Abstract
Interleukin-22 (IL-22) is a Th17 cell hepatoprotective cytokine that is undergoing clinical trials to treat patients with alcoholic hepatitis (AH). Lipopolysaccharide (LPS) activation of macrophage is implicated in hepatocyte cell death and pathogenesis of AH. The role of IL-22 production from macrophage, its regulation by LPS, and effects on alcohol-induced hepatocyte cell death are unexplored and were examined in this study. Low levels of IL-22 mRNA/protein were detected in macrophage but were significantly upregulated by 6.5-fold in response to the tissue reparative cytokine IL-10. Conversely, LPS significantly decreased IL-22 mRNA levels in a temporal and concentration-dependent manner with a maximum reduction of 5-fold. LPS downregulation of IL-22 mRNA levels was rescued in the presence of a pharmacological inhibitor of c-Jun NH2-terminal kinase (JNK) and by JNK knockdown. Next, we explored whether macrophage-derived IL-22 regulated ethanol-induced hepatocyte death. Conditioned media from IL-10-stimulated macrophages attenuated ethanol-induced hepatocyte caspase-3/7 activity, and apoptosis as assessed by fluorometric assay and TdT-mediated dUTP nick-end labeling (TUNEL) staining, respectively. This effect was diminished in conditioned media from macrophages with IL-22 knockdown. Cytokine analysis in sera samples of patients with AH revealed that IL-22 levels were significantly elevated compared with healthy controls and heavy-drinking controls, implying a state of IL-22 resistance in human AH. Macrophage-derived IL-22 protects hepatocytes from ethanol-induced cell death. IL-22 downregulation is a new regulatory target of LPS in the pathogenesis of AH.
Keywords: interleukin-22, macrophage, alcohol, hepatotoxicity, liver, ethanol, immune cells, caspase, apoptosis, TUNEL, Th17, inflammation, alcoholic hepatitis, ASH, AH, JNK, MAPK, SP600125, U0126
alcoholic liver disease is a major cause of morbidity and mortality (7, 22, 27, 31). Gut-derived lipopolysaccharide (LPS) is strongly implicated in this process through stimulation of inflammatory cytokines from macrophages. Recent studies indicate that the Th17 cell-derived cytokine IL-22 plays a protective role in alcohol-induced liver damage by stimulating hepatocyte regeneration, although precise mechanisms by which this occurs remain incompletely defined (16). Given the critical role of macrophages in alcoholic hepatitis (AH) and the putative role of LPS as a mediator of macrophage activation (13, 27, 29), we tested the hypothesis that IL-22 may be produced by macrophages and downregulated by LPS.
IL-22 is a member of the IL-10 family of cytokines and is produced most notably by Th17 cells (5). However, a variety of immune cells have been reported to produce IL-22, including CD4+ T cells (Th1, Th17, and Th22), CD8+ T cells, and even noninnate immune cells such as NKT cells and innate lymphoid cells (5, 34). It was recently described that specific myeloid cells may also produce IL-22 in certain circumstances (5, 11, 34). For example, macrophage-derived IL-22 was produced from human and mouse lung in response to lung injury (9). In addition to their role in tissue inflammation, macrophages are also important for epithelial tissue repair and regeneration. Given the role of IL-22 in epithelial cell repair, we explored the potential role of macrophage-derived IL-22 in alcohol-induced hepatocyte injury in this study.
Based on the premise outlined above, we examine IL-22 production from macrophages in response to LPS and IL-10. We show that regenerative cytokines such as IL-10 can stimulate macrophage-derived IL-22 production. Conversely, LPS downregulates macrophage-derived IL-22 production. The inhibitory effect of LPS requires activation of the multifunctional kinase, c-Jun NH2-terminal kinase (JNK). Furthermore, macrophage-derived IL-22 protects hepatocytes from alcohol-induced cell death. Finally and unexpectedly, we demonstrate that sera IL-22 levels in patients with AH are elevated, suggesting that AH involves a state of IL-22 resistance. Our findings provide new insights into IL-22 function with implications for pathogenesis and treatment of AH.
MATERIALS AND METHODS
Isolation of primary cells.
Lavage fluid from mouse peritoneal cavity was collected and centrifuged at 1,400 rpm for 10 min. Cellular sediments were mixed and then seeded onto 12-well plates in basal DMEM/F-12 (Life Technologies, Grand Island, NY) medium for 4 h to allow the macrophages to adhere to the plates, then refreshed with media with 10% fetal bovine serum (FBS; GIBCO, Grand Island, NY) medium, and cultured overnight. The isolated macrophages were then used for experiments. Kupffer cells were isolated and cultured using the methods described earlier (29).
The use and care of the animals for these experiments were reviewed and approved by the Institutional Animal Care and Use Committee at the Mayo Clinic. Hepatocytes were isolated from wild-type C57BL/6 mice as described previously (19). Briefly, a two-step collagenase perfusion technique was performed. After in situ perfusion of the liver with a calcium-free medium, followed by collagenase D (Roche Diagnostics, Indianapolis, IN), dispersed cell suspensions were purified by Percoll solution (Sigma-Aldrich, St. Louis, MO). The hepatocytes were cultured in DMEM containing 10% FBS and 1% penicillin-streptomycin (GIBCO).
Naïve T cells were isolated from wild-type C57BL/6 mice using EasySep Mouse Naïve CD4+ T Cell Isolation Kit (STEMCELL Technologies, Cambridge, MA). A 96-well plate was precoated with 2.5 µg/ml anti-CD3ε (BioLegend, San Diego, CA) overnight, and then naïve T cells were seeded and cultured with cytokines for 5 days, including 10 µg/ml anti-IL-4 (BioLegend), 25 ng/ml IL-6 (BioLegend), 25 ng/ml IL-23 (PeproTech, Rocky Hill, NJ), and 10 ng/ml transforming growth factor-β (BioLegend) to differentiate naïve T cells into Th17 cells (1, 20).
