Abstract
The mechanisms regulating incretin secretion are not fully known. Human obesity is associated with altered incretin secretion and elevated endocannabinoid levels. Since cannabinoid receptors (CBRs) are expressed on incretin-secreting cells in rodents, we hypothesized that endocannabinoids are involved in the regulation of incretin secretion. We compared plasma glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) responses during oral glucose tolerance test (OGTT) in 20 lean and 20 obese participants from the Baltimore Longitudinal Study of Aging (BLSA). Next, we recruited 20 healthy men to evaluate GIP and GLP-1 responses during OGTT after administering placebo or nabilone (CBR agonist) in a randomized, double-blind, crossover fashion. Compared with the BLSA lean group, the BLSA obese group had significantly higher fasting and post-OGTT GIP levels, but similar fasting GLP-1 and significantly lower post-OGTT GLP-1 levels. In the nabilone vs. placebo study, when compared with placebo, nabilone resulted in significantly elevated post-dose fasting GIP levels and post-OGTT GIP levels, but no change in post-dose fasting GLP-1 levels together with significantly lower post-OGTT GLP-1 levels. Glucose levels were not different with both interventions. We conclude that elevated GIP levels in obesity are likely a consequence of increased endocannabinoid levels. CBRs exert tonic control over GIP secretion, which may have a homeostatic effect in suppressing GLP-1 secretion. This raises the possibility that gut hormones are influenced by endocannabinoids.
Keywords: GIP, GLP-1, incretins, endocannabinoids, obesity
the ability of incretins to enhance insulin secretion in response to nutrients is well described (21, 28). The amount of insulin secreted in response to oral glucose is approximately twice that secreted in response to a similar load of intravenous glucose (27). The augmented insulin secretion from oral vs. intravenous glucose is due to incretins secreted into the circulation in response to food (21, 28). The two known incretins, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1), are synthesized and stored for later stimulus-coupled secretion from enteroendocrine K and L cells, respectively (21, 28). The GIP and GLP-1 secretory responses are in part dependent on nutrient composition and a host of other factors such as age, sex, obesity, and diabetes status among others (13, 33). GLP-1 receptor agonists and inhibitors of GLP-1 degradation by dipeptidyl peptidase 4 (DPP-4) have been developed and approved for treatment of type 2 diabetes. The underlying physiological mechanisms regulating GIP and GLP-1 secretion are complex, fascinating, and an area of active research. Sweet taste receptors and lipid sensing receptors are some of the key molecular nutrient-sensing mechanisms noted in regulating incretin secretion (37). Recently, we and others found that cannabinoid 1 receptors (CB1Rs) are expressed on incretin-secreting cells in rodents and may play a role in the regulation of incretin secretion (15, 29).
The field of cannabinoid research first started with the identification of the principal active ingredient of Cannabis sativa, Δ9-tetrahydrocannabinol (THC), in 1964, and was followed by the search for its receptors and putative endogenous ligands (23). The two most studied endocannabinoids are anandamide (AEA) and 2-arachidonyl glycerol (2-AG). There are two distinct cannabinoid receptors (CBRs): CB1Rs and cannabinoid 2 receptors (CB2Rs), and both are G protein-coupled receptors. CB1Rs are the most abundant G protein-coupled receptors in the brain and are involved in regulating appetite and food intake (39). CB1Rs are also abundant in neurons of the parasympathetic branches of the vagus nerve and in the enteric nervous system where they serve as key components in the regulation of gastric and intestinal motility (42). CB1Rs are also present in hepatocytes, skeletal muscles, and islets of Langerhans (39). CB2Rs are mostly present in the periphery with the majority of them confined to the immune system, with recent evidence suggesting their presence in the liver (7, 39). In recent years, studies have demonstrated the integral role of the endocannabinoid system in energy homeostasis and its dysregulation in obesity (39). Endocannabinoid levels, AEA and 2-AG, have been shown to be elevated in human obesity (5, 12, 24). Furthermore, rodents fed with dietary linoleic acid, which promoted weight gain, were found to have increased endocannabinoid levels in liver and gut (1). Unlike incretins, endocannabinoids are synthesized on an as-needed basis (8); therefore, constitutive endocannabinoid synthesis must also be increased in obesity.
Current literature on incretin levels in obesity remains controversial. Fasting and postprandial GIP levels in obese individuals has been found to be normal or elevated when compared with normal-weight individuals (26). Similarly, data on fasting and postprandial GLP-1 levels in obesity are mixed (13, 33). These discrepancies may be due to several factors: not perfectly matched subjects in terms of age or sex were selected when comparing lean vs. obese individuals; different assays were used in these studies; or different oral stimuli were used such as oral glucose vs. mixed-meal tests.
We hypothesized that the endocannabinoid system serves as one of the modulators of GIP and GLP-1 secretion. In the obese state, the endocannabinoid system is upregulated which in turn increases GIP secretion and decreases GLP-1 secretion. To test our hypothesis that GIP levels are higher and GLP-1 levels are lower in obesity, we performed a case-control study comparing GIP and GLP-1 responses during a 2-h 75-g oral glucose tolerance test (OGTT) between a group of lean (BMI < 25 kg/m2) participants to a group of obese (BMI ≥ 30 kg/m2) participants. To test our hypothesis that increased activation of the endocannabinoid system leads to higher GIP levels and lower GLP-1 levels, we administered nabilone, a CBR agonist approved by the US Food and Drug Administration for treating chemotherapy-induced nausea, to healthy subjects before a 3-h 75-g OGTT. Nabilone is used as a tool to activate the cannabinoid receptors, therefore, simulating elevated endocannabinoid levels in obesity.
