Abstract
Fibroblast growth factor 21 (FGF21) is a potent endocrine regulator with physiological effects on glucose and lipid metabolism and thus garners much attention for its translational potential for the management of obesity and related metabolic syndromes. FGF21 is mainly expressed in several metabolically active tissue organs, such as the liver, adipose tissue, skeletal muscle, and pancreas, with profound effects and therapeutic relevance. Emerging experimental and clinical data point to the demonstrated metabolic benefits of FGF21, which include, but are not limited to, weight loss, glucose and lipid metabolism, and insulin sensitivity. In addition, FGF21 also acts directly through its coreceptor β-klotho in the brain to alter light-dark cycle activity. In this review, we critically appraise current advances in understanding the physiological actions of FGF21 and its role as a biomarker of various metabolic diseases, especially type 2 diabetes mellitus. We also discuss the potentially exciting role of FGF21 in improving our health and prolonging our life span. This information will provide a fuller understanding for further research into FGF21, as well as providing a scientific basis for potentially establishing health care guidelines for this promising molecule.
Keywords: biomarker, circadian, energy homeostasis, life span, lipid metabolism
generally, the fibroblast growth factor (FGF) family of polypeptides acts as mitogens for fibroblasts (28). However, FGF21, atypical for an FGF, does not have mitogen activity and function as an endocrine hormone governing carbohydrate and fatty acid (FA) metabolism. FGF21 is a pleiotropic regulator that elicits a variety of physiological and pathogenic events. Under obesity-related metabolic disorder conditions, systemically administered FGF21 has been shown to induce sustained weight loss, lower blood glucose and triglyceride (TG) levels, improve insulin sensitivity, increase brown adipocyte numbers (43), preserve β-cell function and mass (95), ameliorate hepatic steatosis (99), lower low-density lipoprotein cholesterol (LDL-c), and elevate high-density lipoprotein cholesterol (HDL-c; 44). Meanwhile, thus far, FGF21 therapy does not appear to result in adverse secondary effects, such as hypoglycemia, edema, liver toxicity, or increased mitogenicity (42). The multiple beneficial metabolic effects of FGF21 coupled with its encouraging side effect profile in animals makes it a promising medicine for metabolic syndrome.
In the last decade, since FGF21 has been identified as a pharmacological regulator that may benefit patients with or at risk of diabetes, the volume of published studies examining FGF21 each year has grown 10-fold. Importantly, the more recent expanded research on FGF21 has not only validated its therapeutic potential but also led to greater interest in elucidating its physiological activities, including a suggested role as a prolongevity hormone and its potential to serve as a biomarker for a number of diseases, which will be discussed in this review.
Endogenous FGF21: Its Regulation and Dysregulation
Endogenous FGF21 levels.
FGF21 is widely expressed in metabolic organs including liver, gut, adipose tissue, and pancreas (21). Greater receptor expression, which is related to more effective hormonal function of these tissues, could alleviate a pathophysiological pressure for higher levels of hormones. Levels of FGF21 are increased under a variety of physiological conditions, including starvation (25), overfeeding (58, 91), ketogenic diets (18), low-protein diets (48), and high-carbohydrate diets (79). In humans, prolonged fasting (7 days) led to increased serum FGF21 levels, measured once daily, whereas shorter-term fasting (3 days) did not (25). Because the metabolic rate in mice is ~10-fold higher than that in humans, energy stores are much more rapidly consumed during fasting. Hence the aforementioned 7-day fast in humans should correspond to a fast of ~24 h in mice. A variant in the chromosome 19 loci of humans that is a synonymous single-nucleotide polymorphism located in the first exon of the FGF21 gene was identified in association with decreased protein intake, increased carbohydrate intake, and decreased fat intake in models adjusted for body mass index (BMI). This finding points to the association of the FGF21 variant with macronutrient intake (13, 87). Induction of FGF21 during starvation or adherence to a ketogenic diet appears to be mediated by protein intake restriction in both rodents and humans (48). Low protein-induced increases in FGF21 have been associated with eukaryotic initiation factor 2α, serine/threonine kinase general control nonderepressible-2, and peroxisome proliferator-activated receptor-α (PPARα) signaling in the liver (48). Using the nutritional modeling platform Geometric Framework, Solon-Biet et al. examined how macronutrients interact to influence FGF21 expression and secretion. They found that FGF21 was elevated under low-protein intake conditions and elevated further when low protein was coupled with high carbohydrate intake (79). Their findings indicate that the complex metabolic stresses that elevate FGF21 depend on nutritional context, rather than volume of feeding per se.
Circadian regulation of FGF21.
Like FGF19, which has two peaks during daytime (56), serum FGF21 also exhibits a clear diurnal rhythm. Yu et al. showed that circulating FGF21 concentrations exhibit a characteristic diurnal rhythm in humans and dysregulation of the FGF21 circadian rhythm correlates with obesity-induced lipid disorders (105). They found that normally, FGF21 secretion begins to rise around midnight, reaching a peak at ~5 AM, and then declines to a nadir in the afternoon under both fasting and normal conditions. In a 3-day fasting study, on days 1 and 2 of the fast, Andersen et al. found that plasma FGF21 levels increased during daytime and evening hours, peaking at 2:30 AM, and then decreased to nadir levels at 8.30 AM (2). Gälman et al. found that FGF21 levels were stable in healthy subjects, with only a slight increase from midnight to 1:30 AM in three of five healthy volunteer subjects; however, the sample size was too small to support drawing a strong conclusion (25).
A weak FGF21 circadian rhythm in obese individuals may contribute to obesity-related metabolic dysregulation. Basal serum FGF21 concentrations have been shown to be higher in obese individuals than in lean individuals, with mean amplitudes (peak-to-nadir ratio) in the obese group being significantly smaller than those in the lean group, demonstrating a blunting of the nocturnal FGF21 rise in the obese group (105). Further analysis of the temporal association of serum FGF21 with metabolites indicated that insulin and glucose levels peaked within 30–60 min after each meal during a low point of serum FGF21. Meanwhile, the oscillatory curve of serum cortisol resembles that of FGF21 but peaks 6 h later, suggesting that these two hormones might be controlled by similar sets of circadian genes. Interestingly, the rhythm pattern of serum levels of free FAs resembles that of circulating FGF21, with a peak time ~4 h earlier than that of serum FGF21. An in vitro experiment with human HepG2 hepatocytes showed that FGF21 mRNA was increased maximally 4 h after treatment with the essential FA linoleate (105). Together, these findings suggest that a nocturnal rise in FAs leads to a subsequent increase in circulating FGF21, which, in turn, reduces FA levels through induction of β-oxidation and inhibition of lipolysis in white adipocytes, forming a feedback regulatory loop between FAs and FGF21 (Fig. 1).
Fig. 1.
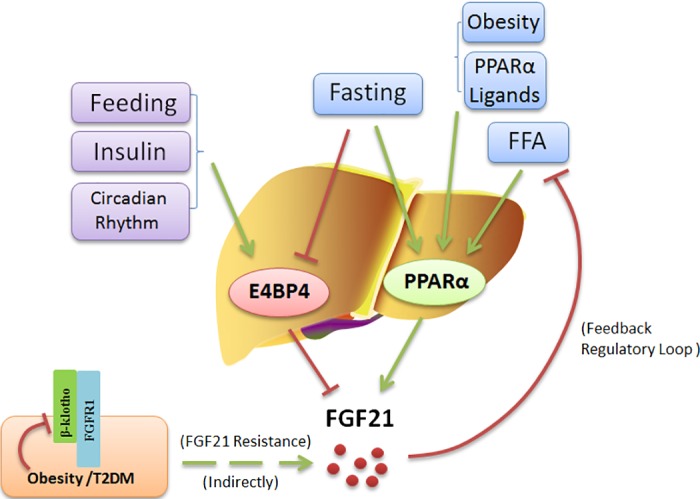
Model for the regulation of hepatic FGF21 expression. Feeding, insulin, and circadian rhythm induce E4BP4 to regulate the FGF21 circadian oscillation. E4BP4 directly binds to the FGF21 promoter to suppress its expression. FGF21 expression in the liver is also induced by fasting, FFAs, obesity, and PPARα ligands in mice. FGF21, in turn, reduces FFA levels through induction of β-oxidation and inhibition of lipolysis in white adipocytes, forming a feedback regulatory loop between FFAs and FGF21. Obesity and T2DM were identified as a state of FGF21 resistance in which FGFR1 and β-klotho are both downregulated to weaken the FGF21 signaling resulting in compensatory FGF21 production.