Cell culture and treatment.
RAW 264.7 [American Type Culture Collection (ATCC), Manassas, VA] macrophage cell line was cultured in RPMI 1640 medium containing 10% FBS and 1% penicillin-streptomycin in a 5% CO2 humidified atmosphere. For transfection experiments, cells were seeded onto six-well plates, and cultured for 24 h to be 50–60% confluent on the day of transfection. The concentrations of compounds included 0–1,000 ng/ml LPS (Sigma-Aldrich), 0–100 ng/ml IL-10 (R&D Systems, Minneapolis, MN), 0–20 μM SP600125 (S-7979; LC, Woburn, MA), 10 μM U0126 (InvivoGen, San Diego, CA), 10 μM SB203580 (InvivoGen), and 50 mM BAY11-7082 (Santa Cruz Biotechnology, Dallas, TX). DMSO (Sigma-Aldrich) was used as a vehicle control.
RNA preparation and quantitative PCR.
An RNeasy kit (QIAGEN, Germantown, MD) was used to extract total RNA from cells according to the manufacturer's instructions. One hundred nanograms of mRNA was used for cDNA synthesis with dNTP and oligo primer using SuperScript III (Invitrogen, Waltham, MA) first-strand synthesis system for reverse transcription PCR (RT-PCR) per the manufacturer's protocol. Real-time PCR was performed with the same amount of cDNA in a total 25-μl-volume reaction using iQ SYBR Green supermix (Bio-Rad, Hercules, CA) and the QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's instructions as previously described (30). Amplification of β-actin was performed in the same reaction for respective samples as internal control. Each experiment was done in triplicates. The cycling conditions were as follows: 2 min at 50°C and 10 min at 95°C followed by 39 cycles at 95°C for 15 s, 60°C for 1 min, 95°C for 10 s, and 65°C for 5 s. Primers used for this study are listed in Table 1. Levels of mRNA were calculated using the 2−ΔΔCt threshold cycle method and normalized to those of β-actin mRNA.
Table 1.
Mouse primers used in RT-qPCR analysis
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| β-Actin | AGAGGGAAATCGTGCGTGAC | CAATAGTGATGACCTGGCCGT |
| IL-22 | CTGAGAAATGCTTGCGTCTG | TCCATTCAGGGATCTTATTGTTCT |
| JNK1 | GGAGGAACGAACTAAGAATGGA | CATTGACAGACGGCGAAGA |
| JNK2 | GGAGGAGCGAACTAAGAATGGA | CATTGACAGACGGCGAAGA |
| RORγt | TGAGGCCATTCAGTATGTGG | CTTCCATTGCTCCTGCTTTC |
Western blot.
Cells were lysed in RIPA buffer (Cell Signaling Technology, Danvers, MA). The samples were mixed with 1× loading buffer [1 M Tris·HCl, pH 6.8, with 2.0% sodium dodecyl sulfate (SDS), 10% glycerol, 0.1 M dithiothreitol, and 0.2% bromophenol blue] and boiled for 15 min, and proteins were separated by SDS-PAGE. The separated proteins in the gels were electrophoretically transferred onto a nitrocellulose membrane at 100 V for 60 min. The blotted membrane was probed with primary antibodies, and GAPDH (Thermo Fisher Scientific) was used as an internal control. Immunoreactive bands were visualized using horseradish peroxidase-conjugated secondary antibody and the enhanced chemiluminescence system (Santa Cruz Biotechnology). All experiments were performed in triplicates, and protein quantitation was done by densitometry.
siRNA knockdown of JNK and IL-22.
Small interfering RNA (siRNA) transfection was performed using mouse ON-TARGETplus MAPK8 (JNK1) or MAPK9 (JNK2) or IL-22 siRNA (Dharmacon, GE Healthcare, Lafayette, CO) according to the manufacturer’s protocol. Knockdown efficiency was determined by real-time PCR for JNK1, JNK2, and IL-22 mRNA or by Western blot analysis.
Caspase-3/7 assay.
Caspase activation and inhibition in response to ethanol and IL-22 were monitored using Apo-ONE (Promega, Fitchburg, WI) kit and using a Synergy H1 multiplate reader (Bio-Tek Instruments, Winooski, VT; Ref. 26).
TUNEL staining.
The isolated primary hepatocytes were seeded onto a Lab-Tek Chamber Slide System (Nunc, Waltham, MA) overnight and then treated with condition media from IL-10-treated macrophages (0.2 ng/ml) alone or combined with ethanol (100 mM) for 48 h. Cells were washed and incubated with 4% paraformaldehyde solution for 1 h at room temperature and permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 2 min on ice. Then, cells were incubated with TdT-mediated dUTP nick-end labeling (TUNEL) reaction mixture (Roche Diagnostics) in a humidified atmosphere for 1 h. The samples were visualized with Zeiss LSM 5 PASCAL confocal microscope, and images were processed with LSM Image software. TUNEL areas were quantified using ImageJ software.
IL-22 receptor neutralization.
IL-22 receptor blocking on hepatocytes was achieved by utilizing neutralizing antibody directed against IL-22 receptor (1 µg/ml; IL-22R1; R&D Systems). IL-22 receptor-specific neutralizing or control IgG antibody was added 4 h before the addition of recombinant IL-22. Hepatocytes were subjected to ethanol treatment, and caspase activation was monitored using the methods described earlier (29).
Human sera IL-22 and other chemokines/cytokines assay.
Samples were collected from Mayo Clinic, Indiana University, and Virginia Commonwealth University through the Translational Research and Evolving Alcoholic Hepatitis Treatment (TREAT) Consortium. Clinical and demographic information about this cohort has been recently published (18). This study obtained ethics approval from the institutional review board, and patients' consents were obtained before study. Sera samples were stored in individual blood collection tubes and kept at −80°C until required. Sera IL-22 levels were assayed by a bead-based multiplex ELISA from patients with alcoholic hepatitis, healthy controls, and chronic heavy alcohol intake but no liver disease.
