Previously, we identified young, healthy persons who suffer compromised exercise tolerance when exercising muscle perfusion pressure is reduced as a result of a lack of compensatory vasodilation. The ability of nitrate supplementation to restore compensatory vasodilation in such noncompensators is unknown. We demonstrated that beetroot juice supplementation led to compensatory vasodilation and restored perfusion and exercise capacity. Elevated plasma nitrite is an effective intervention for correcting the absence of compensatory vasodilation in the noncompensator phenotype.
Keywords: nitrate supplementation, compensatory vasodilation, perfusion pressure
Abstract
Recently, dietary nitrate supplementation has been shown to improve exercise capacity in healthy individuals through a potential nitrate-nitrite-nitric oxide pathway. Nitric oxide has been shown to play an important role in compensatory vasodilation during exercise under hypoperfusion. Previously, we established that certain individuals lack a vasodilation response when perfusion pressure reductions compromise exercising muscle blood flow. Whether this lack of compensatory vasodilation in healthy, young individuals can be restored with dietary nitrate supplementation is unknown. Six healthy (21 ± 2 yr), recreationally active men completed a rhythmic forearm exercise. During steady-state exercise, the exercising arm was rapidly transitioned from an uncompromised (below heart) to a compromised (above heart) position, resulting in a reduction in local pressure of −31 ± 1 mmHg. Exercise was completed following 5 days of nitrate-rich (70 ml, 0.4 g nitrate) and nitrate-depleted (70 ml, ~0 g nitrate) beetroot juice consumption. Forearm blood flow (in milliliters per minute; brachial artery Doppler and echo ultrasound), mean arterial blood pressure (in millimeters of mercury; finger photoplethysmography), exercising forearm venous effluent (ante-cubital vein catheter), and plasma nitrite concentrations (chemiluminescence) revealed two distinct vasodilatory responses: nitrate supplementation increased (plasma nitrite) compared with placebo (245 ± 60 vs. 39 ± 9 nmol/l; P < 0.001), and compensatory vasodilation was present following nitrate supplementation (568 ± 117 vs. 714 ± 139 ml ⋅ min−1 ⋅ 100 mmHg−1; P = 0.005) but not in placebo (687 ± 166 vs. 697 ± 171 min−1 ⋅ 100 mmHg−1; P = 0.42). As such, peak exercise capacity was reduced to a lesser degree (−4 ± 39 vs. −39 ± 27 N; P = 0.01). In conclusion, dietary nitrate supplementation during a perfusion pressure challenge is an effective means of restoring exercise capacity and enabling compensatory vasodilation.
NEW & NOTEWORTHY Previously, we identified young, healthy persons who suffer compromised exercise tolerance when exercising muscle perfusion pressure is reduced as a result of a lack of compensatory vasodilation. The ability of nitrate supplementation to restore compensatory vasodilation in such noncompensators is unknown. We demonstrated that beetroot juice supplementation led to compensatory vasodilation and restored perfusion and exercise capacity. Elevated plasma nitrite is an effective intervention for correcting the absence of compensatory vasodilation in the noncompensator phenotype.
inadequate oxygen delivery (O2D) during exercise can impair muscle contractile function, energy metabolism, and intracellular homeostasis (23–25). The cardiovascular system elicits physiological adjustments during exercise conditions that compromise O2D to deliver oxygen in proportion to metabolic demand. Previously, we explored the impact of reduced perfusion pressure (RPP) on O2D:demand matching during progressive exercise to exhaustion (5). We found that within a healthy, young population, there were individuals who increased vasodilation (compensator phenotype) and thereby prevented a considerable proportion of the compromise to O2D and other individuals who lacked this compensation (noncompensator phenotype). Importantly, the noncompensator’s greater compromise to O2D was that demand matching had, as a consequence, a greater reduction in peak exercise capacity. In a recent follow-up study, we found that the same two phenotypes were evident under conditions of RPP during steady-state exercise (6a). The absence of compensatory vasodilation, once again, prevented restoration of muscle perfusion and led to compromised exercise capacity. As such, the existence of a noncompensator phenotype is functionally relevant for exercise tolerance, and it is therefore important to identify means by which compensatory vasodilation could be established in individuals with a noncompensator phenotype. At present, no such means are known.
The work of Casey and Joyner (9–11) has established an important role for nitric oxide (NO) in compensatory vasodilation in response to hypoperfusion during steady-state forearm exercise. In their model, NO appeared responsible for up to ~40% of the observed compensatory vasodilation, a finding that was also demonstrated during forearm exercise under systemic hypoxia (12). It is important to note that these investigations identified the contribution of an NO pool derived from NO synthase. In contrast, it appears that plasma nitrite is also an important source of NO, particularly under conditions of increased deoxyhemoglobin (HHb) and reduced pH (14, 37). These would be characteristic of the intravascular environment of the exercising muscle when O2D is compromised, thus potentially contributing to compensatory vasodilation. In addition, Umbrello et al. (43) recently published evidence for a direct vasodilatory role of NO metabolites, which could include nitrite in hypoxic vasodilation. Finally, Casey et al. (13) published findings supporting a role for dietary elevation of plasma nitrite concentration ([nitrite]) in enhancing the compensatory vasodilatory response when exercise was initiated under conditions of arterial hypoxemia. However, this was specific to older adults who lacked compensation and not young adults who already demonstrated compensation.
On the basis of the above, we hypothesized that an increase in plasma nitrite, which would be achievable with dietary nitrate (beetroot juice) supplementation (2, 3, 41, 44), would allow for compensatory vasodilation in individuals with a noncompensator phenotype when perfusion was compromised. We also hypothesized that the resulting improvement in exercising muscle perfusion would reverse the exercise tolerance impairment experienced by such individuals.
To test these hypotheses, we conducted a randomized, double-blind, placebo-controlled, crossover experiment in which preidentified individuals, exhibiting a noncompensator phenotype, experienced a sudden reduction in exercising forearm perfusion pressure during steady-state exercise. This perfusion pressure challenge was completed following both beetroot juice supplementation and consumption of a de-nitrated beetroot juice placebo.
METHODS
Participants
Six healthy (21 ± 2 yr), recreationally active men with no history of smoking, cardiovascular disease, hypertension, or specific forearm training, such as racquetball or squash, participated. All participants were previously identified as presenting with a noncompensatory vasodilation phenotype to the imposed perfusion pressure challenge. Interestingly, this was the only difference in the exercise O2D:demand-matching response compared with compensatory vasodilators. Their vasodilatory, hyperemic, and pressor responses were indistinguishable from compensatory vasodilators in terms of both the rate and magnitude of adjustment, in response to incremental increases in oxygen demand to peak, and in terms of the steady-state and kinetic responses during a rest-to-exercise transition in an RPP position. They also demonstrated the same peak vasodilatory capacity. It was only when perfusion is suddenly compromised during steady-state exercise that this unique characteristic of their cardiovascular response becomes apparent (6a). All participants were previously screened to ensure that valid brachial artery blood flow measurements could be obtained. It was confirmed that all participants avoided exercise for 24 h, caffeine for 12 h, and food consumption for 4 h before their laboratory visits. This study was approved by the Health Sciences Research Ethics Board at Queen’s University, according to the terms of the Declaration of Helsinki. Procedures followed were in accordance with institutional guidelines. Each participant provided signed consent after receiving complete verbal and written descriptions of the experimental protocol and potential risks.
Standard anthropometric data.
Participants underwent standard anthropometric assessments. Age, height, weight, and exercising arm forearm girth and volume were measured. A 7-day physical activity recall adapted from Sarkin et al. (39) was completed.
Instrumentation
Forearm blood flow.