Nuclear PPARα has been shown to be an important transcriptional activator of hepatic FGF21 expression; PPARα knockout (KO) mice do not upregulate FGF21 in response to fasting (4). Interestingly, FAs can function as endogenous ligands for PPARα. Hepatic PPARα is also expressed in a circadian manner at the mRNA and protein levels in rats (51) and in mice (66). Stimulation of PPARα with its ligand bezafibrate was shown to induce time-dependent torporlike phenomena (i.e., body temperature decreases). The PPARα activator induced FGF21 expression if injected at night, but not if injected during the day (65). The finding that basal core body temperature in FGF21 transgenic (FGF21-Tg) mice was consistently lower than that of wild-type mice (36) supports the notion that the PPARα-FGF21 pathway may be associated with torporlike phenomena and further confirms that FGF21 expression is regulated in a circadian manner, at least in mice administered a PPARα ligand.
Regarding the circadian regulation of FGF21, it is noteworthy that the mRNA and protein levels of E4-binding protein-4 (E4BP4) oscillate in a circadian fashion (60, 71). E4BP4 is a basic leucine zipper transcription factor that inhibits FGF21 expression by binding to the FGF21 promoter via a D-box binding element (90). In Hepa1c1c-7 cells, E4BP4 knockdown increased basal levels of endogenous FGF21 mRNA by some sevenfold and increased the amplitude of FGF21 mRNA oscillation without affecting its phase. E4BP4 acts as a transcription repressor of FGF21, whose transcription is induced by fasting but repressed by feeding (4, 36) through binding the distal D-box element of FGF21 promoter in fed-mouse liver tissues (90). Insulin effects on FGF21 regulation also appear to be mediated by E4BP4 expression and Akt activation (90). Because E4BP4, a circadian clock mediator, is important for repressing the FGF21 gene during feeding and in response to insulin in mice hepatocytes (90), such findings indicate that FGF21 is another output gene controlled by E4BP4 (Fig. 1).
It has been reported that FGF21 can cross the blood-brain barrier (32) and exist in human cerebrospinal fluid (86). To determine where FGF21 may act centrally, β-Klotho expression in the central nervous system was assessed with laser capture microdissection of divisions of the nervous system and quantitative polymerase chain reaction experiments (7). The results showed that β-klotho was selectively expressed in the suprachiasmatic nucleus (SCN) of hypothalamus, the area postrema and nucleus tractus solitarii of the hindbrain, and the nodose ganglia. On the other hand, the 1c, 2c, and 3c subtypes of the FGF receptor are widely expressed in the nervous system, and FGF21 mRNA was undetectable in the aforementioned β-klotho-expressing regions. FGF21-Tg mice, with plasma FGF21 concentrations in the range of 1,000–2,000 ng/ml (increased to 10–30 ng/ml after ketogenic diet in wild-type mice), showed lower overall activity but more running during the light phase (when mice normally sleep) compared with the FGF21-Tg with β-klotho deletion (7). The β-klotho expression-suppressing experiments indicated that FGF21 effects on insulin levels, growth inhibition, and light-dark cycle activity require FGF21 actions upon the SCN of the hypothalamus and the dorsal vagal complex of the hindbrain (7).
FGF21 changes in diabetes and obesity.
Compared with nondiabetic mice, serum FGF21 levels have been shown to be increased in early-stage diabetes but decreased in late-stage diabetes, indicating that such early-stage increases may be compensatory whereas later decreases may be involved in diabetes-induced tissue damage (106). Regarding human patients, circulating levels of FGF21 have been reported to be elevated in patients diagnosed with obesity (109), type 2 diabetes mellitus (T2DM; 62), coronary heart disease (53), and other metabolic syndromes. Meanwhile, baseline serum FGF21 levels were found to be significantly higher in both obese and T2DM patients, relative to healthy subjects, with FGF21 mRNA expression in visceral fat in obese subjects being double that in healthy group subjects (62). In addition, serum FGF21 levels were found to associate strongly with age, insulin resistance, and adverse lipid profiles (46). Chen et al. also confirmed that fasting FGF21 levels were significantly increased in patients with T2DM compared with healthy controls (10).
The fact that high endogenous FGF21 levels appear to be ineffective whereas high pharmacological doses induce the expected results suggests a state of FGF21 resistance existing in obesity and overt T2DM. The concept of hormone resistance was initially mentioned when insulin resistance was observed in patients with diabetes receiving insulin (55). Succeeding mechanistic research implied that insulin resistance results from downregulation of the insulin receptor (38) and decreased insulin receptor kinase activity (24). What is similar is that obesity was identified as a state of leptin resistance (23) and FGF21 resistance. The mRNA levels of FGF receptor subtype 1c (FGFR1) with the highest binding affinity for FGF21 were reduced in liver, white adipose tissue (19a), and pancreas islets (76) of obese mice. The critical coreceptor β-klotho expression is also greatly reduced in white adipose tissue (30) and islets of obese mice. The downregulation of FGFR1 and β-klotho leads to significant inhibition of FGF21 signaling including decreased phosphorylation of ERK1/2 and the expression of the immediate early genes Egr1 and cFos (19a). Controversially, Markan et al. (58a) found that downregulation of β-klotho expression is not the major reason contributing to alleviate impaired FGF21 signaling in white adipose tissue or increase FGF21 sensitivity in vivo. There may be other mechanisms other than β-klotho expression that are responsible for the development of FGF21 resistance in white adipose tissue of obese mice.
The reason why β-klotho expression is reduced in obese mouse may be PPARγ, the activation of which could result in the downregulation of β-klotho in db/db mice (76). In addition, the expression of PPARγ is decreased in high glucose-treated islets and db/db mouse islets and enhanced by synthetic PPARγ agonists, including troglitazone (Rezulin), rosiglitazone (Avandia), and pioglitazone (Actos), which are approved for use in type 2 diabetic patients (41). Both mRNA and protein expression of β-klotho were increased by PPARγ agonists, and this induction was blocked by PPARγ antagonists in HEK293 cells, suggesting that β-klotho is a target gene of PPARγ (107). Whether PPARγ affects the expression of β-klotho has yet to be determined.
Effects of Exogenous FGF21
Mounting evidence indicates that exogenous administration of FGF21 has beneficial effects, including weight loss and resolution of hepatic TG accumulation, that are essential for a variety of diseases including T2DM, steatohepatitis, and cardiovascular diseases (CVDs). The multitude of beneficial effects of FGF21 that have been observed regarding glucose and lipid homeostasis in experimental animals favors pursuing FGF21 as a potential medicine for metabolic disorders. Because native FGF21 has a brief circulatory half time (0.5–2 h) in vivo, it would be useful to develop longer-acting FGF21 analog molecules. FGF21 analog PF-05231023, with two modified FGF21 molecules linked to a humanized immunoglobulin 1 monoclonal antibody backbone, has been developed (94). Specifically, PF-05231023 appears to exhibit in vivo efficacy comparable to that of native FGF21, including producing improved glucose tolerance in two rodent models of T2DM (34), as well as reducing body weight and improving circulating lipid profiles in obese monkeys and humans (85).