MATERIALS AND METHODS
BLSA case-control study.
For the case-control study, we selected participants from the Baltimore Longitudinal Study of Aging (BLSA), an ongoing, prospective, observational study of normative aging in community-dwelling volunteers established in 1958 (17). The BLSA participants, healthy at study entry, undergo 3 days of medical examination and various testing at the clinical research unit of the National Institute on Aging at predetermined intervals. The BLSA protocol is approved by the Intramural Research Program of the US National Institute on Aging and the Institutional Review Board of the National Institute of Environmental Health Sciences. All participants provided written, informed consent at every visit.
Between April 2013 and March 2015, 740 BLSA participants came for their visit of which 614 were eligible and completed a 75-g oral glucose tolerance test (OGTT). After a 10-h overnight fast, participants consumed a 75-g glucose solution and plasma samples were collected every 20 min for 120 min. A participant was diagnosed as having diabetes if any of the following criteria are met: 1) history of diabetes; 2) taking hypoglycemic medications; or 3) fasting plasma glucose (FPG) ≥ 7.0 mmol/l (126 mg/dl) and/or 2-h OGTT glucose (2hG) ≥ 11.1 mmol/l (200 mg/dl). Among those participants without diabetes (n = 526), we used a random number generator (GraphPad Software) to select 10 men and 10 women from the obese group (BMI ≥ 30 kg/m2, n = 112) and matched them by age and sex to 10 men and 10 women in the lean group (BMI < 25 kg/m2, n = 213). The OGTT samples from these 40 BLSA participants were assayed for glucose, insulin, GIP, and total GLP-1 (tGLP-1).
Human CBR agonist vs. placebo study.
Non-BLSA healthy men between ages of 21 to 55 yr were recruited to study the role of cannabinoids on incretins and metabolic parameters after a 75-g OGTT. They were screened for glucose intolerance using 75-g OGTT. Those with FPG ≥ 5.6 mmol/l (100 mg/dl) or 2hG ≥ 7.8 mmol/l (140 mg/dl) were ineligible for the study. Twenty qualified subjects completed the study. After a 12-h overnight fast, subjects were given nabilone (2 mg orally) or placebo in a randomized, double-blind, crossover fashion, 60 min before administration of OGTT. Blood samples were taken immediately before the ingestion of oral glucose (post-dose fasting; t = 0), then at t = 10, 20, 30, 40, 60, 90, 120, 150, and 180 min. The blood samples were assayed for glucose, insulin, glucagon, GIP, tGLP-1, and active GLP-1 (aGLP-1). Each subject served as his own control and returned several weeks later for the second visit. The CBR agonist/placebo study was approved by the Intramural Research Program of the National Institute on Aging and the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD). All subjects provided written, informed consent.
Sample collection.
For both the BLSA and the CBR agonist/placebo studies, all blood samples were collected into EDTA-coated tubes (1.5 µg/ml blood) containing aprotinin (Trasylol, 40 µg/ml blood; Serological Proteins, Kankakee, IL) and a dipeptidyl peptidase 4 inhibitor (DPP4 inhibitor, 10 µg/ml blood; Millipore, Billerica, MA). Following collection, each sample was centrifuged at 4°C and the plasma was then divided into several aliquots, which were immediately frozen on dry ice and stored at −80°C until analysis.
Plasma hormone and biochemical assays.
We quantified plasma glucose levels using a glucose oxidase analyzer (YSI, Yellow Springs, OH). The plasma hormones were measured by enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA): insulin (ELISA; Mercodia, Winston Salem, NC; intra-assay variation 2.8–4.0%; interassay variation 2.6–3.6%); C-peptide (ELISA; Mercodia, Winston Salem, NC; intra-assay variation 2.9–4.8%; interassay variation 3.0–6.8%); GIP (ELISA; Millipore, Billerica, MA; intra-assay variation 3.0–8.8%; interassay variation 1.8–6.1%); tGLP-1 (ELISA; Alpco Diagnostics, Salem, NH; intra-assay variation 3.7–4.7%; interassay variation 6.2–9.5%); aGLP-1 (ELISA; Alpco Diagnostics, Salem, NH; intra-assay variation 2.5–5.4%; interassay variation 3.9–5.6%); and glucagon (RIA; Millipore, Billerica, MA; intra-assay variation 4.0–6.8%; interassay variation 7.3–13.5%). A1c was measured using Dimension Vista System (Siemens, Camberley, UK).
Statistical analysis.
Results are reported as means ± SE. Area under the curve (AUC) was calculated using the trapezoidal rule and was used as a proxy of the amount of glucose in the circulation or hormones secreted. The Homeostatic Model Assessment 2 (HOMA2) model, an updated version of HOMA which takes into account variations in hepatic and peripheral glucose resistance, was used to calculate insulin resistance (HOMA2-IR) (22). Mann-Whitney test was used to compare the various measures between lean and obese groups in the selected BLSA participants. Wilcoxon matched-pairs sign rank test was used to compare the measures between placebo vs. CBR agonist administration. Pearson correlation was used to confirm that BMI and HOMA2-IR track with GIP and GLP-1 responses in both the BLSA and CBR agonist/placebo studies. Statistical calculations were performed using GraphPad Prism, version 6.03 (GraphPad Software).
RESULTS
BLSA case-control study.