Statistical analysis.
Results are expressed as means ± SE from three or more independent experiments. Two-tailed Student’s t-test or ANOVA was used to test the statistical significance between groups as appropriate. A P value of <0.05 was considered as statistically significant.
RESULTS
Macrophages produce IL-22 with upregulation in response to IL-10.
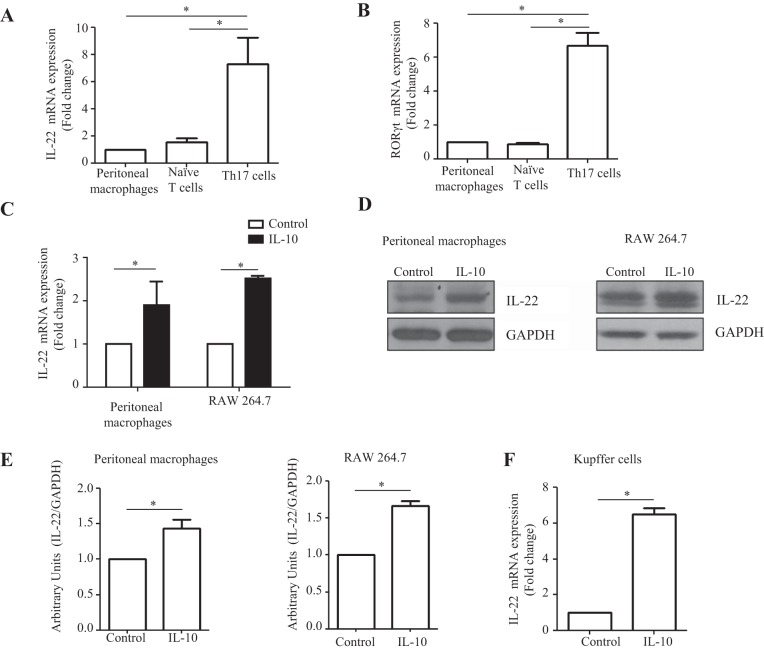
We first examined whether IL-22 production could be detected from macrophages. We isolated mouse peritoneal macrophages, which were recently showed to contribute to the recruited hepatic macrophage pool (32). Induced differentiation of naïve T cells to Th17 cells were used as a positive control for IL-22 production (16). Similar levels of IL-22 mRNA levels were detected in peritoneal macrophages and naïve T cells, which were lower by severalfold compared with Th17 cells (P < 0.05; Fig. 1A). RORγt marker was assayed to confirm Th17 cell phenotype (Fig. 1B). In addition, IL-22 mRNA and protein were detected from both primary murine peritoneal macrophages and macrophage cell line RAW 264.7 cells using PCR and immunoblot analysis, respectively (Fig. 1, C–E). Given IL-10 is a tissue-reparative anti-inflammatory cytokine (14), we treated macrophages with IL-10 recombinant protein (0.2 ng/ml) to determine whether it may upregulate IL-22 production from macrophages. IL-22 expression at mRNA level was twofold higher in IL-10-induced macrophages compared with control macrophages (P < 0.05; Fig. 1C). Similar results were obtained in murine primary Kupffer cells where IL-22 mRNA levels increased sixfold in response to IL-10 stimulation (Fig. 1F). These data indicate that macrophages produce IL-22 and that its production is upregulated by the reparative cytokine IL-10.
Fig. 1.
Macrophages produce IL-22 with upregulation in response to IL-10. A: mouse peritoneal macrophages and naïve T cells were isolated and naïve T cells were differentiated for 5 days in the presence of cytokines that promote differentiation toward Th17 cells. IL-22 mRNA levels were assayed using RT-quantitative PCR (RT-qPCR). The fold change of mRNA levels of IL-22 from macrophages and differentiated Th17 cells was calculated relative to the basal mRNA expression from naïve unstimulated peritoneal macrophage. B: RORγt marker mRNA level was assayed using RT-qPCR. C: mouse peritoneal macrophages were isolated and cultured in 12-well plates for 12 h and then treated with IL-10 (0.2 ng/ml) recombinant protein for 10 h. RAW 264.7 cells were seeded in 6-well plates and cultured overnight and then treated with IL-10 (0.2 ng/ml) protein for 10 h. IL-22 mRNA levels were determined by RT-qPCR. D: IL-22 protein levels were assayed in IL-10-induced macrophages by Western blot. GAPDH was used as an internal control. E: densitometry quantification is shown. F: liver resident primary macrophages were isolated with a 2-step perfusion-based method. After isolation, the macrophages were subjected to treatment with IL-10, where significantly enhanced IL-22 mRNA expression was observed with IL-10 stimulation compared with control as quantified by RT-qPCR (n = 3). Values represent means ± SE; *P < 0.05.
Macrophage-derived IL-22 production is downregulated by LPS stimulation.
