Abstract
The synuclein family has long been associated with Parkinson's disease and dementia. Although the self-assembly of α-synuclein (αS) into oligomers and amyloid fibrils is well established, the aggregation propensity of other members of the family and their role in disease is still under debate. Moriarty et al. now suggest that the pH switching that occurs between different cellular environments could control β-synuclein (βS) aggregation via altering its charge distribution, thus opening new possible roles for βS in Parkinson's and other neurodegenerative diseases.
Introduction
Amyloids are known to be involved in more than 20 fatal diseases. Yet, despite decades of research, there are still many open questions regarding the mechanisms of their formation, their resulting structure, and the basis by which they cause toxicity. This is mostly due to the aggregative and highly polymorphic nature of amyloids that challenges mechanistic and structural investigations. In particular, Parkinson's disease is closely linked to the amyloid-forming protein αS.1 However, there are other members of the synuclein family that possibly also contribute to disease progression. In particular, βS shares 62% sequence identity with αS, and there are known βS mutations associated with dementia (Fig. 1) (1). However, the functional role of βS seems to vary widely from study to study, and many basic details of its structure and activity remain unclear. In their paper, Moriarty et al. (2) now demonstrate a possible explanation for these diverse outcomes, showing that βS fibrillation is regulated by a pH switch, suggesting new fibrillation mechanisms and a more complex in vivo role than formerly anticipated.
Figure 1.
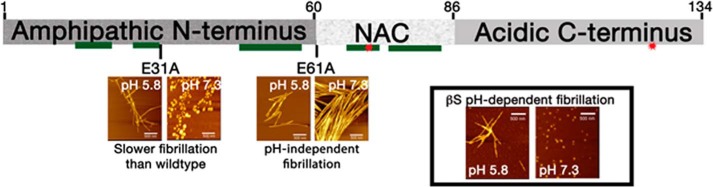
The pH-controlled fibrillation of β-synuclein. The three domains of βS are indicated. The approximate location of two mutations associated with dementia (P123H and V70M) (1) are indicated with red stars. Segments predicted to be amyloidogenic according to the 3D-profile method (10) are indicated by green rectangles below the domains. AFM images shown for wild-type βS (boxed on the right) and its mutants are taken from Ref. 2.
Previous studies have demonstrated that both αS and βS are composed of three domains: an N-terminal amphipathic domain, a central hydrophobic core domain known as the non-amyloid component (NAC), originally reported to be deposited with amyloid-β in the brains of Alzheimer's disease patients, and an acidic C-terminal domain (Fig. 1). The NAC domain, and in particular a central hydrophobic segment within the domain, constitutes the critical determinant of αS fibrillation and toxicity (3). This segment is missing in βS, and as a result, βS was anticipated to lack aggregation propensity. Indeed, βS was suggested to inhibit αS fibrillation and ameliorate disease (4). However, in other studies, fibrillation of βS has been observed and associated with neurodegeneration (5), and the presence of external substances such as environmental pollutants enhanced βS aggregation (6).
Given that synucleins are found in several different cellular compartments where the pH varies and that previous studies showed impacts of changing pH on amyloid protein fibrillation rates, Moriarty et al. (2) tested this effect and found that βS formed fibrils at a slightly acidic pH found along the endosomal pathway (pH 5.8), but not at pH 7.3. The importance of the different domains of βS in fibrillation and pH regulation was investigated by constructing eight chimeras, in which the three domains of αS and βS were swapped. Fibril formation and kinetics were monitored via atomic-force microscopy (AFM) and binding to the amyloid-indicator dye thioflavin T. The chimeras revealed that all three domains regulate fibrillation of βS, although in markedly different ways. An inhibitory effect on fibrillation was attributed to the C-terminal domain, which has the lowest sequence identity (∼30%) to αS. In contrast, the N-terminal domain appeared critical for βS fibrillation. Swapping βS and αS N-terminal domains (90% sequence identity) induced a relatively small effect on fibrillation rate in most chimeras. However, the chimera containing the N-terminal domain of αS with the NAC and C-terminal domains of βS showed no fibrillation, even at acidic pH, which may indicate interdependent relations between the different domains. The role of the N-terminal domain in regulating fibrillation was also supported by computational modeling in which βS was threaded onto the solid-state NMR–based structure of αS (7) and simulated using Rosetta Symmetry (8). The simulations suggested that the N-terminal domain is imperative for the formation and stability of a Greek-key–like architecture observed for αS (2). NMR analysis of the βS monomer also suggested interactions between the N-terminal and NAC domains. Moreover, a glutamate to alanine alteration at position 31 (E31A) in the N-terminal domain delayed fibrillation of βS (Fig. 1), supporting the involvement of this domain in aggregation (2).