To obtain forearm blood flow (FBF), a combination of echo and Doppler ultrasound was used. A 4-MHz-pulsed flat Doppler probe (Model 500V 131 Transcranial Doppler; Multigon Industries, Elmsford, NY) was attached to the skin over the brachial artery, proximal to the ante-cubital fossa of the exercising arm to measure brachial artery blood velocity after the optimal location for the ultrasound signal was determined. A linear echo ultrasound probe positioned over the brachial artery, ~5 cm proximal to the flat Doppler probe, operating at 13 MHz in a two-dimensional mode (Vivid i; GE Medical Systems, London, ONT, Canada), was used to image the diameter in the brachial artery of the exercising arm.
Forearm venous blood sampling.
A 20- or 22-gauge intravenous catheter was inserted retrograde to venous blood flow into the ante-cubital vein. Confirmation that the selected vein drained the active forearm muscle was obtained via echo ultrasound imaging before catheterization. The imaging probe was positioned over the superficial ante-cubital vein so that it provided an image of the junction with the deep vein. The center of the imaging probe was then marked on the skin, providing a reference point for catheter puncture, ensuring that the catheter was threaded into the deep vein. This approach provided blood samples of venous effluent only from the exercising muscle, ensuring that there was not contamination from inactive tissues.
Samples of venous effluents were obtained at specified intervals during exercise and consisted of a discard (2 ml), sample (1 ml), and saline flush (3 ml).
Central hemodynamic measures.
A three-lead electrocardiogram with electrodes attached to the skin in standard CM5 placement was used to measure heart rate. A pulse oximeter (Nellcor N-395; Medtronic, Minneapolis, MN) was placed over the index finger of the nonexercising hand and was used to measure arterial oxygen saturation () during exercise. A finger photoplethysmograph (Finometer MIDI; Finapres Medical Systems, Enschede, The Netherlands) on the middle finger of the nonexercising hand was used to measure arterial blood pressure and provide estimates of stroke volume and cardiac output via Modelflow (Finapres Medical Systems).
Experimental Design
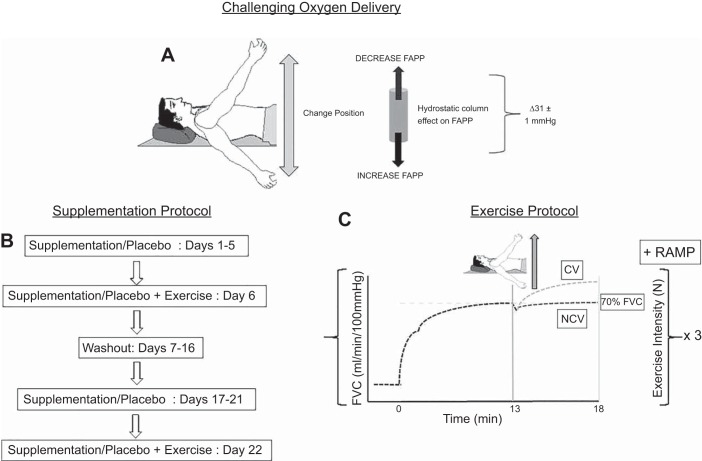
This was a randomized, double-blind, placebo-controlled, crossover experiment, completed over ~3 wk (Fig. 1). Participants completed each of the two data collection sessions at the same time of day; however, time of day varied among participants.
Fig. 1.
Experimental design. A: perfusion pressure manipulation used to compromise oxygen delivery. B: supplementation protocol. C: exercise protocol. In exercise panel, exercise work rate and hyperemic response profiles are demonstrated. FAPP, forearm arterial perfusion pressure; FVC, forearm vascular conductance; CV, compensatory vasodilation; NCV, noncompensatory vasodilation; RAMP, increase in work rate as participants continued exercising as contraction force was increased.
Nitrate or placebo supplementation.
Two separate exercise trials were performed, consisting of identical procedures under differing supplementation conditions. Five days of supplementation were completed with either nitrate-rich beetroot juice containing 0.4 g nitrate/70 ml bottle or nitrate-depleted beetroot juice containing negligible [nitrite] (Beet It Sport; James White Drinks, Ipswich, UK). Beyond [nitrite], both bottles were identical in all external appearances and packaging, volume, taste, smell, and nutrient composition (32). During the 5 days of supplementation, bottles were consumed in the morning and once again in the evening, separated by ~12 h, for a total of 0.8 g nitrate/day. On the day of the experiment, one additional bottle was consumed, such that the experiment would commence ~2 h after consumption. This timing aligns with the presence of peak plasma nitrite following acute exposure (47). After a washout period of 10 days (2, 32, 33), each participant received the opposite supplementation dose. During each supplementation period, participants were instructed to maintain their normal diet. Antibacterial mouthwash and chewing gum were avoided for the duration of the study because of their tendency to destroy the oral bacteria that are necessary for the conversion of nitrate to nitrite (22). Potential ergogenic effects or mechanism of action were not explained to the participants; however, participants were aware of nitrate supplementation during exercise before enrollment in the study.
General experimental conditions.
For all data collection sessions, participants lay supine on a bed with their left arm horizontal at heart level, whereas their right arm was supported on a hinged armrest attached to a pulley system. The pulley system could be raised or lowered to allow for arm/perfusion pressure manipulation. The experiment was performed in a temperature-controlled room (19–21°C) to minimize and stabilize blood flow to the skin so that changes in brachial artery blood flow to the forearm would reflect changes in exercising forearm muscle blood flow.
Perfusion pressure manipulation—compromising O2D.
To compromise O2D, local forearm arterial perfusion pressure (FAPP) was manipulated, as described previously (5, 46). In brief, at rest, the participant’s arm was passively moved to both an above-the-heart RPP position and a below-the-heart control perfusion pressure (CPP) position, such that a measured, 15-mmHg difference from heart level was obtained in each position. Positions were recorded for each participant and used during subsequent visits to compromise O2D. The resulting FAPP challenge was 31 ± 1 mmHg between arm positions. The arm positioning had been previously identified (6a) and was used in the present study.
Exercise protocol.
Participants completed constant load forearm exercise consisting of rhythmic isometric handgrip contraction with a 2-s contraction/2-s relaxation duty cycle at a work rate equivalent to 70% peak virtual forearm vascular conductance (FVCVirtual) for each participant. This work rate was established previously (6a). Exercise began in the CPP position. After 13 min, the arm was raised above the heart during a 2-s contraction to initiate the RPP challenge. Exercise continued for 5 min with the arm in the RPP position. This was followed by a return of the arm to heart level for ~40 min of rest. This process was repeated two more times. Following the completion of the third repeat, participants continued exercising, as contraction force was increased by 24.5 N every 3.5 min until exhaustion. The handgrip force output from an isometric handgrip dynamometer was displayed on a monitor to provide the participant with real-time visual feedback regarding force production, while a metronome was used to provide both visual and auditory cues to guide participant contraction/relaxation duty cycles.
To be confident in our identification of compensatory vasodilation, we repeated measures three times within an individual. With the completion of multiple measures, we were able to confirm response consistency of an individual and thereby confidently classify him as having either a compensatory or noncompensatory vasodilatory response following the completion of each supplementation protocol.
Data Acquisition
All central hemodynamic and FBF responses were obtained on a beat-by-beat basis and averaged into 4 s duty-cycle time bins within each trial. All three trials were averaged to yield one response. Venous effluents were obtained at rest, every 15 s, during the onset of exercise in a given arm position, up to 2 min, and at minutes 3, 4, and 5 within an arm position. All central hemodynamic and FBF responses were computed at the venous sampling intervals. During the progressive exercise portion to exhaustion, all central hemodynamic, FBF responses, and venous effluents were measured during the last 40 s of each completed work rate. As previously described in Bentley et al. (5), mean blood velocity recordings and echo images from the last 10 duty cycles were used in analysis and quantification of the cardiovascular response.
Calculated cardiovascular variables.