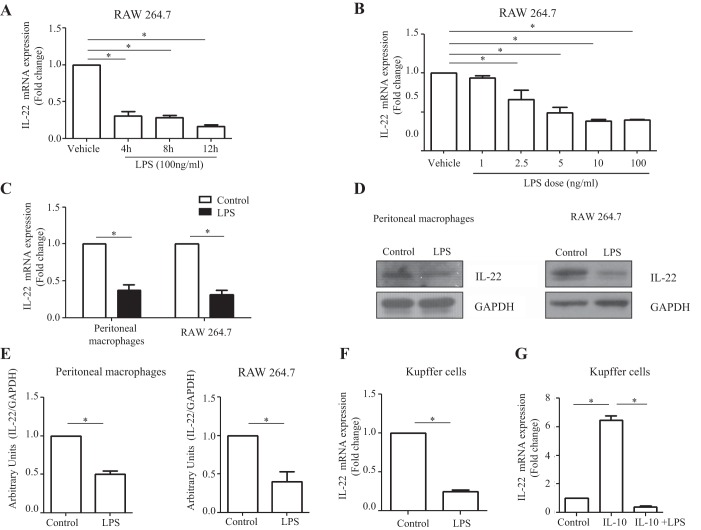
After establishing that macrophages produced IL-22, we next sought to determine whether the proinflammatory LPS, which is commonly elevated and a macrophage activation-associated factor in patients with alcoholic hepatitis, has any effect on IL-22 expression. We examined dose and time kinetics of the IL-22 expression under RAW 264.7 macrophage stimulation with LPS, which revealed a dose- and time-dependent reduction of IL-22 mRNA levels on LPS stimulation (Fig. 2, A and B). Following LPS stimulation, IL-22 expression at both mRNA and protein levels was also significantly decreased in primary peritoneal macrophages (Fig. 2, C–E). We observed similar repression of IL-22 mRNA levels when Kupffer cells were subjected to LPS exposure (Fig. 2F). When IL-10-treated Kupffer cells were further subjected to a secondary challenge with LPS, the IL-10-mediated IL-22 upregulation was lost (Fig. 2G). These results further indicate that LPS downregulates macrophage-derived IL-22 production. Taken together, they also support the concept that modulation of IL-22 with IL-10 and LPS shares a common JNK signaling pathway (n = 3; P < 0.05).
Fig. 2.
Macrophage-derived IL-22 production is downregulated by LPS stimulation. A: RAW 264.7 cells were cultured in 12-well plates overnight and treated with LPS (100 ng/ml) for 4, 8, and 12 h. IL-22 mRNA levels were assayed by RT-qPCR. B: RAW 264.7 cells were seeded in 6-well plates, cultured overnight, and then treated with varying doses of LPS (1, 2.5, 5, 10, and 100 ng/ml) for 8 h. IL-22 mRNA levels were detected by RT-qPCR. C: mouse peritoneal macrophages were isolated and cultured in 12-well plates overnight and then treated with LPS (100 ng/ml) for 10 h. RAW 264.7 cells were seeded in 6-well plates and cultured overnight and then treated with LPS (100 ng/ml) for 10 h. IL-22 mRNA levels were assayed using RT-qPCR. D: IL-22 protein levels were detected in LPS-stimulated macrophages by Western blot. GAPDH was assessed as a leading control. E: densitometric quantification is shown. F: murine Kupffer cells were isolated with a 2-step perfusion-based method and cultured overnight in 24-well plates. Following culture, Kupffer cells were subjected to treatment with LPS (100 ng/ml). LPS treatment significantly reduced IL-22 mRNA expression compared with control cells as quantified by RT-qPCR analysis (n = 3). G: Kupffer cells were stimulated with IL-10 alone or a combination of IL-10 treatment (0.10 ng/ml) followed by LPS (10 ng/ml) treatment. Cells were then cultured for 4 h, and IL-22 mRNA expression was assayed by RT-qPCR. IL-10 treatment significantly enhanced IL-22 mRNA expression; however, LPS stimulation abrogated the effects of IL-10 (n = 3). Values represent means ± SE; *P < 0.05.
IL-22 downregulation in LPS-stimulated macrophage occurs via JNK pathway.
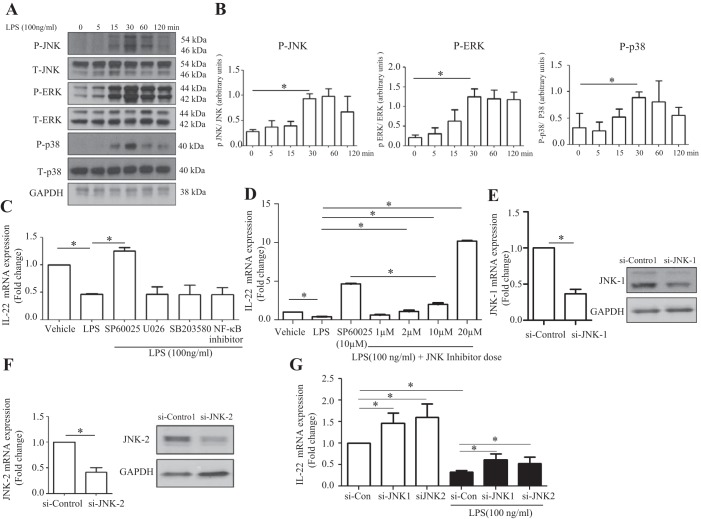
Next, we explored the regulatory mechanism of IL-22 downregulation in the macrophage response to LPS. LPS activates macrophages via Toll-like receptor 4 (TLR4)-mitogen-activated protein kinase (MAPK) pathway (2, 6, 25). To explore whether MAPKs (JNK, ERK, and p38) were involved in LPS attenuation of IL-22, we examined phosphorylation of these MAPK family members. As illustrated in Fig. 3A and B, LPS significantly induced phosphorylation of JNK, ERK, and p38 proteins in RAW 264.7 cells. Furthermore, IL-22 reduction in LPS-induced macrophages was reversed by a pharmacological JNK inhibitor (SP600125; 10 μM) but not by ERK, p38, and NF-κB inhibitors (Fig. 3, C and D). The effects of the JNK inhibitor on IL-22 production were dose-dependent and not only blocked LPS-induced inhibition, but also led to a further 5.1-fold increase in IL-22 production (Fig. 3, C and D). Next, we sought to clarify the JNK isoform involved in this effect on IL-22. JNK1 and JNK2 forms are expressed in many cells, including macrophages (10, 25). To explore the differential effect of JNK1 and JNK2 on IL-22 downregulation in LPS-stimulated macrophages, JNK1 or JNK2 was knocked down in RAW 264.7 cells using siRNA. Figure 3, E and F, confirms JNK1 and JNK2 knockdown at mRNA and protein levels. Figure 3G showed that both JNK1 and JNK2 knockdown significantly reversed IL-22 downregulation in LPS-stimulated RAW 264.7 cells. JNK1 form induced 1.7-fold increase in IL-22 suppression, whereas JNK2 form knockdown achieved a 2.1-fold increase. In summary, these results indicate that both JNK1 and JNK2 mediate the LPS-induced downregulation of IL-22 in macrophages.