There were 10 men and 10 women in each of the lean (BMI < 25 kg/m2) and obese (BMI ≥ 30 kg/m2) groups. The subjects of the two groups were matched for sex and age. HbA1c levels were not significantly different between the two groups. As expected, the fasting glucose, insulin, and HOMA2-IR, a measure of insulin resistance, were significantly higher in the obese group (P = 0.013, P < 0.001, and P < 0.001, respectively). Fasting GIP levels were significantly higher in the obese group (P < 0.001). However, the fasting tGLP-1 levels were comparable between the two groups. Table 1 summarizes the results.
Table 1.
Characteristics of the selected BLSA cohort
| Variables | Lean | Obese | P Value |
|---|---|---|---|
| Men/women, n/n | 10/10 | 10/10 | |
| Age, year | 68.5 ± 2.9 | 68.5 ± 2.9 | 0.984 |
| BMI, kg/m2 | 23.6 ± 0.3 | 35.6 ± 0.9 | <0.001 |
| A1c, % | 5.5 ± 0.1 | 5.6 ± 0.1 | 0.123 |
| Fasting glucose, mmol/l | 5.2 ± 0.1 | 5.5 ± 0.1 | 0.013 |
| (mg/dl) | (93.2 ± 1.5) | (98.3 ± 1.2) | |
| Fasting insulin, pmol/l | 34.7 ± 2.8 | 86.1 ± 10.4 | <0.001 |
| (µIU/l) | (5.0 ± 0.4) | (12.4 ± 1.5) | |
| Fasting GIP, pmol/l | 44.2 ± 3.4 | 82.8 ± 10.7 | <0.001 |
| Fasting tGLP-1, pmol/l | 3.3 ± 0.4 | 3.9 ± 0.5 | 0.425 |
| HOMA2-IR | 0.77 ± 0.07 | 1.87 ± 0.21 | <0.001 |
| Glucose AUC, mmol·l−1·min | 778 ± 32 | 919 ± 28 | 0.002 |
| (mg·dl−1·min) | (14,000 ± 579) | (16,545 ± 499) | |
| Insulin AUC, pmol·l−1·min | 31,361 ± 3,720 | 75,944 ± 9,202 | <0.001 |
| (µIU·l−1·min) | (4,519 ± 536) | (10,943 ± 1,326) | |
| GIP AUC, pmol·l−1·min | 22,063 ± 1,261 | 27,228 ± 1,649 | 0.043 |
| tGLP-1 AUC, pmol·l−1·min | 1,150 ± 90 | 831 ± 117 | 0.019 |
Data are presented as means ± SE.
Compared with the lean group, the post-OGTT glucose and insulin excursions were significantly elevated in the obese group, as expected (Fig. 1, A and B). Interestingly, the post-OGTT GIP excursions were significantly elevated while the post-OGTT tGLP-1 excursions were significantly decreased in the obese group (Figs. 1, C and D). The findings were quantified using AUCs of post-OGTT glucose, insulin, GIP, and tGLP-1 (Table 1).
Fig. 1.
The excursion profile of plasma glucose (A), insulin (B), GIP (C), and tGLP-1 (D) after oral glucose ingestion in 2 groups of selected BLSA cohort: lean (blue circle) and obese (red square). Data are presented as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Human CBR agonist vs. placebo study.
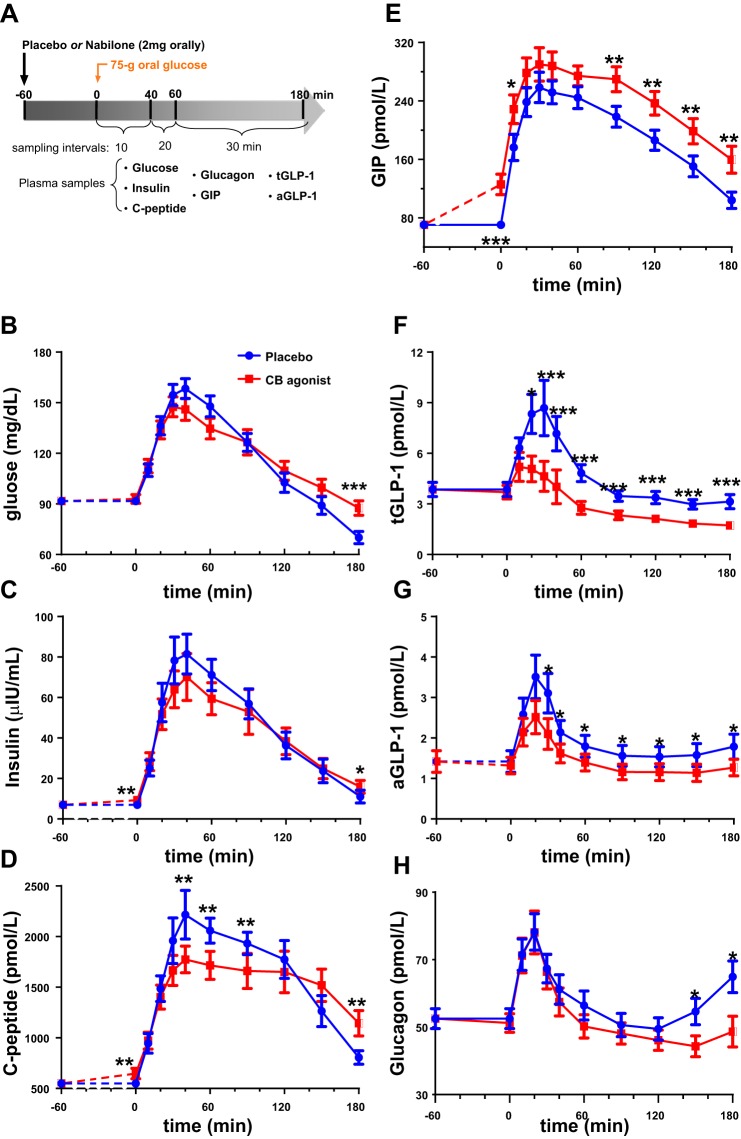
The study schema is shown in Fig. 2A, where nabilone (2 mg orally) or placebo was administered in a randomized, double-blind, crossover fashion. The characteristics of the 20 subjects in the CBR agonist/placebo study and the study results are summarized in Table 2. When compared with placebo, nabilone (a CBR agonist), 60 min after administration, did not affect post-dose fasting glucose, glucagon, tGLP-1, or aGLP-1 levels but induced a highly significant elevation (80%) in post-dose fasting GIP levels from 70 to 126 pmol/l (P < 0.001). Interestingly, post-dose fasting insulin levels were also significantly elevated. The average post-dose fasting insulin level was 65 pmol/l compared with 49 pmol/l for placebo (P = 0.009). This increase in post-dose fasting insulin was not associated with any change in glucose levels, thus resulting in a significant elevation in hepatic insulin resistance, as measured by HOMA-2-IR (P = 0.013) (Table 2). C-peptide levels were also significantly increased in the post-dose fasting state (P = 0.004), in keeping with insulin levels.
