it is well known that athletes have a resting sinus bradycardia—their resting heart rate can be half the normal value (7)— and this is normally attributed to high vagal tone (3). This is a logical assumption, because we have known since 1921 from the work of the physiologist Otto Loewi that stimulating the vagus nerve decreases the heart rate (8a). However, we argue here that the bradycardia is not the result of vagal tone and instead is the result of an electrical remodeling of the sinus node. In exercise-trained rats and mice, we have shown a downregulation of ion channels, intracellular Ca2+-handling molecules, Na+-K+ pump subunits, and gap junction channels of the pacemaker of the heart, the sinus node (5). In particular, we have shown a downregulation of the HCN4 pacemaker channel and the corresponding ionic current (funny current, If), and block of If abolishes the difference in heart rate between trained and untrained animals (5). More recently, we have shown that upregulation of a microRNA (miR-423–5p) prevents the downregulation of HCN4 and If and the consequent bradycardia (6).
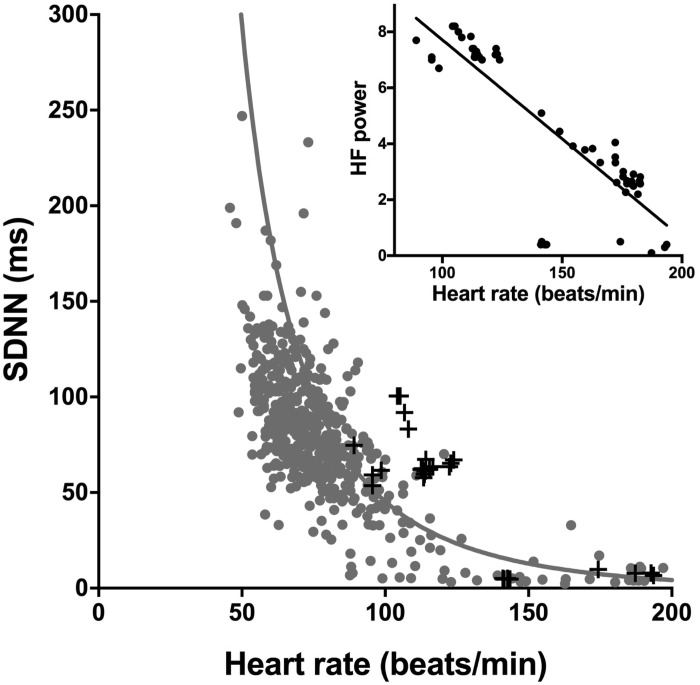
Much of the evidence for the high vagal tone hypothesis concerns heart rate variability (1). For example, Billman et al. (3) argued that exercise training-induced bradycardia in the dog is the result of high vagal tone based partly on low heart rate variability after exercise training. In general, it is well established that heart rate variability is low in the athlete and this is taken as evidence of high vagal tone (1). However, based on the underlying biophysics, Zaza’s group (12, 16) and we (11) have shown that heart rate variability is primarily a nonlinear surrogate of heart rate itself and cannot be used in any simple manner as a measure of autonomic nerve activity to the heart. Billman et al. (3) report two measures of heart rate variability, the standard deviation of normal-to-normal beats (SDNN) and high frequency power. The gray points in Fig. 1 show the relationship between SDNN and heart rate from a wide range of published studies (from different species, preparations, and conditions) collected and previously published by us (11). The gray points show that regardless of the source of the data there is a unique exponential-like relationship between SDNN and heart rate, and the solid line, which is a good fit to the experimental data, is the relationship between SDNN and heart rate as predicted by a simple biophysical model (11). The crosses show all available data from Billman et al. (3). They are consistent with the other data shown as well as the predicted relationship between SDNN and heart rate. Based on all the data available in the paper from Billman et al. (3), the inset in Fig. 1 shows the relationship between high frequency power and the corresponding heart rates—over the range of heart rates in their study, there is an excellent linear relationship between the two (R2 = 0.73; P < 0.0001). It is concluded that the changes in heart rate variability in the study of Billman et al. (3) are largely the result of the changes in heart rate rather than changes in autonomic tone. It is possible changes in autonomic tone do result in changes in heart rate variability, but any such changes will be almost impossible to distinguish from the overriding effect of heart rate.
Fig. 1.
