We demonstrate that anatomic dead space ventilation increases significantly over time in mice in response to mechanical ventilation. The novel functional lung-imaging techniques applied here yield sensitive measures of airway volumes that may have wide applications.
Keywords: anatomic dead space, mechanical ventilation, four-dimensional computed tomography, velocimetry
Abstract
Increased dead space is an important prognostic marker in early acute respiratory distress syndrome (ARDS) that correlates with mortality. The cause of increased dead space in ARDS has largely been attributed to increased alveolar dead space due to ventilation/perfusion mismatching and shunt. We sought to determine whether anatomic dead space also increases in response to mechanical ventilation. Mice received intratracheal lipopolysaccharide (LPS) or saline and mechanical ventilation (MV). Four-dimensional computed tomography (4DCT) scans were performed at onset of MV and after 5 h of MV. Detailed measurements of airway volumes and lung tidal volumes were performed using image analysis software. The forced oscillation technique was used to obtain measures of airway resistance, tissue damping, and tissue elastance. The ratio of airway volumes to total tidal volume increased significantly in response to 5 h of mechanical ventilation, regardless of LPS exposure, and airways demonstrated significant variation in volumes over the respiratory cycle. These findings were associated with an increase in tissue elastance (decreased lung compliance) but without changes in tidal volumes. Airway volumes increased over time with exposure to mechanical ventilation without a concomitant increase in tidal volumes. These findings suggest that anatomic dead space fraction increases progressively with exposure to positive pressure ventilation and may represent a pathological process.
NEW & NOTEWORTHY We demonstrate that anatomic dead space ventilation increases significantly over time in mice in response to mechanical ventilation. The novel functional lung-imaging techniques applied here yield sensitive measures of airway volumes that may have wide applications.
acute respiratory distress syndrome (ARDS) is a severe, life-threatening form of respiratory failure characterized by its acute onset, diffuse lung inflammation, and severe hypoxemia (19, 27). Many physiological abnormalities are associated with ARDS, including poor gas exchange and decreased lung compliance. Increased dead space is another well-recognized physiological disturbance in the ARDS lung (14, 15, 23), and recent studies have demonstrated that it is an early prognostic marker of mortality (5, 14, 15, 23). Increased dead space in ARDS is primarily attributable to increased alveolar dead space through ventilation/perfusion heterogeneity and shunt (6, 28). However, positive pressure ventilation and positive end-expiratory pressure (PEEP) increase anatomic dead space, which is the volume of air in the upper airways that does not participate in gas exchange (6, 9, 30). Therefore the effects of mechanical ventilation on anatomic dead space might play a role in the increased dead space of ARDS, although increased anatomic dead space has not yet been linked to increased mortality in this disease.
In vivo studies of small animal airway morphology and responses of the airways to mechanical ventilation are challenging, and novel approaches have been used to overcome the size limitations. Sinclair et al. (32) ventilated rats using tantalum dust to provide contrast during microfocal X-ray imaging of the airways and demonstrated increased airway distension and strain with increasing tidal volumes and PEEP. Nickles et al. (22) used a flat-panel volume micro-CT scanner to acquire high-spatial resolution images of the trachea, the right and left main bronchi, and the large segmental bronchi of mice and demonstrated a similar, large increase in upper airway volumes with mechanical ventilation compared with spontaneous breathing. These authors also elegantly demonstrated an increase in inflammatory cytokine production in isolated tracheas exposed to ventilation. However, in vivo responses of airways to prolonged exposure to mechanical ventilation have not been examined to date.
The aim of this study was to determine how the large airways respond over time to positive pressure ventilation. We addressed this by applying four-dimensional computed tomography (4DCT) to mice in a model of prolonged (5 h) mechanical ventilation. We used intensity-based thresholding and skeletonization-based image-processing techniques to analyze CT images to directly measure the airway volumes. We applied X-ray velocimetry to the same image data to generate detailed three-dimensional tissue expansion maps (7, 10, 21, 25), which allowed us to precisely measure lung tidal volumes. Combining these measurements, we estimated the relative contribution of airway volumes to the total tidal volume, Vaw/Vt, and discovered that this ratio increases over time in response to mechanical ventilation.
METHODS
Animals.
Eight-week-old BALB/c female mice (n = 11) were obtained from Monash Animal Research Platform (Monash University, Melbourne, VIC, Australia). All experiments were approved by the local Animal Ethics Committee of Monash University (Melbourne, VIC, Australia) and conducted in accordance with the guidelines set out in the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Administration of intratracheal reagents.
Mice were transiently anesthetized with isofluorane (Piramal Healthcare, Bethlehem, PA) and suspended from their incisors for intratracheal instillation of lipopolysaccharide (LPS, Escherichia coli; 0111:B4, ultrapure; InvivoGen, San Diego, CA; n = 6) or saline alone (Pfizer, Bentley, WA, Australia; n = 5). LPS was diluted in saline (100 μg/ml), and both groups received 2 μl/g body wt of solution intratracheally (total volumes ranged from 45 to 60 µl). We demonstrated in a prior study that this dose of intratracheal LPS (0.2 mg/kg) does not cause lung injury alone, but can lead to lung injury in conjunction with injurious mechanical ventilation (13). Following instillation, mice were allowed to recover from anesthesia and returned to their cages.
