Abstract
The syndecan family of heparan sulfate proteoglycans contributes to cell adhesion and communication by serving as co-receptors for cell signaling and extracellular matrix molecules. Syndecan-2 is located at the cell surface, and we previously reported that it induces matrix metalloproteinase-7 (MMP-7) expression in colon cancer cells. However, the underlying regulatory mechanisms are unknown. Here, we report that overexpression of syndecan-2 in HT-29 colon cancer cells increases the phosphorylation of focal adhesion kinase (FAK) and ERK in parallel with up-regulated MMP-7 expression, but a syndecan-2 mutant lacking the cytoplasmic domain showed significant reductions in these effects. Consistent with this observation, FAK inhibition via FAK-related non-kinase expression or inhibition of ERK with the ERK1/2 inhibitor SCH772984 diminished the syndecan-2–mediated up-regulation of MMP-7. Activation of PKC enhanced syndecan-2–mediated MMP-7 expression, whereas inhibition of PKC had the opposite effect. Of note, the exogenous expression of syndecan-2 triggered localization of PKCγ to the membrane. Expression of syndecan-2 harboring a phosphomimetic (S198E) mutation of the variable region of the cytoplasmic domain enhanced MMP-7 expression and FAK phosphorylation. Finally, experimental suppression of shedding of the syndecan-2 extracellular domain did not significantly affect the syndecan-2–mediated up-regulation of MMP-7 in the early period after syndecan-2 overexpression. Taken together, these findings suggest that syndecan-2's cytoplasmic domain up-regulates MMP-7 expression in colon cancer cells via PKCγ-mediated activation of FAK/ERK signaling.
Keywords: extracellular signal-regulated kinase (ERK), matrix metalloproteinase (MMP), protein kinase C (PKC), PTK2 protein tyrosine kinase 2 (PTK2) (focal adhesion kinase) (FAK), receptor protein serine/threonine kinase, receptor regulation, signal transduction, syndecan
Introduction
The members of the syndecan family of heparan sulfate proteoglycans contribute to cell adhesion and cell communication by serving as co-receptors for cell signaling and extracellular matrix (ECM)2 molecules (1). Similar to many other receptors, the syndecans contain three domains (2) that have unique but related functions in signal transduction. The extracellular domain interacts with various extracellular ligands, including growth factors, ECM molecules, cytokine, chemokines, and enzymes (1), to accommodate the functional needs of the cells (3). These interactions initiate signal transduction to regulate cellular functions both inside and outside cells. In particular, extracellular ligand binding induces clustering of syndecan transmembrane domains to initiate intracellular signaling events. Notably, syndecans contain a GXXXG motif that promotes self-association with the core protein (4). In the context of intracellular signaling, however, the cytoplasmic domain sequence is the most critical for regulating syndecan-mediated adhesion and cytoskeletal organization. The cytoplasmic domain of syndecan is essentially composed of two regions of conserved amino acid sequences, C1 and C2. The C1 region participates in direct interactions with the ezrin-radixin-moesin protein, tubulin, cortactin, and Src (5, 6); in syndecan-1, this domain is involved in regulating endocytosis (7). The C2 region contains a PDZ-binding motif and is responsible for regulating syndecan recycling and exosome formation (8, 9). The C1 and C2 regions are separated by a central variable sequence of amino acids that is distinct for each family member (V) and provides the unique function of each syndecan (10). The V region of syndecan-1 is known to be involved in the regulation of cell spreading and actin–fascin bundling (11). The syndecan-4 V region contains an NPXY sequence that binds to phosphatidylinositol 4,5-bisphosphate and regulates syndecan-4 cytoplasmic domain oligomerization and PKCα activation (12–14). In syndecan-2, the V region has the sequence RKPSSAA, in which the two serine residues can be phosphorylated by PKCα, -β, and -γ (15–17). Indeed, the associations of the syndecan-2 cytoplasmic domain with PKCs are important for syndecan-2–mediated functions. For example, PKC-βII directly interacts with syndecan-2, enhances its membrane localization, and increases melanin synthesis in melanoma cells (18). Moreover, PKCγ has been shown to mediate the development of the left–right axis in the Xenopus heart (15). These previous results support the unique functional roles of the cytoplasmic domain of syndecan-2 and show that these functions may be mediated through serine phosphorylation.
In colon cancers, various matrix metalloprotease (MMPs) are overexpressed, and their increased expressions and activations have been correlated with cancer activity and poor prognosis (19, 20). Human colon cancer cells reportedly overexpress MMP-1, -2, -3, -7, -9, -10, -11, -12, -13, and -14 (21). Of them, MMP-7 is consistently expressed in colon cancer cells (22), and its enzymatic activity has been closely associated with colon cancer progression and advanced clinical stages (23). MMP-7 is well-known to directly cleave various ECM substrates, including elastin, type II collagen, fibronectin, vitronectin, aggrecan, and proteoglycan (24–26). When promoting cancer activity, MMP-7 also cleaves cell surface molecules; it has been shown to promote the shedding of pro-TNF-α, Fas ligand, and heparan sulfate proteoglycans in various cancers (27–29). We previously reported that syndecan-2 expression is increased in colon cancer cells (30) and that increased syndecan-2 expression enhances the tumorigenic potential of colon cancer cells (30–33). Therefore, the regulation of MMP-7 expression might be critical to colon cancer progression.
We previously reported that syndecan-2 interacts with pro-MMP-7 at the cell surface and that elevated syndecan-2 expression critically activates the processing of pro-MMP-7 into the active form (30). Accordingly, we proposed that syndecan-2 could function as a docking receptor for pro-MMP-7. However, it remained unknown how syndecan-2 might regulate the expression of MMP-7 as a cell surface receptor. Here, we analyzed the ability of the syndecan-2 cytoplasmic domain to act as a cell surface receptor in regulating MMP-7 expression.
Results
Syndecan-2 promotes MMP-7 expression by activating the focal adhesion kinase (FAK)/ERK pathway
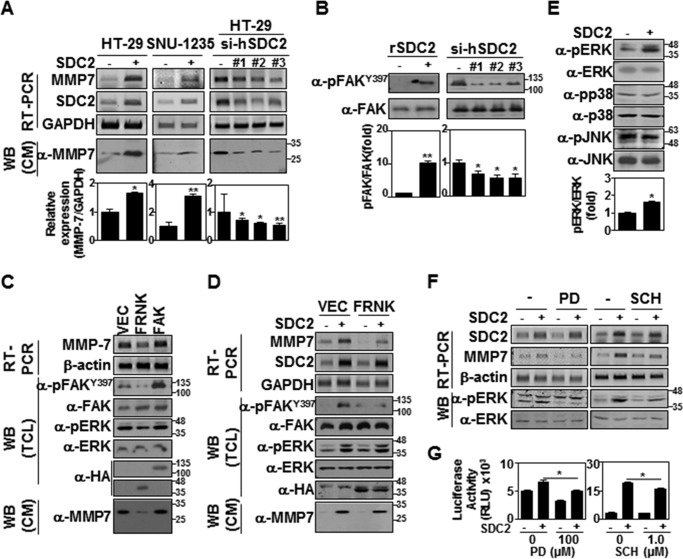
We previously reported that syndecan-2 expression triggers MMP-7 expression in HT-29 colon cancer cells (30). To further explore the mechanism through which syndecan-2 regulates MMP-7 expression, we examined how the stable expression of rat syndecan-2 affected human colon carcinoma cell lines HT-29 and SNU-1235 (Fig. 1). Consistent with our previous results (30), both HT-29 and SNU-1235 cells overexpressing rat syndecan-2 showed an increase in the mRNA expression levels of MMP-7, and knockdown of syndecan-2 expression by target-specific siRNA reduced expression levels of MMP-7 in HT-29 cells (Fig. 1A), confirming involvement of syndecan-2 in MMP-7 expression. To begin investigating the regulatory mechanism underlying this effect in HT-29 cells, we examined the effect of human syndecan-2 expression on tyrosine phosphorylation of FAK, which is a major regulator of cell adhesion signaling (34, 35), and its activation critically involves autophosphorylation at tyrosine 397 (36, 37). As shown in Fig. 1B, our analysis revealed that syndecan-2 expression increased the phosphorylation of FAK tyrosine 397, and knockdown of syndecan-2 expression by target-specific siRNA reduced the phosphorylation of FAK tyrosine 397 in HT-29 cells (Fig. 1B). Consistently, transfection of HA-tagged FAK-related non-kinase (HA-FRNK), which is known to have a dominant negative effect on FAK signaling (39, 40), reduced both the basal (Fig. 1C) and syndecan-2–induced (Fig. 1D) expression levels of MMP-7 in HT-29 cells, suggesting that syndecan-2 regulates MMP-7 expression through the FAK signaling pathway.