Fig. 3.
IL-22 downregulation in LPS-stimulated macrophages occurs via JNK pathway. A: RAW 264.7 cells were cultured in 6-well plates overnight and then treated with LPS (100 ng/ml) for 0, 5, 15, 30, 60, and 120 min. Total (T-) and phosphorylated (P-) JNK, ERK, and p38 were assayed by Western blot. GAPDH was detected as a loading control. B: densitometric quantification from multiple experiments showed the changes of phosphorylation of JNK, ERK, and p38. C: RAW 264.7 cells were seeded in 6-well plates, cultured overnight, and then pretreated with JNK, ERK, p38, or NF-κB inhibitors for 1 h. After pretreatment, cells were coincubated with LPS (100 ng/ml) for 5 h. RT-qPCR was used to determine IL-22 mRNA levels. D: RAW 264.7 cells were seeded in 6-well plates, cultured overnight, and then pretreated with JNK inhibitor (1, 2, 10, or 20 μM) for 1 h. After pretreatment with JNK inhibitor, cells were coincubated with LPS (100 ng/ml) for 5 h. IL-22 mRNA levels were assayed by RT-qPCR. E–G: RAW 264.7 cells were cultured in 6-well plates for 8 h and then transfected with JNK1 or JNK2 siRNA (si-) or control siRNA. Thirty-six hours posttransfection, LPS (100 ng/ml) was added into the culture media and cells were coincubated with LPS for 10 h. JNK1 and JNK2 mRNA and protein levels were assayed by RT-qPCR and Western blot, respectively. IL-22 mRNA levels were determined by RT-qPCR. Values represent means ± SE; *P < 0.05.
Macrophage-derived IL-22 protects hepatocytes from alcohol-induced damage.
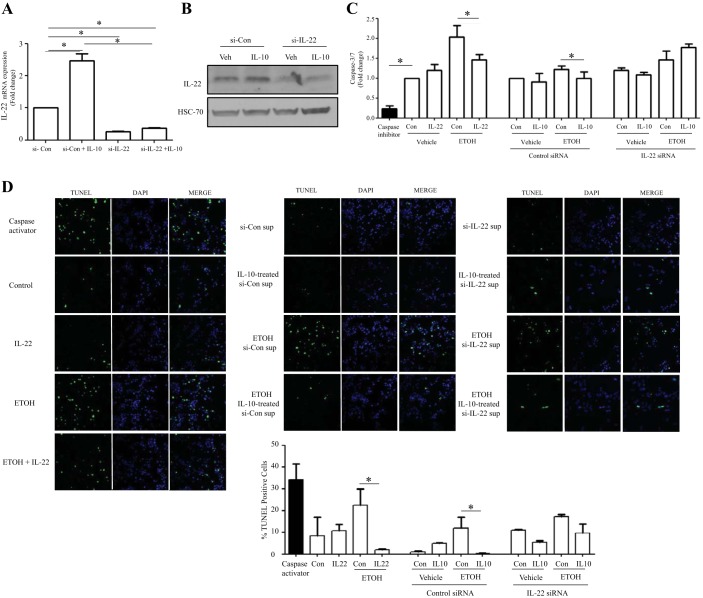
Next, we examined whether macrophage-derived IL-22 can play a hepatoprotective role against alcohol-induced damage in vitro, especially hepatocyte apoptosis. For these experiments, we harvested conditioned media from macrophages in which IL-22 production was manipulated by IL-22 siRNA and/or IL-10 stimulation. Indeed, IL-22 siRNA markedly reduced IL-22 mRNA and protein levels from RAW 264.7 cells at basal levels and after IL-10 stimulation (Fig. 4, A and B). Next, we tested caspase-3/7 activity and TUNEL expression in alcohol-induced hepatocytes incubated with the various macrophage-derived conditioned media. As illustrated in Fig. 4, C and D, incubation of hepatocytes with recombinant IL-22 protein (10 ng/ml) markedly reduced alcohol-induced caspase-3/7 activity and TUNEL-positive hepatocytes as a positive control, indicating that IL-22 protects against alcohol-induced hepatocyte death. Conditioned media from IL-10-treated macrophages with control siRNA transfection also attenuated alcohol-induced hepatocyte caspase-3/7 activity and TUNEL staining (Fig. 4, B and C). This effect on hepatocytes was diminished when using conditional media from IL-10-treated-macrophages with IL-22 knockdown (Fig. 4, C and D). These results indicate that macrophage-derived IL-22 can protect hepatocytes from alcohol-induced apoptosis and increased survival.
Fig. 4.
Macrophage-derived IL-22 protects hepatocytes from alcohol-induced death. A: RAW 264.7 cells were seeded in 60-mm dishes and transfected with IL-22 siRNA or control siRNA (si-Con). Twenty-four hours posttransfection, IL-10 (0.2 ng/ml) was added into the culture media, cells were coincubated for 36 h, cell lysates were collected for IL-22 analysis, and supernatants were stored for primary hepatocyte incubation. IL-22 mRNA level was assayed by RT-qPCR. B: IL-22 protein level was detected by Western blot. HSC70 was detected as a loading control. Veh, vehicle control. C: mouse primary hepatocytes were isolated, seeded in 96-well plates, and cultured overnight. Cells were treated with the recombinant IL-22 protein or conditional media and/or coincubated with ethanol (ETOH; 100 mM) for 24 h. Caspase inhibitor was used as a negative control. Caspase-3/7 activity was detected using Apo-ONE kit. D: primary mouse hepatocytes were seeded in 8-well chamber slides, cultured overnight, and then treated with the recombinant IL-22 protein or conditional media and/or coincubated with ethanol (100 mM) for 48 h. Caspase activator was used as a positive control. In situ cell death detection kit was used to detect TUNEL expression. Slides were analyzed by laser confocal microscopy; 15–20 images in each well were taken and then quantified and analyzed. Values represent means ± SE; *P < 0.05. DAPI, 4′,6′-diamidino-2-phenylindole; sup, supernatant.