Tracheostomy and mechanical ventilation.
Ninety minutes after intratracheal LPS or saline, mice were anesthetized with intraperitoneal injections of a mix of ketamine (Parnell Australia, Alexandria, NSW, Australia) and xylazine (Xylazil-20; Troy Laboratories, Smithfield, NSW, Australia) at doses of 150 and 10 mg/kg, respectively. Each mouse was securely restrained in a custom-built acrylic chassis (7) in a supine position, and a surgical tracheostomy was performed as previously described (35). Mice were then ventilated using pressure control ventilation on a mouse ventilator (4Dx; Melbourne, VIC, Australia) with an inspiratory pressure of 20 cmH2O, zero positive end-expiratory pressure (PEEP), and inspiratory and expiratory times of 300 ms each (a respiratory rate of 100 breaths/min). Tidal volumes with these settings were ~250 µl for a 20-g mouse, or 12.5 µl/g (12.5 ml/kg). Mice underwent mechanical ventilation (MV) for a total of 5 h and were imaged at the onset of MV and after 5 h of MV.
Subcutaneous saline (500 µl) was administered at the onset of MV and after 3 h of MV. Repeat doses of anesthesia were administered as subcutaneous injections as needed (usually every 2–3 h). Mice were kept warm using pocket warmers wrapped around the lower abdomen and legs.
Imaging protocol.
Imaging was conducted in the Laboratory for Dynamic Imaging at Monash University (Melbourne, VIC, Australia). The X-ray-imaging setup consisted of a high-brightness microfocus (15-µm spot size) X-ray source (Excillum, Kista, Sweden; 16, 17). This X-ray source (70 kV, 250 W) produces a spectrum with a characteristic peak at 25 keV. A high-speed complementary metal-oxide semiconductor (CMOS) flat-panel detector (PaxScan; Varian Medical Systems, Palo Alto, CA) with an isotropic pixel size of 0.194 mm was used to capture images at a frame rate of 30 Hz and an exposure time of 18 ms. The system enables appreciable propagation-based phase contrast, which results in lung tissue speckle patterns, based on the contrast between air and soft tissue (17). The mouse was positioned in the acrylic chassis in front of the X-ray beam in the upright position. A high-precision rotary stage (Zaber Technologies, Vancouver, BC, Canada) was used to rotate the mice 360 deg under mechanical ventilation for the 4DCT scan. The imaging was synchronized with ventilation and gated to obtain 800 projection images of the lungs at end-expiration and end-inspiration for CT reconstruction. The radiation dose delivered (equating to 1,600 projections with 18-ms exposure times and an air kerma rate of 5.01 mGy/s) was measured to be 120 mGy, which is 1.8% of the LD50/30 (the dose of radiation expected to cause death to 50% of an exposed population within 30 days; ~7 Gy) for BALB/c mice (24).
A calibration scan of an acrylic cylinder with fiducials (34) was performed before and after the mouse scans. This process captures the tilt angle and center of rotation of the scan necessary for accurate CT reconstruction results. The source-to-isocenter of the rotation stage and source-to-detector distances were 374 and 3,315 mm, respectively, resulting in an effective isotropic voxel size of 21.9 µm for the entire imaging system.
Image analysis.
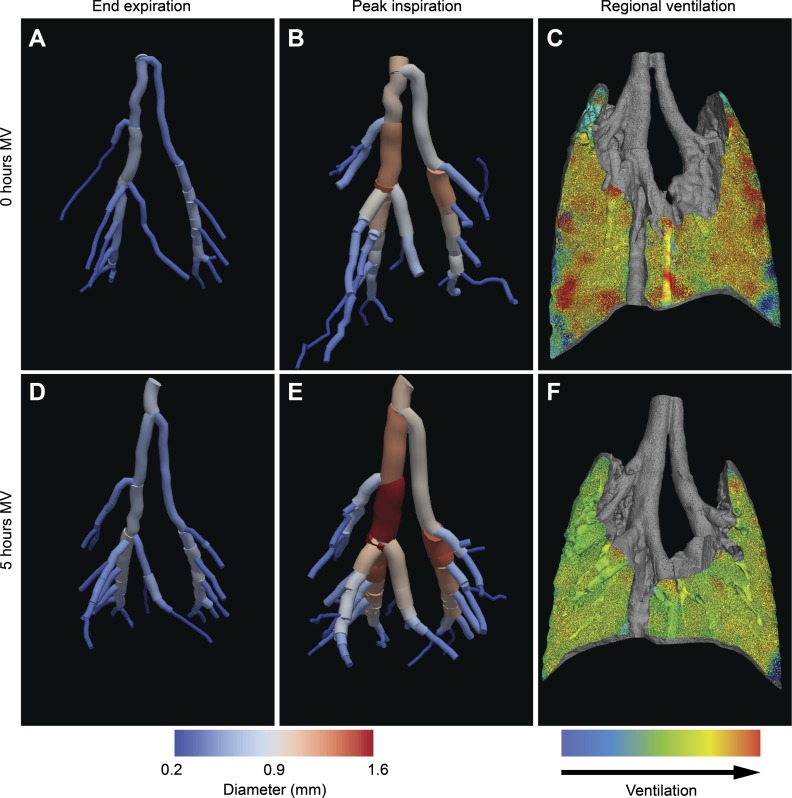
The airway trees at the end-expiratory (Fig. 1, A and D) and end-inspiratory (Fig. 1, B and E) phases of the respiratory cycle were extracted in a two-stage process. Using Avizo (FEI Visualization Sciences Group, Mérignac, France) 3D visualization and analysis software, the airways were first segmented using a threshold-based flood fill technique. The root of the airway tree was then specified manually, and the centerline tree was extracted using a skeletonization procedure. We included airways with diameters ≥0.2 mm for analysis as this was the smallest airway diameter that could be reliably resolved (data not shown).