Figure 1.
The syndecan-2 cytoplasmic domain regulates MMP-7 expression via FAK/ERK signaling. A, cells were transfected with either a rat syndecan-2 cDNA or oligomer si-syndecan-2 (si-hSDC2). The expression levels of the target mRNAs were analyzed by both RT-PCR (top) and quantitative real-time PCR (bottom). Data are shown as mean ± S.D. (error bars) (n = 3), normalized to GAPDH expression. *, p < 0.05; **, p < 0.01 versus control. B, control HT-29 and HT-29 cells stably overexpressing syndecan-2 were lysed with RIPA buffer, and equal amounts of total cell lysates (TCL) were subjected to immunoblotting with the indicated antibodies (left). HT-29 cells were transfected with oligomer si-syndecan-2 for 48 h. Total cell lysates were subjected to immunoblotting with the indicated antibodies (right). Quantitative analysis of three independent experiments was performed. Data are shown as mean ± S.D. (n = 3), normalized to total FAK expression. *, p < 0.05; **, p < 0.01 versus control. C and D, HT-29 cells (C) or HT-29 cells stably expressing syndecan-2 (D) were transfected with 1 μg of control vector (VEC) or vectors encoding FRNK or FAK. After 24 h, the expression levels of the target mRNAs were analyzed by RT-PCR (top). TCL were subjected to immunoblotting with the indicated antibodies. HA was detected as the transfection control. β-Actin was detected as a control (middle). Conditioned media (CM) were immunoblotted with the MMP-7-specific antibody (bottom). E, equal amounts of TCL from HT-29 cells were subjected to immunoblotting with the indicated antibodies. Data are shown as mean ± S.D. (n = 3), normalized to total ERK expression. *, p < 0.05 versus control. F, cells were treated with either 100 μm PD98059 or 1 μm SCH772984. After 5 h, the expression levels of the target mRNAs were analyzed by RT-PCR. Both expression and phosphorylation of Erk1/2 were examined by Western blotting in triplicate. G, HT-29 cells stably expressing syndecan-2 were co-transfected with PGL-3 basic or the MMP-7 promoter expression vector together with pCMV/β-galactosidase. After 24 h, the cells were treated with 100 μm PD98059 or 1 μm SCH772984. After 5 h, luciferase activity and β-galactosidase activity were measured. Data are shown as mean ± S.D. (n = 3). *, p < 0.05 versus control.
Consistent with previous reports that FAK can activate the mitogen-activated protein kinase cascade during adhesion-mediated cell signaling (38), we observed increased phosphorylation of ERK, but neither p38 nor JNK, in HT-29 cells stably expressing syndecan-2 (Fig. 1E). The syndecan-2–mediated induction of MMP-7 expression was inhibited in cells treated with the potent MEK inhibitor, PD98059, and the specific ERK1/2 inhibitor, SCH772984 (Fig. 1F). Furthermore, syndecan-2 expression increased the activity of the MMP-7 promoter, but both PD98059 and SCH772984 reduced this effect (Fig. 1G). Taken together, these data suggest that syndecan-2 expression up-regulates MMP-7 by activating the FAK/ERK signaling pathway.
The cytoplasmic domain of syndecan-2 is crucial for its ability to induce MMP-7 expression
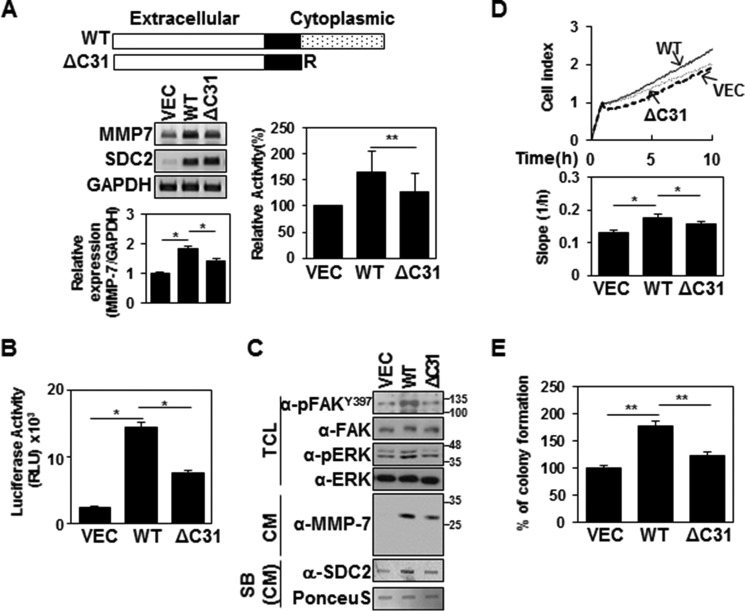
We next investigated whether the cytoplasmic domain of syndecan-2 was involved in its ability to induce MMP-7 expression. We expressed wild-type syndecan-2 or a syndecan-2 mutant lacking the entire cytoplasmic domain (ΔC31). As expected, the exogenous expression of syndecan-2 increased the expression of MMP-7 and the proteolytic activity of MMP-7 in the HT-29 conditioned media (Fig. 2A). In contrast, ΔC31 showed much lower expression levels of MMP-7 and the proteolytic activity of MMP-7 in the HT-29 conditioned media (Fig. 2A). Similar results were observed when we monitored MMP-7 promoter activation; ΔC31 expression triggered much less MMP-7 promoter activity than wild-type syndecan-2 (Fig. 2B). Parallel with reduced expression levels of both syndecan-2 and MMP-7 in the HT-29 conditioned media, ΔC31 showed much less phosphorylation of FAK or ERK (Fig. 2C). These findings indicate that the cytoplasmic domain is important for the ability of syndecan-2 to mediate MMP-7 expression. Consistent with this notion, compared with wild-type syndecan-2, ΔC31 expression triggered much less cell migration activity (as monitored using an xCELLigence system; Fig. 2D) and colony formation (as assessed using a soft agar colony forming assay; Fig. 2E). These data suggest that the syndecan-2 cytoplasmic domain is essential for the ability of syndecan-2 expression to increase MMP-7 expression in HT-29 colon cancer cells.
Figure 2.
The cytoplasmic domain of syndecan-2 is critical for its ability to up-regulate MMP-7. A, schematic representations of WT syndecan-2 and the syndecan-2 mutant lacking the entire cytoplasmic domain (ΔC31) (top). HT-29 cells were stably transfected with the vector encoding the syndecan-2 or ΔC31 mutant, and the expression levels of the target mRNAs were analyzed by RT-PCR and qPCR (bottom left). Data are shown as mean ± S.D. (error bars) (n = 3), normalized to total ERK expression. *, p < 0.05. Proteolytic activity of MMP-7 in the conditioned media was evaluated by quenched fluorescence peptide cleavage assay (bottom right). Data are shown as mean ± S.D. (n = 3). **, p < 0.01 versus SDC2. B, HT-29 cells stably expressing syndecan-2 or the ΔC31 mutant were lysed with luciferase lysis buffer, and luciferase activity and β-galactosidase activity were measured. Data are shown as mean ± S.D. (n = 3). *, p < 0.05. C, HT-29 cells stably expressing syndecan-2 or the ΔC31 mutant were lysed with RIPA buffer. TCL and CM were immunoblotted with the indicated antibodies. CM were subjected to slot blotting (SB) with anti-syndecan-2 antibody (bottom). D, cells were plated on RTCA CIM-plates and cultured for the indicated times, and cell migration was monitored using the xCELLigence system. Representative results from three independent experiments are shown as mean ± S.D. (n = 3). *, p < 0.05. E, cells were seeded on soft agar. After 17 days, colonies were stained with 0.005% crystal violet and counted. Data are shown as mean ± S.D. (n = 3). **, p < 0.01.
Phosphorylation of serine 198 in the variable region is required for the ability of syndecan-2 to induce MMP-7 expression
The variable cytoplasmic domain is important for the functional specificity of each syndecan (15, 41, 42). As the ability to regulate MMP-7 expression is a unique feature of syndecan-2, we examined the possible involvement of the cytoplasmic domain in this effect. We generated truncated cytoplasmic domain mutants lacking the C-terminal EFYA sequence (ΔC4), the central region and the EFYA sequence (ΔC14) or the entire cytoplasmic domain (ΔC31) (Fig. 3A). HT-29 cells were transfected with vectors encoding the wild-type or mutant versions of syndecan-2, and the mRNA expression of MMP-7 was assessed by RT-PCR and quantitative real-time PCR. Compared with vector-transfected cells, those expressing wild-type syndecan-2 exhibited enhanced expression of the MMP-7 mRNA. The mutant syndecan-2 harboring deletion of four amino acid residues at the C terminus (the ΔC4 mutant) also increased MMP-7 expression, whereas the other two deletion mutants failed to enhance MMP-7 expression (Fig. 3A). The same two deletion mutants, ΔC14 and ΔC31, both failed to induce MMP-7 promoter activity (Fig. 3B) or increase colony-forming activity (Fig. 3C). These findings suggest that the central variable region of the cytoplasmic domain is important for the ability of syndecan-2 to increase MMP-7 expression.