FGF21, unlike other FGF family members, does not require heparin for receptor binding. Instead, FGF21 binding of FGF receptors requires a coreceptor called “β-klotho.” β-Klotho is a single-pass transmembrane protein whose enriched expression in metabolic organs such as the liver, the pancreas, and adipose tissue enables FGF21 to target these tissues selectively. In adult mice, β-klotho expression is seen in adipose and nervous tissue in addition to enterohepatic tissues, including the liver, gall bladder, colon, and pancreas (7, 21). In humans, β-klotho expression is also rich in white adipocytes and liver (3, 33, 50), lower in pancreatic islets (1), and detectable but low in muscle biopsies (59). The β-klotho-expressing tissues are implicated inherently as sites of FGF21 action. FGF21 activates multiple FGF receptors, and it binds FGFR1 with much higher affinity than other FGF receptor subtypes in the presence of β-klotho. Studies show that FGFR1 is expressed in human and mouse liver (96, 97). Xu et al. (100) show that in the liver of C57BL/6 mice, FGF21 administration could stimulate the phosphorylation of ERK1/2, which is a well-known FGF21 signaling pathway, indicative of the direct action of FGF21 on liver. In FGF21 KO mice, hepatic FA activation and β-oxidation were reduced, and levels of free fatty acids (FFAs) were increased; when subcutaneous infusion of FGF21 was given to FGF21 KO mice for 4 wk, methionine-and choline-deficient diet could reduce steatosis and peroxidative damage compared with those mice not receiving FGF21, suggestive of the indirect effect of FGF21 on the liver and hepatocytes (20). Indeed, FGFR4 is the major hepatic FGF receptor isoform and is known for the hepatic effects of FGF15 (74). The interaction of FGF21 with FGFR4-β-klotho is very weak even at high concentration, and thus it could be negligible at physiological concentration (101).
When FGF21 binds with its receptor, the receptor dimerizes and autophosphorylates receptor tyrosine residues, activating FGF receptor substrate 2α (FRS2α). FRS2α transduces FGF21 signals to ERK1/2 and protein kinase B (Akt) signaling pathways, thereby initiating the transcription of immediate early genes (19a). Findings from our laboratory have shown that FGF21 signaling is enhanced by PPARγ activation (76) and inhibits growth hormone (GH) signaling (77) in mice islets. Recent results from our group, which should be further confirmed, also indicate that FGF21 can reduce lipotoxicity-induced lipid accumulation and increase the expression of FA oxidation-related genes (not published). FGF21 has been shown to decrease acetyl-CoA carboxylase protein expression in mouse islets (83); acetyl-CoA carboxylase produces malonyl-CoA, a key substrate for FA biosynthesis. Considering that FGF21 reduces circulating TG levels and can enable resolution of fatty liver, it is of great interest to determine whether FGF21 reduces lipid accumulation within pancreatic islets.
FGF21 and Food Intake and Preference
Carbohydrates, proteins, and fats are the primary macronutrients that provide humans with the bulk of their energy needs. Carbohydrates represent a major source of food for many mammals, though excessive carbohydrate consumption may cause obesity, liver steatosis, and other metabolic diseases. FGF21 (or FGF21 analog) has been found to reduce preferences for sweets and alcohol in mice as well as the preference for sweets in cynomolgus monkeys, and that decrease in preference correlated with reductions in dopamine concentrations in the nucleus accumbens (NAc; 84). Hence FGF21 may affect sweet preference by affecting the dopamine signaling that coordinates reward behavior. Consistent with this possibility, Talukdar et al. found that 2 wk of FGF21 administration significantly decreased dopamine levels in the NAc and changed the expression of dopamine-related genes in the ventral tegmental area and caudate putamen (84). In addition to mice and nonhuman primates, it has recently been reported that circulating FGF21 may regulate sweet intake in adult humans, as indicated by levels of FGF21 after a 12-h fast being 51% higher in sweet-dislikers than in sweet-likers. This research is based on the genotyped variants and their ingestive behaviors of 6,514 participants from the Danish Inter99 cohort. Individuals with particular variants of the FGF21 gene, FGF21 rs838133, were ~20% more likely to prefer sweet snacking and nominally associated with increased alcohol intake and daily smoking (78).
Interestingly, alcoholics show a particularly strong preference for sweet solutions (22), and sweetness cravings appear to share neural receptors and pathways with alcohol dependence (39). It is found that FGF21 may control reward behavior through direct actions on dopaminergic neurons (85). Compared with wild-type mice, FGF21-Tg mice showed a significantly decreased preference for drinking water with sucrose or saccharin. Exogenous FGF21 suppressed the sweet preference in wild-type mice but had no effect in mice lacking β-klotho selectively in the central nervous system. Preferences for FA and bitter tastes were unaffected by these manipulations (85).
There is great interest in clarifying the mechanisms that regulate carbohydrate intake, with the aim of preventing the negative effects of excessive carbohydrate consumption. Sweets are a major source of carbohydrates that can be readily oxidized or stored as energy. FGF21 KO mice eat more sugars, including sucrose, glucose, and fructose, than wild-type mice. Nevertheless, FGF21-Tg and FGF21-treated mice show a selective reduction in sweet food intake (84, 93). The transcription factor carbohydrate response element-binding protein (ChREBP) has been reported to regulate FGF21 levels in vitro in response to high carbohydrate concentrations (35). Experiments with ChREBP KO mice indicate that sugar intake activates FGF21 expression by stimulating ChREBP signaling in the liver. Mice with selective KO of β-klotho in the paraventricular nucleus (PVN) or SCN were generated; the former showed a doubling in preference for a high-sucrose diet compared with control mice, demonstrating that FGF21 may reduce sugar intake via signaling in the PVN of the hypothalamus. These data are consistent with a negative feedback loop within a novel liver-to-brain hormonal axis wherein FGF21 acts in an endocrine manner in the PVN to alter food preferences (Fig. 2). Decreasing carbohydrate intake should help to limit lipid accumulation by decreasing de novo lipogenesis, which may help prevent adverse events associated with overactive lipid oxidation (19), excessive ketosis, or reactive oxygen species production. In this regard, FGF21 analogs represent an interesting possibility as a potential means of treating obesity and T2DM via a qualitatively improved diet.
Fig. 2.
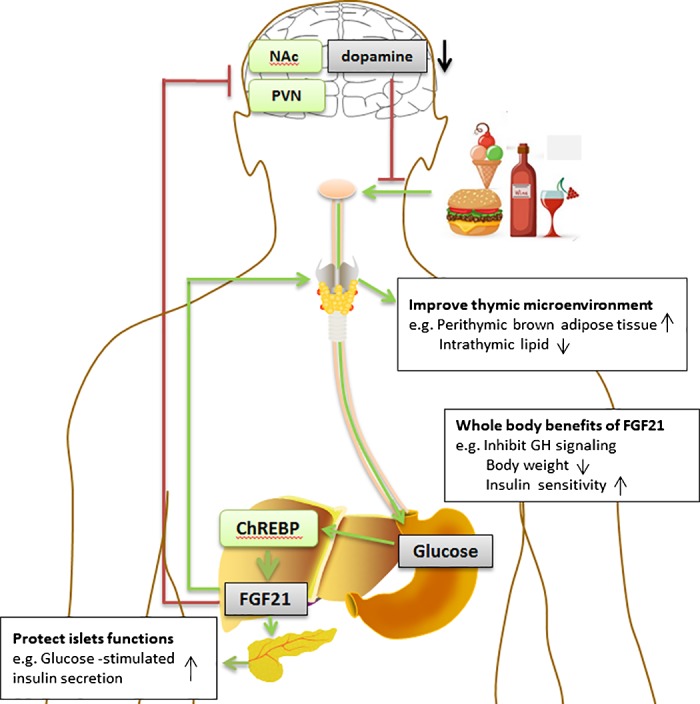
Role of FGF21 in metabolism and food preference. Dietary intake of carbohydrates activates ChREBP in the liver, which leads to the increased expression of FGF21 and its release into circulation. In the paraventricular nucleus of the hypothalamus (PVN), FGF21 acts as an endocrine factor to inhibit the consumption of carbohydrates and reduce levels of the neurotransmitter dopamine in the nucleus accumbens (NAc), forming a potential negative feedback loop and restricting the reward derived from intake of sugars. Meanwhile, FGF21 shows beneficial immunoregulatory effects and regulates peripheral T cell homeostasis by preventing age-related thymic involution. From a whole body viewpoint, FGF21 inhibits GH signaling and reduces body weight to improve longevity and health status.