Fig. 1.
Analysis of large airway volumes and regional ventilation. Three-dimensional reconstructions of the larger airways (>0.2 mm) of each mouse were performed at end-expiration (A and D) and end-inspiration (B and E), and regional tidal ventilation (C and F) was measured for scans performed at the onset of mechanical ventilation (0-h MV; A–C) and after 5 h of mechanical ventilation (5-h MV, D–F). Only airways that were determined to be present on all four airway images were selected for volume measurements.
Two investigators (H. D. Jones and E. H. Kim) independently calculated the volumes for each airway tree using the open-source software ParaView (1). Given the diameter threshold, not all subsegmental airways were present on all airway tree reconstructions for the same mouse, and therefore only airways that were present on all four airway trees (0-h MV and 5-h MV end-expiratory and end-inspiratory scans) were measured. We compared the volume measurements between the two investigators and found excellent interobserver agreement (intraclass correlation coefficients of 0.97–1.0).
We used the term “airway tidal volume” (ΔVaw) to denote the difference between the end-inspiratory airway tree volume and the end-expiratory airway tree volume. Lung parenchymal tidal volumes were calculated using velocimetry analysis (7, 8; Fig. 1, C and F). Total lung tidal volumes (Vt) were calculated by adding airway volumes and parenchymal tidal volumes. The anatomic dead space fraction was calculated as the ratio of airway volume to total tidal volume (Vaw/Vt).
Lung function.
Lung mechanics were assessed using a modification of the forced oscillation technique as described previously (36). Briefly, during 6-s pauses in ventilation at elastic equilibrium lung volume, an oscillatory signal, delivered from a loudspeaker via a wave tube, was introduced via the tracheal cannula. The oscillatory signal contained nine frequencies ranging from 4 to 38 Hz and was used to calculate the respiratory system impedance spectrum (Zrs). A four-parameter model with constant phase tissue impedance (11) was fitted to the Zrs to calculate the airway resistance (Raw), tissue damping (G), and tissue elastance (H). These measurements were made at baseline, 2.5 h, and 5 h into the ventilation period.
Statistics.
Between-group comparisons for airway volumes (Vaw), airway tidal volumes (ΔVaw), total lung tidal volumes (Vt), Vaw-to-Vt ratios, and lung function were made using a two-way repeated-measures ANOVA (treatment × time) with Holm-Sidak post hoc tests. Data are presented as means (SD), and P < 0.05 was considered statistically significant. Relationships between Vaw/Vt and lung mechanics (Raw, G, and H) were assessed by Pearson correlation.
Intraclass correlation coefficients (ICC) were calculated considering a two-way mixed model to evaluate the interobserver agreement for airway measurements for four time points as well as their respective 95% confidence interval. Values close to 1 indicate excellent agreement, and values close to 0 indicate poor agreement. In addition, an F-test was computed to evaluate the null hypothesis of ICC = 0; a P value <0.05 rejects this hypothesis.
RESULTS
Anatomic dead space fraction increases with mechanical ventilation.