Evidence against the vagal tone hypothesis: changes in heart rate variability in the study of Billman et al. (3) are primarily determined by heart rate. Main graph shows the relationship between SDNN and heart rate in the study of Billman et al. (3); black points show data from Billman et al. (3); gray points show data from a wide range of published studies (11); the gray line shows the relationship between SDNN and heart rate as determined by a simple biophysical model (11). Inset shows the relationship between high frequency (HF) power and heart rate in the study of Billman et al. (3).
A second line of evidence for the high vagal tone hypothesis concerns the “intrinsic heart rate” measured in vivo after complete autonomic blockade (usually by atropine and propranolol). For example, Billman et al. (3) report that the training-induced bradycardia in dogs is abolished after complete autonomic blockade and cite this as evidence for the high vagal tone hypothesis. However, contrary to this, Billman et al. (3) also report that complete block of vagal nerve activity alone had no effect on the bradycardia: the baseline heart rate was 18.7 beats/min lower in the trained dogs; after block of vagal nerve activity by atropine, the heart rate was still 18.5 beats/min lower. Billman et al. (3) cite three studies in which they say autonomic blockade does abolish the difference in heart rate between exercise-trained and sedentary individuals: a review (15), one study on human subjects (13), and one on mice (8). The review (15) does not contain data relating to heart rate, and the study on human subjects from Shi et al. (13) does not bear scrutiny: the intrinsic heart rate after complete autonomic blockade in eight untrained subjects 22–34 yr of age is reported to be 85 ± 3 beats/min. In contrast, Jose and Collison (9) reported that the intrinsic heart rate after complete autonomic blockade is 105.5 ± 0.7 beats/min based on data from 152 subjects 20–30 yr of age. The intrinsic heart rate reported by Shi et al. (13) is more than two standard deviations from the value reported by Jose and Collison (9), and we conclude that the data are flawed and cannot be used. However, the study on mice from De Angelis et al. (8) cannot be challenged on technical grounds and there is no obvious explanation why De Angelis et al. (8) observed that the training-induced bradycardia in mice was abolished after complete autonomic blockade—in contrast, we have reported that the training-induced bradycardia in mice is not abolished after complete autonomic blockade (5). The weight of evidence suggests that autonomic blockade has little effect on training-induced bradycardia: all studies on human subjects that bear scrutiny show that the bradycardia in athletes is little affected or even greater after complete autonomic blockade (4). A recent study measured the training-induced bradycardia in 20 young and 31 old subjects and the bradycardia was approximately the same under baseline conditions (5.9 and 4.8 beats/min) and after the block of vagal tone by atropine (6.0 and 5.9 beats/min) (2). The majority of animal studies also show that the resting bradycardia is little affected or even greater after complete autonomic blockade (4). Finally, in our work we measure the intrinsic heart rate in the isolated sinus node (in which autonomic blockade has no effect), and this preparation also shows that the intrinsic heart rate is lower in trained rats and mice (5). Analogous data have been obtained from the rabbit (14).
Billman et al. (3) claim that after heart transplantation (and cardiac denervation) there is no training-induced bradycardia and they cite various studies. However, one study of heart transplant patients reports that there is a training-induced bradycardia— in eight highly compliant patients there was a decrease in resting heart rate of ~11 beats/min (10). However, even where there is no training-induced bradycardia after heart transplantation, it does not necessarily mean that the training-induced bradycardia is the result of high vagal tone. We believe that the bradycardia is the result of a remodeling of ion channels in the sinus node (5) and, although our latest work suggests that this is driven by microRNAs (6), we do not know the ultimate stimulus for the remodeling. One possibility is that it is the undoubted changes in autonomic tone during exercise, such as the increase in sympathetic activity, that stimulates the remodeling of the sinus node.
In conclusion, the most likely mechanism underlying the resting sinus bradycardia in athletes is a remodeling of ion channels in the sinus node.
GRANTS
This work was supported by the British Heart Foundation (RG/11/18/29257).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.R.B. prepared figures; M.R.B. drafted manuscript; M.R.B., Y.W., S.N., J.A., G.H., O.M., and A.D. edited and revised manuscript; M.R.B., Y.W., S.N., J.A., G.H., O.M., and A.D. approved final version of manuscript.