Metabolic Diseases Associated with Altered FGF21
Serum FGF21 levels in patients with nonalcoholic fatty liver disease (NAFLD) have been shown to be significantly higher than those in control subjects in both Chinese (52) and Caucasian study cohorts (18, 103). Moreover, FGF21 mRNA expression levels correlated with degree of steatosis in human liver tissues and FGF21 protein concentrations in the liver correlated with intrahepatic TG levels (52). Consistent with previous reports, a significant positive association exists between FGF21 levels and several parameters of obesity in patients with NAFLD, including BMI, waist circumference, and body fat percentage. Increased FGF21 expression in NAFLD might be counteracting metabolic stress or simply be a compensatory upregulation response to decreased FGF21 sensitivity. With respect to steatosis monitoring, it is important to note that FGF21 measurement may be helpful for detection of mild steatosis. The diagnostic sensitivity of ultrasonography decreases sharply in cases in which the degree of steatosis is <30% on biopsy (52). FGF21 mRNA expression and protein levels in livers affected by grade 1 steatosis were more than 4-fold and 2.25-fold higher than those in grade 0 samples, respectively (52). Yang et al. showed that compared with levels in wild-type counterparts, FGF21 was increased multifold in the hepatocytes of mice affected by genetically induced hepatocarcinogenesis due to disruptions in genes such as LKB1, p53, MST1/2, SAV1, and PTEN (102). Conversely, FGF21 protein levels were decreased in DEN-OVE26 mice (a type 1 diabetes model chemically induced to develop hepatocellular carcinoma) compared with untreated OVE26 controls, which exhibit cancerous hyperproliferation and aberrant p53 and transforming growth factor-β/Smad signaling during cancer development (108). These apparently paradoxical results may be due to different carcinogenesis induction methods and need further confirmation.
Similarly, serum FGF21 is increased in several CVDs (coronary heart disease, atherosclerosis, myocardial ischemia, and cardiac hypertrophy), so serum FGF21 levels might be regarded as a potential biomarker for CVDs as well as for metabolic disorders. Given that CVDs are attributed to lipotoxicity or a lipid metabolic disorder, it is noteworthy that FGF21 can improve lipid metabolic disorders and insulin resistance (26, 99). Indeed, the mechanisms of FGF21 actions are directly therapeutically relevant to CVDs (11). Administration of exogenous FGF21 can improve lipid profiles dramatically, including decreasing serum LDL-c levels and increasing serum HDL-c levels (44), measures that form a cornerstone in the management of patients with atherosclerotic CVD. High levels of FGF21 are associated with adverse lipid profiles in coronary heart disease (CHD) and coronary artery disease patients (53, 73). Indeed, median circulating FGF21 levels were threefold higher in CHD patients than in control subjects after adjustment for BMI. Serum FGF21 levels in patients with CHD comorbid with diabetes, hypertension, or both were higher than those in patients with CHD without these complications (53). Meanwhile, atherosclerosis is a progressive disease characterized by the accumulation of lipids and white blood cells in the artery wall. Exogenous FGF21 ameliorated dyslipidemia in apoE−/− mice by decreasing the number and area of aortic plaques, while also reducing the number of apoptotic cells and the expression of endoplasmic reticulum stress proteins. Simultaneously, endogenous expression of FGF21 and its receptor were upregulated in the mice (98). In a human study, elevated serum FGF21 levels were found to be associated with increased carotid intima-media thickness (12). Cardiac hypertrophy is characterized by an increase in cardiomyocyte size, enhanced protein synthesis, a greater degree of sarcomere organization, and a shift from FAs to glucose as an energy source (92). Mice lacking FGF21 were found to be more prone to cardiac hypertrophy, and treatment with FGF21 reversed these effects (68). This inhibitory action of FGF21 on cardiac hypertrophy as well as on inflammation was associated with induction of PPARγ coactivator-1α, a repressor of the NF-κB proinflammatory pathway. However, it has also been found that FGF21 is necessary for the cardiac remodeling and physiological cardiac hypertrophy during late pregnancy (69), which suggests the different roles of FGF21 in diverse physiological conditions. Likewise, studies suggested that FGF21 protects from lipid- or diabetes-induced cardiac apoptosis by stimulating the ERK-MAPK-AMPK pathway (106) and preserves the heart from oxidative stress by preventing induction of prooxidative pathways under inflammatory or hypertrophic conditions (67).
FGF21 has also been found to be related to renal dysfunction in end-stage chronic kidney disease (CKD). Clinical studies indicate that plasma FGF21 levels become increased with the development of early- to end-stage CKD and are independently associated with loss of renal function (54). Median circulating FGF21 levels in end-stage CKD patients were 10-fold higher than normal, ~3.5-fold higher than in early-stage CKD, and ~1.5-fold higher than in middle-stage CKD, after adjusting for age, sex, and BMI. Left ventricular hypertrophy is a common manifestation of CVD and an independent risk factor for mortality in patients with CKD. Plasma FGF21 levels were found to be significantly higher in CKD patients with left ventricular hypertrophy vs. in than those without it (54). CKD patients with left ventricular hypertrophy have been shown to have higher plasma FGF21 levels, which may be due to the severe FGF21 resistance caused by complications. There is no evidence indicating that FGF21 can cause damage to the heart; on the contrary, previous studies have shown that FGF21 protects the heart from stress-induced apoptosis and oxidative stress (67, 106).
It is important to identify those diabetic subjects at high risk of progressive diabetic nephropathy as early as possible to prevent grave disease progression. Serum FGF21 increases may also be a useful biomarker for detecting early-stage nephropathy in diabetic patients (49). In a long-term study (median follow-up, 4 yr), serum FGF21 levels were shown to associate independently with estimated glomerular filtration rate, a key parameter of renal function (81). In concordance, FGF21 expression in the mesangial cells of the kidneys in diabetic mice was elevated 20-fold relative to that in control mice (49). Additionally, FGF21 levels have been reported to be increased in patients with clear cell primary renal tumors, with greater serum FGF21 levels being associated with shorter disease-free survival (46).
Elevated serum FGF21 is also receiving attention as a potential prognostic biomarker for polycystic ovary syndrome (27), mitochondrial disease (15), preeclampsia during pregnancy (80), and Cushing syndrome (17). In contrast, FGF21 level decreased in anorexia nervosa patients with prominent reduction in body weight (16). Findings pointing to FGF21 as a possible disease biomarker are summarized in Table 1. Taking all these diseases together, it is interesting to find that FGF21 is not the only factor elevated: serum levels of TG, FA, HDL-c, and LDL-c; BMI; waist circumference; and homeostatic model assessment of insulin resistance (HOMA-IR) are all greatly increased in these diseases. The mentioned indexes are closely related to obesity. Hepatic expression and circulating levels of FGF21 are generally induced by the high concentration of serum FAs. The underlying mechanism is that PPARα agonist and FAs stimulate the activation of PPARα in the liver, the predominant place for FGF21 production, and then FGF21 is induced in large part in a PPARα-dependent manner. Actually, FGF21 could facilitate the removal of the surplus serum lipids which are found within the chylomicron/VLDL fraction under normal physiological conditions (4). Combining FGF21 levels with other lipid profile parameters including BMI, serum TG, and HOMA-IR may represent a more reasonable perspective for the diagnosis of these metabolic diseases. Since an FGF21 assay is simple and noninvasive, it may be a promising test for population-based screening for the aforementioned diseases or for identifying those who are at high risk of developing these diseases.
Table 1.
FGF21 concentrations in different metabolic diseases
| FGF21 Levels, ng/l |
||||
|---|---|---|---|---|
| Pathological Category | Patients | Healthy Controls | P Value | Reference |
| Overweight/obese | 291.8 (144.5–512.0) | 208.7 (94.4–325.7) | <0.001 | (109) |
| Type 2 diabetes mellitus | 395 ± 56 | 213 ± 23 | <0.05 | (62) |
| Nonalcoholic fatty liver disease | 402.38 (242.03, 618.25) | 198.62 pg/ml (134.96, 412.62) | <0.01 | (52) |
| Coronary heart disease | 362.5 (210.6–661.9) | 131.0 (70.4 to 249.5) | <0.001 | (53) |
| Chronic kidney disease | 1,447.9 ± 6,146.5 | 147.36 ± 12.25 | <0.0001 | (54) |
| Clear cell renal cell carcinoma | 219.57 (28.55–2,224.88) | 76.86 (0–425.60) | <0.0001 | (46) |
| Coronary heart disease | 362.5 (210.6–661.9) | 131.0 (70.4–249.5) | <0.001 | (53) |
| Polycystic ovary syndrome | 99.5 (173.7) | 52.0 (88.0) | <0.05 | (27) |
| Preeclampsia | 309.6 | 105.2 | <0.001 | (80) |
| Anorexia nervosa | 112.4 ± 23.44 | 272.3 ± 40.04 | <0.05 | (16) |
Values are means ± SD or medians with interquartile range in parentheses.