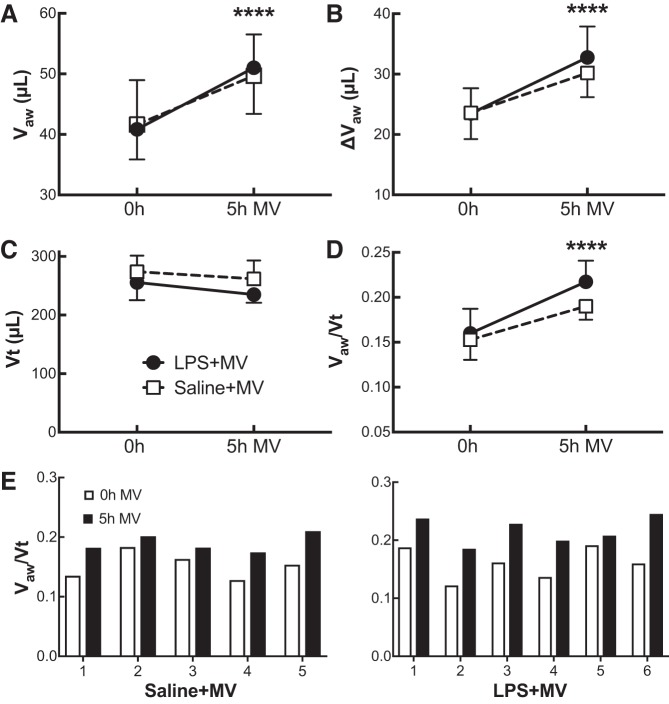
Five hours of mechanical ventilation led to increases in both the large airway volumes, Vaw (P < 0.0001; Fig. 2A), and the change in volume between end-expiration and end-inspiration, which we termed the airway tidal volume, ΔVaw (P < 0.0001; Fig. 2B). In contrast, mechanical ventilation had minimal effect on total tidal volume, Vt (P = 0.09; Fig. 2C), such that the ratio of Vaw to Vt, or the anatomic dead space fraction, increased after 5 h of ventilation compared with baseline (P < 0.0001; Fig. 2D). There was no difference between the LPS- and saline-treated mice for any of these parameters (airway volumes, P = 0.94; ΔVaw, P = 0.63; Vt, P = 0.12; Vaw/Vt, P = 0.19). Individual mice demonstrated a consistent increase in Vaw/Vt for both saline+MV and LPS+MV conditions (Fig. 2E).
Fig. 2.
Airway volume-to-total tidal volume ratios. Airway volumes (Vaw; A), airway “tidal volumes” (ΔVaw; B), total tidal volumes (Vt; C), and the ratios of airway volume to total tidal volume (Vaw/Vt; D) were measured for mice that received either lipopolysaccharide (LPS; ●) or saline (□) at 0 and 5 h of mechanical ventilation (MV). After 5 h of MV we observed a significant increase in airway volumes, airway tidal volumes, and airway volume-to-total tidal volume ratios in both groups. Vaw-to-Vt ratios (E) for individual saline- and LPS-treated mice at 0 and 5 h of MV demonstrate the same trend in each mouse as evident in C. Data are presented as means + SD; n = 5–6 mice per group. ****P < 0.0001 for effect between 0 and 5 h of MV.
Tissue elastance increases in response to mechanical ventilation.
We measured airway resistance (Raw), tissue damping (G), and tissue elastance (H) to determine whether a change in any lung function parameters mirrored the changes seen in Vaw and Vaw/Vt in response to mechanical ventilation. There was no effect of LPS challenge (P = 0.69) or mechanical ventilation (P = 0.54) on Raw (Fig. 3A). H increased by the end of the ventilation period (P = 0.04), but there was no difference between LPS+MV and saline+MV treatment groups (P = 0.35; Fig. 3B). In contrast, whereas LPS challenge had no effect on baseline G (P = 0.47), ventilation increased G in the LPS+MV group (5 h, P = 0.002) such that G was significantly higher by the end of the ventilation period in the LPS+MV group compared with the saline+MV group (P = 0.008; Fig. 3C). Whereas no correlation was seen between Raw and Vaw/Vt (r = −0.22, P = 0.44) or between H and Vaw/Vt (r = 0.38, P = 0.19), G showed a significant correlation with Vaw/Vt (r = 0.72, P = 0.003).
Fig. 3.
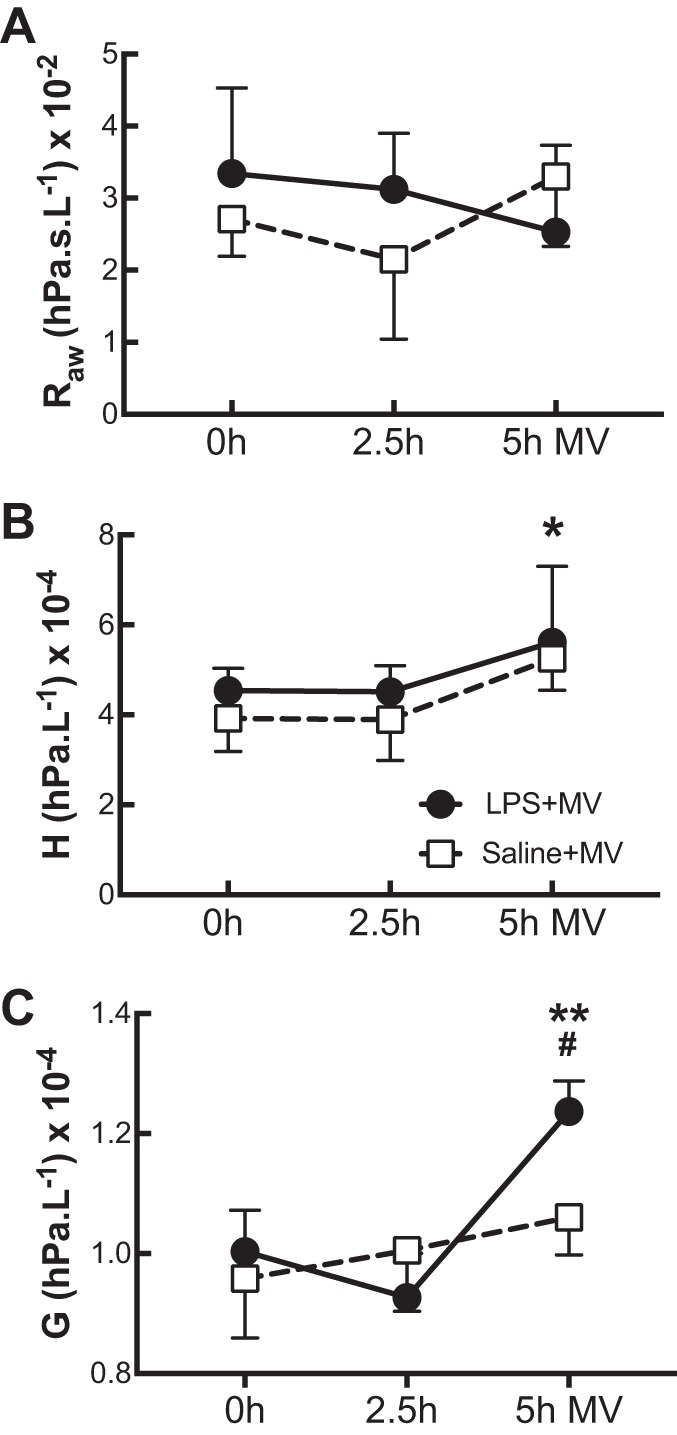
Lung mechanics during prolonged mechanical ventilation. Lung mechanics of mice that received either lipopolysaccharide (LPS; ●) or saline (□) were assessed using a forced oscillation technique (see methods) at 0, 2.5, and 5 h of mechanical ventilation (MV). Airway resistance (Raw; A), tissue elastance (H; B), and tissue damping (G; C) are shown. Data are presented as means + SD; n = 3–4 mice per group. *P < 0.05 for both LPS and saline groups at 0 and 5 h of MV; **P < 0.005 for LPS mice at 5 h of MV; #P < 0.05 for LPS vs. saline groups at 5 h of MV.