FBF was calculated as the following: {mean blood velocity (centimeters per second) · 60 s/min· π [brachial artery diameter (centimeters)/2]2}. FVCVirtual, which is termed “virtual,” as it represents the pressure/flow relationship in a vascular bed, resulting from the interaction of vasodilation and mechanical forces of muscle contraction, was calculated as FBF/FAPP · 100 mmHg, whereas FVCRelax, which quantifies the pressure/flow relationship during relaxation between contractions, resulting from vasodilation, was calculated as FVCRelax/FAPP · 100 mmHg, as described previously (46). FVCVirtual (70%) was calculated as (Peak FVCVirtual – Resting FVCVirtual) × 0.7 + resting FVCVirtual (40). Arterial oxygen concentration () was calculated from (·[Hb]·1.34) + 0.003· , where is arterial hemoglobin saturation (fraction), [Hb] is hemoglobin concentration (grams per milliliter) in the blood, 1.34 is the mean volume of O2 that can be bound to 1 g normal Hb when fully saturated, 0.003 is the solubility of O2 in human plasma, and is the partial pressure of O2 in the arterial blood. was assumed to be 100 mmHg. The values obtained from the venous effluent allowed for the calculation of venous oxygen saturation (). Oxygen consumption (V̇o2) was calculated using the Fick equation as FBF (–). O2D was calculated from FBF·.
Venous effluent—blood gases and electrolytes.
All venous effluents were analyzed with a blood gas analyzer (Stat Profile Prime Blood Gas Analyzer; Nova Biomedical, Mississauga, ON, Canada) for standard blood gases and electrolytes.
Venous effluent—plasma [nitrite].
Venous effluent was sampled into 4.5 ml lithium-heparin vacutainers at resting baseline, and during steady-state exercise in both CPP and RPP positions. Samples were inverted 8–10 times and centrifuged within ~1 min at 1,050 g and 4°C for 10 min. Plasma was extracted, aliquoted, and frozen immediately at −80°C for subsequent analysis. All plasma nitrite analysis was completed in triplicate, and a mean was obtained. Potassium iodide in acetic acid was used for nitrite analysis. Following the reaction equilibration, head-space samples were obtained with an air-tight syringe and injected into an NO analyzer (Sievers NOA 280i; GE Analytical Instruments, Boulder, CO) for quantification of NO via chemiluminescence (7).
Post hoc identification of supplementation effects—compensatory vs. noncompensatory vasodilation.
To identify compensatory vasodilation in response to a sudden reduction of forearm perfusion pressure following supplementation, we compared the vasodilatory responses during exercise in the CPP and RPP positions. Steady-state vasodilation from the last 10 duty cycles within each perfusion pressure position were compared using a one-tailed paired Student’s t-test with the a priori alternative hypothesis that vasodilation would be greater in RPP compared with CPP exercise. Greater vasodilation in the RPP position compared with the CPP position would reflect compensatory vasodilation, whereas similar vasodilation in both positions would reflect the absence of a compensatory response and therefore a noncompensatory response.
Statistical Analysis
A one-tailed paired Student’s t-test was completed for each participant using FVCRelax measures from the last 10 duty cycles in each arm position to determine the presence or absence of compensatory vasodilation. A paired Student’s t-test was completed for each participant using mean FBF measures from the last 10 duty cycles to determine the effect of perfusion pressure position on muscle blood flow (see Table 2).
Table 2.
Compensatory vasodilation identification—vasodilatory and flow responses following a perfusion pressure compromise and their resulting level of significance
| Subject | FVCRelax, min−1 ⋅ 100 mmHg−1 | FVCRelax, P | FBF, ml/min | FBF, P |
|---|---|---|---|---|
| Placebo, n = 6 | ||||
| E | 556 vs. 547 | 0.23 | 338 vs. 313 | 0.06 |
| H | 987 vs. 988 | 0.46 | 667 vs. 506 | 3.61E-03 |
| O | 630 vs. 640 | 0.56 | 366 vs. 329 | 0.10 |
| R | 520 vs. 532 | 0.60 | 290 vs. 207 | 9.88E-04 |
| U | 777 vs. 786 | 0.42 | 401 vs. 380 | 0.16 |
| W | 686 vs. 681 | 1.00 | 435 vs. 376 | 1.3E-03 |
| Average ± SD | 688 ± 166 vs. 697 ± 171 | 0.42 | 415 ± 131 vs. 353 ± 98* | 0.03 |
| Nitrate, n = 6 | ||||
| E | 543 vs. 647 | 1.54E-05 | 350 vs. 353 | 0.87 |
| H | 758 vs. 898 | 1.35E-06 | 437 vs. 406 | 0.07 |
| O | 478 vs. 557 | 6.55E-05 | 316 vs. 324 | 0.25 |
| R | 431 vs. 604 | 3.40E-07 | 221 vs. 246 | 0.03 |
| U | 545 vs. 855 | 6.37E-05 | 308 vs. 335 | 5.33E-03 |
| W | 643 vs. 713 | 0.02 | 396 vs. 383 | 0.35 |
| Average ± SD | 568 ± 117 vs. 714 ± 139* | 5.0E-03 | 338 ± 71 vs. 341 ± 55 | 0.69 |
Values presented for each participant are the means of the 3 trials. E-03–E-07, exponents to negative 3–7, respectively.
Statistical significant difference between reduced perfusion pressure and control perfusion pressure positions (P < 0.05).
To compare the cardiovascular responses from CPP exercise at steady state and RPP exercise at steady state between supplementation conditions, a two-way, repeated-measures ANOVA was used (Figs. 2B, 3, and 4). A paired Student’s t-test was used to compare the mean differences from CPP to RPP exercise between supplementation conditions (Figs. 2B, 3, and 4). A two-way, repeated-measure ANOVA was used to assess the effect of supplementation at rest and CPP and RPP steady-state exercise and to assess peak exercise capacity both within and between supplementation conditions for each perfusion pressure position (Fig. 5).
Fig. 2.
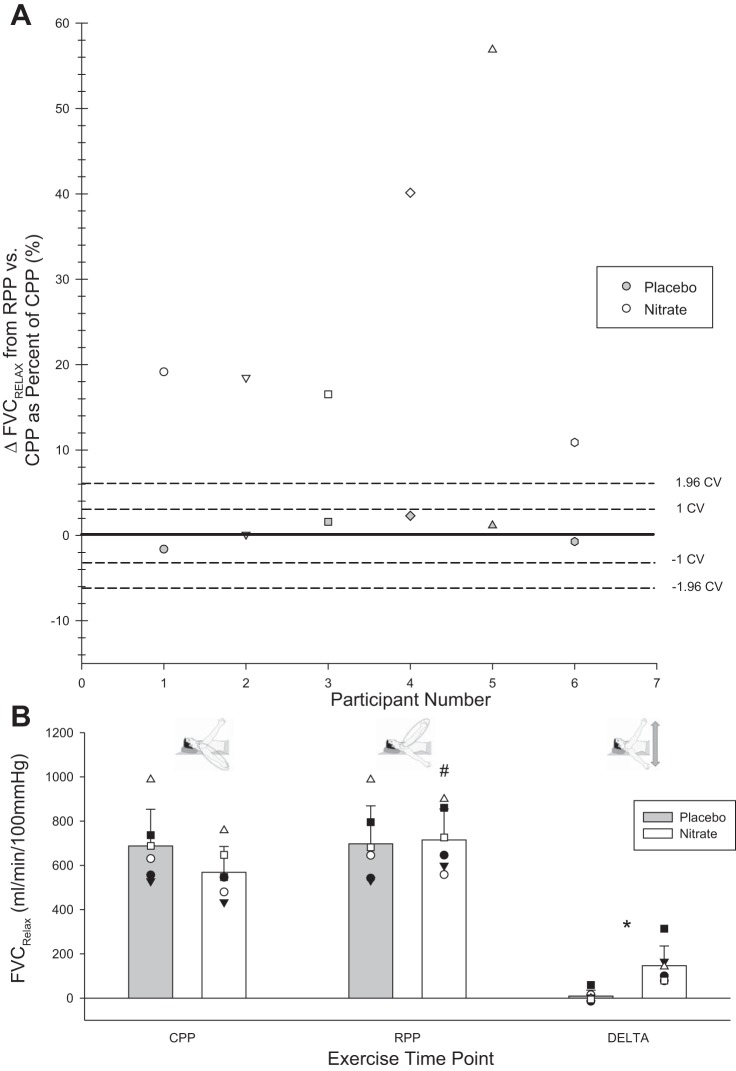
Compensatory vasodilation identification. A: individual responses—change (Δ) in FVCRelax from control perfusion pressure (CPP) to reduced perfusion pressure (RPP) steady state under placebo and nitrate conditions. Coefficient of variation (CV) of means stemming from averaging 3 trials in phenotype identification is identified by dashed lines. Variability in compensatory vasodilation in nitrate and placebo of these means was computed with a modified Monte Carlo simulation for each participant. Data points within 1.96 CV can be expected by chance. Data points above this threshold are deemed to reflect existence of compensatory vasodilation, given the present level of variability in triplicate assessment of compensatory vasodilation. B: FVCRelax. Mean responses denoted by vertical bars; individual responses denoted by symbols. *Statistically significant difference between nitrate and placebo (P < 0.05); #statistically significant difference between RPP and CPP positions within a supplementation condition (P < 0.05). CPP, exercise condition steady state; RPP, exercise condition steady state; DELTA, change from RPP to CPP steady-state exercise.