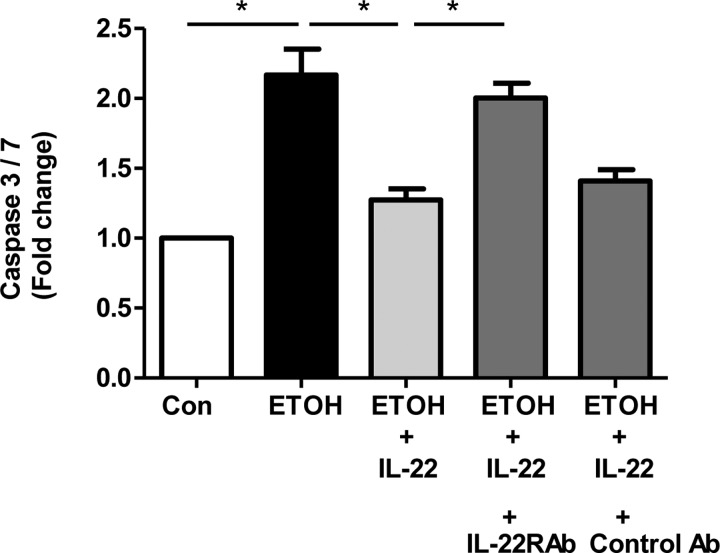
To determine whether the caspase inhibitory function of IL-22 is specific and dependent on binding of IL-22 with its receptor, we utilized a neutralizing antibody directed against IL-22 receptor. We subjected mouse primary hepatocytes to ethanol/IL-22 treatment. Ethanol treatment was associated with significantly enhanced caspase-3/7 activation compared with control cells, whereas pretreatment of cells with recombinant IL-22 abolished caspase activation by ethanol (Fig. 5). In presence of IL-22 neutralizing antibody, IL-22-mediated caspase inhibitory function was lost in the ethanol-treated cells (Fig. 5). These observations taken together suggest that IL-22-mediated protective functions in hepatocytes are specifically mediated by binding with its receptor.
Fig. 5.
IL-22 receptor neutralization blocks IL-22 protection from alcohol-induced injury. Mouse primary hepatocytes were isolated and cultured in a 96-well plate overnight. Primary hepatocytes treated with ethanol (100 mM) exhibited significantly enhanced caspase-3/7 activation compared with control cells as determined using Apo-ONE Homogeneous Caspase-3/7 activity kit. When hepatocytes were pretreated with recombinant IL-22, there was a significant reduction of ethanol-induced caspase activation (*P < 0.05). IL-22 receptor-α (IL-22R1) blockage with neutralizing antibody (Ab) demonstrated that the caspase inhibitory effect of IL-22 in ethanol-treated cells was lost (n = 3; *P < 0.05).
IL-22 levels are increased in patients with AH.
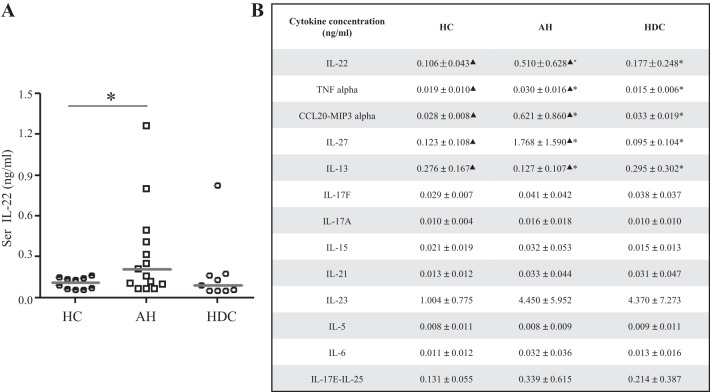
Finally, we sought to examine serum levels of IL-22 in patients with AH. Samples were collected from normal controls, heavy drinkers without liver disease, and patients with AH. Clinical and demographic information about this cohort has been recently published (18). Sera IL-22 levels were assayed by multiplex ELISA (Fig. 6A). Unexpectedly, sera IL-22 levels were significantly increased in patients with AH compared with heavy drinkers and healthy control. IL-22 levels increased about sixfold in patients with severe AH compared with healthy control patients and over threefold compared with heavy drinkers without liver disease (Fig. 6A). No significant differences in IL-22 levels were observed between alcoholics and normal healthy controls. Notable from the multiplex assay was that TNFα levels were also significantly higher in severe AH compared with healthy control and heavy drinkers (Fig. 6B). We also found that the lymphocyte regulator IL-27 and chemokine CCL20/MIP3α were significantly increased in the severe AH group, whereas the anti-inflammatory cytokine IL-13 was significantly decreased (Fig. 6B). IL-10 was also examined, although most samples were below the detection range in the conditions we used for the assay. In summary, these results indicate that in patients with AH, not only are inflammatory cytokines increased, but the reparative cytokine IL-22 is elevated as well, suggestive of deficiency in IL-22 binding with its receptor.
Fig. 6.
IL-22 levels are increased in patients with AH (alcoholic hepatitis). A: sera (Ser) IL-22 levels in AH (n = 15), heavy drinkers control (HDC; n = 9), and healthy control (HC; n = 10) were detected by multiplex ELISA. Values represent means ± SE, *P < 0.05. B: additional inflammation-related cytokines in AH, HDC, and HC were measured by multiplex ELISA. Values represent means ± SE, ▲P < 0.05 or *P < 0.05. ▲Statistical difference between AH and HC. *Statistical difference between group AH and HDC.