DISCUSSION
We used a mouse model to determine whether mechanical ventilation, with or without LPS challenge, impacted anatomic dead space. The main finding of this report is that the anatomic dead space fraction, Vaw/Vt, is significantly increased in response to 5 h of mechanical ventilation. Additionally, airway excursion during inspiration, which we termed the airway “tidal volume,” also increases in response to prolonged mechanical ventilation. To our knowledge, this is the first time that airway volumes have been shown to increase over time on mechanical ventilation.
This study builds on prior work characterizing airway volumes during mechanical ventilation (22, 32). We took advantage of the enhanced contrast between air and soft tissue that is possible with phase contrast X-ray technology (due to the refractive index between the 2 materials) to obtain detailed images of the airways and lung parenchyma. We used 4DCTs to capture dynamic information about airway distension during the respiratory cycle that might have been lost by comparing static inspiratory and expiratory CTs, which are susceptible to gas redistribution during the required breath holds (26). Our technique improves on prior imaging techniques by providing higher resolution of airways compared with flat-plate micro-CT (22), and our image analysis with thresholding and skeletonization allowed continuous measurements of all subsegmental airways >0.2 mm compared with focal measurements at specified points in the airways (22, 32). Furthermore, we demonstrated that the airway volumes continue to increase over time in response to mechanical ventilation, which suggests dynamic changes in the anatomic dead space in response to this stimulus, and has not been previously reported.
There is strong evidence from older literature (6, 9, 30) showing that anatomic dead space increases with positive pressure ventilation. For example, anatomic dead space was shown to increase in direct relationship to increasing levels of PEEP in dogs, an effect ascribed to increased functional residual capacity (from increased PEEP) and the mechanical effects of increased lung volumes on conducting airway caliber (6, 9). Indeed, Severinghaus and Stupfel demonstrated a linear relationship between anatomic dead space and functional residual capacity and also between anatomic dead space and tidal volumes (30). In the same report, the authors described an increase in anatomic dead space with conditions that caused decreased compliance and noted that “under the conditions of constant ventilation, a decreased compliance is followed by increased positive pressure during inspiration, and it is this pressure which distends the anatomic dead space” (30). These same authors demonstrated a substantial (30%) increase in anatomic dead space in human subjects injected with atropine and concluded that the bronchomotor tone controls the volume of anatomic dead space (31) suggesting that airway stiffness is a determinant of the response.