Fig. 3.
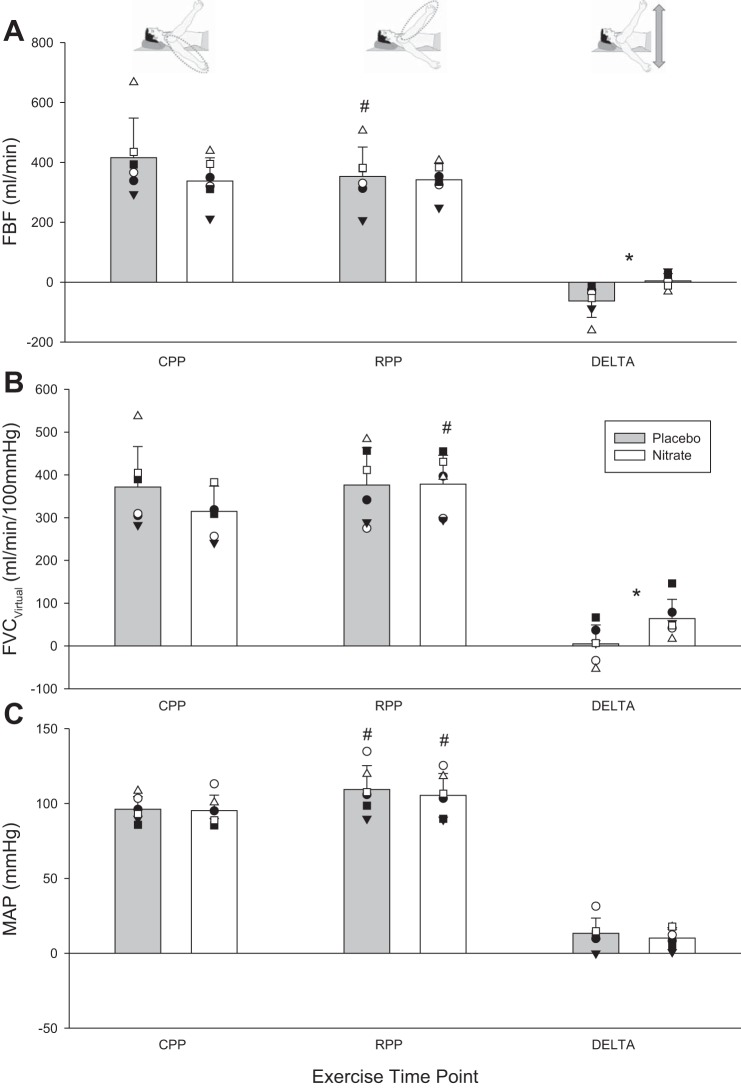
FBF and its determinants during exercise at key exercising time points. A: FBF. B: FVCVirtual. C: mean arterial blood pressure (MAP). Mean responses denoted by vertical bars; individual responses denoted by symbols. *Statistically significant difference between nitrate and placebo (P < 0.05); #statistically significant difference between reduced perfusion pressure (RPP) and control perfusion pressure (CPP) positions within a supplementation condition (P < 0.05). CPP, exercise condition steady state; RPP, exercise condition steady state; DELTA, change from RPP to CPP steady-state exercise.
Fig. 4.
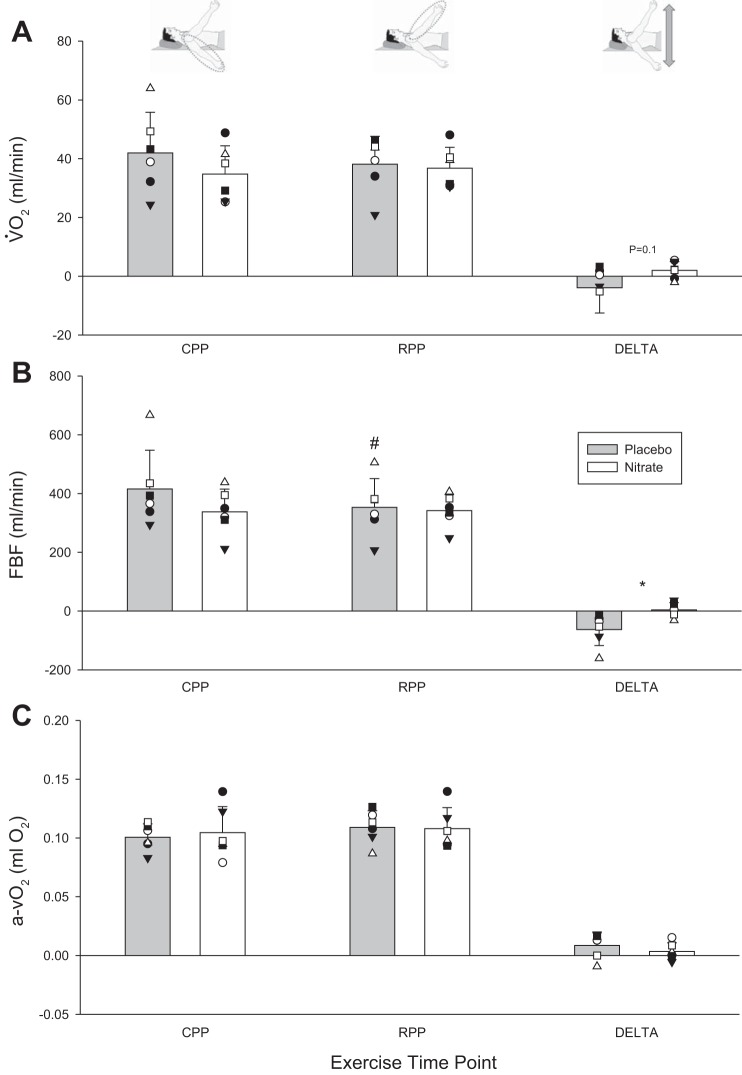
V̇o2 and its Fick equation components during exercise at key exercising time points. A: V̇o2. B: FBF. C: arterial-venous oxygen (a-vO2) content difference. Mean responses denoted by vertical bars; individual responses denoted by symbols. *Statistically significant difference between nitrate and placebo (P < 0.05); #statistically significant difference between reduced perfusion pressure (RPP) and control perfusion pressure (CPP) positions within a supplementation condition (P < 0.05). CPP, exercise condition steady state; RPP, exercise condition steady state; DELTA, change from RPP to CPP steady-state exercise.