Fig. 2.
A: study design. Each fasted participant took either placebo or nabilone (CBR agonist) orally in a randomized, crossover fashion on 2 different days several weeks apart. A 75-g OGTT was administered 1 h after the placebo/CBR agonist was given. Blood samples were collected over 3 h to measure various factors associated with glucose metabolism. When compared with placebo (blue circle), nabilone (red square) administration 60 min before a 75-g OGTT is associated with comparable glucose excursion (B), significant elevation in fasting insulin level 1 h after dosing and then comparable insulin excursion post-OGTT (C), significant elevation in fasting C-peptide level 1 h after dosing (D), significant elevation in fasting GIP levels 1 h after dosing and elevated levels that persisted throughout the OGTT (E), significant suppression of tGLP-1 (F) and aGLP-1 (G) throughout the OGTT, and significant lowering of glucagon levels 2.5 to 3 h after glucose load (H). The post-dose fasting levels of glucose and respective hormones at t = 0 under placebo condition were plotted at t = −60 min, and dashed lines (t = −60 to 0 min) were used for illustrative purposes only. Data are presented as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 2.
Placebo vs. CBR agonist
| Variables | Placebo | CBR Agonist | P Value |
|---|---|---|---|
| N | 20 | ||
| Age, yr | 38.3 ± 1.9 | ||
| BMI, kg/m2 | 27.1 ± 0.4 | ||
| A1c, % | 5.5 ± 0.1 | ||
| *Fasting glucose, mmol/l | 5.1 ± 0.1 | 5.2 ± 0.1 | 0.351 |
| (mg/dl) | (91.7 ± 1.3) | (93.0 ± 2.6) | |
| *Fasting insulin, pmol/l | 48.6 ± 4.2 | 64.6 ± 7.6 | 0.009 |
| (µIU/l) | (7.0 ± 0.6) | (9.3 ± 1.1) | |
| *Fasting C-peptide, pmol/l | 548.4 ± 37.3 | 647 ± 52.0 | 0.004 |
| *Fasting glucagon, pmol/l | 52.5 ± 3.0 | 51.2 ± 2.8 | 0.898 |
| *Fasting GIP, pmol/l | 70.4 ± 4.9 | 125.6 ± 14.1 | <0.001 |
| *Fasting tGLP-1, pmol/l | 3.9 ± 0.4 | 3.7 ± 0.4 | 0.654 |
| *Fasting aGLP-1, pmol/l | 1.4 ± 0.3 | 1.3 ± 0.2 | 0.998 |
| HOMA2-IR | 1.05 ± 0.09 | 1.39 ± 0.17 | 0.013 |
| Glucose AUC, mmol·l−1·min | 1,173 ± 38 | 1,185 ± 41 | 0.898 |
| (mg·dl−1·min) | (21,134 ± 675) | (21,349 ± 744) | |
| Insulin AUC, pmol·l−1·min | 57,776 ± 7,584 | 53,803 ± 7,278 | 0.701 |
| (µIU·l−1·min) | (8,319 ± 1,092) | (7,747 ± 1,048) | |
| C-peptide AUC, pmol·l−1·min | 292,608 ± 22,012 | 275,273 ± 22,309 | 0.294 |
| Glucagon AUC, pmol·l−1·min | 10,379 ± 650 | 9,418 ± 608 | 0.105 |
| GIP AUC, pmol·l−1·min | 35,191 ± 2,188 | 43,324 ± 2,725 | <0.001 |
| tGLP-1 AUC, pmol·l−1·min | 822.1 ± 78.7 | 511.2 ± 60.3 | <0.001 |
| aGLP-1 AUC, pmol·l−1·min | 342.9 ± 49.1 | 259.8 ± 37.5 | 0.033 |
Data are presented as means ± SE.
These fasting levels were obtained 60 min after administration of nabilone/placebo but before oral glucose solution.