Applying these observations to our model, we cannot attribute the increased airway tidal volumes to increased functional residual capacity because we did not apply PEEP. Furthermore, we used a pressure control mode of ventilation and did not observe significant changes in tidal volumes after 5 h of MV (Fig. 2B). We observed decreased parenchymal compliance, reflected as increased elastance (Fig. 3B) in both groups in response to 5 h of MV, which is consistent with findings from prior studies of lung mechanics in ventilated mice (3). However, we cannot attribute the increased airway volumes to decreased compliance as per the mechanism postulated by Severinghaus and Stupfel (30), because we used pressure control ventilation and hence the positive pressure was fixed in our study. Furthermore, total tidal volumes did not decrease, suggesting a minimal effect of the changes in compliance. We observed an increase in tissue damping that was restricted to the LPS-challenged mice (Fig. 3C) and suggests that LPS exposure in this model has resulted in peripheral airway inhomogeneity (18). However, this finding is unlikely to play a role in the airway dilatation as it was only found in LPS-exposed mice, whereas both saline- and LPS-exposed mice had similar increases in airway volumes.
Another possible mechanism of increased anatomic dead space in this study is increased airway compliance, either from decreased bronchomotor tone due to factors secreted in response to mechanical ventilation or from mechanical disruption of airway structural integrity with continuous exposure to positive pressure ventilation. The prior work by Sinclair et al. (32) and Nickles et al. (22) sets the stage for understanding how this might occur. Sinclair et al. demonstrated significant increases in airway strain with the application of positive pressure ventilation, and Nickles et al. showed that inflammatory cytokines such as IL-1β, which are known to affect cell-cell tight junctions (2, 4), are secreted by isolated trachea in response to ventilation. These observations suggest that the changes we observed over time in airway volumes in response to mechanical ventilation may represent a pathological response of the airways to prolonged exposure to positive pressure.
A further consideration is the possibility that the intrabronchial airway surface fluid lining becomes thinner over the course of 5-h MV from the drying effects of the nonhumidified air used in mouse ventilators. Indeed, a high-resolution phase contrast X-ray-imaging approach has previously been used to specifically examine the airway surface liquid layer and to visualize mucocilliary transport of debris within a live mouse airway to a resolution of 2–5 μm (33). However, the resolution of our technique is ~50–60 μm (29; unpublished data), which is below the range of airway surface liquid layer thicknesses in mice of 10–30 μm (20), and therefore we would be unable to detect changes in the airway surface liquid layer in our study. In any case, a thinning of the airway surface liquid layer would not explain the changes in airway volumes over the respiratory cycle because the thickness of the airway surface liquid layer would not change from inspiration to expiration. Hence the dynamic changes we detect over the course of 5-h MV likely reflect actual airway excursion rather than thinning of the fluid lining the airways due to drying.
Our study has several limitations. The first and most obvious is that mouse airways are orders of magnitude smaller than human airways, and it is unlikely that the trachea and mainstem bronchi in humans demonstrate marked dilatation over time on MV. However, these structures have intact cartilaginous rings, and although the mouse trachea also has cartilaginous rings, the murine main bronchi and segmental airways do not and therefore are more comparable to distal airways in humans (12). It is plausible to consider that the progressive effects of mechanical ventilation that we observed in the mouse airways may have corollaries in the noncartilaginous airways in humans, and this question needs to be the subject of future study.
We did not observe increased elastance in response to LPS exposure compared with saline exposure, which was unexpected. In prior work, we found that intratracheal LPS plus volume control mechanical ventilation in male C57BL/6 mice caused significantly worse lung injury compared with saline plus mechanical ventilation controls (13). However, in this study we used female BALB/c mice and pressure control ventilation, and it is likely that strain, sex, and ventilation mode played a significant role in susceptibility to acute lung injury under these conditions.
In summary, we applied a novel functional lung-imaging technique to image the motion of lung tissue and airways in mechanically ventilated mice and detected a significant increase in airway volumes and airway expansion over time on mechanical ventilation. This report builds on prior studies showing increased airway volumes during positive pressure ventilation, and we have demonstrated that airway volumes continue to increase with prolonged exposure to positive pressure ventilation in the absence of increased tidal volumes. Mechanisms of this progressive increase in airway volumes remain to be elucidated but may represent pathological responses to mechanical ventilation. Furthermore, our novel imaging technique demonstrates a degree of resolution of airway morphology and volume measurements heretofore not possible, and the ability to image the airways in mice in vivo with this degree of resolution opens the door to many further applications in airway and other lung diseases.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant 125806 (H. D. Jones), National Health and Medical Research Council Project Grant 1077905 (G. R. Zosky), NHMRC Development Grant APP1055116 (A. Fouras), and a Multi-modal Australian Sciences Imaging and Visualization Environment grant (MASSIVE Project NCIy40; A. Fouras).
DISCLOSURES
A. Fouras is founder and CEO of 4Dx. C. R. Samarage and R. Carnibella are employees and have stock ownership in 4Dx. H. D. Jones has stock ownership in 4Dx.