Figure 3.
A serine residue in the variable region of the syndecan-2 cytoplasmic domain is important for its ability to upregulate MMP-7. A, schematic representations of the studied versions of syndecan-2. The full-length syndecan-2 protein (WT), a mutant lacking the C-terminal EFYA sequence (ΔC4), a mutant lacking half of the variable cytoplasmic domain and EFYA (ΔC14), and a mutant lacking the entire cytoplasmic domain (ΔC31) are shown (top). Cells were stably transfected with the indicated mutant constructs, as described under “Experimental procedures.” Total RNA was extracted, and the expression levels of syndecan-2 and MMP-7 were analyzed by RT-PCR (middle) and RT-qPCR (bottom). Data are shown as mean ± S.D. (error bars) (n = 3), normalized to GAPDH expression. *, p < 0.05. B, the indicated cells were lysed with luciferase lysis buffer, and luciferase activity and β-galactosidase activity were measured. Data are shown as mean ± S.D. (n = 3). *, p < 0.05. C, the indicated cells were seeded on soft agar. After 17 days, colonies were stained with 0.005% crystal violet and counted. Data are shown as mean ± S.D. (n = 3). *, p < 0.05. D, schematic representation of the studied versions of the syndecan-2 cytoplasmic domain. Shown are the WT syndecan-2 cytoplasmic domain and mutants in which serines 197 and 198 were changed to alanine and glutamic acid, respectively (AE); glutamic acid and alanine, respectively (EA); alanine and alanine (AA); and glutamic acid and glutamic acid (EE) (top). Cells were stably transfected with the indicated mutant constructs. After 24 h, the expression levels of the target mRNAs were analyzed by RT-PCR and RT-qPCR (bottom left). TCL were subjected to immunoblotting with the indicated antibodies, and quantitative analysis of three independent experiments was performed (bottom right). Quantitative analysis of three independent experiments was performed and normalized to GAPDH or total FAK expression. Data are shown as mean ± S.D. (n = 3). *, p < 0.05. E, the indicated cells (1 × 105 cells/well) were seeded on soft agar. After 17 days, colonies were stained with 0.005% crystal violet and counted. Data are shown as mean ± S.D. (n = 3); *, p < 0.05.
Because the syndecan-2 cytoplasmic domain contains unique serine residues within the variable region, we hypothesized that serine phosphorylation might be involved in the ability of syndecan-2 to regulate MMP-7 expression. To test this possibility, we replaced serine 197 and/or 198 with either alanine (to prevent potential phosphorylation) or glutamic acid (to act as a phosphomimetic of phosphoserine) (Fig. 3D). Similar to the results shown in Figs. 1 and 2, syndecan-2 overexpression increased MMP-7 expression and the phosphorylations of FAK tyrosine 397 and ERK. Interestingly, syndecan-2 mutants substituting serine 197 and 198 with alanine (AA) or glutamic acids (EE) did not activate MMP-7 expression as much as wild-type syndecan-2. However, syndecan-2 mutant AE, in which serine 197 was mutated to alanine and serine 198 was mutated to glutamic acid, triggered higher-level MMP-7 expression than wild-type syndecan-2, whereas syndecan-2 mutant EA, in which serine 197 was mutated to glutamic acid and serine 198 was replaced with alanine, showed much reduced MMP-7 expression activity (Fig. 3D). These findings suggest that phosphorylation of serine 198 is crucial for the ability of syndecan-2 to trigger MMP-7 expression. Consistent with the above results, AE, but not EA, induced phosphorylation of FAK tyrosine 397 (Fig. 3D) and enhanced the soft agar colony-forming activity of HT-29 cells (Fig. 3E). Together, these data suggest that phosphorylation of syndecan-2 serine 198 in the cytoplasmic domain is essential for the ability of syndecan-2 to induce MMP-7 expression in HT-29 colon cancer cells.
Syndecan-2 requires PKCγ to up-regulate MMP-7
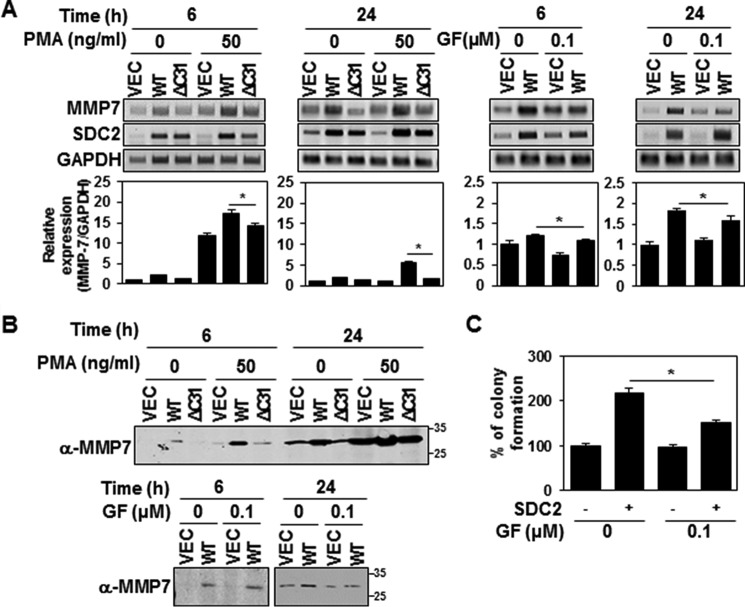
It has been shown that the syndecan-2 cytoplasmic domain can be serine-phosphorylated by PKCα, -β, and -γ (15, 16). To explore the potential involvement of PKCs in the syndecan-2–mediated up-regulation of MMP-7, we treated HT-29 cells with either phorbol 12-myristate 13-acetate (PMA; a highly potent PKC activator) or bisindolylmaleimide I (GF 109203X; a competitive PKC inhibitor) and examined MMP-7 expression over time (Fig. 4). Our results revealed that PMA enhanced the basal expression level of MMP-7 in vector control cells and further enhanced the syndecan-2–mediated up-regulation of MMP-7 (Fig. 4, A and B). The syndecan-2–induced increase in MMP-7 expression was greatly reduced in cells expressing the cytoplasmic domain deletion mutant (ΔC31; Fig. 4, A and B). Moreover, treatment with GF 109203X reduced syndecan-2–mediated MMP-7 expression at 6 h and completely inhibited it after 24 h (Fig. 4, A and B). Consistent with this finding, inhibition of PKC activity blocked the ability of syndecan-2 to increase the soft agar colony-forming activity of HT-29 cells (Fig. 4C). Together, these data suggest that PKC activity is necessary for the ability of syndecan-2 to induce MMP-7 expression.
Figure 4.
PKC activity is required for the ability of syndecan-2 to up-regulate MMP-7. A and B, HT-29 cells stably expressing syndecan-2 or the ΔC31 mutant were treated with 50 ng/ml PMA or 0.1 μm GF109203X. At 6 and 24 h post-treatment, the expression levels of the target mRNAs were analyzed by RT-PCR and RT-qPCR. Data are shown as mean ± S.D. (error bars) (n = 3), normalized to GAPDH expression. *, p < 0.05. A, conditioned media were collected from the indicated cells and immunoblotted with the MMP-7–specific antibody (B). C, HT-29 cells with or without stable expression of syndecan-2 were treated with 0.1 μm GF109203X. After 24 h, the cells were seeded on soft agar and grown for 17 days. The colonies were then stained with 0.005% crystal violet and counted. Data are shown as mean ± S.D. (n = 3). *, p < 0.05.