Except for the higher fasting insulin levels, nabilone did not impact the insulin excursion after glucose load (Fig. 2C). Higher circulating GIP levels persisted throughout the post-glucose load period but no further increase in GIP secretion beyond that contributed by glucose alone occurred (Fig. 2E). Even though fasting tGLP-1 levels were not affected with CBR agonist administration, their post-OGTT levels were significantly lower throughout (25–40%) (Fig. 2F). We also assayed for aGLP-1 levels to examine if CBR agonist has any effect on degradation of tGLP-1 by DPP-4. Both post-dose fasting and post-OGTT aGLP-1 levels responded in a similar fashion compared with those of tGLP-1, which were significantly lower with CBR agonist compared with placebo (Fig. 2G). These results were quantified using AUCs of post-OGTT glucose, insulin, C-peptide, GIP, tGLP-1, aGLP-1, and glucagon (Table 2).
To confirm that BMI and HOMA2-IR track with GIP and GLP-1 responses, we performed a final observational analysis using Pearson correlation for both the BLSA case-control study and the CBR agonist/placebo study (Table 3). In the obese group, HOMA2-IR has a significantly positive correlation with fasting GIP whereas BMI has a significantly negative correlation with fasting tGLP-1 and with AUC of post-OGTT tGLP-1, as expected. In the CBR agonist/placebo study, nabilone administration significantly increased the association between HOMA2-IR and fasting GIP, confirming our hypothesis.
Table 3.
Pearson correlation between BMI and HOMA2-IR with fasting and post-OGTT incretin levels
| Fasting GIP |
Fasting GLP-1 |
GIP AUC |
tGLP-1 AUC |
|||||
|---|---|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | r | P value | |
| BLSA case-control study | ||||||||
| Lean | ||||||||
| BMI | 0.416 | 0.068 | −0.112 | 0.638 | 0.409 | 0.073 | −0.016 | 0.948 |
| HOMA2-IR | 0.277 | 0.236 | −0.272 | 0.246 | 0.357 | 0.123 | −0.045 | 0.852 |
| Obese | ||||||||
| BMI | 0.132 | 0.580 | −0.494 | 0.027 | 0.262 | 0.265 | −0.591 | 0.006 |
| HOMA2-IR | 0.665 | 0.001 | 0.036 | 0.879 | 0.395 | 0.085 | −0.168 | 0.478 |
| Human CBR agonist vs. placebo study | ||||||||
| Placebo | ||||||||
| BMI | −0.262 | 0.264 | 0.344 | 0.137 | −0.236 | 0.317 | 0.177 | 0.455 |
| HOMA2-IR | −0.341 | 0.142 | 0.196 | 0.408 | 0.465 | 0.039 | 0.182 | 0.441 |
| CB agonist | ||||||||
| BMI | 0.038 | 0.872 | 0.024 | 0.919 | 0.104 | 0.663 | −0.194 | 0.413 |
| HOMA2-IR | 0.653 | 0.002 | 0.319 | 0.171 | 0.434 | 0.056 | 0.383 | 0.095 |
DISCUSSION
We conclude that, in humans, incretin secretion is in part regulated by the endocannabinoid system. Data from the BLSA cohort showed that obesity alone is associated with significantly higher fasting and post-OGTT GIP levels and significantly lower post-OGTT GLP-1 levels when age and sex were taken into account. Next, we showed that a CBR agonist (nabilone) has clear effects on incretin secretion in humans. At a usual oral dose of 2 mg, routinely prescribed for therapeutic purposes, a CBR agonist increased circulating GIP levels by ~80% in the fasting (nonstimulated) state within 1 h of ingestion. The differential secretion of GIP between placebo and CBR agonist administration remained constant for the duration of a glucose stimulus suggesting that glucose-mediated GIP secretion was not enhanced by the CBR agonist.
Contrary to its effect on GIP, nabilone did not have an impact on fasting GLP-1 levels but did result in decreased GLP-1 secretion in response to oral glucose. Furthermore, it induced a significant increase in fasting insulin and C-peptide levels without any alteration in fasting glucose levels, which resulted in a significant increase in insulin resistance, most likely at the level of the liver. These findings mirror the incretin profile during both fasting and post-OGTT of individuals with obesity when compared with lean individuals. Therefore, it is possible the incretin changes seen in the obese state are due to altered endocannabinoid levels.
These results were collaborated by the significant correlation found between HOMA2-IR and fasting GIP levels in the BLSA obese group and when nabilone was administered to healthy nonobese subjects. In the CBR agonist/placebo study, there was significant correlation between HOMA2-IR and fasting GIP with CBR agonist administration but not with placebo administration. These results were consistent with the hypothesis that modulation of the endocannabinoid system can affect incretin secretion. The significant correlation observed between BMI and GLP-1 (both fasting and post-OGTT AUC) in the obese state has been documented in several studies (30, 40). We did not see a significant change in fasting GLP-1 with CBR agonist compared with placebo, suggesting that the regulation of GLP-1 during the fasted state may be through other mechanisms and not through the endocannabinoid system.
To our knowledge, this is the first time any compound has been shown to have such profound and contrasting effects on incretin secretion in humans. We can only speculate as to how nabilone, a CBR agonist, may be influencing constitutive (non-nutrient-stimulated) release of GIP from K cells. Since circulating GIP levels were increased within 1 h of nabilone ingestion without nutrient stimulation, we can assume it is not due to new synthesis of GIP. Isolated mouse K cells have been reported to contain CB1Rs (29). Furthermore, methanandamide, a CB1R agonist, was reported to inhibit GIP secretion from isolated K cells and to also inhibit glucose-mediated GIP secretion in vivo in rats (29). The influence of a CBR agonist on incretin secretion in humans, as we have shown here, is clearly not the same as in rodents. There are many other instances of disparate results between rodents and humans from putative incretin secretagogues (9). To our knowledge, no one has yet reported the presence of CBR in human incretin-secreting cells.