Fig. 5.
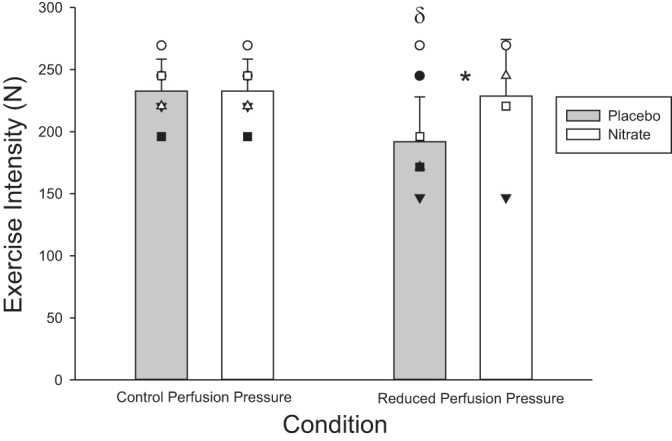
Peak exercise capacity. Peak exercise capacity in control perfusion pressure (CPP) and reduced perfusion pressure (RPP) conditions in both nitrate and placebo. Mean responses denoted by vertical bars; individual responses denoted by symbols. *Statistically significant difference between nitrate and placebo (P < 0.05); δstatistically significant difference between control and RPP conditions within a supplementation condition (P < 0.05).
Repeatability of Multiple Exercise Trials
Coefficient of variation (CV) for each subject for each exercise time point was calculated by dividing the SD of the three trials by the mean of the three trials and then multiplying by 100 (26). A modified Monte Carlo simulation was completed to determine the effects of triplicate assessment on CV (Fig. 2A). A two-way, repeated-measures ANOVA was used to determine if the exercising work rate was different between supplementation conditions.
For all analyses, the level of significance was set at P < 0.05. Only significant F-statistics within the ANOVA were further assessed using Tukey’s post hoc tests. All assumptions of the repeated-measures ANOVA were met. In instances when the assumption of sphericity was violated, a Greenhouse-Geisser correction was applied when interpreting the F-statistic. Statistics were calculated using a combination of SigmaPlot 12.0 (Systat Software, San Jose, CA) and SPSS 20 (IBM, Armonk, NY). One participant mistakenly received nitrate supplementation on what should have been his placebo trial (participant H) and was exercising at an intensity that only elicited 50%, as opposed to the target 70%, peak exercise FVCVirtual. Therefore, for participant H, we used his nonsupplementation, noncompensatory vasodilation response from the phenotype identification study (6a) to represent his placebo response in the current study. All results are presented as means ± SD unless noted otherwise.
RESULTS
Anthropometric Measurements
Anthropometric measures are presented in Table 1.
Table 1.
Anthropometric measures
| Variable | Value |
|---|---|
| Age, yr | 21 ± 2 |
| Height, cm | 185 ± 4 |
| Weight, kg | 76 ± 5 |
| Forearm volume, ml | 1,079 ± 122 |
| Forearm girth, cm | 27 ± 1 |
| 7 day PAR score, METs/wk | 274 ± 25 |
Values are means ± SD. PAR, physical activity readiness; METs, metabolic equivalents.
Effect of Dietary Nitrate Supplementation
Nitrate-rich beetroot juice resulted in a robust increase in plasma [nitrite] compared with control at resting baseline (245 ± 60 vs. 39 ± 9 nmol/l; P < 0.001). This six and one-half-fold increase compared with placebo was consistent during steady exercise in the CPP (284 ± 85 vs. 41 ± 13 nmol/l; P < 0.001) and RPP (280 ± 89 vs. 43 ± 11 nmol/l; P < 0.001) positions.
Reproducibility of Multiple Exercise Trial Repeats
The effects of nitrate supplementation and placebo on compensatory vasodilation were quantified within an individual as the mean response of three trials for each condition. With the use of each mean and the trial-to-trial CV for a given participant, we ran a modified Monte Carlo simulation to determine the variability in mean responses stemming from three repeats. This provided an indication of response consistency across the means of three trials, given the present degree of variability. This produced a mean CV for FVCRelax in the CPP position of 3.1 ± 0.2% and 2.9 ± 0.2% for the RPP (Fig. 2). There was no difference in the work rate completed during exercise between CPP and RPP positions (158 ± 18 vs. 157 ± 14 N) and work rate within RPP (155 ± 18 vs. 157 ± 20 vs. 158 ± 21 N) and CPP (158 ± 20 vs. 156 ± 17 vs. 157 ± 19 N) positions or between nitrate and placebo (160 ± 21 vs. 154 ± 16 N; all P > 0.05).
Perfusion Pressure Reduction—Compensatory vs. Noncompensatory Vasodilation Identification
A sudden compromise in perfusion yielded two clear and disparate vasodilatory responses. Whereas no compensatory vasodilation was evident with placebo in the noncompensator phenotype, it did occur with nitrate supplementation (Table 2 and Fig. 2). In this way, the compromise to FBF, observed in placebo (−63 ± 55 ml/min), was prevented with nitrate supplementation (2 ± 23 ml/min; P = 0.01 vs. placebo; Fig. 3). O2D was trending in a similar direction (−10 ± 12 vs. −0.3 ± 4 ml O2/min; P = 0.06). V̇o2 in CPP and RPP steady state was not different between placebo and nitrate nor was the change from CPP to RPP steady state (Fig. 4).
The pressor response to hypoperfusion was not different between placebo and nitrate (13 ± 10 and 9 ± 7 mmHg; P = 0.4). Cardiac output was elevated during steady-state exercise in the RPP position compared with the CPP position in placebo, but there were no differences between nitrate and placebo at CPP (6.3 ± 1.0 vs. 7.1 ± 1.3 l/min; P = 0.6) or RPP (6.8 ± 1.6 vs. 7.9 ± 1.3 l/min; P = 0.7).
Peak exercise capacity with the arm in an RPP position was reduced in the placebo (−39 ± 27 N; P = 0.03), whereas it was maintained in nitrate (−4 ± 39 N; P = 0.8; Fig. 5).
Venous Effluent—Venous Saturation and [Lactate]
At rest and CPP and RPP position steady state, there were no differences between nitrate and placebo in absolute venous saturation (68 ± 10 vs. 73 ± 5%, 42 ± 12 vs. 46 ± 7%, and 41 ± 9 vs. 43 ± 8%) or [lactate] (0.7 ± 0.2 vs. 0.9 ± 0.3 mmol/l, 1.3 ± 0.5 vs. 1.7 ± 0.9 mmol/l, and 1.4 ± 0.6 vs. 2.0 ± 0.6 mmol/l; all P > 0.05).
DISCUSSION
Dietary nitrate supplementation has been shown previously to increase exercise performance in healthy participants (3, 31, 45). We recently established the existence of a noncompensator vasodilator phenotype in young, healthy individuals in response to a sudden compromise in muscle perfusion during exercise (6a). This noncompensation was functionally significant in that these individuals experienced compromised exercise capacity. In this study, we asked the question, “Can elevating plasma nitrite via consumption of beetroot juice restore compensatory vasodilation, and thereby exercise capacity, in these individuals?” The primary findings of the study were as follows: 1) dietary nitrate supplementation did indeed lead to compensatory vasodilation, whereas placebo had no effect; 2) the compensatory vasodilation resulted in a restoration of exercising FBF; and 3) this perfusion restoration was accompanied by abolishment of impaired exercise capacity under RPP conditions. These findings are the first to identify an important role for increased plasma nitrite in enabling compensatory vasodilation in the noncompensator phenotype.