AUTHOR CONTRIBUTIONS
M.P., R.C., A.F., G.R.Z., and H.D.J. conceived and designed research; E.H.K., M.P., R.C., E.B., and H.D.J. performed experiments; E.H.K., M.P., R.C., C.R.S., E.B., M.A.D., A.F., G.R.Z., and H.D.J. analyzed data; E.H.K., M.P., R.C., E.B., M.A.D., G.R.Z., and H.D.J. interpreted results of experiments; M.P., R.C., C.R.S., G.R.Z., and H.D.J. prepared figures; M.P., G.R.Z., and H.D.J. drafted manuscript; E.H.K., M.P., R.C., C.R.S., M.A.D., A.F., G.R.Z., and H.D.J. edited and revised manuscript; E.H.K., M.P., R.C., C.R.S., E.B., M.A.D., A.F., G.R.Z., and H.D.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. William Parks and Dr. Wolfgang Kuebler for thoughtful discussions and editorial advice.
REFERENCES
- 1.Ahrens J, Geveci B, Law C. ParaView: an end-user tool for large data visualization. In: Visualization Handbook, edited by Hansen CD and Johnson CR. New York: Elsevier, 2005, p. 717–731. doi: 10.1016/B978-012387582-2/50038-1. [DOI] [Google Scholar]
- 2.Al-Sadi R, Ye D, Dokladny K, Ma TY. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J Immunol 180: 5653–5661, 2008. doi: 10.4049/jimmunol.180.8.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannizzaro V, Hantos Z, Sly PD, Zosky GR. Linking lung function and inflammatory responses in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 300: L112–L120, 2011. doi: 10.1152/ajplung.00158.2010. [DOI] [PubMed] [Google Scholar]
- 4.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta 1788: 864–871, 2009. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charron C, Repesse X, Bouferrache K, Bodson L, Castro S, Page B, Jardin F, Vieillard-Baron A. PaCO2 and alveolar dead space are more relevant than PaO2/FiO2 ratio in monitoring the respiratory response to prone position in ARDS patients: a physiological study. Crit Care 15: R175, 2011. doi: 10.1186/cc10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffey RL, Albert RK, Robertson HT. Mechanisms of physiological dead space response to PEEP after acute oleic acid lung injury. J Appl Physiol Respir Environ Exerc Physiol 55: 1550–1557, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Dubsky S, Hooper SB, Siu KK, Fouras A. Synchrotron-based dynamic computed tomography of tissue motion for regional lung function measurement. J R Soc Interface 9: 2213–2224, 2012. doi: 10.1098/rsif.2012.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubsky S, Jamison RA, Higgins SP, Siu KK, Hourigan K, Fouras A. Computed tomographic X-ray velocimetry for simultaneous 3D measurement of velocity and geometry in opaque vessels. Exp Fluids 52: 543–554, 2012. doi: 10.1007/s00348-010-1006-x. [DOI] [Google Scholar]
- 9.Dueck R, Wagner PD, West JB. Effects of positive end-expiratory pressure on gas exchange in dogs with normal and edematous lungs. Anesthesiology 47: 359–366, 1977. doi: 10.1097/00000542-197710000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Fouras A, Allison BJ, Kitchen MJ, Dubsky S, Nguyen J, Hourigan K, Siu KK, Lewis RA, Wallace MJ, Hooper SB. Altered lung motion is a sensitive indicator of regional lung disease. Ann Biomed Eng 40: 1160–1169, 2012. doi: 10.1007/s10439-011-0493-0. [DOI] [PubMed] [Google Scholar]
- 11.Hantos Z, Adamicza A, Govaerts E, Daróczy B. Mechanical impedances of lungs and chest wall in the cat. J Appl Physiol (1985) 73: 427–433, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Hyde DM, Hamid Q, Irvin CG. Anatomy, pathology, and physiology of the tracheobronchial tree: emphasis on the distal airways. J Allergy Clin Immunol 124, Suppl: S72–S77, 2009. doi: 10.1016/j.jaci.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Jones HD, Crother TR, Gonazalez-Villalobos RA, Jupelli M, Chen S, Dagvadorj J, Arditi M, Shimada K. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol Biol, 50: 270–280, 2014. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallet RH, Alonso JA, Pittet JF, Matthay MA. Prognostic value of the pulmonary dead-space fraction during the first 6 days of acute respiratory distress syndrome. Respir Care 49: 1008–1014, 2004. [PubMed] [Google Scholar]
- 15.Kallet RH, Zhuo H, Liu KD, Calfee CS, Matthay MA; National Heart Lung and Blood Institute ARDS Network Investigators . The association between physiologic dead-space fraction and mortality in subjects with ARDS enrolled in a prospective multi-center clinical trial. Respir Care 59: 1611–1618, 2014. doi: 10.4187/respcare.02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitchen MJ, Buckley GA, Leong AF, Carnibella RP, Fouras A, Wallace MJ, Hooper SB. X-ray specks: low dose in vivo imaging of lung structure and function. Phys Med Biol 60: 7259–7276, 2015. doi: 10.1088/0031-9155/60/18/7259. [DOI] [PubMed] [Google Scholar]