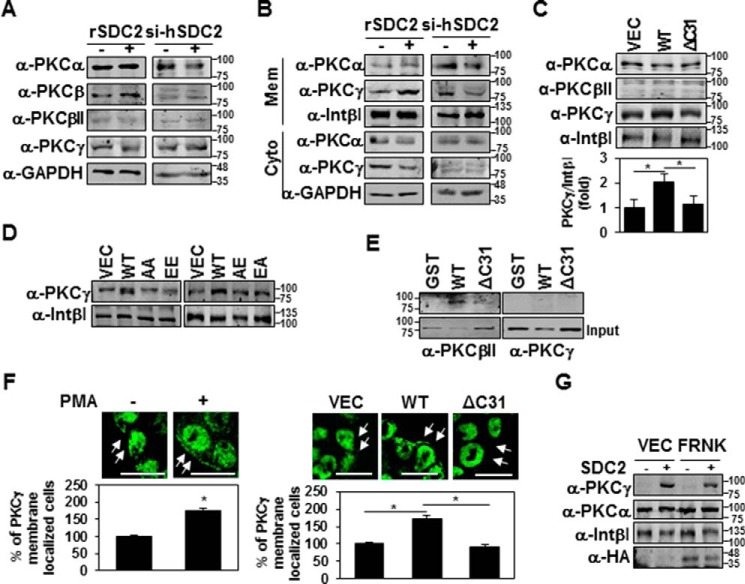
To further investigate which PKCs are involved in the syndecan-2 cytoplasmic domain-mediated up-regulation of MMP-7 expression, we examined PKC isotypes PKCα, -β, and -γ, which are known to phosphorylate serine residues in the syndecan-2 cytoplasmic domain (Fig. 5). As shown in Fig. 5A, HT-29 cells expressed all three of these PKC isotypes. Both syndecan-2 overexpression and siRNA-mediated syndecan-2 knockdown failed to affect the levels of these PKC isotypes in HT-29 cells (Fig. 5A). Interestingly, however, the overexpression of syndecan-2 specifically induced the membrane localization of PKCγ, but not PKCα or PKCβ, and syndecan-2 knockdown reduced the membrane localization of PKCγ (Fig. 5B). These findings suggest that PKCγ is involved in the syndecan-2 cytoplasmic domain–mediated induction of MMP-7. Consistent with these findings, the syndecan-2 cytoplasmic domain deletion mutant (ΔC31) could not induce the membrane localization of PKCγ (Fig. 5C). Interestingly, none of the syndecan-2 mutants (AE, AA, EE, or EA) induced the membrane localization of PKCγ (Fig. 5D), suggesting that PKCγ localizes to the membrane but might separate from the membrane following the phosphorylation of Ser-198.
Figure 5.
Syndecan-2 expression increases membrane localization of PKCγ. A and B, HT-29 cells stably transfected with syndecan-2 or si-syndecan-2 (si-hSDC2) oligomer. After 48 h, the PKC isotypes in TCL were determined by immunoblotting with isotype-specific antibodies. GAPDH was used as a loading control (A). The indicated cells were fractionated, and the amounts of the different PKC isotypes in the membrane fraction were analyzed by immunoblotting with isotype-specific PKC antibodies. Integrin β1 was used as a loading control (B). C and D, HT-29 cells stably expressing the syndecan-2, ΔC31, AA, EE, AE, or EA mutant constructs were subjected to membrane fractionation, and the amount of PKCγ in the membrane fraction was analyzed by immunoblotting with an isotype-specific PKC antibody. Integrin β1 was used as a loading control. Data are shown as mean ± S.D. (error bars) (n = 3), normalized to total FAK expression. *, p < 0.05. E, purified GST-SDC2 was incubated with rat brain PKCs for 2 h. Bound proteins were immunoblotted with anti-PKC antibodies. F, HT-29 cells were treated with 20 nm PMA for 4 h and immunostained with the PKCγ antibody. The percentage of PKCγ membrane localization (cells showing membrane localization/total cells) is presented (left). HT-29 cells with or without stable expression of syndecan-2 were immunostained with the PKCγ antibody. The percentage of PKCγ membrane localization (cells showing membrane localization/total cells) is presented (right). Scale bars, 20 μm. Data are shown as mean ± S.D. (n = 3). *, p < 0.05. G, HT-29 cells stably expressing syndecan-2 were transfected with either control (VEC) or an expression vector encoding FRNK. After 24 h, the amount of PKCs in the membrane fraction was analyzed by immunoblotting with an isotype-specific PKC antibody. Integrin β1 was used as a loading control.
To further analyze the interaction between syndecan-2 and PKCγ, we performed a GST-pulldown assay with purified GST-tagged syndecan-2 protein and purified rat brain PKCs. The GST-pulldown assay showed that, unlike PKCβII, which was previously known to interact with syndecan-2 (18), we could not detect direct interaction between recombinant syndecan-2 and PKCγ under our experimental conditions (Fig. 5E), suggesting indirect involvement of syndecan-2 on PKCγ membrane localization. In addition, immunostaining of HT-29 cells with PKCγ-specific antibody indicated that expression of wild-type syndecan-2 increased the membrane localization of PKCγ, as also seen in HT-29 cells treated with PMA (Fig. 5F). In addition, FRNK-mediated inhibition of FAK activity did not affect the membrane localization of PKCγ (Fig. 5G), suggesting that this localization is upstream of FAK activation. Taken together, these results suggest that the syndecan-2 cytoplasmic domain-mediated activation of PKCγ is crucial for the ability of syndecan-2 to induce MMP-7 expression.
Syndecan-2 cytoplasmic domain induces MMP-7 expression independent of extracellular domain shedding
We previously showed that 1) the extracellular domain of syndecan-2 can be shed from the cell surface (33); 2) MMP-7 mediates the extracellular domain shedding of syndecan-2 (32); and 3) the shed syndecan-2 fragment enhances MMP-7 expression (33). We also proposed that syndecan plays a dual regulatory role as a cell surface receptor and docking receptor and that this dual regulatory function enables syndecan-2 to regulate both intracellular and extracellular activities (43). Therefore, we speculated herein that syndecan-2 might regulate MMP-7 expression through both cytoplasmic domain–mediated signal transduction (as a cell surface receptor) and shed syndecan-2–mediated signal transduction (as an extracellular ligand). To clarify the potential cell surface receptor–related effect of syndecan-2 on MMP-7, we generated a non-cleavable syndecan-2 mutant (NC) (33) and an NC lacking the entire cytoplasmic domain (NCΔC31) (Fig. 6A). We then transfected cells with vectors encoding the wild-type and mutant versions of syndecan-2 and examined MMP-7 expression over time. At 24 h post-transfection, cells expressing wild-type syndecan-2 and the NC mutant showed comparable enhancements of MMP-7 expression, whereas NCΔC31 failed to induce MMP-7 expression (Fig. 6B). At 48 h post-transfection, the NC mutant-induced MMP-7 expression was significantly lower than induced by wild-type syndecan-2 (Fig. 6C). This suggests that syndecan-2 induces MMP-7 expression mainly via the cytoplasmic domain soon after transfection and that shed syndecan-2 mediates MMP-7 expression later on, after MMP-7 has accumulated to a sufficient degree. Consistent with this notion, the levels of shed syndecan-2 were higher in the conditioned media of HT-29 cells transfected with a syndecan-2–expressing construct compared with vector-transfected cells, but the observed increases were progressively less dramatic in cells expressing the NC and NCΔC31 mutants (Fig. 6C).
Figure 6.
The syndecan-2 cytoplasmic domain induces MMP-7 expression independent of extracellular domain shedding. A, schematic representations of the studied syndecan-2 proteins rat syndecan-2 WT, the L149I non-cleavable mutant (NC), the syndecan-2 mutant lacking the whole cytoplasmic domain (ΔC31), and the L149I non-cleavable mutant lacking the whole cytoplasmic domain (NCΔC31). B, HT-29 cells were transfected with 1 μg of vectors encoding WT syndecan-2 or the ΔC31, NC, or NCΔC31 mutants. Total RNA was extracted, and the expression levels of the target mRNAs were analyzed by RT-PCR. GAPDH was used as a loading control (top left). The expressions of MMP-7 were analyzed by RT-qPCR (bottom left). TCL were subjected to immunoblotting with the indicated antibodies. GAPDH was detected as a control (top right), and quantitative analysis of three independent experiments was performed (bottom right). Data are shown as mean ± S.D. (error bars) (n = 3), normalized to GAPDH expression or total FAK expression. *, p < 0.05 versus SDC2. C, HT-29 cells were transfected with 1 μg of vectors encoding WT syndecan-2 or the NC or NCΔC31 mutants. After the indicated times, the expression levels of the target mRNAs were analyzed by RT-PCR (top), and conditioned media were subjected to slot blotting (SB) with anti-syndecan-2 antibody (bottom). D, HT-29 cells were transfected with 1 μg of vectors encoding WT syndecan-2 or the ΔC31, NC, or NCΔC31 mutants and plated on RTCA CIM-plates. After the indicated times, cell migration was monitored using an xCELLigence system. Representative results from three independent experiments are shown as mean ± S.D. *, p < 0.05 versus SDC2. E, the indicated cells (1 × 105 cells/well) were seeded on soft agar. After 17 days, colonies were stained with 0.005% crystal violet and counted. Shown are representative results from three independent experiments. Data are shown as mean ± S.D. (n = 3). *, p < 0.05 versus SDC2.