Efficacy of Nitrate Supplementation in Elevating Plasma [Nitrite]
Our 5-day supplementation protocol produced a six and one-half-fold increase in plasma [nitrite], whereas placebo had no effect on plasma [nitrite]. This increase is in line with previous studies using an ~5-day supplementation period (2, 3, 41, 44) and mirrors previous findings, demonstrating a large increase in plasma [nitrite] compared with placebo, following both acute and chronic supplementation periods of differing durations and doses (27, 31–33). These large increases in plasma [nitrite] were associated with various metabolic improvements (2, 3, 32, 33, 44), as well as increases in exercise performance (3, 27, 31, 45).
Mechanism of Action for Nitrite-Mediated Compensatory Vasodilation
The present study demonstrated that dietary nitrate supplementation, which resulted in large increases in plasma [nitrite], produces compensatory vasodilation in previously established noncompensators. The end product we believe is NO, all stemming from consumption of oral inorganic nitrate. Within the mouth, ~25% of consumed nitrate enters into the enterosalivary circulation, where it is concentrated in saliva and converted into nitrite by bacteria on the tongue (17, 34). The produced nitrite has two fates once swallowed: 1) some of the nitrite enters the stomach, where it is reduced within the acidic environment (35), and 2) some of the nitrite is absorbed within the plasma (15, 34), where it elicits positive effects on the vasculature through various metabolic potential pathways.
Cosby et al. (14) demonstrated an HHb-nitrite-NO relationship during forearm exercise, confirming an hypothesis first proposed by Doyle et al. (16). Exercise produced a drop in partial pressure of oxygen and corresponding increases in iron-nitrosylated hemoglobin, S-nitroso-hemoglobin, and vasodilation. In addition to nitrite eliciting vasodilation via low exercising skeletal muscle partial pressure of oxygen and NO intermediaries, rat models have demonstrated that under hypoxic conditions, nitrite can directly facilitate ATP synthesis and ATP release from red blood cells (RBCs) (8). This proposes an NO-independent pathway, in which ATP itself is responsible for vasodilation in response to plasma elevations in nitrite. ATP has been noted for its role in exercise hyperemia and vasodilation (20, 29) and now potentially within the mechanism of nitrite-mediated vasodilation (8). Lastly, Umbrello et al. (43) recently published evidence for a direct vasodilatory role of NO metabolites, which could include nitrite, in mediating hypoxic vasodilation.
In all proposed pathways involving nitrite-mediated vasodilation, an hypoxic environment is a common denominator. Whereas we were not able to establish directly the intravascular oxygen environment of the active skeletal muscle, we were, however, able to obtain various indicators from exercising muscle venous effluent that would allow us to infer the presence of a particular environment. We believe that transitioning the arm from a CPP position to an RPP position produces an intravascular environment conducive to NO production. Regardless of the supplementation condition, following the immediate switch from CPP to RPP during forearm exercise, FBF is compromised (349 ± 110 vs. 235 ± 87 ml/min averaged across both nitrate and placebo). Due to the reduction in FBF in the face of a constant oxygen demand, absolute venous saturation decreases from 44 ± 10 to 38 ± 8%. This reflects the maintenance of V̇o2 under reduced convective O2D, thereby increasing [HHb]. Coupled with the reduction in flow and increased [HHb], venous pH was reduced in the RPP compared with the CPP position (7.37 ± 0.21 vs. 7.35 ± 0.15), although not to the magnitude previously identified by Modin et al. (37). Both the increased HHb and decreased pH represent conditions that facilitate nitrite conversion to NO. Lastly, [lactate] was increased in the RPP position compared with the CPP position (1.7 ± 0.6 vs. 1.5 ± 0.7 mmol). Lactate increases have an associated hydrogen ion accumulation and a reduction in pH (42). Taken together with the aforementioned variables, these indirect measures of the intravascular environment at the active skeletal muscle from venous effluents suggest an intravascular environment necessary for preferential nitrite-mediation vasodilation.
In characterizing the noncompensatory phenotype, it appears that hypoperfusion-induced increases in HHb do not result in a functionally significant recruitment of the available nitrite pool to elicit vasodilation. Our previous work (6a) showed no differences in venous effluent plasma [nitrite] between compensator and noncompensator phenotypes at rest or during exercise, yet our compensators presented with vasodilation suggest that basal nitrite levels are likely not the reason for the presence of compensatory vasodilation. However, when we substantially augment plasma nitrite levels, we now observe vasodilation in response to that previous desaturation stimulus. This implies that nitrite availability may mediate an aspect of the noncompensator phenotype, despite not being the primary mechanism for noncompensation. In both conditions, the manipulation of the arm into the RPP position increases desaturation; however, there is a restoration of saturation, as exercise in the RPP position is sustained. It can be appreciated that the potential mechanism/pathway(s) of nitrite-mediated vasodilation can be extremely complex and possibly interconnected. Regardless of the exact processes involved, it could simply be that by substantially increasing the nitrite pool, a similar cellular environment of hypoxia and modestly lowered pH could be resulting in greater NO production, simply due to a greater concentration of the precursor substrate.
Perfusion and Vasodilatory Effects of Nitrate Supplementation
Forearm exercise in the CPP position produced similar vasodilatory and perfusion responses in both nitrate supplementation and placebo. Interestingly, it is only when perfusion is suddenly perturbed by manipulating the arm that we see additional nitrite-mediated vasodilation following nitrate supplementation. Our observation of an unaffected “normal” hyperemic response following supplementation adds to the growing body of evidence from previous work in humans (13, 14, 28), suggesting that plasma nitrite elevation does not enhance exercising muscle hyperemia. During normal exercise, the cardiovascular system may effectively match O2D to metabolic demand (1, 38). Exercise increases metabolic demand, which evokes a hyperemic response that is coordinated by feedforward and feedback mechanisms, including RBC desaturation and metabolic production of vasodilatory metabolites. These mechanisms are some of the many integrated yet redundant vasodilatory pathways that facilitate vasodilation even when a given pathway is blocked (30). In this scenario, any increases in nitrite-mediated vasodilation or perfusion could effectively hyperperfuse the skeletal muscle and result in an O2D:demand “excess.” Gonzalez-Alonso et al. (21) actually demonstrated hyperoxic-mediated vasoconstriction within active leg skeletal muscle to prevent such instances from occurring. It appears that in the rest-to-exercise transition, nitrite is not obligatory to vasodilation.
When we suddenly transition the arm into an RPP position, however, we observe a role for nitrite in vasodilation. Nitrate supplementation effectively creates a vasodilatory response that restores skeletal muscle perfusion. In the face of a constant oxygen demand, feedforward mechanisms are not contributing to exercise hyperemia. Rather, it is more likely to be the integration of feedback vasodilator mechanisms pertaining to the oxygenation status of the skeletal muscle and additional RBC desaturation. In this new environment, nitrite appears to be no longer redundant and actively contributes to vasodilation to facilitate O2D:demand matching. The observation of nitrite-mediated vasodilation in perfusion scenarios considered outside the realm of normal aligns with previous work by Casey et al. (13).
Our previous work (6a) demonstrated a pressor response in response to a perfusion compromise. This pressor response was unaffected by compensatory vasodilation or the lack thereof, as the magnitude was similar in both compensatory and noncompensatory phenotypes. Similarly, in the present investigation, we also observed a pressor response that was unaffected by degree of perfusion compensation and therefore, nitrate vs. placebo. Once again, it appears that compensatory vasodilation appears to be the primary pathway for re-establishing adequate perfusion. Importantly, although perfusion was not restored to the same level as CPP exercise, the observed pressor response did partially restore perfusion in placebo, albeit restoration was not complete until accompanied by compensatory vasodilation in nitrate supplementation.