FGF21 and Life Span
Human life span has been extended and aging seemingly postponed as human societies have entered civilized times. Nevertheless, aging is still a major factor in metabolic decline and associated diseases. T2DM and CVDs are high-incidence aging-related diseases. Insulin resistance and obesity constitute the major components of metabolic syndrome and are also common in older persons. FGF21 has been shown to have reliable beneficial effects, including weight loss and improved insulin sensitivity, in subjects with obesity or T2DM (5, 8, 99). Beyond metabolic functions, recent studies suggest that FGF21 has life-prolonging effects.
The thymus produces naive T lymphocytes and establishes the initial peripheral T cell pool (31). The thymus regulates the homeostatic balance of the immune system, and thymus involution with aging is characterized by the generation of less effective T cells and reduced emigration of naive T cells to the periphery (57). Thymic decline in the elderly leads to increased risk of infection and cancers (89). Thus protecting thymopoietic activity could potentially improve longevity. Dixit and colleagues showed that FGF21, β-klotho, and FGFR1 mRNA are principally expressed in thymic stromal cells (104). Accordingly, thymic FGF21 declines with age, while β-klotho and FGFR1 expression increase (19a). Gain-of-function experiments demonstrated that FGF21-Tg mice have increased brown adipose tissue adjacent to the thymus, reminiscent of FGF21-induced thermogenic gene expression in adipose tissue and browning of white adipose tissue to adapt to chronic cold exposure in mice (20a). Meanwhile, FGF21-Tg mice also have reduced macrophages with large crystals and age-related increases in thymic fibroblasts and inflammation. All of these effects contribute to improving the thymic microenvironment during aging by lowering lipid-derived damage-associated elements and preserving thymic epithelial cell function. Conversely, global FGF21-deficient mice show accelerated thymic involution and loss of naive T cells. The mortality of FGF21-deficient mice undergoing irradiation and hematopoietic stem cell transplantation was also increased compared with wild-type mice because of their reduced thymic reconstitution. Collectively, this research demonstrates a direct action of FGF21 on the thymus and suggests that FGF21 acts as a prothymic molecule critical for immune-metabolic interactions that maintain homeostasis and protect against tissue damage (104). Additionally, Nakayama et al. suggested the role of FGF21 as one of the intrathymic cytokines in the juvenile stage of mice, indispensable for thymocyte development in a β-klotho-independent manner (64).
FGF21 has been shown to inhibit skeletal growth and reduce GH insensitivity during prolonged undernutrition (47). Many anabolic actions of GH are mediated by insulin-like growth factor 1 (IGF-1). GH induces IGF-1 transcription through a complex cascade that is initiated when GH binds its cell surface receptor. Levels of GH decline with age, and this decline is commonly accompanied by a concomitant decline in IGF-1. Animal models of congenital GH deficiency show greatly increased life span, and humans with congenital GH deficiency may have decreased rates of age-related diseases such as diabetes and cancer (70). Thus it is assumed that inhibition of GH action might delay senescence. Attenuated insulin/IGF-1 signaling has been reported to extend life span in rodents (72, 88) and humans (82). An FGF21-Tg mouse line has been made wherein FGF21 is selectively expressed in hepatocytes under the control of the apoE promoter, resulting in circulating FGF21 levels 5–10 times higher than those under fasting (37). After a 4-h fast, plasma glucose and IGF-1 levels in advanced-age fasting-induced FGF21-Tg mice were lower than those in their wild-type littermates. Interestingly, longevity was extended in these FGF21-Tg mice, whose median survival time was 36% longer than that of their wild-type littermates.
The results of a microarray study of RNA samples from three tissues of mice subjected to either caloric restriction or a 24-h fast suggested that FGF21 may extend life span by regulating a small subset of genes, namely, Fom3, Igfals, and so on, which are also regulated by caloric restriction in liver. FGF21 has also been reported to modulate the AMPK-sirtuin-mechanistic target of rapamycin (mTOR) pathway (9); increases in AMPK and sirtuin activity and decreases in mTOR activity have been reported to be associated with increased longevity (6, 40). These results suggest that FGF21 may extend life span by suppressing the GH/IGF-1 signaling axis in the liver in a manner similar to that of caloric restriction (110; Fig. 3). The aforementioned fast-induced transgenic mice were found to have increased GH concentrations but reduced IGF-1 transcription (by ~30%) in the liver, the main site of IGF-1 generation. At the same time, FGF21 induced hepatic expression of IGF-1 binding protein-1 and suppressor of cytokine signaling 2, which were negative regulators of GH signaling (29, 75). Collectively, the evidence suggests that FGF21 causes GH resistance by reducing concentrations of active forms of GH mediators, including Janus kinase 2 and signal transducer and activator of transcription 5, which inhibit GH signaling (37).
Fig. 3.
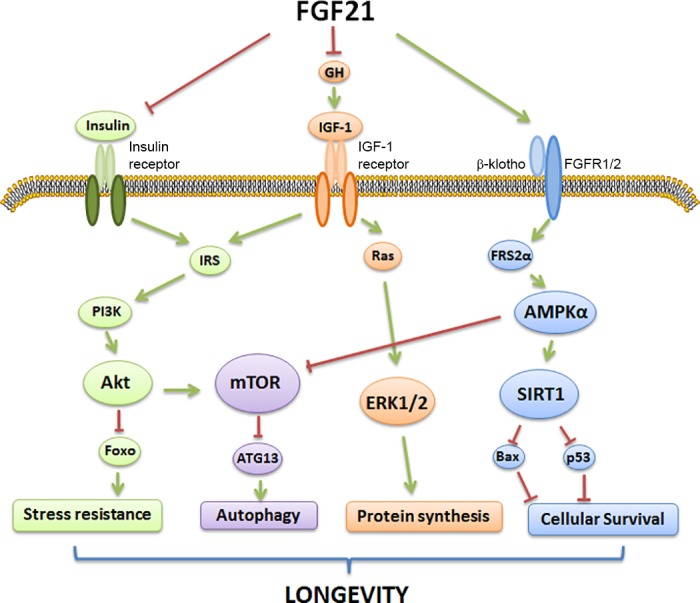
Metabolic pathways changed by FGF21 to regulate mammalian longevity. FGF21 exerts many beneficial metabolic effects including extended life span in mammals. Because of the action of FGF21, numerous downstream cellular pathways are activated, including sirtuin 1 (SIRT1) activation (blue), phosphatidylinositol 3-kinase (PI3K)/Akt signaling (green), AMPK/mTOR signaling (purple), and ERK1/2 signaling (orange). Stimulation of the above pathways by FGF21 is believed to improve cellular longevity through activation of autophagy, stress resistance, and survival signals while attenuating cellular growth and protein synthesis. Arrows indicate a stimulatory effect, whereas blunt-ended lines indicate an inhibitory effect. IRS, insulin receptor substrate; ATG13, autophagy-related protein-13; Foxo, forkhead box protein; FGFR1/2, fibroblast growth factor receptor 1/2.