What is the role of the NAC domain? The chimeras constructed by Moriarty et al. reinforced the critical role of this domain in αS fibrillation regardless of the tested pH and the identity of the other domains. Moreover, the chimeras containing the NAC domain of αS exhibited faster fibrillation than all their equivalent chimeras containing the NAC domain of βS. Importantly, the chimeras that included the NAC domain of βS fibrillated only at acidic pH, suggesting that a pH switch is likely dependent on this domain. Accordingly, mutation of the first residue at the NAC domain, which is occupied by a glutamate residue in both αS and βS, into an alanine (E61A) abolished the pH switch and resulted in rapid and robust fibrillation of βS at both neutral and acidic pH. Because βS lacks the critical hydrophobic stretch that is present in the NAC domain of αS (3), fibrillation might be nucleated via alternative core segments, either in the NAC or N-terminal domains. Interestingly, an amyloid-like structure was determined for a 10-residue segment from the N-terminal domain of αS that included the A53T mutation observed in families with inherited forms of Parkinson's disease (9). The equivalent segment in βS, 47GVVQGVASVA56, is predicted to be highly amyloidogenic according to the 3D-profile method (Fig. 1) (10), with a higher amyloid-forming propensity score than that of the equivalent αS wild-type segment. It puts forward the possible involvement of this region in nucleating fibrillation of βS.
The results of Moriarty et al. suggest a possible biological and pathophysiological role of βS fibrils in specific microenvironments and inside organelles. The authors postulated that the pH switch stems from rearrangement of charge distribution involving altered protonation of side chains that regulate fibrillation propensities via “polar zippers.” It is striking that residue Glu-61, which appears to be critical for controlling the pH switch, is conserved in αS, which already fibrillates at both pH values. This highlights the complex regulation of these two synuclein homologs and supports the possibility of different nucleation regions. Regardless of whether the Greek-key–like architecture of αS is preserved in βS, it is very likely that the regions forming β-sheet interactions differ between αS and βS. Thus, elucidating the high-resolution structure of the βS fibril core will provide important information into how this polymorphism may occur. What about γ-synuclein (γS), another member of the family? Fibrillation should be expected because it shows high sequence conservation between the N-terminal and NAC domains with αS (67% sequence identity) but has a significantly shorter and variable C-terminal domain. Yet, previous results show lack of fibrillation (4), which might be related to the microenvironment tested, as shown for βS, or to a critical inhibitory role of the short C-terminal domain. Overall, this manuscript further demonstrates the complexity of the amyloid fold and its vast polymorphism. It will be intriguing to figure out how this complex regulation of fibrillation evolved in a specific protein family and whether this was a dedicated process or a mishap leading to human diseases.
The author declares that she has no conflicts of interest with the contents of this article.
- αS
- α-synuclein
- βS
- β-synuclein
- γS
- γ-synuclein
- NAC
- non-amyloid component
- AFM
- atomic force microscopy.
References
- 1. Ohtake H., Limprasert P., Fan Y., Onodera O., Kakita A., Takahashi H., Bonner L. T., Tsuang D. W., Murray I. V., Lee V. M., Trojanowski J. Q., Ishikawa A., Idezuka J., Murata M., Toda T., Bird T. D., Leverenz J. B., Tsuji S., and La Spada A. R. (2004) Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology 63, 805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moriarty G. M., Olson M. P., Atieh T. B., Janowska M. K., Khare S. D., and Baum J. (2017) A pH -dependent switch promotes β-synuclein fibril formation via glutamate residues. J. Biol. Chem. 292, 16368–16379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giasson B. I., Murray I. V., Trojanowski J. Q., and Lee V. M. (2001) A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J. Biol. Chem. 276, 2380–2386 [DOI] [PubMed] [Google Scholar]
- 4. Uversky V. N., Li J., Souillac P., Millett I. S., Doniach S., Jakes R., Goedert M., and Fink A. L. (2002) Biophysical properties of the synucleins and their propensities to fibrillate: Inhibition of α-synuclein assembly by β- and γ-synucleins. J. Biol. Chem. 277, 11970–11978 [DOI] [PubMed] [Google Scholar]
- 5. Taschenberger G., Toloe J., Tereshchenko J., Akerboom J., Wales P., Benz R., Becker S., Outeiro T. F., Looger L. L., Bähr M., Zweckstetter M., and Kügler S. (2013) β-Synuclein aggregates and induces neurodegeneration in dopaminergic neurons. Ann. Neurol. 74, 109–118 [DOI] [PubMed] [Google Scholar]
- 6. Yamin G., Munishkina L. A., Karymov M. A., Lyubchenko Y. L., Uversky V. N., and Fink A. L. (2005) Forcing nonamyloidogenic β-synuclein to fibrillate. Biochemistry 44, 9096–9107 [DOI] [PubMed] [Google Scholar]
- 7. Tuttle M. D., Comellas G., Nieuwkoop A. J., Covell D. J., Berthold D. A., Kloepper K. D., Courtney J. M., Kim J. K., Barclay A. M., Kendall A., Wan W., Stubbs G., Schwieters C. D., Lee V. M. Y., George J. M., and Rienstra C. M. (2016) Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 23, 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiMaio F., Leaver-Fay A., Bradley P., Baker D., and André I. (2011) Modeling symmetric macromolecular structures in Rosetta3. PloS One 6, e20450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez J. A., Ivanova M. I., Sawaya M. R., Cascio D., Reyes F. E., Shi D., Sangwan S., Guenther E. L., Johnson L. M., Zhang M., Jiang L., Arbing M. A., Nannenga B. L., Hattne J., Whitelegge J., Brewster A. S., Messerschmidt M., Boutet S., Sauter N. K., Gonen T., and Eisenberg D. S. (2015) Structure of the toxic core of α-synuclein from invisible crystals. Nature 525, 486–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldschmidt L., Teng P. K., Riek R., and Eisenberg D. (2010) Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc. Natl. Acad. Sci. U.S.A. 107, 3487–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]