Because both syndecan-2 and shed syndecan-2 have been shown to regulate cell migration (31–33), we performed a real-time migration assay using an xCELLigence system (Fig. 6D). Over 19 h, overall cell migration was markedly higher in HT-29 cells expressing wild-type syndecan-2 than in cells expressing any of the tested mutants (NC, ΔC31, and NCΔC31; Fig. 6D). At 5 h after plating, however, the NC mutant (but not ΔC31 or NCΔC31) was associated with an increase of cell migration comparable with that seen in cells expressing wild-type syndecan-2 (Fig. 6D). This suggests that syndecan-2–induced cell migration is dependent on the syndecan-2 cytoplasmic domain at the early stage of syndecan-2 expression. Similarly, HT-29 cells expressing the syndecan-2 mutants showed much less colony-forming activity compared with those expressing wild-type syndecan-2 (Fig. 6E). Taken together, our findings strongly suggest that the syndecan-2 cytoplasmic domain induces MMP-7 expression via FAK/ERK signaling and independent of extracellular domain shedding.
Discussion
Although syndecans are highly conserved among family members and species, each syndecan has one or more unique functional roles that are critically governed by the variable cytoplasmic domain. The syndecan-2 cytoplasmic domain is known to be involved in regulating MMP-7 expression, but the underlying mechanism was previously unknown. Here, we investigated the regulatory role of the syndecan-2 cytoplasmic domain in detail and show that the syndecan-2 cytoplasmic domain regulates MMP-7 expression through the PKCγ-mediated activation of FAK/ERK signaling. Our results revealed that overexpression of syndecan-2 up-regulated MMP-7, enhanced the membrane localization of PKCγ (Fig. 5), and increased the phosphorylations of FAK tyrosine 397 and ERK (Fig. 1). In contrast, deletion of either the entire cytoplasmic domain or the serine residue–containing variable region diminished the ability of syndecan-2 to enhance MMP-7 expression, suggesting that the variable region of the cytoplasmic domain plays an important role in this effect. Because syndecan-2 is the only syndecan known to regulate MMP-7 expression as a cell surface receptor, this involvement of the variable region is not unexpected. Syndecan-2 is unique among the syndecans in that its cytoplasmic domain contains two serine residues in the variable region. Therefore, it is highly plausible that the regulatory function of syndecan-2 could be closely related to serine phosphorylation. A previous study showed that PKCγ mediates the phosphorylation of the syndecan-2 cytoplasmic domain to regulate syndecan-2–mediated inside-out signaling during the left–right development of Xenopus (15). PKCβII was shown to be involved in regulating syndecan-2–mediated melanogenesis in melanocyte-derived cells (18), and PKCδ was found to regulate syndecan-2–induced apoptosis in osteoblasts (44). In addition, Oh et al. (41) and Prasthofer et al. (17) reported that classical PKC directly phosphorylates serine residues in the variable region of the syndecan-2 cytoplasmic domain. Our present findings suggest that PKCγ is a major regulator of syndecan-2 cytoplasmic domain serine phosphorylation, because syndecan-2 expression in HT-29 cells increased the membrane localization of PKCγ but not PKCα (Fig. 5). In addition, our experiments using a phosphomimetic mutant showed that phosphorylation of serine 198 in the variable region of syndecan-2 was involved in its ability to activate FAK and subsequently increase MMP-7 expression (Fig. 3). This may explain the specific ability of syndecan-2 to regulate MMP-7 expression. Interestingly, however, neither of the phosphomimetic mutants for serine 197 (EA or EE) increased the expression of MMP-7; instead, cells expressing these mutants showed less induction of MMP-7 than those expressing wild-type syndecan-2 (Fig. 3). This suggests that phosphorylation is specifically required for the syndecan-2–mediated up-regulation of MMP-7 expression. Furthermore, our data indicate that excess phosphorylation at serine 197 may negatively regulate the ability of syndecan-2 to regulate MMP-7 expression. Our findings thus confirm the specific role of the cytoplasmic domain and suggest that the PKC isotypes play different regulatory roles in different cell types and for different functions.
Along with PKC, FAK is an important regulator of adhesion-mediated signaling. Syndecan-2 has been reported to regulate FAK activity in fibrosarcoma and melanoma cells (45, 46). Here, we show that syndecan-2 expression enhances FAK tyrosine 397 phosphorylation and the activity of ERK, which is a downstream effector of FAK, in colon cancer cells (Fig. 1, B–E). In colon cancer cells, the ERK MAPK pathway is critically important for intestinal epithelial differentiation, tumor progression, and oncogenic behaviors (47). Here, we found that inhibition of ERK reduced the syndecan-2–triggered expression of MMP-7 in HT-29 cells (Fig. 1, F and G), suggesting that the FAK/ERK pathway is an important regulator of syndecan-2–induced MMP-7 expression. Interestingly, inhibition of FAK activity via the application of FRNK diminished the syndecan-2–mediated up-regulation of MMP-7 (Fig. 1D) but did not alter the syndecan-2-induced membrane localization of PKCγ (Fig. 5G). This suggests that the membrane localization of PKCγ is upstream of FAK phosphorylation.
We also previously showed that MMP-7 can induce shedding of the extracellular domain of syndecan-2 (32) and that shed syndecan-2 can induce MMP-7 expression as a cell surface ligand (33). To analyze the role of the syndecan-2 extracellular domain, we explored and compared the effects of wild-type syndecan-2, a non-cleavable syndecan-2 mutant (NC), and an NC mutant lacking the cytoplasmic domain (NCΔC31; Fig. 6). Our data revealed that wild-type syndecan-2 and the NC mutant induced MMP-7 expression to comparable degrees at an early stage of syndecan-2 transfection, whereas the NCΔC31 mutant induced much less MMP-7 expression at this point (Fig. 6). This confirmed that the cytoplasmic domain of syndecan-2 is important for its ability to up-regulate MMP-7. Interestingly, the NCΔC31 mutant, but not the NC mutant, significantly reduced syndecan-2–mediated cell migration and colony-forming activity (Fig. 6, D and E). This was somewhat unexpected, as we previously showed that syndecan-2–mediated cell migration was reduced in cells expressing the NC mutant (33). The present findings suggest that MMP-7 is likely to be important to the ability of syndecan-2 to regulate cancer cell activity. Because syndecan-2–induced cell migration could reflect the abilities of syndecan-2 to up-regulate MMP-7 as both a cell surface receptor and a shed ligand, we monitored cell migration in real time (Fig. 6D). Interestingly, at an early stage of cell transfection, cell migration was dependent on the expression of syndecan-2 (Fig. 6C), presumably reflecting its action as a “cell surface receptor.” Later, however, shed syndecan-2 appeared to play a more important role in regulating cell migration and other cancer activities. We speculate that significant levels of MMP-7 are present by this time, due to the actions of the syndecan-2 cytoplasmic domain as a cell surface receptor. Moreover, syndecan-2 shedding is increased at this point. Thus, we believe that syndecan-2 regulates MMP-7 expression and colon cancer activity as both a cell surface receptor and a soluble ligand. In addition, our findings indicate that syndecan-2–mediated MMP-7 expression is critical at the early stage of cancer progression, whereas MMP-7–mediated syndecan-2 shedding is more important at the later stage. Thus, our study suggests that anti-cancer efforts targeting such signaling molecules may need to use different strategies during different stages of cancer progression.
Our experiments further reveal that when syndecan-2 expression is increased in colon cancer cells, its cytoplasmic domain activation triggers the membrane localization of PKCγ, which may then induce the activating phosphorylation of syndecan-2 serine 198. The activated syndecan-2 cytoplasmic domain then activates FAK/ERK signaling to up-regulate MMP-7 expression. Based on our further findings, we propose that after syndecan-2 acts as a cell surface receptor to induce MMP-7 expression, the generated MMP-7 promotes the extracellular shedding of syndecan-2, and the shed syndecan-2 acts as a soluble ligand to additionally enhance the expression of MMP-7 (Fig. 7). To our knowledge, this is the first study examining the molecular mechanisms that regulate syndecan-2–mediated MMP-7 expression in colon cancer cells that model an early stage of cancer progression.
Figure 7.

Syndecan-2 cytoplasmic domain regulates MMP-7 expression via protein kinase Cγ–mediated FAK/ERK signaling pathway.