REFERENCES
- 1.Aubert AE, Seps B, Beckers F. Heart rate variability in athletes. Sports Med 33: 889–919, 2003. doi: 10.2165/00007256-200333120-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bahrainy S, Levy WC, Busey JM, Caldwell JH, Stratton JR. Exercise training bradycardia is largely explained by reduced intrinsic heart rate. Int J Cardiol 222: 213–216, 2016. doi: 10.1016/j.ijcard.2016.07.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billman GE, Cagnoli KL, Csepe T, Li N, Wright P, Mohler PJ, Fedorov VV. Exercise training-induced bradycardia: evidence for enhanced parasympathetic regulation without changes in intrinsic sinoatrial node function. J Appl Physiol (1985) 118: 1344–1355, 2015. doi: 10.1152/japplphysiol.01111.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyett MR, D’Souza A, Zhang H, Morris GM, Dobrzynski H, Monfredi O. Viewpoint: is the resting bradycardia in athletes the result of remodeling of the sinoatrial node rather than high vagal tone? J Appl Physiol (1985) 114: 1351–1355, 2013. doi: 10.1152/japplphysiol.01126.2012. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza A, Bucchi A, Johnsen AB, Logantha SJ, Monfredi O, Yanni J, Prehar S, Hart G, Cartwright E, Wisloff U, Dobryznski H, DiFrancesco D, Morris GM, Boyett MR. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun 5: 3775, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza A, Gill E, Cox C, Dobrzynski H, Cartwright E, Oceandy D, Boyett MR. MicroRNA control of the pacemaker channel and training-induced bradycardia. Circ Res 117, 2015. [Google Scholar]
- 7.D’Souza A, Sharma S, Boyett MR. CrossTalk opposing view: bradycardia in the trained athlete is attributable to a downregulation of a pacemaker channel in the sinus node. J Physiol 593: 1749–1751, 2015. doi: 10.1113/jphysiol.2014.284356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Angelis K, Wichi RB, Jesus WR, Moreira ED, Morris M, Krieger EM, Irigoyen MC. Exercise training changes autonomic cardiovascular balance in mice. J Appl Physiol (1985) 96: 2174–2178, 2004. doi: 10.1152/japplphysiol.00870.2003. [DOI] [PubMed] [Google Scholar]
- 8a.Hurst JW, Fye WB, Zimmer HG. Otto Loewi and the chemical transmission of vagus stimulation in the heart. Clin Cardiol 29: 135–136, 2006. doi: 10.1002/clc.4960290313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res 4: 160–167, 1970. doi: 10.1093/cvr/4.2.160. [DOI] [PubMed] [Google Scholar]
- 10.Kavanagh T, Yacoub MH, Mertens DJ, Kennedy J, Campbell RB, Sawyer P. Cardiorespiratory responses to exercise training after orthotopic cardiac transplantation. Circulation 77: 162–171, 1988. doi: 10.1161/01.CIR.77.1.162. [DOI] [PubMed] [Google Scholar]
- 11.Monfredi O, Lyashkov AE, Johnsen AB, Inada S, Schneider H, Wang R, Nirmalan M, Wisloff U, Maltsev VA, Lakatta EG, Zhang H, Boyett MR. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension 64: 1334–1343, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocchetti M, Malfatto G, Lombardi F, Zaza A. Role of the input/output relation of sinoatrial myocytes in cholinergic modulation of heart rate variability. J Cardiovasc Electrophysiol 11: 522–530, 2000. doi: 10.1111/j.1540-8167.2000.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 13.Shi X, Stevens GH, Foresman BH, Stern SA, Raven PB. Autonomic nervous system control of the heart: endurance exercise training. Med Sci Sports Exerc 27: 1406–1413, 1995. doi: 10.1249/00005768-199510000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Such L, Rodriguez A, Alberola A, Lopez L, Ruiz R, Artal L, Pons I, Pons ML, García C, Chorro FJ. Intrinsic changes on automatism, conduction, and refractoriness by exercise in isolated rabbit heart. J Appl Physiol (1985) 92: 225–229, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Zanesco A, Antunes E. Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol Ther 114: 307–317, 2007. doi: 10.1016/j.pharmthera.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Zaza A, Lombardi F. Autonomic indexes based on the analysis of heart rate variability: a view from the sinus node. Cardiovasc Res 50: 434–442, 2001. doi: 10.1016/S0008-6363(01)00240-1. [DOI] [PubMed] [Google Scholar]