Prospects for Future Studies
Although our understanding of FGF21 as a major metabolic regulator has been accelerating in the past decade, some aspects of FGF21 biology remain controversial and need to be prioritized. FGF21 signaling is not limited to a single tissue or physiological process. A comprehensive network of metabolic elements converges on FGF21 and regulates its expression in response to various physiological and pathological stimuli. In fact, FGF21 can have opposing physiological effects under different circumstances, such as inhibition of lipolysis in adipose tissue during fasting and promotion of FA oxidation in the liver during energy surplus conditions. Despite the paradoxical roles of FGF21 in metabolism, it is gaining interest as a potential biomarker for many diseases and as a potential medicine for treating T2DM, obesity, and related syndromes. This work provides insights into the physiological and pathophysiological roles of FGF21 based on recent findings. More research is required to clarify FGF21 actions in different tissues and under different environmental stress conditions. Long-term, FGF21-related strategies may be developed to delay the progression of age-related diseases and improve quality of life.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.X. and P.S.L. prepared figures; T.X. drafted manuscript; T.X. and P.S.L. edited and revised manuscript; T.X. and P.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
This work was supported by the General Research Fund of the Research Grants Council of the Hong Kong Special Administrative Region, China (CUHK 14107415, awarded to P. S. Leung).
REFERENCES
- 1.Abramovitz L, Rubinek T, Ligumsky H, Bose S, Barshack I, Avivi C, Kaufman B, Wolf I. KL1 internal repeat mediates klotho tumor suppressor activities and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin Cancer Res 17: 4254–4266, 2011. doi: 10.1158/1078-0432.CCR-10-2749. [DOI] [PubMed] [Google Scholar]
- 2.Andersen B, Beck-Nielsen H, Højlund K. Plasma FGF21 displays a circadian rhythm during a 72-h fast in healthy female volunteers. Clin Endocrinol (Oxf) 75: 514–519, 2011. doi: 10.1111/j.1365-2265.2011.04084.x. [DOI] [PubMed] [Google Scholar]
- 3.Arner P, Pettersson A, Mitchell PJ, Dunbar JD, Kharitonenkov A, Rydén M. FGF21 attenuates lipolysis in human adipocytes: a possible link to improved insulin sensitivity. FEBS Lett 582: 1725–1730, 2008. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 4.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150: 4084–4093, 2009. doi: 10.1210/en.2009-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet 8: 835–844, 2007. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 7.Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19: 1147–1152, 2013. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camporez JP, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, Shulman GI. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology 154: 3099–3109, 2013. doi: 10.1210/en.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc Natl Acad Sci USA 107: 12553–12558, 2010. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WW, Li L, Yang GY, Li K, Qi XY, Zhu W, Tang Y, Liu H, Boden G. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with Type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 116: 65–68, 2008. doi: 10.1055/s-2007-985148. [DOI] [PubMed] [Google Scholar]
- 11.Cheng P, Zhang F, Yu L, Lin X, He L, Li X, Lu X, Yan X, Tan Y, Zhang C. Physiological and pharmacological roles of FGF21 in cardiovascular diseases. J Diabetes Res 2016: 1540267, 2016. doi: 10.1155/2016/1540267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow WS, Xu A, Woo YC, Tso AW, Cheung SC, Fong CH, Tse HF, Chau MT, Cheung BM, Lam KS. Serum fibroblast growth factor-21 levels are associated with carotid atherosclerosis independent of established cardiovascular risk factors. Arterioscler Thromb Vasc Biol 33: 2454–2459, 2013. doi: 10.1161/ATVBAHA.113.301599. [DOI] [PubMed] [Google Scholar]
- 13.Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Qi Q, Curhan GC, Rimm EB, Hunter DJ, Pasquale LR, Ridker PM, Hu FB, Chasman DI, Qi L; CHARGE Nutrition Working Group; DietGen Consortium . Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet 22: 1895–1902, 2013. doi: 10.1093/hmg/ddt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis RL, Liang C, Edema-Hildebrand F, Riley C, Needham M, Sue CM. Fibroblast growth factor 21 is a sensitive biomarker of mitochondrial disease. Neurology 81: 1819–1826, 2013. doi: 10.1212/01.wnl.0000436068.43384.ef. [DOI] [PubMed] [Google Scholar]
- 16.Dostálová I, Kaválková P, Haluzíková D, Lacinová Z, Mráz M, Papezová H, Haluzík M. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab 93: 3627–3632, 2008. doi: 10.1210/jc.2008-0746. [DOI] [PubMed] [Google Scholar]
- 17.Durovcová V, Marek J, Hána V, Matoulek M, Zikán V, Haluzíková D, Kaválková P, Lacinová Z, Krsek M, Haluzík M. Plasma concentrations of fibroblast growth factors 21 and 19 in patients with Cushing’s syndrome. Physiol Res 59: 415–422, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 139: 456–463, 2010. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr 57, Suppl: 779S–785S, 1993. [DOI] [PubMed] [Google Scholar]
- 19a.Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59: 2781–2789, 2010. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher FM, Chui PC, Nasser IA, Popov Y, Cunniff JC, Lundasen T, Kharitonenkov A, Schuppan D, Flier JS, Maratos-Flier E. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology 147: 1073–1083, 2014. doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281, 2012. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 24: 2050–2064, 2010. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortuna JL. Sweet preference, sugar addiction and the familial history of alcohol dependence: shared neural pathways and genes. J Psychoactive Drugs 42: 147–151, 2010. doi: 10.1080/02791072.2010.10400687. [DOI] [PubMed] [Google Scholar]
- 23.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1: 1311–1314, 1995. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 24.Freidenberg GR, Henry RR, Klein HH, Reichart DR, Olefsky JM. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest 79: 240–250, 1987. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gälman C, Lundåsen T, Kharitonenkov A, Bina HA, Eriksson M, Hafström I, Dahlin M, Åmark P, Angelin B, Rudling M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 8: 169–174, 2008. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Gimeno RE, Moller DE. FGF21-based pharmacotherapy: potential utility for metabolic disorders. Trends Endocrinol Metab 25: 303–311, 2014. doi: 10.1016/j.tem.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Gorar S, Culha C, Uc ZA, Dellal FD, Serter R, Aral S, Aral Y. Serum fibroblast growth factor 21 levels in polycystic ovary syndrome. Gynecol Endocrinol 26: 819–826, 2010. doi: 10.3109/09513590.2010.487587. [DOI] [PubMed] [Google Scholar]
- 28.Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature 249: 123–127, 1974. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- 29.Greenhalgh CJ, Bertolino P, Asa SL, Metcalf D, Corbin JE, Adams TE, Davey HW, Nicola NA, Hilton DJ, Alexander WS. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b). Mol Endocrinol 16: 1394–1406, 2002. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- 30.Hale C, Chen MM, Stanislaus S, Chinookoswong N, Hager T, Wang M, Véniant MM, Xu J. Lack of overt FGF21 resistance in two mouse models of obesity and insulin resistance. Endocrinology 153: 69–80, 2012. doi: 10.1210/en.2010-1262. [DOI] [PubMed] [Google Scholar]
- 31.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol 18: 529–560, 2000. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 32.Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide FGF21 can enter brain from blood. Peptides 28: 2382–2386, 2007. doi: 10.1016/j.peptides.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 75: 503–533, 2013. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Ishino T, Chen G, Rolzin P, Osothprarop TF, Retting K, Li L, Jin P, Matin MJ, Huyghe B, Talukdar S, Bradshaw CW, Palanki M, Violand BN, Woodnutt G, Lappe RW, Ogilvie K, Levin N. Development of a novel long-acting antidiabetic FGF21 mimetic by targeted conjugation to a scaffold antibody. J Pharmacol Exp Ther 346: 270–280, 2013. doi: 10.1124/jpet.113.204420. [DOI] [PubMed] [Google Scholar]