Exercise Capacity During Compromises in O2D: Vasodilatory or Pressor Compensation
In the present study, we observed an increase in exercise capacity with dietary nitrate supplementation, such that perfusion pressure-induced compromises to peak work rate were abolished. Two potential pathways underlie the proposed performance benefits of nitrate supplementation: 1) dietary nitrate has been shown to reduce to oxygen cost of exercise (3), and 2) skeletal muscle perfusion has been shown to be improved postsupplementation (13, 19). With regard to the former and the latter, McDonagh et al. (36) recently observed both improved quadriceps muscle oxygenation and reduced oxygen cost in cycling exercise, potentially implicating both as contributors to exercise performance benefits in their exercise model. In the present study, in an isolated forearm exercise model, the data set did not reveal a statistically detectable difference in V̇o2 under nitrate supplementation in the CPP steady state, and this was especially so in the RPP steady state (see Fig. 4). Therefore, we do not believe that a reduction in the oxygen cost associated with exercise was contributing to the improvement in exercise capacity. However, it is acknowledged that the magnitude of effect on V̇o2 may be beyond the detection capabilities of our blood sampling across a very small muscle mass. We did, however, observe a restoration of muscle blood flow with supplementation, which was absent in placebo, where in the face of reduced O2D and maintained V̇o2, [ADP] and [π] would be greater, stimulation of glycolysis would be increased, and the rate of lactate formation would increase as a result (42). Due to the impaired perfusion, as seen with placebo, contractile performance would be compromised as a result of inadequate removal of fatigue-inducing byproducts (4), and therefore, exercise capacity would also be compromised. In contrast, the perfusion increase with restored compensatory vasodilation in nitrate supplementation would better protect skeletal muscle cell oxygenation and therefore allow lowered [ADP] and [π] to maintain a given V̇o2 compared with placebo (45).
Our model demonstrated a role of nitrate supplementation in mediating compensatory vasodilation in otherwise healthy, young, preidentified noncompensators and the establishment of adequate perfusion following a sudden compromise during exercise. Clinical conditions in which systemic perfusion is compromised provide additional support for the beneficial role of nitrate supplementation. Peripheral artery disease represents a model in which perfusion and therefore O2D to the lower limb are often compromised, whereas chronic heart failure with preserved ejection fraction typically presents with impaired systemic perfusion due to noncardiac factors. In both of these models, a perfusion disturbance is present, akin to our noncompensatory vasodilators following arm manipulation. Kenjale et al. (27) demonstrated improved exercise capacity by ~18% and reduced gastrocnemius deoxygenation during a maximal graded exercise test in patients with peripheral artery disease. Similarly, although measures of perfusion were not established, Eggebeen et al. (18) observed improved submaximal exercise performance, measured via an increase in time to exhaustion, in chronic heart failure patients. Despite the observed benefits in certain O2D disturbances that require increased perfusion to compensate, individuals with type II diabetes do not have improvements in a 6-min walk time postsupplementation (41). Although certain clinical situations (peripheral artery disease and chronic heart failure) also observe improvements in perfusion with dietary nitrate supplementation, we would point out that the perfusion compromise in those situations is considerably different from the acute reduction stimulus used in the present model.
Experimental Considerations
In our previous study, we identified eight individuals who responded with noncompensatory vasodilation during our perfusion pressure perturbation (6a). Of the eight, six were available to return for the present investigation. A potential concern with our small sample size is the inability to detect differences in our variables of interest. Our primary outcome was the ability to establish compensatory vasodilation in the noncompensatory phenotype following dietary nitrate supplementation. Importantly, our dietary nitrate supplementation resulted in a robust elevation in plasma [nitrite]. Under these conditions, we clearly demonstrated the presence of compensatory vasodilation, the impact of compensatory vasodilation on muscle blood flow, and the resulting impact on exercise capacity. The only variable that was underpowered to detect a difference was O2D, which was trending toward significance (P = 0.06). However, given the clear impact on flow and exercise capacity, we do not feel that a small sample size compromises the significance of the study or the clear establishment that dietary nitrate induces compensatory vasodilation in instances of RPP.
It may be suggested as a potential concern that FBF and FVC (Fig. 3) group means are lower in nitrate by ~17–19% in CPP steady state, inferring that nitrate reduced dilation and blood flow. Whereas this was statistically not significant (P = 0.1 and P = 0.15, respectively), in small sample-size studies, this can be due to a lack of statistical power. However, examination of individual data shows that this difference in means is driven by a single subject. Interestingly, this is the subject identified in the Repeatability of Multiple Exercise Trial Repeats section who received nitrate on both his nitrate and placebo experimental day in the current study. Therefore, we used his nonsupplementation response from our phenotype identification study (6a) to quantify the response without supplementation. This may explain specifically for this subject why there is a greater difference in his values. However, it must again be emphasized that in an experimental design, where there is a within-subject manipulation, it is the pre- vs. postmanipulation comparison that identifies a manipulation effect. For this subject, there was a compromise to exercise capacity when no vasodilatory compensation occurred, and this was eliminated when nitrate resulted in compensatory vasodilation being enabled (Fig. 5). These findings support the key interpretations that noncompensation compromises exercise capacity and that nitrate enables compensation and thereby restores exercise capacity.
Conclusion
Dietary nitrate supplementation resulted in a six and one-half-fold increase in plasma [nitrite] compared with placebo. Our group is the first to have identified a noncompensatory vasodilation phenotype in young, healthy adults under sudden reductions in exercising muscle perfusion pressure (6a). These individuals all exhibit normal vasodilatory and O2D:demand-matching responses across exercise intensities, normal kinetic and steady-state responses during rest-to-exercise transitions, and normal peak vasodilatory capacity compared with the compensator phenotype. In the present study, we demonstrate that in such noncompensators, nitrate supplementation clearly enabled compensatory vasodilation in response to a perfusion pressure-induced compromise to O2D. This compensatory response restored exercising muscle blood flow and eliminated any perfusion pressure-induced impairments in exercise capacity. These findings have important implications for these individuals in activity situations where O2D:demand matching is disrupted via compromised muscle blood flow, as could happen with physical tasks in the workplace where arm position changes or potentially, where the nature of the task results in reduced time between contractions, which we have shown also to impair muscle blood flow (6). Thus dietary nitrate can have a therapeutic application in an identified noncompensatory vasodilator response phenotype by restoring the ability to compensate for compromised exercise hyperemia.
GRANTS
Support for this project was provided by Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant 250367-11 and Research Tools and Instruments Grant EQPEQ0407690-11, as well as infrastructure grants from the Canadian Foundation for Innovation and the Ontario Innovation Trust (to M. E. Tschakovsky). Support for R. F. Bentley was provided by an NSERC Postgraduate Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.F.B. and M.E.T. conceived and designed research; R.F.B., J.J.W., P.J.D., A.V., S.J.K., and A.M.F. performed experiments; R.F.B. analyzed data; R.F.B. and M.E.T. interpreted results of experiments; R.F.B. prepared figures; R.F.B. drafted manuscript; R.F.B., J.J.W., P.J.D., A.V., S.J.K., A.M.F., and M.E.T. edited and revised manuscript; R.F.B., J.J.W., P.J.D., A.V., S.J.K., A.M.F., and M.E.T. approved final version of manuscript.