- 17.Larsson DH, Lundström U, Westermark UK, Arsenian Henriksson M, Burvall A, Hertz HM. First application of liquid-metal-jet sources for small-animal imaging: high-resolution CT and phase-contrast tumor demarcation. Med Phys 40: 021909, 2013. doi: 10.1118/1.4788661. [DOI] [PubMed] [Google Scholar]
- 18.Lutchen KR, Hantos Z, Peták F, Adamicza A, Suki B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol (1985) 80: 1841–1849, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan KS, Donnelley M, Paganin DM, Fouras A, Yagi N, Suzuki Y, Takeuchi A, Uesugi K, Boucher RC, Parsons DW, Siu KK. Measuring airway surface liquid depth in ex vivo mouse airways by X-ray imaging for the assessment of cystic fibrosis airway therapies. PLoS One 8: e55822, 2013. doi: 10.1371/journal.pone.0055822. [Erratum. PLoS One 9 (May): e97829, 2014. doi:.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng I, Paganin DM, Fouras A. Optimization of in-line phase contrast particle image velocimetry using a laboratory X-ray source. J Appl Phys 112: 074701, 2012. doi: 10.1063/1.4757407. [DOI] [Google Scholar]
- 22.Nickles HT, Sumkauskaite M, Wang X, Wegner I, Puderbach M, Kuebler WM. Mechanical ventilation causes airway distension with proinflammatory sequelae in mice. Am J Physiol Lung Cell Mol Physiol 307: L27–L37, 2014. doi: 10.1152/ajplung.00288.2013. [DOI] [PubMed] [Google Scholar]
- 23.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346: 1281–1286, 2002. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 24.Okunieff P, Wu T, Huang K, Ding I. Differential radioprotection of three mouse strains by basic or acidic fibroblast growth factor. Br J Cancer Suppl 27: S105–S108, 1996. [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons DW, Morgan K, Donnelley M, Fouras A, Crosbie J, Williams I, Boucher RC, Uesugi K, Yagi N, Siu KK. High-resolution visualization of airspace structures in intact mice via synchrotron phase-contrast X-ray imaging (PCXI). J Anat 213: 217–227, 2008. doi: 10.1111/j.1469-7580.2008.00950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paula LF, Wellman TJ, Winkler T, Spieth PM, Güldner A, Venegas JG, Gama de Abreu M, Carvalho AR, Vidal Melo MF. Regional tidal lung strain in mechanically ventilated normal lungs. J Appl Physiol (1985) 121: 1335–1347, 2016. doi: 10.1152/japplphysiol.00861.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS; ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin definition. JAMA 307: 2526–2533, 2012. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 28.Robertson HT, Swenson ER. What do dead-space measurements tell us about the lung with acute respiratory distress syndrome? Respir Care 49: 1006–1007, 2004. [PubMed] [Google Scholar]
- 29.Samarage CR, Carnibella R, Preissner M, Jones HD, Pearson JT, Fouras A, Dubsky S. Technical Note: contrast free angiography of the pulmonary vasculature in live mice using a laboratory X-ray source. Med Phys 43: 6017–6023, 2016. doi: 10.1118/1.4964794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Severinghaus JW, Stupfel M. Alveolar dead space as an index of distribution of blood flow in pulmonary capillaries. J Appl Physiol 10: 335–348, 1957. [DOI] [PubMed] [Google Scholar]
- 31.Severinghaus JW, Stupfel M. Respiratory dead space increase following atropine in man, and atropine, vagal or ganglionic blockade and hypothermia in dogs. J Appl Physiol 8: 81–87, 1955. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair SE, Molthen RC, Haworth ST, Dawson CA, Waters CM. Airway strain during mechanical ventilation in an intact animal model. Am J Respir Crit Care Med 176: 786–794, 2007. doi: 10.1164/rccm.200701-088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siu KK, Morgan KS, Paganin DM, Boucher R, Uesugi K, Yagi N, Parsons DW. Phase contrast X-ray imaging for the non-invasive detection of airway surfaces and lumen characteristics in mouse models of airway disease. Eur J Radiol 68, Suppl: S22–S26, 2008. doi: 10.1016/j.ejrad.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Yang K, Kwan AL, Miller DF, Boone JM. A geometric calibration method for cone beam CT systems. Med Phys 33: 1695–1706, 2006. doi: 10.1118/1.2198187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zosky GR, Cannizzaro V, Hantos Z, Sly PD. Protective mechanical ventilation does not exacerbate lung function impairment or lung inflammation following influenza A infection. J Appl Physiol (1985) 107: 1472–1478, 2009. doi: 10.1152/japplphysiol.00393.2009. [DOI] [PubMed] [Google Scholar]
- 36.Zosky GR, Janosi TZ, Adamicza A, Bozanich EM, Cannizzaro V, Larcombe AN, Turner DJ, Sly PD, Hantos Z. The bimodal quasi-static and dynamic elastance of the murine lung. J Appl Physiol (1985) 105: 685–692, 2008. doi: 10.1152/japplphysiol.90328.2008. [DOI] [PubMed] [Google Scholar]