DISCUSSION
The major findings in the present study are that 1) macrophages produce IL-22 especially in response to protective cytokines such as IL-10, 2) IL-22 is downregulated by LPS in a JNK-dependent manner, and 3) macrophage-derived IL-22 protects hepatocytes from ethanol-induced apoptosis. Figure 6 summarizes these key findings, which are discussed in greater detail below.
First, we demonstrate that IL-22 is produced by mouse primary macrophages. Although T cells can produce high levels of IL-22, resident and recruited macrophages may account for ≤20% of nonparenchymal cells in the liver, and thus their contribution to local IL-22 production could be significant (23). Further experiments indicate that IL-22 expression is downregulated in LPS-stimulated macrophage in a dose-dependent manner. Thus, in AH, gut-derived LPS may limit IL-22 production and abolish the hepatoprotective function of macrophage-derived IL-22, thereby promoting liver injury (12, 17, 24). Conversely, IL-22 is upregulated in IL-10-induced macrophages, and IL-10 is one of the most important anti-inflammatory cytokines and its production may promote liver repair (4, 21). We anticipate that reparative macrophages could enhance anti-inflammatory capability and hepatic protection from alcoholic injury in part through IL-22 production.
LPS activates macrophages through the TLR4 pathway with downstream activation of MAPKs (3, 6, 25). Our study demonstrates that LPS promotes the phosphorylation of multiple MAPKs (ERK, JNK, and p38) but that IL-22 reduction in response to LPS is reversed only by a pharmacological JNK inhibitor and not by ERK, p38, and NF-κB inhibitors. JNK has three isoforms; JNK1 and JNK2 are expressed in liver parenchymal cells, whereas JNK3 is restricted to the central nervous system (10, 25). Current controversies relate to redundant vs. distinct functions of JNK isoforms (33). In our study, silencing of JNK1 or JNK2 could each partially yet significantly prevent LPS-induced IL-22 inhibition. This would suggest both redundant and distinct functions of the isoforms in the JNK-induced regulation of macrophage IL-22.
Hepatocyte apoptosis plays a central role in hepatocyte death in alcoholic liver disease (8, 35). IL-22 is believed to activate hepatic signal transducer and activator of transcription 3 (STAT3) and thereby facilitate proliferation and antiapoptosis responses in hepatocytes and ameliorate alcohol-induced liver injury (15). Our study verifies that treatment of IL-22 recombinant protein promotes antiapoptosis in ethanol-induced hepatic injury and, more importantly, demonstrates that conditioned media from IL-10-stimulated macrophages can attenuate ethanol-induced hepatocyte apoptosis in an IL-22-dependent manner. This finding provides evidence that macrophage-derived IL-22 production prevents ethanol-induced hepatocyte apoptosis and may be biologically relevant and potentially beneficial in alcoholic liver disease.
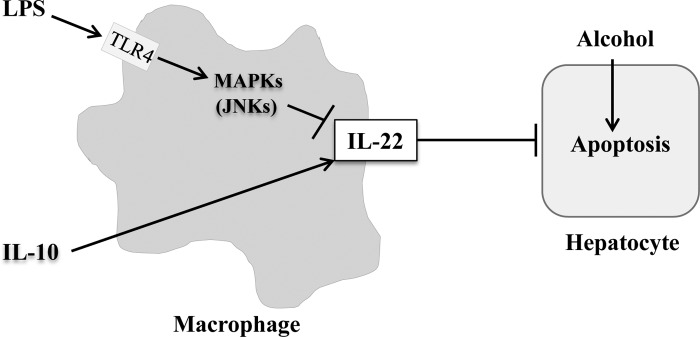
In the present study, we unexpectedly found sera IL-22 levels are elevated in patients with AH compared with healthy controls and heavy drinkers. Additional analyses from our multiplex ELISA demonstrate that TNFα, IL-27, and CCL20/MIP3α are significantly increased in patients with AH compared with healthy controls and heavy drinkers. Why are IL-22 levels elevated in patients with AH despite the fact that LPS downregulates IL-22 and is thought to drive disease pathogenesis in these patients? First, severe AH is associated with bacterial infection, and it is known bacterial infection elevates IL-22 levels (28). Second, it is possible that IL-10-stimulated M2 macrophages may predominate in AH, contributing to increased IL-22 levels (12, 23, 31). A model of macrophage-derived IL-22 in attenuating alcohol-induced hepatocyte cell death is represented in Fig. 7. Finally, and not mutually exclusively, the elevated sera IL-22 levels in patients with AH may indicate that AH is an IL-22-resistant state. Our ongoing clinical trial (NCT02655510 at https://clinicaltrials.gov/) will further explore how sera IL-22 levels undergo changes in patients with AH and effects on clinical phenotype, following recombinant IL-22 treatment and/or other therapies.
Fig. 7.
Model of macrophage-derived IL-22 in attenuating alcohol-induced hepatocyte cell death. Macrophages can produce IL-22, which is reciprocally regulated by IL-10 and LPS. LPS downregulates IL-22 in macrophages via JNK-dependent manner. Macrophage-derived IL-22 protects hepatocytes from alcohol-induced cell death.
In summary, our study elucidates new regulatory mechanisms and potential hepatoprotective functions of macrophage-derived IL-22 and provides a framework for further work on IL-22 in both human and animal studies.
GRANTS
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grants U01-AA-021788 and R01-AA-21171 (to V. H. Shah). We thank the generous support of the National Institute of Diabetes and Digestive and Kidney Diseases-funded Mayo Clinic Center for Cell Signaling (P30-DK-084567), in particular, the Clinical Core and the Optical Microscopy Core for their support. Funding support from Center for Regenerative Medicine, Mayo Clinic to V. K. Verma is acknowledged.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.L., V.K.V., G.J.G., B.G., and V.H.S. conceived and designed research; Y.L. and V.K.V. performed experiments; Y.L. and V.K.V. analyzed data; Y.L., V.K.V., and V.H.S. interpreted results of experiments; Y.L. and V.K.V. prepared figures; Y.L. drafted manuscript; Y.L., V.K.V., H.M., G.J.G., B.G., and V.H.S. edited and revised manuscript; P.S.K., A.S., and N.C. provided study material; Y.L., V.K.V., and V.H.S. approved final version of manuscript.