Experimental procedures
Antibodies and materials
An antibody capable of recognizing human, rat, and mouse syndecan-2 was produced by AdipoGen Inc. (Incheon, Korea) using the Fc-fused extracellular domain of syndecan-2. Monoclonal anti-MMP-7 was obtained from Abcam (Cambridge, UK). Monoclonal anti-phosphotyrosine (4G10) was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). The polyclonal antibodies against site-specific phosphorylated FAK (FAK-Tyr(P)397), FAK, phospho-p38, phospho-JNK, p38, and JNK were obtained from Cell Signaling (Danvers, MA). Monoclonal anti-ERK, anti-phospho-ERK, anti-β-actin, anti-GST, and anti-HA and polyclonal anti-PKCα, anti-PKCβII, and anti-integrin β1 were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX). Monoclonal anti-PKCγ was purchased from BD Biosciences. PD98059 was purchased from Cell Signaling. SCH772984 and PMA was purchased from Sigma. GF109203X was purchased from Millipore (Billerica, MA).
Cell culture and transfection
The HT-29 human colon adenocarcinoma cell line (ATCC® HTB-38TM) was purchased from ATCC (Manassas, VA), and the SNU-1235 human colon adenocarcinoma cell line was purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). HT-29 cells were maintained in McCoy's 5A complete medium (Welgene, Daegu, Korea), and SNU-1235 cells were maintained in Roswell Park Memorial Institute 1640 complete medium (RPMI 1640) (Welgene, Daegu, Korea) supplemented with 10% (v/v) FBS (Hyclone, Logan, UT) and gentamycin (50 g/ml; Sigma) at 37 °C in a 5% CO2-containing humidified atmosphere. Transfections were performed using the Viva Magic transfection reagent (Vivagen, Gyeonggi-Do, Korea) according to the manufacturer's instructions. HT-29 cells (4.0 × 105 cells/well) and SNU-1235 cells (3.0 × 106 cells/well) were plated on 6-well plates, incubated at 37 °C for 24 h, and then transfected with the generated expression vectors. siRNA transfection was performed in 60-mm dishes using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Vector construction and generation of stable cell lines
Expression vectors encoding HA epitope–tagged WT FAK (pKH3-HA-FAK) and FRNK (pKH3-HA-FRNK) were kindly provided by Dr. Jun-Lin Guan (Cornell University, Ithaca, NY). The cytoplasmic deletion mutant of rat syndecan-2, the ΔC4 construct deletion of amino acids 198–201, the ΔC14 construct deletion of amino acids 188–201, and the ΔC31 construct deletion of amino acids 181–211 were constructed by PCR amplification (31). Double point mutants in the cytoplasmic domain of syndecan-2 (i.e. AA (S197A/S198A), EE (S197E/S198E), AE (S197A/S198E), and EA (S197E/S198A)) were constructed by commercial gene synthesis (Bioneer, Daejeon, Korea). The deletion or point mutants were inserted into the pcDNA3.1 expression vector (Invitrogen). To generate cell lines stably expressing the various versions of syndecan-2, HT-29 cells (1 × 105) were transfected with 1 μg of empty pcDNA3.1 (control), pcDNA3.1-FLAG-Syndecan-2 (wild type), ΔC4, ΔC14, ΔC31, AA, EE, AE, or EA and then selected in medium containing 400 μg/ml G418 (EMD Biosciences, San Diego, CA) for 4 weeks. The surviving clones were individually isolated and analyzed by FACS and RT-PCR.
RT-PCR
Total RNA was extracted from cells and reverse transcribed. Aliquots of the resulting cDNAs were amplified using the following primers: rat syndecan-2, 5′-ATGCGGGTACGAGCCACGTC-3′ (forward) and 5′-CGGGAGCAGCACTAGTGAGG-3′ (reverse); human MMP-7, 5′-GGTCACCTACAGGATCGTATCATAT-3′ (forward) and 5′-CATCACTGCATTAGGATCAGAGGAA-3′ (reverse); human β-actin, 5′-TGGAATCCTGTGGCATCCATGAAA-3′ (forward) and 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ (reverse); human GAPDH, 5′-CCACCCATGGCAAATTCCATGGCA-3′ (forward) and 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ (reverse); and human syndecan-2, 5′-ACATCTCCCCTTTGCTAACGGC-3′ (forward) and 5′-TAACTCCATCTCCTTCCCCAGG-3′ (reverse). After an initial denaturation at 94 °C for 5 min, the samples were subjected to 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 60 s, and extension at 72 °C for 60 s. Human GAPDH and human β-actin were amplified as internal controls. The generated PCR products were separated by 1% agarose gel electrophoresis.
Quantitative real-time PCR
Quantitative real-time PCR was performed using the CFX96TM real-time PCR detection system (Bio-Rad) in a two-step procedure using SensiFASTTM SYBR® Hi-ROX kit (BioLine, London, UK). Amplification of GAPDH was performed to standardize the amount of sample cDNA. Primer sequences were as follows: GAPDH, 5′-CCTCAAGATCATCAGCAAT-3′ (forward) and 5′-CCATCCACAGTCTTCTGGGT-3′ (reverse); human syndecan-2, 5′-CTGGGAATATCCGTGCCAGG-3′ (forward) and 5′-AGTTGAAGAAAACTGGATGTCAGTC-3′ (reverse); human MMP-7, 5′-GGCTTTAAACATGTGGGGCA-3′(forward) and 5′-GGCCCATCAAATGGGTAGGA-3′ (reverse). All reactions were performed in a 96-well plate using the following cycling conditions: 40 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C 1 min. Using the ΔΔCT method, the value of each control sample was set at 1 and used to calculate the -fold change of target genes.
siRNAs
Two human syndecan-2 siRNA constructs, number 1 (catalog no. 1134290) (sense, 5′-CAGCAAACCCAAAGAUGUU-3′; antisense, 5′-AACAUCUUUGGGUUUGCUG-3′) and number 2 (catalog no. 1134292) (sense, 5′-CUCAGAUUGACCUUACCAA-3′; antisense, 5′-UUGGUAAGGUCAAUCUGAG-3′) were obtained from a predesigned siRNA library from Bioneer. An additional oligonucleotide (number 3) was purchased from Dharmacon, Inc. The oligonucleotide sequence was as follows: 5′-GATCCCCTGACGATGACTACGCTTCTTTCAAGAGAACTGCTACTGATGCGAAGATTTTTGGAAA-3′ (sense) and 5′-AGCTTTTCCAAAAATGACGATGACTACGCTTCTTCTCTTGAAACTGCTACTGATGCGAAGAGGG-3′ (antisense).
MMP enzyme assay
Catalytic activity of MMP-7 was analyzed by a peptide cleavage assay using a quenched fluorescent peptide, 7-methoxycoumarin-4-yl–acetyl-Pro-Leu-Gly-Leu–N-3(2,4-dinitrophenyl)-l-2,3-diaminopropionyl–Ala-Arg–NH2 (catalog no. M-1895, Bachem), as a substrate (49). The reactions were performed in a final volume of 100 μl of MMP assay buffer (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 5 mm CaCl2, 0.5 mm ZnCl2, 0.001% Brij35) in the presence of 1 μm oligopeptide and 50 μl of conditioned media for 4 h at 37 °C. The reaction was stopped by the addition of 0.1 m (final concentration) sodium acetate, pH 4.0. Fluorescence was determined at an excitation wavelength of 328 nm and at an emission wavelength of 393 nm with a KONTRON SFM 25 fluorometer (50).
Immunoblotting
Cultures were washed twice with PBS, and the cells were lysed with RIPA buffer (50 mm Tris, pH 8.0, 150 mm NaCl, 1% Nonidet P-40, 10 μm NaF, and 2 μm Na3VO4) containing a protease inhibitor mixture (1 μg/ml aprotinin, 1 μg/ml antipain, 5 μg/ml leupeptin, 1 μg/ml pepstatin A, and 20 μg/ml phenylmethylsulfonyl fluoride). Cell lysates were clarified by centrifugation at 13,000 rpm for 15 min at 4 °C, denatured with SDS-PAGE sample buffer, boiled, and analyzed by SDS-PAGE. Proteins were transferred to 0.45-μm nitrocellulose blotting membranes (GE Healthcare) and probed with the appropriate antibodies. Signals were detected using an Odyssey CLx imager (LI-COR Biosciences, Lincoln, NE), and the data were analyzed with the Image Studio Lite software (LI-COR Biosciences).
Cellular fractionation
Culture plates were washed twice with PBS and then treated with a hypo-osmotic solution (20 mm Tris-HCl, pH 7.5, 2 mm 2-mercaptoethanol, 5 mm EGTA, 2 mm EDTA) containing a protease inhibitor mixture. The cells were then scraped off of the plates and homogenized on ice, and the homogenate was centrifuged at 13,000 × g for 15 min at 4 °C. To collect the membrane fraction, the remaining pellet was solubilized in RIPA buffer containing a protease inhibitor mixture. Equal amounts of lysates were resolved by SDS-PAGE, transferred to NC membranes, and probed with the indicated antibodies.