A plausible scenario to account for the increased fasting GIP levels after CBR agonist administration is as follows: GIP release from K cells is under tonic inhibition, possibly through a Gαq-mediated signaling pathway such as fatty acid receptors (35), Gαi-mediated signaling such as somatostatin receptors (29), or by the enteric nervous system (38), and nabilone administration resulted in release of this inhibitory brake. CB1R agonists are known to decrease gut motility thus slowing the transit of food through the gut which can potentially increase GIP secretion because of increased contact time of nutrient with enteroendocrine cells (19). However, changes in gut motility cannot explain increased GIP levels with nabilone administered in the fasted state where gut motility should be minimal. Furthermore, the introduction of glucose into the gut 1 h after nabilone was given, did not further increase GIP secretion beyond that attained by glucose alone. Note that the post-OGTT GIP levels from the placebo and CBR agonist administration paralleled each other. Therefore, the influence of nabilone is on the non-nutrient-stimulated, constitutive GIP release in humans. The differential secretion of GIP between placebo and CBR agonist administration remained constant for the duration of a glucose stimulus suggesting that glucose-mediated GIP secretion was not enhanced by the CBR agonist but was additive to the baseline CBR-associated constitutive (fasting) GIP release. Furthermore, the difference in GIP levels between placebo and CBR agonist was still significant 3-h post-OGTT suggesting that the CBR was still activated 4 h after nabilone administration (nabilone was administered 1 h before OGTT). The elimination half-life of nabilone is ~2 h (25); hence, the CBR may still be activated four to five half-lives later. Another possible explanation could be that nabilone induced central GIP secretion in the brain, which in turn may have stimulated peripheral GIP release. GIP receptor transcript and protein are found in rat and human brain (14) and centrally administered GIP via intracerebroventricular infusion stimulated peripheral GIP release in nonhuman primates (18).
How do we account for reduced post-OGTT GLP-1 secretion in response to nabilone while nabilone had no impact on fasting GLP-1 levels? CBR agonists may regulate nutrient-stimulated GLP-1 secretion through either direct or indirect mechanisms. The decrease in post-OGTT GLP-1 levels after nabilone administration is not likely due to inhibitory effects of nabilone on gut motility because slowing motility through the duodenum, thereby increasing nutrient intestinal transit time, should increase and not decrease GLP-1 secretion, as shown by acarbose administration which delayed gastric emptying and enhanced GLP-1 release in healthy subjects (11, 36). If the effect of nabilone on incretins was due to altered gut motility in response to glucose, changes in incretin secretion would be predicted to be in the same direction for both GIP and GLP-1 which is clearly not the case. We had previously reported that exogenous GIP administration in humans was associated with significantly reduced GLP-1 secretion in response to a food stimulus (4). Therefore, it is possible that the nabilone-induced elevation in fasting GIP levels and the corresponding differential increase in glucose-mediated GIP secretion were responsible for the significantly suppressed post-OGTT GLP-1 levels, and not a direct effect of nabilone on CBRs in L cells. To our knowledge, there is only one other human study that examined the effect of exogenous GIP infusion on GLP-1 secretion, and the GIP infusion was carried on during hyperglycemic clamp experiments. As expected, no changes in GLP-1 levels were found because there was no stimulus for GLP-1 secretion, such as oral nutrients, given in the study (32). However, GIP has been shown to stimulate GLP secretion using in situ models of isolated perfused rat ileum preparation and isolated perfused segments of porcine ileum, and in vitro studies using GLP-1 release assay based on primary canine intestinal L-cells and GLUTag cells line (3, 6, 16, 17). However, in situ and in vitro models are not representative of the actual human physiology, which involves a complex interplay of endocrine, paracrine, and neuronal pathways. How elevation in GIP levels suppresses GLP-1 secretion in humans is not clear and needs further research.
Elevated endocannabinoids levels, as occur in obesity, are thought to play a role in insulin resistance because endocannabinoids are known negative regulators of insulin action, especially in liver (20, 34). In our CBR agonist/placebo study, 1 h after oral nabilone but still in a fasted state, the subjects became significantly more insulin resistant, as reflected in the significant increase in HOMA2-IR. HOMA2-IR is derived from fasting plasma glucose and insulin levels, and primarily reflects hepatic insulin resistance (41). The elevated GIP levels were unlikely to be the direct, immediate cause of the increase in fasting insulin levels after oral nabilone because GIP-mediated insulin secretion is glucose-dependent, in that rising circulating glucose levels are required for GIP to enhance insulin secretion (10). The fasting plasma glucose levels with placebo or nabilone were not significantly different. Additionally, elevated insulin levels would be expected to lower fasting glucose levels unless there was concomitant insulin resistance, as we have shown in this case. However, it is also possible that elevated circulating GIP levels may be an additive factor in hepatic insulin resistance because there is experimental evidence from mice suggesting that GIP can cause reduced insulin action (31). Hepatic insulin resistance induced by nabilone administration would also explain the elevation in fasting C-peptide levels. Fasting and post-OGTT glucagon levels for the first 2 h were not different between placebo and nabilone. With placebo, glucagon levels began to rise 2.5 h post-OGTT when plasma glucose dropped below fasting levels of ∼5 mmol/l (90 mg/dl). With nabilone, glucagon levels did not rise because glucose levels dropped at a slower rate and did not drop below fasting levels. This observation likely reflects reduced insulin action in liver in the presence of nabilone.