- 35.Iizuka K, Takeda J, Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett 583: 2882–2886, 2009. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 36.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5: 415–425, 2007. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8: 77–83, 2008. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kahn CR, Neville DM Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem 248: 244–250, 1973. [PubMed] [Google Scholar]
- 39.Kampov-Polevoy A, Garbutt JC, Janowsky D. Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry 154: 269–270, 1997. doi: 10.1176/ajp.154.2.269. [DOI] [PubMed] [Google Scholar]
- 40.Kenyon CJ. The genetics of ageing. Nature 464: 504–512, 2010. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 41.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature 405: 421–424, 2000. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 42.Kharitonenkov A, Shanafelt AB. FGF21: a novel prospect for the treatment of metabolic diseases. Curr Opin Investig Drugs 10: 359–364, 2009. [PubMed] [Google Scholar]
- 43.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest 115: 1627–1635, 2005. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148: 774–781, 2007. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- 46.Knott ME, Minatta JN, Roulet L, Gueglio G, Pasik L, Ranuncolo SM, Nuñez M, Puricelli L, De Lorenzo MS. Circulating fibroblast growth factor 21 (Fgf21) as diagnostic and prognostic biomarker in renal cancer. J Mol Biomark Diagn 7, Suppl 2: 15, 2016. doi: 10.4172/2155-9929.S2-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubicky RA, Wu S, Kharitonenkov A, De Luca F. Role of fibroblast growth factor 21 (FGF21) in undernutrition-related attenuation of growth in mice. Endocrinology 153: 2287–2295, 2012. doi: 10.1210/en.2011-1909. [DOI] [PubMed] [Google Scholar]
- 48.Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Münzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest 124: 3913–3922, 2014. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee CH, Hui EY, Woo YC, Yeung CY, Chow WS, Yuen MM, Fong CH, Xu A, Lam KS. Circulating fibroblast growth factor 21 levels predict progressive kidney disease in subjects with type 2 diabetes and normoalbuminuria. J Clin Endocrinol Metab 100: 1368–1375, 2015. doi: 10.1210/jc.2014-3465. [DOI] [PubMed] [Google Scholar]
- 50.Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes 38: 170–176, 2014. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemberger T, Saladin R, Vázquez M, Assimacopoulos F, Staels B, Desvergne B, Wahli W, Auwerx J. Expression of the peroxisome proliferator-activated receptor alpha gene is stimulated by stress and follows a diurnal rhythm. J Biol Chem 271: 1764–1769, 1996. doi: 10.1074/jbc.271.3.1764. [DOI] [PubMed] [Google Scholar]
- 52.Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K, Xu A, Jia W. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol 53: 934–940, 2010. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S, Xiao J, Wang X, Feng W, Li X. Serum levels of FGF-21 are increased in coronary heart disease patients and are independently associated with adverse lipid profile. PLoS One 5: e15534, 2010. doi: 10.1371/journal.pone.0015534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin Z, Zhou Z, Liu Y, Gong Q, Yan X, Xiao J, Wang X, Lin S, Feng W, Li X. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One 6: e18398, 2011. doi: 10.1371/journal.pone.0018398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowell FC. Immunologic studies in insulin resistance I. Report of a case exhibiting variations in resistance and allergy to insulin. J Clin Invest 23: 225–231, 1944. doi: 10.1172/JCI101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lundåsen T, Gälman C, Angelin B, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med 260: 530–536, 2006. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 57.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol 30: 366–373, 2009. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63: 4057–4063, 2014. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58a.Markan KR, Naber MC, Small SM, Peltekian L, Kessler RL, Potthoff MJ. FGF21 resistance is not mediated by downregulation of beta-klotho expression in white adipose tissue. Mol Metab 6: 602–610, 2017. doi: 10.1016/j.molmet.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, Chibalin AV, Moller DE, Kharitonenkov A, Krook A. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev 27: 286–297, 2011. doi: 10.1002/dmrr.1177. [DOI] [PubMed] [Google Scholar]
- 60.Mitsui S, Yamaguchi S, Matsuo T, Ishida Y, Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev 15: 995–1006, 2001. doi: 10.1101/gad.873501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mottillo EP, Desjardins EM, Fritzen AM, Zou VZ, Crane JD, Yabut JM, Kiens B, Erion DM, Lanba A, Granneman JG, Talukdar S, Steinberg GR. FGF21 does not require adipocyte AMP-activated protein kinase (AMPK) or the phosphorylation of acetyl-CoA carboxylase (ACC) to mediate improvements in whole-body glucose homeostasis. Mol Metab 6: 471–481, 2017. doi: 10.1016/j.molmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mraz M, Bartlova M, Lacinova Z, Michalsky D, Kasalicky M, Haluzikova D, Matoulek M, Dostalova I, Humenanska V, Haluzik M. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 71: 369–375, 2009. doi: 10.1111/j.1365-2265.2008.03502.x. [DOI] [PubMed] [Google Scholar]
- 64.Nakayama Y, Masuda Y, Ohta H, Tanaka T, Washida M, Nabeshima YI, Miyake A, Itoh N, Konishi M. Fgf21 regulates T-cell development in the neonatal and juvenile thymus. Sci Rep 7: 330, 2017. doi: 10.1038/s41598-017-00349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oishi K, Uchida D, Ishida N. Circadian expression of FGF21 is induced by PPARalpha activation in the mouse liver. FEBS Lett 582: 3639–3642, 2008. doi: 10.1016/j.febslet.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 66.Patel DD, Knight BL, Wiggins D, Humphreys SM, Gibbons GF. Disturbances in the normal regulation of SREBP-sensitive genes in PPAR α-deficient mice. J Lipid Res 42: 328–337, 2001. [PubMed] [Google Scholar]
- 67.Planavila A, Redondo-Angulo I, Ribas F, Garrabou G, Casademont J, Giralt M, Villarroya F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res 106: 19–31, 2015. doi: 10.1093/cvr/cvu263. [DOI] [PubMed] [Google Scholar]
- 68.Planavila A, Redondo I, Hondares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, van Bilsen M, Villarroya F. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun 4: 2019, 2013. doi: 10.1038/ncomms3019. [DOI] [PubMed] [Google Scholar]
- 69.Redondo-Angulo I, Mas-Stachurska A, Sitges M, Tinahones FJ, Giralt M, Villarroya F, Planavila A. Fgf21 is required for cardiac remodeling in pregnancy. Cardiovasc Res, 2017. doi: 10.1093/cvr/cvx088. [DOI] [PubMed] [Google Scholar]
- 70.Rincon M, Muzumdar R, Atzmon G, Barzilai N. The paradox of the insulin/IGF-1 signaling pathway in longevity. Mech Ageing Dev 125: 397–403, 2004. doi: 10.1016/j.mad.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 71.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet 38: 369–374, 2006. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 72.Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol 8: 681–691, 2007. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 73.Shen Y, Ma X, Zhou J, Pan X, Hao Y, Zhou M, Lu Z, Gao M, Bao Y, Jia W. Additive relationship between serum fibroblast growth factor 21 level and coronary artery disease. Cardiovasc Diabetol 12: 124, 2013. doi: 10.1186/1475-2840-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shin DJ, Osborne TF. FGF15/FGFR4 integrates growth factor signaling with hepatic bile acid metabolism and insulin action. J Biol Chem 284: 11110–11120, 2009. doi: 10.1074/jbc.M808747200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silha JV, Murphy LJ. Insights from insulin-like growth factor binding protein transgenic mice. Endocrinology 143: 3711–3714, 2002. doi: 10.1210/en.2002-220116. [DOI] [PubMed] [Google Scholar]