TCA precipitation and slot blotting
Conditioned media were collected, and proteins were concentrated by TCA precipitation (48). Conditioned medium samples were applied to the membrane on a Bio-Dot SF apparatus (Bio-Rad). The membrane was stained with Ponceau S (as a control) and then blocked in 0.05% Tween 20 in TBS (TBST; 50 mm Tris-HCl, pH 7.4, 150 mm NaCl) containing 5% skim milk. The membrane was washed with TBST and probed with the appropriate primary antibody (MMP-7 mAb or syndecan-2 mAb) for 24 h at 4 °C. The blots were then probed with a species-specific secondary antibody of IRDye 800CW-conjugated goat anti-mouse, and the signals were detected with an Odyssey CLx imager (LI-COR Biosciences).
Luciferase assay
HT-29 cells stably expressing the various versions of syndecan-2 and the corresponding control cells were plated to 6-well plates. After 24 h, the cells were co-transfected with 1 μg of pGL3-basic vector or MMP-7(−2344) reporter constructs (a generous gift from Dr. Seung-Teak Lee at Yonsei University) together with 0.1 μg of pCMV/β-galactosidase (Clontech, Palo Alto, CA) (normalizing control), using the Viva Magic transfection reagent (Vivagen). Twenty-four hours post-transfection, the cells were washed with PBS and lysed with the buffer provided in the utilized luciferase assay kit (Promega, Madison, WI). Soluble extracts were harvested and assayed for luciferase and β-galactosidase activities, according to the manufacturer's instructions. To normalize the luciferase values for transfection efficiency, the results are presented as relative luciferase units (luciferase activity/β-galactosidase activity).
Cell migration and colony-forming assays
Cell migration was monitored in real time using an xCELLigence system (Roche Diagnostics GmbH, Basel, Switzerland). The lower chambers of a CIM-plate 16 (8-μm pore size) were filled with fresh medium containing 10% FBS, the upper chambers were filled with serum-free medium (30 μl/well), and the plate was incubated at 37 °C in 5% CO2 for 1 h. The background was measured using an RTCA DP Analyzer (RTCA software version 1.2, ACEA Biosciences). Transfected HT-29 cells (4 × 104 cells/well) were added to each well, and the plate was incubated at 25 °C. After 30 min, the plate was assembled onto the RTCA DP Analyzer, and cell migration was assessed at 5-min intervals for 48 h under conditions of 37 °C and 5% CO2. The data obtained were analyzed using the provided RTCA software. To assess colony formation, 6-well culture plates were coated with 3 ml/well bottom agar mixture (McCoy's 5A, 10% FBS, 0.6% agar). After the bottom layer had solidified, 1 ml of top agar mixture (McCoy's 5A, 10% FBS, 0.3% agar) containing HT-29 cells (1 × 105 cells/well) was added to each well, and the cultures were incubated at 37 °C in a 5% CO2 atmosphere. Colony formation was monitored daily with a light microscope. After 17 days, the colonies were stained with 0.005% crystal violet and photographed with a digital camera.
Immunofluorescence analysis
Cells cultured in coverslips in 12-well plates were fixed with 3.5% paraformaldehyde for 10 min. The cells were then washed with PBS, blocked with 0.5% BSA, and incubated overnight with anti-PKCγ antibodies at 4 °C. For detection of intracellular proteins, the cells were permeabilized with 0.5% Triton X-100 in PBS for 10 min before the blocking step. After a further wash with PBS, the cells were incubated with fluorescent dye-conjugated secondary antibodies (Invitrogen) for 1 h at 25 °C. The coverslips were mounted on glass slides with mounting solution, and the results were imaged under a confocal fluorescence microscope (Carl Zeiss, Gottingen, Germany).
GST pull-down assay
Rat brain PKCs was purified by ion exchange chromatography as described previously (51). Recombinant GST–syndecan-2 proteins were purified on glutathione-agarose beads as described previously (14). Bead-bound proteins were mixed with purified PKCβ or -γ and incubated at 4 °C on a rotator for 2 h. The precipitated complexes were eluted with SDS sample buffer, resolved by SDS-PAGE, and immunoblotted with anti-PKC antibodies.
Statistical analysis
Data are presented as the means from at least three independent experiments. Statistical analysis was performed using an unpaired Student's t test. A p value < 0.05 or < 0.01 was considered statistically significant.
Author contributions
B. J. and E. S. O. conceived and coordinated the study and wrote the paper. B. J., H. J., S. C., Y. H. L., S.-T. L., and E.-S. O. performed the experiments and analyzed the data. All authors reviewed the results and approved the final version of the manuscript.
This research was supported by National Research Foundation of Korea (NRF) Grants 2014K1A3A7A03075056 and 2016R1D1A1B03934873 funded by the Korean government (MSIP) and the Seabury Foundation. The authors declare that they have no conflicts of interest with the contents of this article.
- ECM
- extracellular matrix
- SDC2
- syndecan-2
- CM
- conditioned media
- ΔC
- cytoplasmic deletion
- FRNK
- FAK-related non-kinase
- MMP
- matrix metalloproteinase
- FAK
- focal adhesion kinase
- PMA
- phorbol 12-myristate 13-acetate
- RIPA
- radioimmune precipitation
- TCL
- total cell lysates
- qPCR
- quantitative PCR.
References
- 1. Carey D. J. (1997) Syndecans: multifunctional cell-surface co-receptors. Biochem. J. 327, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tkachenko E., Rhodes J. M., and Simons M. (2005) Syndecans: new kids on the signaling block. Circ. Res. 96, 488–500 [DOI] [PubMed] [Google Scholar]
- 3. Afratis N. A., Nikitovic D., Multhaupt H. A., Theocharis A. D., Couchman J. R., and Karamanos N. K. (2017) Syndecans: key regulators of cell signaling and biological functions. FEBS J. 284, 27–41 [DOI] [PubMed] [Google Scholar]
- 4. Mendrola J. M., Berger M. B., King M. C., and Lemmon M. A. (2002) The single transmembrane domains of ErbB receptors self-associate in cell membranes. J. Biol. Chem. 277, 4704–4712 [DOI] [PubMed] [Google Scholar]
- 5. Kinnunen T., Kaksonen M., Saarinen J., Kalkkinen N., Peng H. B., and Rauvala H. (1998) Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J. Biol. Chem. 273, 10702–10708 [DOI] [PubMed] [Google Scholar]
- 6. Granés F., Berndt C., Roy C., Mangeat P., Reina M., and Vilaró S. (2003) Identification of a novel Ezrin-binding site in syndecan-2 cytoplasmic domain. FEBS Lett. 547, 212–216 [DOI] [PubMed] [Google Scholar]
- 7. Chen K., and Williams K. J. (2013) Molecular mediators for raft-dependent endocytosis of syndecan-1, a highly conserved, multifunctional receptor. J. Biol. Chem. 288, 13988–13999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grootjans J. J., Zimmermann P., Reekmans G., Smets A., Degeest G., Dürr J., and David G. (1997) Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. U.S.A. 94, 13683–13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsueh Y.-P., Yang F.-C., Kharazia V., Naisbitt S., Cohen A. R., Weinberg R. J., and Sheng M. (1998) Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J. Cell Biol. 142, 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Couchman J. R. (2003) Syndecans: proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 4, 926–937 [DOI] [PubMed] [Google Scholar]
- 11. Chakravarti R., Sapountzi V., and Adams J. C. (2005) Functional role of syndecan-1 cytoplasmic V region in lamellipodial spreading, actin bundling, and cell migration. Mol. Biol. Cell 16, 3678–3691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim S.-T., Longley R. L., Couchman J. R., and Woods A. (2003) Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase Cα (PKCα) increases focal adhesion localization of PKCα. J. Biol. Chem. 278, 13795–13802 [DOI] [PubMed] [Google Scholar]
- 13. Murakami M., Horowitz A., Tang S., Ware J. A., and Simons M. (2002) Protein kinase C (PKC) δ regulates PKCα activity in a syndecan-4-dependent manner. J. Biol. Chem. 277, 20367–20371 [DOI] [PubMed] [Google Scholar]
- 14. Oh E.-S., Woods A., and Couchman J. R. (1997) Syndecan-4 proteoglycan regulates the distribution and activity of protein kinase C. J. Biol. Chem. 272, 8133–8136 [DOI] [PubMed] [Google Scholar]