Strengths of our studies include the availability and ability to weave together results from a human observation study and a human intervention study. Furthermore, we eliminated age and sex as confounders when comparing the lean with the obese group; therefore, we showed that with obesity, fasting and glucose-stimulated plasma GIP levels were significantly higher and glucose-stimulated GLP-1 levels significantly lower. This observation leads us to conclude that endocannabinoids, either in circulation and/or synthesized in the gut, may be involved in regulating constitutive GIP and nutrient-stimulated GLP-1 release. However, there are certain limitations. First, the BLSA cohort is older; therefore, we cannot make a definitive conclusion that endocannabinoids are responsible for the altered incretin levels in obesity. The data with nabilone, however, are clear. Second, nabilone binds to both CB1Rs and CB2Rs (2); therefore, although we cannot discern which receptors the observed effects are working through, CB2R has never been shown to be present in hormone-secreting cells of rodent or humans, and it is reported to be confined to the immune system in the periphery. Last, HOMA2-IR is a proxy for insulin resistance and has its limitations (43).
In summary, we have shown that, in humans, the endocannabinoid system is likely involved in the regulation of incretin secretion. In the obese state, elevation in endocannabinoid levels may at least partly account for the observed altered regulation of incretin secretion.
GRANTS
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.W.C. and J.M.E. conceived and designed research; C.W.C. and O.D.C. performed experiments; C.W.C., O.D.C., D.D.L., I.G.-M., S.S.-C.C., and J.M.E. analyzed data; C.W.C., O.D.C., D.D.L., I.G.-M., S.S.-C.C., and J.M.E. interpreted results of experiments; C.W.C. prepared figures; C.W.C. drafted manuscript; C.W.C., O.D.C., D.D.L., I.G.-M., S.S.-C.C., and J.M.E. edited and revised manuscript; C.W.C., O.D.C., D.D.L., I.G.-M., S.S.-C.C., and J.M.E. approved final version of manuscript.
REFERENCES
- 1.Alvheim AR, Torstensen BE, Lin YH, Lillefosse HH, Lock EJ, Madsen L, Frøyland L, Hibbeln JR, Malde MK. Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids 49: 59–69, 2014. doi: 10.1007/s11745-013-3842-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashton JC, Wright JL, McPartland JM, Tyndall JD. Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists. Curr Med Chem 15: 1428–1443, 2008. doi: 10.2174/092986708784567716. [DOI] [PubMed] [Google Scholar]
- 3.Brubaker PL, Schloos J, Drucker DJ. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology 139: 4108–4114, 1998. doi: 10.1210/endo.139.10.6228. [DOI] [PubMed] [Google Scholar]
- 4.Chia CW, Carlson OD, Kim W, Shin YK, Charles CP, Kim HS, Melvin DL, Egan JM. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 58: 1342–1349, 2009. doi: 10.2337/db08-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Côté M, Matias I, Lemieux I, Petrosino S, Alméras N, Després JP, Di Marzo V. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 31: 692–699, 2007. doi: 10.1038/sj.ijo.0803539. [DOI] [PubMed] [Google Scholar]
- 6.Damholt AB, Buchan AM, Kofod H. Glucagon-like-peptide-1 secretion from canine L-cells is increased by glucose-dependent-insulinotropic peptide but unaffected by glucose. Endocrinology 139: 2085–2091, 1998. doi: 10.1210/endo.139.4.5921. [DOI] [PubMed] [Google Scholar]
- 7.De Gottardi A, Spahr L, Ravier-Dall’Antonia F, Hadengue A. Cannabinoid receptor 1 and 2 agonists increase lipid accumulation in hepatocytes. Liver Int 30: 1482–1489, 2010. doi: 10.1111/j.1478-3231.2010.02298.x. [DOI] [PubMed] [Google Scholar]
- 8.Di S, Boudaba C, Popescu IR, Weng FJ, Harris C, Marcheselli VL, Bazan NG, Tasker JG. Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J Physiol 569: 751–760, 2005. doi: 10.1113/jphysiol.2005.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drucker DJ. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab 24: 15–30, 2016. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, Habener JF, Andersen DK. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept 51: 63–74, 1994. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 11.Enç FY, Imeryüz N, Akin L, Turoğlu T, Dede F, Haklar G, Tekeşin N, Bekiroğlu N, Yeğen BC, Rehfeld JF, Holst JJ, Ulusoy NB. Inhibition of gastric emptying by acarbose is correlated with GLP-1 response and accompanied by CCK release. Am J Physiol Gastrointest Liver Physiol 281: G752–G763, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 54: 2838–2843, 2005. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Færch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T, Sandbæk A, Holst JJ, Jørgensen ME. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The ADDITION-PRO study. Diabetes 64: 2513–2525, 2015. doi: 10.2337/db14-1751. [DOI] [PubMed] [Google Scholar]
- 14.Figueiredo CP, Antunes VL, Moreira EL, de Mello N, Medeiros R, Di Giunta G, Lobão-Soares B, Linhares M, Lin K, Mazzuco TL, Prediger RD, Walz R. Glucose-dependent insulinotropic peptide receptor expression in the hippocampus and neocortex of mesial temporal lobe epilepsy patients and rats undergoing pilocarpine induced status epilepticus. Peptides 32: 781–789, 2011. doi: 10.1016/j.peptides.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 15.González-Mariscal I, Krzysik-Walker SM, Kim W, Rouse M, Egan JM. Blockade of cannabinoid 1 receptor improves GLP-1R mediated insulin secretion in mice. Mol Cell Endocrinol 423: 1–10, 2016. doi: 10.1016/j.mce.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen L, Holst JJ. The effects of duodenal peptides on glucagon-like peptide-1 secretion from the ileum. A duodeno--ileal loop? Regul Pept 110: 39–45, 2002. doi: 10.1016/S0167-0115(02)00157-X. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann-Rinke C, Vöge A, Hess M, Göke B. Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol 147: 25–31, 1995. doi: 10.1677/joe.0.1470025. [DOI] [PubMed] [Google Scholar]