REFERENCES
- 1.Bedoya SK, Wilson TD, Collins EL, Lau K, Larkin J 3rd. Isolation and Th17 differentiation of naïve CD4 T lymphocytes. J Vis Exp 79: e50765, 2013. doi: 10.3791/50765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boonstra A, Rajsbaum R, Holman M, Marques R, Asselin-Paturel C, Pereira JP, Bates EE, Akira S, Vieira P, Liu YJ, Trinchieri G, O’Garra A. Macrophages and myeloid dendritic cells, but not plasmacytoid dendritic cells, produce IL-10 in response to MyD88- and TRIF-dependent TLR signals, and TLR-independent signals. J Immunol 177: 7551–7558, 2006. doi: 10.4049/jimmunol.177.11.7551. [DOI] [PubMed] [Google Scholar]
- 3.Cubero FJ, Zoubek ME, Hu W, Peng J, Zhao G, Nevzorova YA, Al Masaoudi M, Bechmann LP, Boekschoten MV, Muller M, Preisinger C, Gassler N, Canbay AE, Luedde T, Davis RJ, Liedtke C, Trautwein C. Combined activities of JNK1 and JNK2 in hepatocytes protect against toxic liver injury. Gastroenterology 150: 968–981, 2016. doi: 10.1053/j.gastro.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol 5: 683, 2015. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33: 747–785, 2015. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol 27, Suppl 2: 89–93, 2012. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585, 2011. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut 54: 1024–1033, 2005. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansson M, Silverpil E, Lindén A, Glader P. Interleukin-22 produced by alveolar macrophages during activation of the innate immune response. Inflamm Res 62: 561–569, 2013. doi: 10.1007/s00011-013-0608-1. [DOI] [PubMed] [Google Scholar]
- 10.Himes SR, Sester DP, Ravasi T, Cronau SL, Sasmono T, Hume DA. The JNK are important for development and survival of macrophages. J Immunol 176: 2219–2228, 2006. doi: 10.4049/jimmunol.176.4.2219. [DOI] [PubMed] [Google Scholar]
- 11.Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, Misic AM, Bartow-McKenney C, Larson DM, Pavan WJ, Eberl G, Grice EA, Veiga-Fernandes H. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535: 440–443, 2016. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol 34: 216–223, 2013. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju C, Mandrekar P. Macrophages and alcohol-related liver inflammation. Alcohol Res 37: 251–262, 2015. [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaratani H, Tsujimoto T, Douhara A, Takaya H, Moriya K, Namisaki T, Noguchi R, Yoshiji H, Fujimoto M, Fukui H. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm 2013: 495156, 2013. doi: 10.1155/2013/495156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52: 1291–1300, 2010. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong X, Feng D, Mathews S, Gao B. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol 28, Suppl 1: 56–60, 2013. doi: 10.1111/jgh.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11: 750–761, 2011. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 18.Liangpunsakul S, Puri P, Shah VH, Kamath P, Sanyal A, Urban T, Ren X, Katz B, Radaeva S, Chalasani N, Crabb DW; Translational Research and Evolving Alcoholic Hepatitis Treatment Consortium . Effects of age, sex, body weight, and quantity of alcohol consumption on occurrence and severity of alcoholic hepatitis. Clin Gastroenterol Hepatol 14: 1831–1838.e3, 2016. doi: 10.1016/j.cgh.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut 56: 1124–1131, 2007. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol 9: 641–649, 2008. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23: 549–555, 2002. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 22.Menon KV, Gores GJ, Shah VH. Pathogenesis, diagnosis, and treatment of alcoholic liver disease. Mayo Clin Proc 76: 1021–1029, 2001. doi: 10.4065/76.10.1021. [DOI] [PubMed] [Google Scholar]
- 23.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 43, Suppl 1: S54–S62, 2006. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 24.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50: 638–644, 2009. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology 143: 307–320, 2012. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo YS, Kwon JH, Yaqoob U, Yang L, De Assuncao TM, Simonetto DA, Verma VK, Shah VH. HMGB1 recruits hepatic stellate cells and liver endothelial cells to sites of ethanol-induced parenchymal cell injury. Am J Physiol Gastrointest Liver Physiol 305: G838–G848, 2013. doi: 10.1152/ajpgi.00151.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 12: 387–400, 2015. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 28.Valeri M, Raffatellu M. Cytokines IL-17 and IL-22 in the host response to infection. Pathog Dis 74: ftw111, 2016. doi: 10.1093/femspd/ftw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, Gores GJ, Shah VH. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol 64: 651–660, 2016. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma VK, Taneja V, Jaiswal A, Sharma S, Behera D, Sreenivas V, Chauhan SS, Prasad HK. Prevalence, distribution and functional significance of the −237C to T polymorphism in the IL-12Rβ2 promoter in Indian tuberculosis patients. PLoS One 7: e34355, 2012. doi: 10.1371/journal.pone.0034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, Lotersztajn S, Pavoine C. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 59: 130–142, 2014. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Kubes P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165: 668–678, 2016. doi: 10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol 19: 142–149, 2007. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Witte E, Witte K, Warszawska K, Sabat R, Wolk K. Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev 21: 365–379, 2010. doi: 10.1016/j.cytogfr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, Trinchet JC, Beaugrand M, Guillet JG, Guettier C. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol 34: 254–260, 2001. doi: 10.1016/S0168-8278(00)00047-7. [DOI] [PubMed] [Google Scholar]