REFERENCES
- 1.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol (1985) 109: 135–148, 2010. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 107: 1144–1155, 2009. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 4.Barclay JK. A delivery-independent blood flow effect on skeletal muscle fatigue. J Appl Physiol (1985) 61: 1084–1090, 1986. [DOI] [PubMed] [Google Scholar]
- 5.Bentley RF, Kellawan JM, Moynes JS, Poitras VJ, Walsh JJ, Tschakovsky ME. Individual susceptibility to hypoperfusion and reductions in exercise performance when perfusion pressure is reduced: evidence for vasodilator phenotypes. J Appl Physiol (1985) 117: 392–405, 2014. doi: 10.1152/japplphysiol.01155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bentley RF, Poitras VJ, Hong T, Tschakovsky ME. Characteristics and effectiveness of vasodilatory and pressor compensation for reduced relaxation time during rhythmic forearm contractions. Exp Physiol 102: 621–634, 2017. doi: 10.1113/EP086069. [DOI] [PubMed] [Google Scholar]
- 6a. Bentley RF, Walsh JJ, Drouin PJ, Velickovic A, Kitner SJ, Fenuta AM, Tschakovsky ME. Absence of compensatory vasodilation with perfusion pressure challenge in exercise: evidence for and implications of the non-compensator phenotype. J Appl Physiol (1985). First published July 13, 2017; 10.1152/japplphysiol.00952.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brien JF, McLaughlin BE, Nakatsu K, Marks GS. Chemiluminescence headspace-gas analysis for determination of nitric oxide formation in biological systems. Methods Enzymol 268: 83–92, 1996. doi: 10.1016/S0076-6879(96)68011-2. [DOI] [PubMed] [Google Scholar]
- 8.Cao Z, Bell JB, Mohanty JG, Nagababu E, Rifkind JM. Nitrite enhances RBC hypoxic ATP synthesis and the release of ATP into the vasculature: a new mechanism for nitrite-induced vasodilation. Am J Physiol Heart Circ Physiol 297: H1494–H1503, 2009. doi: 10.1152/ajpheart.01233.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey DP, Joyner MJ. Contribution of adenosine to compensatory dilation in hypoperfused contracting human muscles is independent of nitric oxide. J Appl Physiol (1985) 110: 1181–1189, 2011. doi: 10.1152/japplphysiol.00836.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey DP, Joyner MJ. NOS inhibition blunts and delays the compensatory dilation in hypoperfused contracting human muscles. J Appl Physiol (1985) 107: 1685–1692, 2009. doi: 10.1152/japplphysiol.00680.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey DP, Joyner MJ. Prostaglandins do not contribute to the nitric oxide-mediated compensatory vasodilation in hypoperfused exercising muscle. Am J Physiol Heart Circ Physiol 301: H261–H268, 2011. doi: 10.1152/ajpheart.00222.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey DP, Madery BD, Curry TB, Eisenach JH, Wilkins BW, Joyner MJ. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J Physiol 588: 373–385, 2010. doi: 10.1113/jphysiol.2009.180489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey DP, Treichler DP, Ganger CT IV, Schneider AC, Ueda K. Acute dietary nitrate supplementation enhances compensatory vasodilation during hypoxic exercise in older adults. J Appl Physiol (1985) 118: 178–186, 2015. doi: 10.1152/japplphysiol.00662.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 15.Dejam A, Hunter CJ, Schechter AN, Gladwin MT. Emerging role of nitrite in human biology. Blood Cells Mol Dis 32: 423–429, 2004. doi: 10.1016/j.bcmd.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem 256: 12393–12398, 1981. [PubMed] [Google Scholar]
- 17.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1: 546–551, 1995. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 18.Eggebeen J, Kim-Shapiro DB, Haykowsky M, Morgan TM, Basu S, Brubaker P, Rejeski J, Kitzman DW. One week of daily dosing with beetroot juice improves submaximal endurance and blood pressure in older patients with heart failure and preserved ejection fraction. JACC Heart Fail 4: 428–437, 2016. doi: 10.1016/j.jchf.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591: 547–557, 2013. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002. doi: 10.1161/01.RES.0000044939.73286.E2. [DOI] [PubMed] [Google Scholar]
- 21.González-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 19: 333–337, 2008. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Hogan MC, Arthur PG, Bebout DE, Hochachka PW, Wagner PD. Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol (1985) 73: 728–736, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol (1985) 86: 1367–1373, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Hogan MC, Richardson RS, Kurdak SS. Initial fall in skeletal muscle force development during ischemia is related to oxygen availability. J Appl Physiol (1985) 77: 2380–2384, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Hopkins WG. Measures of reliability in sports medicine and science. Sports Med 30: 1–15, 2000. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol (1985) 110: 1582–1591, 2011. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JK, Moore DJ, Maurer DG, Kim-Shapiro DB, Basu S, Flanagan MP, Skulas-Ray AC, Kris-Etherton P, Proctor DN. Acute dietary nitrate supplementation does not augment submaximal forearm exercise hyperemia in healthy young men. Appl Physiol Nutr Metab 40: 122–128, 2015. doi: 10.1139/apnm-2014-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirby BS, Crecelius AR, Richards JC, Dinenno FA. Sources of intravascular ATP during exercise in humans: critical role for skeletal muscle perfusion. Exp Physiol 98: 988–998, 2013. doi: 10.1113/expphysiol.2012.071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamb IR, Murrant CL. Potassium inhibits nitric oxide and adenosine arteriolar vasodilatation via K(IR) and Na(+)/K(+) ATPase: implications for redundancy in active hyperaemia. J Physiol 593: 5111–5126, 2015. doi: 10.1113/JP270613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, Gilchrist M, Benjamin N, Jones AM. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc 43: 1125–1131, 2011. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 32.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol (1985) 110: 591–600, 2011. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 33.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 34.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med 37: 395–400, 2004. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol 2: 593–602, 2004. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 36.McDonagh ST, Vanhatalo A, Fulford J, Wylie LJ, Bailey SJ, Jones AM. Dietary nitrate supplementation attenuates the reduction in exercise tolerance following blood donation. Am J Physiol Heart Circ Physiol 311: H1520–H1529, 2016. doi: 10.1152/ajpheart.00451.2016. [DOI] [PubMed] [Google Scholar]
- 37.Modin A, Björne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand 171: 9–16, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol (1985) 75: 1911–1916, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Sarkin J, Campbell J, Gross L, Roby J, Bazzo S, Sallis J, Calfas K. Project GRAD Seven-Day Physical Activity Recall Interviewer’s Manual. Med Sci Sports Exerc 29, Suppl: S91–S102, 1997. [Google Scholar]
- 40.Saunders NR, Pyke KE, Tschakovsky ME. Dynamic response characteristics of local muscle blood flow regulatory mechanisms in human forearm exercise. J Appl Physiol (1985) 98: 1286–1296, 2005. doi: 10.1152/japplphysiol.01118.2004. [DOI] [PubMed] [Google Scholar]
- 41.Shepherd AI, Gilchrist M, Winyard PG, Jones AM, Hallmann E, Kazimierczak R, Rembialkowska E, Benjamin N, Shore AC, Wilkerson DP. Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled crossover trial. Free Radic Biol Med 86: 200–208, 2015. doi: 10.1016/j.freeradbiomed.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Spriet LL, Howlett RA, Heigenhauser GJ. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med Sci Sports Exerc 32: 756–763, 2000. doi: 10.1097/00005768-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Umbrello M, Dyson A, Pinto BB, Fernandez BO, Simon V, Feelisch M, Singer M. Short-term hypoxic vasodilation in vivo is mediated by bioactive nitric oxide metabolites, rather than free nitric oxide derived from haemoglobin-mediated nitrite reduction. J Physiol 592: 1061–1075, 2014. doi: 10.1113/jphysiol.2013.255687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol 299: R1121–R1131, 2010. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 45.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J Physiol 589: 5517–5528, 2011. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker KL, Saunders NR, Jensen D, Kuk JL, Wong SL, Pyke KE, Dwyer EM, Tschakovsky ME. Do vasoregulatory mechanisms in exercising human muscle compensate for changes in arterial perfusion pressure? Am J Physiol Heart Circ Physiol 293: H2928–H2936, 2007. doi: 10.1152/ajpheart.00576.2007. [DOI] [PubMed] [Google Scholar]
- 47.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 115: 325–336, 2013. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]