- 18.Higgins PB, Shade RE, Rodríguez-Sánchez IP, Garcia-Forey M, Tejero ME, Voruganti VS, Cole SA, Comuzzie AG, Folli F. Central GIP signaling stimulates peripheral GIP release and promotes insulin and pancreatic polypeptide secretion in nonhuman primates. Am J Physiol Endocrinol Metab 311: E661–E670, 2016. doi: 10.1152/ajpendo.00166.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol 141: 1335–1345, 2004. doi: 10.1038/sj.bjp.0705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim W, Doyle ME, Liu Z, Lao Q, Shin YK, Carlson OD, Kim HS, Thomas S, Napora JK, Lee EK, Moaddel R, Wang Y, Maudsley S, Martin B, Kulkarni RN, Egan JM. Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes 60: 1198–1209, 2011. doi: 10.2337/db10-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60: 470–512, 2008. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21: 2191–2192, 1998. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 23.Maccarrone M, Bab I, Bíró T, Cabral GA, Dey SK, Di Marzo V, Konje JC, Kunos G, Mechoulam R, Pacher P, Sharkey KA, Zimmer A. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 36: 277–296, 2015. doi: 10.1016/j.tips.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matias I, Gonthier M-P, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V. Regulation, function, and dysregulation of endocannabinoids in models of adipose and β-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91: 3171–3180, 2006. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 25.McGilveray IJ. Pharmacokinetics of cannabinoids. Pain Res Manag 10, Suppl A: 15A–22A, 2005. doi: 10.1155/2005/242516. [DOI] [PubMed] [Google Scholar]
- 26.McIntosh CHS, Widenmaier S, Kim SJ. Glucose‐dependent insulinotropic polypeptide (gastric inhibitory polypeptide; GIP). In: Vitamins & Hormones. New York: Academic, 2009, chapt. 15, p. 409–471. doi: 10.1016/S0083-6729(08)00615-8 [DOI] [PubMed] [Google Scholar]
- 27.McIntyre N, Holdsworth CD, Turner DS. New Interpretation of Oral Glucose Tolerance. Lancet 284: 20–21, 1964. doi: 10.1016/S0140-6736(64)90011-X. [DOI] [PubMed] [Google Scholar]
- 28.Meier JJ, Nauck MA. Glucagon-like peptide 1(GLP-1) in biology and pathology. Diabetes Metab Res Rev 21: 91–117, 2005. doi: 10.1002/dmrr.538. [DOI] [PubMed] [Google Scholar]
- 29.Moss CE, Marsh WJ, Parker HE, Ogunnowo-Bada E, Riches CH, Habib AM, Evans ML, Gribble FM, Reimann F. Somatostatin receptor 5 and cannabinoid receptor 1 activation inhibit secretion of glucose-dependent insulinotropic polypeptide from intestinal K cells in rodents. Diabetologia 55: 3094–3103, 2012. doi: 10.1007/s00125-012-2663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes 57: 1340–1348, 2008. doi: 10.2337/db07-1315. [DOI] [PubMed] [Google Scholar]
- 31.Nasteska D, Harada N, Suzuki K, Yamane S, Hamasaki A, Joo E, Iwasaki K, Shibue K, Harada T, Inagaki N. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes 63: 2332–2343, 2014. doi: 10.2337/db13-1563. [DOI] [PubMed] [Google Scholar]
- 32.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91: 301–307, 1993. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 54: 10–18, 2011. doi: 10.1007/s00125-010-1896-4. [DOI] [PubMed] [Google Scholar]
- 34.Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, Bátkai S, Marsicano G, Lutz B, Buettner C, Kunos G. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest 118: 3160–3169, 2008. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52: 289–298, 2009. doi: 10.1007/s00125-008-1202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranganath L, Norris F, Morgan L, Wright J, Marks V. Delayed gastric emptying occurs following acarbose administration and is a further mechanism for its anti-hyperglycaemic effect. Diabet Med 15: 120–124, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Reimann F, Gribble FM. Mechanisms underlying glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 secretion. J Diabetes Investig 7, Suppl 1: 13–19, 2016. doi: 10.1111/jdi.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimann F, Tolhurst G, Gribble FM. G-protein-coupled receptors in intestinal chemosensation. Cell Metab 15: 421–431, 2012. doi: 10.1016/j.cmet.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 39.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab 17: 475–490, 2013. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86: 3717–3723, 2001. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 41.Tripathy D, Almgren P, Tuomi T, Groop L. Contribution of insulin-stimulated glucose uptake and basal hepatic insulin sensitivity to surrogate measures of insulin sensitivity. Diabetes Care 27: 2204–2210, 2004. doi: 10.2337/diacare.27.9.2204. [DOI] [PubMed] [Google Scholar]
- 42.Vianna CR, Donato J Jr, Rossi J, Scott M, Economides K, Gautron L, Pierpont S, Elias CF, Elmquist JK. Cannabinoid receptor 1 in the vagus nerve is dispensable for body weight homeostasis but required for normal gastrointestinal motility. J Neurosci 32: 10331–10337, 2012. doi: 10.1523/JNEUROSCI.4507-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]