- 15. Kramer K. L., Barnette J. E., and Yost H. J. (2002) PKCγ regulates syndecan-2 inside-out signaling during Xenopus left-right development. Cell 111, 981–990 [DOI] [PubMed] [Google Scholar]
- 16. Itano N., Oguri K., Nagayasu Y., Kusano Y., Nakanishi H., David G., and Okayama M. (1996) Phosphorylation of a membrane-intercalated proteoglycan, syndecan-2, expressed in a stroma-inducing clone from a mouse Lewis lung carcinoma. Biochem. J. 315, 925–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prasthofer T., Ek B., Ekman P., Owens R., Hook M., and Johansson S. (1995) Protein kinase C phosphorylates two of the four known syndecan cytoplasmic domains in vitro. Biochem. Mol. Biol. Int. 36, 793–802 [PubMed] [Google Scholar]
- 18. Jung H., Chung H., Chang S. E., Choi S., Han I. O., Kang D. H., and Oh E. S. (2014) Syndecan-2 regulates melanin synthesis via protein kinase C βII-mediated tyrosinase activation. Pigment Cell Melanoma Res. 27, 387–397 [DOI] [PubMed] [Google Scholar]
- 19. Imai K., Hiramatsu A., Fukushima D., Pierschbacher M. D., and Okada Y. (1997) Degradation of decorin by matrix metalloproteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-β1 release. Biochem. J. 322, 809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sternlicht M. D., and Werb Z. (2001) How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17, 463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sengupta N., and MacDonald T. (2007) The role of matrix metalloproteinases in stromal/epithelial interactions in the gut. Physiology 22, 401–409 [DOI] [PubMed] [Google Scholar]
- 22. Adachi Y., Yamamoto H., Itoh F., Arimura Y., Nishi M., Endo T., and Imai K. (2001) Clinicopathologic and prognostic significance of matrilysin expression at the invasive front in human colorectal cancers. Int. J. Cancer. 95, 290–294 [DOI] [PubMed] [Google Scholar]
- 23. Masaki T., Matsuoka H., Sugiyama M., Abe N., Goto A., Sakamoto A., and Atomi Y. (2001) Matrilysin (MMP-7) as a significant determinant of malignant potential of early invasive colorectal carcinomas. Br. J. Cancer 84, 1317–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson C. L., and Matrisian L. M. (1996) Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int. J. Biochem. Cell Biol. 28, 123–136 [DOI] [PubMed] [Google Scholar]
- 25. Nelson A. R., Fingleton B., Rothenberg M. L., and Matrisian L. M. (2000) Matrix metalloproteinases: biologic activity and clinical implications. J. Clin. Oncol. 18, 1135–1149 [DOI] [PubMed] [Google Scholar]
- 26. Shiomi T., and Okada Y. (2003) MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. 22, 145–152 [DOI] [PubMed] [Google Scholar]
- 27. Gearing A. J., Beckett P., Christodoulou M., Churchill M., Clements J., Davidson A. H., Drummond A. H., Galloway W. A., Gilbert R., and Gordon J. L. (1994) Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 370, 555–557 [DOI] [PubMed] [Google Scholar]
- 28. Vargo-Gogola T., Crawford H. C., Fingleton B., and Matrisian L. M. (2002) Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch. Biochem. Biophys. 408, 155–161 [DOI] [PubMed] [Google Scholar]
- 29. Li Q., Park P. W., Wilson C. L., and Parks W. C. (2002) Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 111, 635–646 [DOI] [PubMed] [Google Scholar]
- 30. Ryu H.-Y., Lee J., Yang S., Park H., Choi S., Jung K.-C., Lee S.-T., Seong J.-K., Han I.-O., and Oh E.-S. (2009) Syndecan-2 functions as a docking receptor for pro-matrix metalloproteinase-7 in human colon cancer cells. J. Biol. Chem. 284, 35692–35701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee H., Kim Y., Choi Y., Choi S., Hong E., and Oh E.-S. (2011) Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem. Biophys. Res. Commun. 409, 148–153 [DOI] [PubMed] [Google Scholar]
- 32. Choi S., Kim J.-Y., Park J. H., Lee S.-T., Han I.-O., and Oh E.-S. (2012) The matrix metalloproteinase-7 regulates the extracellular shedding of syndecan-2 from colon cancer cells. Biochem. Biophys. Res. Commun. 417, 1260–1264 [DOI] [PubMed] [Google Scholar]
- 33. Choi S., Choi Y., Jun E., Kim I.-S., Kim S.-E., Jung S.-A., and Oh E.-S. (2015) Shed syndecan-2 enhances tumorigenic activities of colon cancer cells. Oncotarget 6, 3874–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schlaepfer D. D., and Hunter T. (1998) Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 8, 151–157 [DOI] [PubMed] [Google Scholar]
- 35. Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., and Schlaepfer D. D. (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2, 249–256 [DOI] [PubMed] [Google Scholar]
- 36. Hamadi A., Bouali M., Dontenwill M., Stoeckel H., Takeda K., and Rondé P. (2005) Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J. Cell Sci. 118, 4415–4425 [DOI] [PubMed] [Google Scholar]
- 37. McLean G. W., Carragher N. O., Avizienyte E., Evans J., Brunton V. G., and Frame M. C. (2005) The role of focal-adhesion kinase in cancer: a new therapeutic opportunity. Nat. Rev. Cancer 5, 505–515 [DOI] [PubMed] [Google Scholar]
- 38. Parsons J. T. (2003) Focal adhesion kinase: the first ten years. J. Cell Sci. 116, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 39. Schaller M. D., Borgman C. A., and Parsons J. T. (1993) Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol. Cell. Biol. 13, 785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hauck C. R., Sieg D. J., Hsia D. A., Loftus J. C., Gaarde W. A., Monia B. P., and Schlaepfer D. D. (2001) Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res. 61, 7079–7090 [PubMed] [Google Scholar]
- 41. Oh E.-S., Couchman J. R., and Woods A. (1997) Serine phosphorylation of syndecan-2 proteoglycan cytoplasmic domain. Arch. Biochem. Biophys. 344, 67–74 [DOI] [PubMed] [Google Scholar]
- 42. Essner J. J., Chen E., and Ekker S. C. (2006) Syndecan-2. Int. J. Biochem. Cell Biol. 38, 152–156 [DOI] [PubMed] [Google Scholar]
- 43. Kwon M.-J., Jang B., Yi J. Y., Han I.-O., and Oh E. S. (2012) Syndecans play dual roles as cell adhesion receptors and docking receptors. FEBS Lett. 586, 2207–2211 [DOI] [PubMed] [Google Scholar]
- 44. Orosco A., Fromigué O., Haÿ E., Marie P. J., and Modrowski D. (2006) Dual involvement of protein kinase C δ in apoptosis induced by syndecan-2 in osteoblasts. J. Cell. Biochem. 98, 838–850 [DOI] [PubMed] [Google Scholar]
- 45. Park H., Han I., Kwon H. J., and Oh E.-S. (2005) Focal adhesion kinase regulates syndecan-2–mediated tumorigenic activity of HT1080 fibrosarcoma cells. Cancer Res. 65, 9899–9905 [DOI] [PubMed] [Google Scholar]
- 46. Lee J.-H., Park H., Chung H., Choi S., Kim Y., Yoo H., Kim T.-Y., Hann H.-J., Seong I., Kim J., Kang K. G., Han I. O., and Oh E. S. (2009) Syndecan-2 regulates the migratory potential of melanoma cells. J. Biol. Chem. 284, 27167–27175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fang J. Y., and Richardson B. C. (2005) The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 6, 322–327 [DOI] [PubMed] [Google Scholar]
- 48. Jang B., Jung H., Chung H., Moon B.-I., and Oh E.-S. (2016) Syndecan-2 enhances E-cadherin shedding and fibroblast-like morphological changes by inducing MMP-7 expression in colon cancer cells. Biochem. Biophys. Res. Commun. 477, 47–53 [DOI] [PubMed] [Google Scholar]
- 49. Jo Y., Yeon J., Kim H.-J., and Lee S.-T. (2000) Analysis of tissue inhibitor of metalloproteinases-2 effect on pro-matrix metalloproteinase-2 activation by membrane-type 1 matrix metalloproteinase using baculovirus/insect-cell expression system. Biochem. J. 345, 511–519 [PMC free article] [PubMed] [Google Scholar]
- 50. Koo H., Kim J.-H., Hwang I., Lee S.-J., Kim T.-H., Rhee K.-H., and Lee S.-T. (2002) Refolding of the catalytic and hinge domains of human MT1-mMP expressed in Escherichia coli and its characterization. Mol. Cells 13, 118–124 [PubMed] [Google Scholar]
- 51. Marais R. M., and Parker P. J. (1989) Purification and characterisation of bovine brain protein kinase C isotypes α, β and γ. Eur. J. Biochem. 182, 129–137 [DOI] [PubMed] [Google Scholar]