- 76.So WY, Cheng Q, Chen L, Evans-Molina C, Xu A, Lam KS, Leung PS. High glucose represses β-klotho expression and impairs fibroblast growth factor 21 action in mouse pancreatic islets: involvement of peroxisome proliferator-activated receptor γ signaling. Diabetes 62: 3751–3759, 2013. doi: 10.2337/db13-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.So WY, Cheng Q, Xu A, Lam KS, Leung PS. Loss of fibroblast growth factor 21 action induces insulin resistance, pancreatic islet hyperplasia and dysfunction in mice. Cell Death Dis 6: e1707, 2015. doi: 10.1038/cddis.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Søberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, von Holstein-Rathlou S, Grevengoed TJ, Christensen KB, Bredie WL, Potthoff MJ, Solomon TP, Scheele C, Linneberg A, Jørgensen T, Pedersen O, Hansen T, Gillum MP, Grarup N. FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab 25: 1045–1053.e6, 2017. doi: 10.1016/j.cmet.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Solon-Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, McMahon AC, Warren A, Durrant-Whyte J, Walters KA, Krycer JR, Ponton F, Gokarn R, Wali JA, Ruohonen K, Conigrave AD, James DE, Raubenheimer D, Morrison CD, Le Couteur DG, Simpson SJ. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab 24: 555–565, 2016. doi: 10.1016/j.cmet.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Stepan H, Kley K, Hindricks J, Kralisch S, Jank A, Schaarschmidt W, Schrey S, Ebert T, Lössner U, Kratzsch J, Blüher M, Stumvoll M, Richter J, Fasshauer M. Serum levels of the adipokine fibroblast growth factor-21 are increased in preeclampsia. Cytokine 62: 322–326, 2013. doi: 10.1016/j.cyto.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 81.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 82.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA 105: 3438–3442, 2008. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun MY. The Role of FGF21 in Pancreatic Islet Metabolism (MS thesis). Toronto: Univ. of Toronto, 2011. [Google Scholar]
- 84.Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, Bernardo B, Müller CP, Tang H, Mangelsdorf DJ, Goodwin B, Kliewer SA. FGF21 regulates sweet and alcohol preference. Cell Metab 23: 344–349, 2016. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM, Brenner MB, Trimmer JK, Gropp KE, Chabot JR, Erion DM, Rolph TP, Goodwin B, Calle RA. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab 23: 427–440, 2016. doi: 10.1016/j.cmet.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Tan BK, Hallschmid M, Adya R, Kern W, Lehnert H, Randeva HS. Fibroblast growth factor 21 (FGF21) in human cerebrospinal fluid: relationship with plasma FGF21 and body adiposity. Diabetes 60: 2758–2762, 2011. doi: 10.2337/db11-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, Mikkilä V, Renstrom F, Sonestedt E, Zhao JH, Chu AY, Qi L, Chasman DI, de Oliveira Otto MC, Dhurandhar EJ, Feitosa MF, Johansson I, Khaw KT, Lohman KK, Manichaikul A, McKeown NM, Mozaffarian D, Singleton A, Stirrups K, Viikari J, Ye Z, Bandinelli S, Barroso I, Deloukas P, Forouhi NG, Hofman A, Liu Y, Lyytikäinen LP, North KE, Dimitriou M, Hallmans G, Kähönen M, Langenberg C, Ordovas JM, Uitterlinden AG, Hu FB, Kalafati IP, Raitakari O, Franco OH, Johnson A, Emilsson V, Schrack JA, Semba RD, Siscovick DS, Arnett DK, Borecki IB, Franks PW, Kritchevsky SB, Lehtimäki T, Loos RJ, Orho-Melander M, Rotter JI, Wareham NJ, Witteman JC, Ferrucci L, Dedoussis G, Cupples LA, Nettleton JA. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr 97: 1395–1402, 2013. doi: 10.3945/ajcn.112.052183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science 299: 1346–1351, 2003. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 89.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev 205: 72–93, 2005. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 90.Tong X, Muchnik M, Chen Z, Patel M, Wu N, Joshi S, Rui L, Lazar MA, Yin L. Transcriptional repressor E4-binding protein 4 (E4BP4) regulates metabolic hormone fibroblast growth factor 21 (FGF21) during circadian cycles and feeding. J Biol Chem 285: 36401–36409, 2010. doi: 10.1074/jbc.M110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uebanso T, Taketani Y, Yamamoto H, Amo K, Ominami H, Arai H, Takei Y, Masuda M, Tanimura A, Harada N, Yamanaka-Okumura H, Takeda E. Paradoxical regulation of human FGF21 by both fasting and feeding signals: is FGF21 a nutritional adaptation factor? PLoS One 6: e22976, 2011. doi: 10.1371/journal.pone.0022976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Bilsen M, van der Vusse GJ, Reneman RS. Transcriptional regulation of metabolic processes: implications for cardiac metabolism. Pflugers Arch 437: 2–14, 1998. doi: 10.1007/s004240050739. [DOI] [PubMed] [Google Scholar]
- 93.von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, Potthoff MJ. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab 23: 335–343, 2016. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weng Y, Chabot JR, Bernardo B, Yan Q, Zhu Y, Brenner MB, Vage C, Logan A, Calle R, Talukdar S. Pharmacokinetics (PK), pharmacodynamics (PD) and integrated PK/PD modeling of a novel long acting FGF21 clinical candidate PF-05231023 in diet-induced obese and leptin-deficient obese mice. PLoS One 10: e0119104, 2015. doi: 10.1371/journal.pone.0119104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55: 2470–2478, 2006. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 96.Woolsey SJ, Beaton MD, Mansell SE, Leon-Ponte M, Yu J, Pin CL, Adams PC, Kim RB, Tirona RG. A fibroblast growth factor 21-pregnane X receptor pathway downregulates hepatic CYP3A4 in nonalcoholic fatty liver disease. Mol Pharmacol 90: 437–446, 2016. doi: 10.1124/mol.116.104687. [DOI] [PubMed] [Google Scholar]
- 97.Wu S, Levenson A, Kharitonenkov A, De Luca F. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. J Biol Chem 287: 26060–26067, 2012. doi: 10.1074/jbc.M112.343707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu X, Qi YF, Chang JR, Lu WW, Zhang JS, Wang SP, Cheng SJ, Zhang M, Fan Q, Lv Y, Zhu H, Xin MK, Lv Y, Liu JH. Possible role of fibroblast growth factor 21 on atherosclerosis via amelioration of endoplasmic reticulum stress-mediated apoptosis in apoE(-/-) mice. Heart Vessels 30: 657–668, 2015. doi: 10.1007/s00380-014-0557-9. [DOI] [PubMed] [Google Scholar]
- 99.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, Busby J, Hecht R, Li YS, Li Y, Lindberg R, Véniant MM. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models: association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab 297: E1105–E1114, 2009. doi: 10.1152/ajpendo.00348.2009. [DOI] [PubMed] [Google Scholar]
- 101.Yang C, Jin C, Li X, Wang F, McKeehan WL, Luo Y. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One 7: e33870, 2012. doi: 10.1371/journal.pone.0033870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang C, Lu W, Lin T, You P, Ye M, Huang Y, Jiang X, Wang C, Wang F, Lee MH, Yeung SC, Johnson RL, Wei C, Tsai RY, Frazier ML, McKeehan WL, Luo Y. Activation of liver FGF21 in hepatocarcinogenesis and during hepatic stress. BMC Gastroenterol 13: 67, 2013. doi: 10.1186/1471-230X-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yilmaz Y, Eren F, Yonal O, Kurt R, Aktas B, Celikel CA, Ozdogan O, Imeryuz N, Kalayci C, Avsar E. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest 40: 887–892, 2010. doi: 10.1111/j.1365-2362.2010.02338.x. [DOI] [PubMed] [Google Scholar]
- 104.Youm Y-H, Horvath TL, Mangelsdorf DJ, Kliewer SA, Dixit VD. Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proc Natl Acad Sci USA 113: 1026–1031, 2016. doi: 10.1073/pnas.1514511113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu H, Xia F, Lam KS, Wang Y, Bao Y, Zhang J, Gu Y, Zhou P, Lu J, Jia W, Xu A. Circadian rhythm of circulating fibroblast growth factor 21 is related to diurnal changes in fatty acids in humans. Clin Chem 57: 691–700, 2011. doi: 10.1373/clinchem.2010.155184. [DOI] [PubMed] [Google Scholar]
- 106.Zhang C, Huang Z, Gu J, Yan X, Lu X, Zhou S, Wang S, Shao M, Zhang F, Cheng P, Feng W, Tan Y, Li X. Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia 58: 1937–1948, 2015. doi: 10.1007/s00125-015-3630-8. [DOI] [PubMed] [Google Scholar]
- 107.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N. Klotho is a target gene of PPAR-γ. Kidney Int 74: 732–739, 2008. doi: 10.1038/ki.2008.244. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Q, Li Y, Liang T, Lu X, Liu X, Zhang C, Jiang X, Martin RC, Cheng M, Cai L. Loss of FGF21 in diabetic mouse during hepatocellular carcinogenetic transformation. Am J Cancer Res 5: 1762–1774, 2015. [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, Wong RL, Chow WS, Tso AW, Lam KS, Xu A. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 57: 1246–1253, 2008. doi: 10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, Yu RT, Evans RM, Kliewer SA, Mangelsdorf DJ. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1: e00065, 2012. doi: 10.7554/eLife.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]


