the expression of yeast coenzyme QH2-cytochrome c reductase. J. Biol. Chem. 264, 11122–11130 [PubMed] [Google Scholar]
- 23. Gruschke S., Kehrein K., Römpler K., Gröne K., Israel L., Imhof A., Herrmann J. M., and Ott M. (2011) Cbp3-Cbp6 interacts with the yeast mitochondrial ribosomal tunnel exit and promotes cytochrome b synthesis and assembly. J. Cell Biol. 193, 1101–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schägger H., and von Jagow G. (1987) Tricine-SDS polyacrylamide gel electrophoresis for the separation of proteins in the range from 1–100 kDa. Anal. Biochem. 166, 368–379 [DOI] [PubMed] [Google Scholar]
- 25. Iwata S., Ostermeier C., Ludwig B., and Michel H. (1995) (1995) Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature 376, 660–669 [DOI] [PubMed] [Google Scholar]
- 26. Taanman J. W., and Capaldi R. (1992) A purification of yeast cytochrome c oxidase with a subunit composition resembling the mammalian enzyme. J. Biol. Chem. 267, 22481–22485 [PubMed] [Google Scholar]
- 27. George-Nascimento C., and Poyton R. O. (1981) Further analysis of the polypeptide subunits of yeast cytochrome c oxidase. Isolation and characterization of subunits III, V, and VII. J. Biol. Chem. 256, 9363–9370 [PubMed] [Google Scholar]
- 28. Power S. D., Lochrie M. A., Sevarino K. A., Patterson T. E., and Poyton R. O. (1984) The nuclear-coded subunits of yeast cytochrome c oxidase: I. fractionation of the holoenzyme into chemically pure polypeptides and the identification of two new subunits using solvent extraction and reversed phase high performance liquid chromatography. J. Biol. Chem. 259, 6564–6570 [PubMed] [Google Scholar]
- 29. Tzagoloff A., and Dieckmann C. L. (1990) PET genes of Saccharomyces cerevisiae. Microbiol. Rev. 54, 211–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LaMarche A. E., Abate M. I., Chan S. H., and Trumpower B. L. (1992) Isolation and characterization of COX12, the nuclear gene for a previously unrecognized subunit of Saccharomyces cerevisiae cytochrome c oxidase. J. Biol. Chem. 267, 22473–22480 [PubMed] [Google Scholar]
- 31. Vukotic M., Oeljeklaus S., Wiese S., Vögtle F. N., Meisinger C., Meyer H. E., Zieseniss A., Katschinski D. M., Jans D. C., Jakobs S., Warscheid B., Rehling P., and Deckers M. (2012) Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab. 15, 336–347 [DOI] [PubMed] [Google Scholar]
- 32. Myers A. M., Pape L. K., and Tzagoloff A. (1985) Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 4, 2087–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hill J. E., Myers A. M., Koerner T. J., and Tzagoloff A. (1986) Yeast/E.coli shuttle vectors with multiple unique restriction sites. Yeast 2, 163–167 [DOI] [PubMed] [Google Scholar]
- 34. Rothstein R. J. (1983) One-step gene disruption in yeast. Methods Enzymol. 101, 202–211 [DOI] [PubMed] [Google Scholar]
- 35. Herrmann J. M., Foelsch H., Neupert W., and Stuart R. A. (1994) Isolation of yeast mitochondria and study of mitochondrial protein translation. In Cell Biology: A Laboratory Handbook (Celis J. E., ed) Vol. I, pp. 538–544, Academic Press, San Diego [Google Scholar]
- 36. Sambrook J., Fritsch E. F., and Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 37. Schiestl R. H., and Gietz R. D. (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16, 339–346 [DOI] [PubMed] [Google Scholar]
- 38. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 39. Wittig I., Braun H. P., and Schägger H. (2006) Blue native PAGE. Nat. Protoc. 1, 418–428 [DOI] [PubMed] [Google Scholar]
- 40. Lowry O. H., Rosebrough N. J., Farr A. L., and Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]